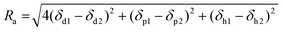How to make membrane distillation greener: a review of environmentally friendly and sustainable aspects
Emilia
Gontarek-Castro
 *a and
Roberto
Castro-Muñoz
b
*a and
Roberto
Castro-Muñoz
b
aDepartment of Environmental Technology, Faculty of Chemistry, University of Gdansk, 80-308 Gdansk, Poland. E-mail: emilia.gontarek-castro@ug.edu.pl
bFaculty of Civil and Environmental Engineering, Department of Sanitary Engineering, Gdansk University of Technology, 11/12 Narutowicza St., 80-233 Gdansk, Poland
First published on 16th November 2023
Abstract
There is an urgent need for the development of new water resources in order to solve the problem of the world's growing demand for clean water. Membrane distillation (MD) is a promising alternative to conventional seawater desalination. Although MD itself is often defined as sustainable desalination technology, there are many aspects within the membrane manufacture and process operation that make it far from being green. For instance, non-biodegradable polymers, toxic solvents and fluoroalkyl silanes are typical chemicals that unfortunately are used in membrane fabrication protocols. Additionally, the huge amount of wastewater generated from membrane fabrication processes makes solvent-free methods more attractive and desirable for extensive investigation. Apart from this, the low energy efficiency of the MD process can be effectively overcome by integrating MD systems with low-grade waste heat. This review critically addresses and discusses the recent advances in methods and strategies to improve the sustainability of MD technology, which is not a common scope of study among the research community. Here, our attention has been devoted to the main aspects of MD membrane fabrication, such as polymers, solvents (and their costs), nonsolvents, additives, solvent-free fabrication procedures, fluoro-free post-modification, and MD operation (energy consumption). This review intends to introduce inspiration for membrane scientists for the development of the next-generation MD process, by promoting the sustainable transformation of today's approaches into a greener way. In this latter scenario, we provide some timely considerations that could be followed by the researchers in the field.
1. Introduction
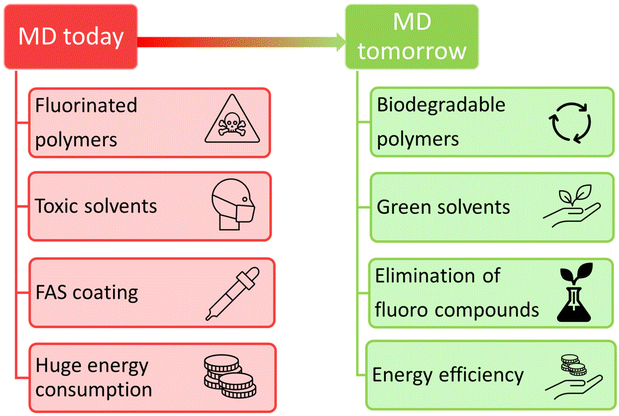
Over the past century, the population of the world has increased, as has the demand for water. This global challenge for water supply will become even more serious due to further expansion in the population and economic growth, followed by a continuing increase in demands on water resources. The World Water Council estimated that by 2030, a few billion people will suffer from water scarcity or poor water quality.1 Thus, it is not surprising that governments and industry have tried to develop alternative water sources, water recycling, water imports, and desalination to face the concern of the world's growing demand for clean water. In general, desalination techniques can be divided into two groups: thermal-based and membrane-based. The former ones, thermal technologies, including multi-stage flash, multi-effect distillation, and vapor compression distillation,2 require the supply of thermal energy to induce the evaporation of water molecules from seawater; the vapor is subsequently condensed to give drinking water. Despite its versatility and ease of operation, thermal desalination has one significant disadvantage, its high energy consumption, as it is a costly and environmentally unfriendly method. For this reason, alternative methods to thermal desalination are sought, mainly to reduce the costs of the process.Currently, among the most dynamically developing desalination techniques are membrane-based technologies, which are becoming more popular due to their lower energy expenditure, compact modular construction, possibility of scaling-up, lower environmental footprint, and the possibility of spending investment recovery.3 Among membrane-based technologies, the dominant one used for desalination (in terms of the amount of treated water) is reverse osmosis, which, according to the literature, produces up to 60% of the world's desalted water;4 however, both costly installation and maintenance represent the main drawbacks of this technology. This has encouraged researchers to seek new membrane desalination alternatives, such as membrane distillation (MD), which is a promising technique for seawater desalination. The driving force of the process is based on a vapor pressure gradient across the membrane. This gradient is induced by the temperature difference between the feed and permeate solutions. The solutions are separated by a hydrophobic microporous membrane that allows the diffusion of vapor while preventing the permeation of the aqueous phase. Water evaporates at the solid/liquid interface on the heated feed side of the membrane, then diffuses through the air trapped in the membrane pores, and finally condenses at the cooler permeate side. Simultaneously, non-volatile substances remain in the aqueous feed solution. MD seems to be an effective method of seawater desalination as it successfully deals with high salinity content, high oil content, and high surfactant content feed. Despite the great progress in MD membrane preparation and performance improvement, their fabrication approaches and implementation procedures still need to be revised in terms of toxicity and pollution generation.5,6 For instance, the typical materials used for membrane manufacture are perfluorocarbon-based non-biodegradable polymers, while toxic organic solvents are mostly applied at several membrane manufacturing stages, producing more than 50 billion liters of contaminated wastewater yearly and contributing to various health and environmental risks. Due to the fundamentals of the MD process, more and more emphasis is being placed on the fabrication of superhydrophobic or even omniphobic membranes; however, typical substances applied for the post-fabrication modification of membrane surface properties are toxic fluoroalkyl silanes (FAS). Another aspect is the energy expenditure; the low energy efficiency of the standalone MD system significantly reduces its potential application. Due to the constant growth of environmental pollution problems, improving the sustainability of MD and developing green manufacturing protocols are a must around all aspects. Fig. 1 illustrates a comparison of the current scenario of MD and the aim of pursuing the green and sustainable scenario soon. This latter panorama can be reached by following the updated “twelve principles of green chemistry”, developed by Anastas and Warner7 and proposed by the United States Environmental Protection Agency. The idea represents the design of safer chemical processes and products that reduce or eliminate the use or generation of hazardous chemicals.
In the light of green chemistry principles, the most often used definition of sustainable development is the one proposed by the Brundtland Commission in 1987 as “development that meets the needs of the present without compromising the ability of future generations to meet their own needs”.8 Thus, to be really “sustainable”, the MD process should not only be free of the use of hazardous substances in membrane fabrication but also in the operating step process. We believe that these aspects need to be reviewed and discussed exhaustively, and to the best of our knowledge, there has been no report discussing these aspects to date. Therefore, in this review, the latest advances related to the greener approaches of MD are described in detail. We highlight different ways to improve the sustainability of the membrane manufacturing process, such as the use of biodegradable polymers, green solvents and FAS-free modification. The strategies for the solvent-free fabrication are discussed together with the possibility of reducing the amount of solvents at various manufacturing stages. Finally, to reduce the energy consumption and make the MD process more competitive with reverse osmosis, the energy efficient routes offered by integrating MD systems are also addressed.
2. Polymer membrane fabrication
2.1. Biodegradable and perfluorocarbon-free polymers
For the MD process, only water-repellent membranes, such as the ones made of petroleum-based polymers (polytetrafluorethylene (PTFE) and polyvinylidene difluoride (PVDF)), or coated with perfluorosilanes, have been extensively investigated.9,10 In the case of fluorocarbon membranes, they are expensive and prone to partial wetting during long-term use, due to the interactions between the membrane surface and contaminants.11–13 These membranes owe their commercial interest to their suitable properties in terms of their high porosities, low thermal conductivities, stability and hydrophobic nature.14 Despite their many advantages, one of the main concerns of using and disposing of these polymer materials is their low biodegradability; this refers to materials which, once disposed of in nature, undergo degradation via microorganisms. Owing to their high thermal and chemical stability, they are resistant to microbial attack as their carbon linkages cannot be broken by enzymes and microorganisms. On the other hand, biodegradable polymers originate from three sources, plants (for example cellulose acetate and starch), animals (collagen and sericin), and sometimes synthetic resources (poly(butylene succinate) or poly(ε-caprolactone)). The application of these materials is rapidly growing, especially in the packaging industry; however, an increased interest is also observed in biodegradable membrane fabrication.15 Although efficient MD membranes made from biodegradable and low-cost materials are highly desired, they are still not commercially available.Recently, a few studies have proved the possibility of using cellulose for MD membrane preparation.16 Cellulose, which is a renewable polymer and found abundantly on Earth, can be extracted from various sources, including underutilized biomass feedstocks such as agricultural residues, recycled cellulosic products, and industrial waste. These fibers possess unique structural and mechanical properties, and they are abundant, cost-effective, and environmentally friendly. Thus, it has been found attractive to explore the use of cellulose fibers as a material to create permeable MD membranes. The presence of hydroxyl groups on the cellulose surface makes it hydrophilic; therefore, a major concern in applying this material for MD membrane preparation is to create a hydrophobic surface with a green and sustainable approach. Several studies demonstrate different approaches for the creation of hydrophobic cellulosic surfaces, such as chemical modification with fluoropolymers or silanes, and processing methods, namely chemical vapor deposition, plasma treatment, and electrospinning (see Table 1).
| Form of cellulose used | Fabrication method | Pore size (μm) porosity (%) | Thickness (μm) | Contact angle (deg) | Flux rejection | Pros and cons | Ref. |
|---|---|---|---|---|---|---|---|
| Microfibrillated cellulose | Vacuum filtration of microfibrillated cellulose with additives followed by hydrophobic/hydrophilic treatment | 0.50 | 295 ± 12 | 147 | 23.0 ± 0.06 kg L m−2 h−1 | + Membrane manufacturing based on papermaking techniques | 25 |
| 55.7% | 97.5% | - Large average pore size | |||||
| Cellulose acetate | Electrospinning of cellulose nanofibers followed with silica grafting and surface fluorination | 1 | 52 | 150.6 ± 4.0 | 46.3 L m−2 h−1 | + High permeate flux | 26 |
| 90.2% | ∼100% | + Strong antiwetting properties | |||||
| Cellulose nanocrystals | Electrospinning of PVDF-HFP/cellulose solution and hot pressing | ∼0.3 | 200–300 | 132.2 | 11.5 L m−2 h−1 | + Tensile strength improvement | 21 |
| 60–75% | 99% | - Porosity and hydrophobicity reduction | |||||
| Cellulose sheet | Polymer solution casting on cellulose sheet | ∼0.005 nm | 200 | 112 | 6.9 L m−2 h−1 | + Excellent long-term rejection | 23 |
| 75.1% | 99.9% | - Utilization of fluorinated polymers | |||||
| Microcrystalline cellulose | Coating electrospun polymer membranes with cellulose-based solution | 0.2–0.3 | 86–105 | 0 | 20.52 L m−2 h−1 | + High oil and salt rejection | 24 |
| 49.71% | 100% | - Multi-step preparation procedure | |||||
| - Nanoparticle leaching problem | |||||||
| Bacterial nanocellulose (BNC) | Hydrophobization of desired BNC gels via CVD using trichlorosilane | 0.115 | 257 ± 45 | 156 | 22.92 L m−2 h−1 | + High porosity | 17 |
| 98.0% | 99.95 | + Low thermal conductivity | |||||
| Wood cellulose fibers | The treatment of wood slices followed by their fluorination | 0.28 | 502 ± 35 | 144 | 20.8 ± 0.8 L m−2 h−1 | + Membrane preparation directly from wood | 22 |
| 89% | 99.8% | - High thickness |
For instance, Leitch et al.17 fabricated a novel fibrous nanocellulose aerogel membrane from bacterial nanocellulose, which met the requirements of the MD process (e.g., high porosity >98%, and low thermal conductivity <0.03 W m−1 K−1). Due to their isotropic fibrous structure and easily tunable fiber diameter between 15 nm and 7 μm, these biomembranes may become a natural substitute for electrospun polymer membranes.18,19 The authors compared the direct contact membrane distillation (DCMD) performance of nanocellulose aerogel membrane and commercial PVDF membrane. The high void fraction of nanocellulose aerogel membrane led to the reduction of conducted heat flux, thus improved membrane thermal efficiency and temperature polarization coefficient (see Fig. 2a and b). On the other hand, smaller average pore diameter and higher thickness, compared with the commercial PVDF membrane, increased the mass transport resistance of the nanocellulose aerogel membrane. Dizge et al.26 fabricated a superhydrophobic and oleophobic membrane by a three-step process: the preparation of cellulose nanofibers by electrospinning, modification with silica and surface fluorination. The membrane system displayed exceptional resistance to wetting and maintained a high and stable performance. The water vapor permeability against 1 M NaCl feed solution was found to be as high as 46.3 L m−2 h−1, which was 1.7 and 1.6 higher when compared with the water vapor flux rate of commercial available PVDF and PTFE membranes, respectively.
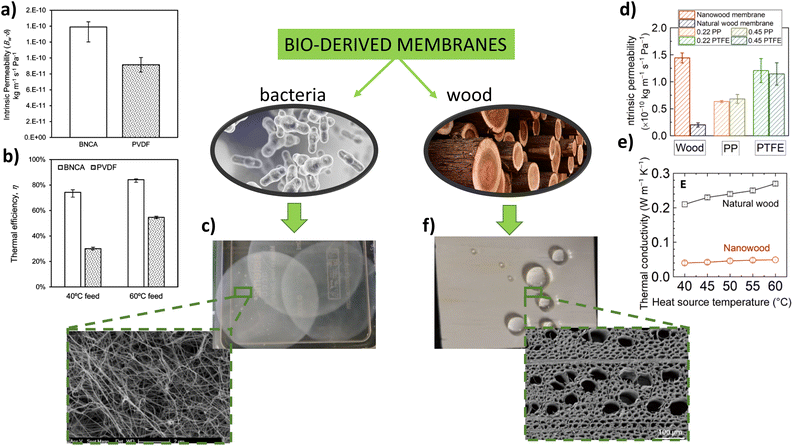 | ||
| Fig. 2 Bio-derived MD membranes and their performance, (a) enhanced permeability of bacteria-derived membranes over commercial phase-inversion PVDF membranes, (b) high thermal efficiency of bacteria-derived membranes, (c) bacteria-derived membrane and its cross section, (d) permeability of the nanowood and commercial membranes, (e) thermal conductivity of the wood and nanowood membranes, (f) nanowood membrane and its cross section. a–c reproduced from ref. 17 with permission from American Chemical Society, copyright 2016. d–f reproduced from ref. 22 with permission from American Association for the Advancement of Science, copyright 2019. | ||
Cellulose itself is the basic building block of plant fibers, proving mechanical support to specific growing parts of the plants. This molecule also provides mechanical integrity, which makes it an attractive material for membrane reinforcement.20 Lalia et al.21 fabricated polyvinylidenefluoride-co-hexafluoropropylene membranes containing different loadings of nanocrystalline cellulose (NCC) by the electrospinning technique. The polymer membrane modified with NCC was found to have 30% higher tensile strength and 45% higher Young's modulus values than that of the nonmodified one. Additionally, the incorporation of NCC into the membrane resulted in enhancement of its liquid entry pressure (LEP) from 19 psi up to 27 psi. This high LEP led to the effective DCMD performance with a water flux of 11.5 L m−2 h−1 and salt rejection of 99%. Hou et al.22 have fabricated a cost-effective membrane directly from a sustainable wood material. In contrast to nanocellulose-based membranes, this membrane was made by directly removing lignin and hemicellulose from the wood samples via chemical treatment and freeze-drying. The prepared membrane had high porosity (∼90%) and hierarchical pore structure, while the presence of crystalline cellulose nanofibrils and xylem vessels and channels across the structure led to the facilitated water vapor transport. This innovative plant-based material demonstrated potential for MD water desalination, and the performance comparable to or better than that of commercially available membranes derived from fossil resources. For instance, the intrinsic permeability of nanowood membrane was 2.5 times higher than that of commercial polypropylene (PP) membrane, with exceptional thermal efficiency exceeding 70% (see Fig. 2c and d). Although nanowood membrane can be fabricated by a scalable top-down approach, it needed the FAS (perfluorodecyltriethoxysilane) treatment to become hydrophobic.
Recent studies proved the possibility of using cellulose for fabricating dual-layer membranes combining both hydrophobic and hydrophilic polymers. This type of membrane is supposed to eventually reduce the vapor transport path, thus improve the mass transport across the membrane. Owing to their surface properties, these membranes can be used for treating oily waste waters. For instance, Arumugham et al.23 fabricated a dual-layered membrane made by coating the cellulose substrate with novel perfluorooctanoic acid-modified melamine nanofillers embedded in PVDF. The long perfluoro chain in hydrophobic fillers increased membrane surface roughness by randomly overlapping with the PVDF backbone, resulting in a higher water contact angle. Results indicated that the 1% PFOM membrane demonstrated high flux and rejection ratio, making it a promising candidate for seawater desalination.
Nassrullah et al.24 used zeolite nanoparticles to open up the cellulose structure, and thus to increase the porosity in dual-layered membrane. Their process initially involves the preparation of casting solution using nano zeolite and microcrystalline cellulose, and the subsequent utilization of this solution to coat electrospun polymer membrane. Finally, each of the cellulose-coated membranes is physically stacked on the top of an unmodified electrospun membrane to form a double-layer membrane. The results showed that the addition of nano zeolite to the cellulose coating can effectively enhance the membrane performance without compromising the membrane selectivity. In the study of Joshi et al.,25 superhydrophobic cellulose-based membranes containing a dual-layered structure have been fabricated using a straightforward method. The superhydrophobic properties have been achieved by the surface microstructure modification with inorganic filler, followed by a hydrophobic sizing agent treatment, which is a common additive in papermaking. Cellulosic membrane was tested for desalination of water using the DCMD configuration and exhibited high water flux (23.0 ± 0.06 kg L m−2 h−1) and high salt rejection (97.5%), comparable to the performance of commercial PTFE membranes.
Das et al. proposed the application of perfluorocarbon-free MD membranes derived from silica and poly(methyl methacrylate) (PMMA) that are both water-wet materials. Inspired by the cuticles of springtails and hairs of sea skaters, they developed a two-step drilling process to create biomimetic gas-entrapping membranes (GEMs). Interestingly, the resulting GEMs comprised vertically aligned cylindrical pores with re-entrant inlets and outlets, which effectively entrapped air on the membrane surface upon immersion in wetting liquids and gave it omniphobic character. To some extent, MD testing proved a robust separation of salt solutions from deionized water, yielding a stable and high flux of desalinated water permeation of 1 L m−2 h−1 for over 12 h.
Remark. A significant number of publications have been devoted to minimizing the environmental impacts of membrane manufacture, and to developing polymers with environmental sustainability and minor disposal concerns. Nevertheless, the work on bio-based and biodegradable membranes is much more widespread for membrane processes other than MD. Polylactide, poly(lactide-co-glycolide), polyhydroxyalkanoates, chitosan and chitin, starch and poly(vinyl alcohol) are common biodegradable membrane materials that have been extensively studied towards enhanced membrane separation via microfiltration, ultrafiltration, nanofiltration, electrodialysis, and reverse osmosis.15 Although the low cost of bio-based membranes may facilitate the dissemination of technology for water desalination in various areas, it is important to notice that for MD operation, one side of the membrane must be modified to become hydrophobic. So far, the chemical vapor deposition method, to cover the hydrophilic fibers with hydrophobic silane,9 and FAS (perfluorodecyltriethoxysilane) treatment22 have been proposed. Therefore, it is apparent that the abovementioned membrane preparation procedures are often not environmentally friendly. The development of more environmentally friendly methods for tailoring the structure of biodegradable membranes should be explored in detail.
2.2. Sustainable and green approaches for porous membrane preparation
Porous MD membranes can be prepared by various methods, including stretching, template leaching, track etching, phase inversion, and electrospinning, which are widely described and explained elsewhere.19,27,28 Among them, phase inversion stands out as the most applied for the manufacture of commercially available membranes, and it attracts continuously growing attention from researchers, as reflected in the growing number of publications (see Fig. 3a). The basis of the phase inversion process is the preparation of a thermodynamically stable polymer solution which is subsequently transformed from a liquid into a solid state (coagulation) in a controlled manner. This coagulation is preceded by a so-called liquid–liquid demixing. In general, a polymeric solution is used to form the shape of the membrane, which is followed by membrane immersion into a liquid coagulant also called nonsolvent. Then the demixing starts, separating the polymer solution into a polymer-rich and a polymer-lean phase. The higher polymer concentration phase starts to coagulate and leads to the solid membrane matrix, while the polymer-lean phase leads to pore formation. The membrane immersion in a nonsolvent bath is called a non-solvent induced phase separation (NIPS); however, the demixing process may also be induced by controlled evaporation of the volatile solvent from the polymer solution (evaporation induced phase separation, EIPS), thermally induced phase separation (TIPS) or by placing the cast film in a vapor phase consisting of nonsolvent – vapor induced phase separation (VIPS).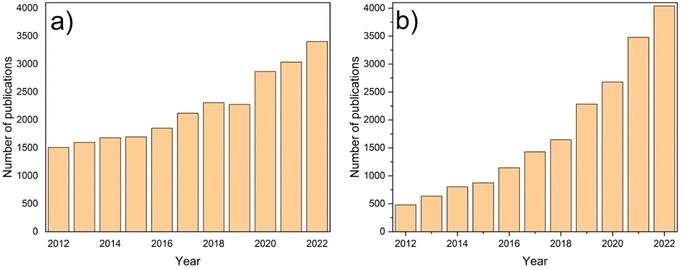 | ||
| Fig. 3 Publications on (a) phase inversion membranes, and (b) electrospinning membranes. Data taken from sciencedirect.com (30.08.2023). | ||
The other membrane preparation method is electrospinning. It is known as a simple and reliable technique for nanofiber preparation from a variety of polymers and is extensively used by the research community for the design of nanostructured matrices for different applications (see Fig. 3b). It involves the application of a high electric field to create nanofibers from a charged polymer solution or melt. By varying electrospinning parameters and polymer solution properties, different morphologies of the membrane can be produced. Despite the universality of phase inversion and electrospinning methods, their ease and the feasibility of producing membranes with the intended properties, they require the preparation of a polymer dope solution in order to form a membrane shape. Usually, hydrophobic polymers, such as PTFE, PVDF, PP and/or polyethylene (PE),29 exhibit low polarity, requiring them to be dissolved in conventional non-polar (or nearly non-polar) solvents, such as N-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF) and N,N-dimethylacetamide (DMAc). Although these chemicals are considered as fine universal solvents, with great abilities to dissolve both amorphous and semi-crystalline polymeric materials, they have an adverse effect on living organisms due to their serious toxicity according to multiple reports (see Table 2).30 For instance, DMF is considered as a carcinogenic compound that, according to the International Agency for Research on Cancer, gives rise to mutations in mammalian somatic cells. On the other hand, DMAc is deemed to be a human reproductive toxicant and responsible for the promotion of congenital malformation in the fetus, while NMP brings an adverse effect on human fertility or on the unborn child.
| Solvent | Chastrette's report classification31 | Pfizer classification – reason32 | General classification33 |
|---|---|---|---|
| Abbreviations: CMR – carcinogenic, mutagenic, or toxic for reproduction, HAP – hazardous air pollutant. | |||
| DMF | Aprotic highly dipolar | Undesirable – toxicity, classified as a HAP in the US, strongly regulated by EU solvent directive | Hazardous |
| DMAc | Aprotic highly dipolar | Undesirable – strongly regulated by EU solvent directive, toxicity | Hazardous |
| NMP | Aprotic highly dipolar | Undesirable – strongly regulated by EU solvent directive, toxicity | Hazardous |
| Tetrahydrofuran | Hydrogen bonding | Usable | Problematic/hazardous |
| Chloroform | Miscellaneous | Undesirable – carcinogen, classified as a HAP in the US | Highly hazardous |
| 1,4-Dioxane | Electron pair donor | Undesirable – carcinogen (CMR category 3), classified as HAP in the US | Hazardous |
| Toluene | Aromatic apolar | Usable | Problematic |
Therefore, to fabricate sustainable membranes, one must refrain from using these toxic and dangerous solvents by applying one of two common approaches. One of them is to employ green solvents for polymer solution preparation (as discussed in the next subsection), and the second requires the use of a solvent-free method.
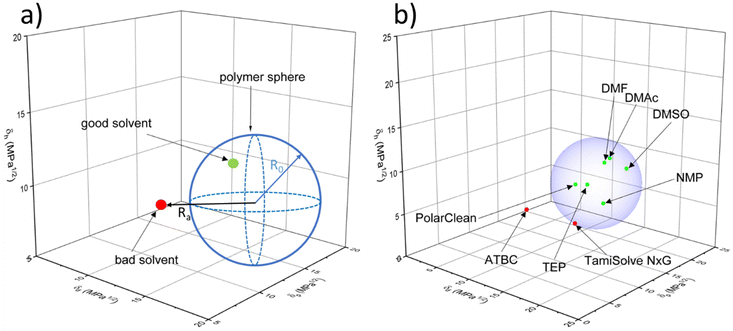
where δd, δp, and δh are the parameters for the dispersion, polar and hydrogen bonding interactions, respectively. The Ra value must not exceed the R0 value of the polymer if solubility has to be maintained. These dependencies are often presented graphically in the form of the Hansen solubility sphere of the polymer (based on the R0 value) in a three-dimensional coordinate system. Knowing the Hansen solubility parameter values of the solvents, it is possible to assess whether the solvent will be suitable for a given polymer, as described in Fig. 4a. Due to its simplicity, this method has been widely used in combination with experimental results. For instance, Fig. 4b shows the three-dimensional Hansen solubility parameter sphere of a common polymer used for MD membrane preparation – PVDF, and the position of solvents (both conventional and green ones) reported in the literature to dissolve this polymer.
To date, several green solvents have been widely employed for porous MD membrane preparation with good affinity for water as well (see Table 3). Most of the reports concern PVDF membranes35–38; however, poly(ethylene-chlorotrifluoroethylene) (ECTFE),39,40 PP,41,42 and PU43 MD membranes were also prepared by employing harmless solvents. For instance, Ding et al.43 developed environmentally friendly PU/PTFE nanofiber membranes via electrospinning using ethanol and diacetone alcohol as a polymer solvent. Zou et al.44 fabricated porous PVDF membranes via the NIPS method using PolarClean. The membrane was tested in various MD systems and presented higher flux than that found in the literature, indicating that this green membrane showed good MD performance towards saline water and food extracts. Triethyl phosphate (TEP), as a green solvent, has been used to prepare both flat sheet and hollow fiber membranes.45–47 Since TEP has a good affinity with PVDF (see Fig. 4b), the research on this green solvent has basically focused on PVDF membrane fabrication. For example, Chang et al.47 fabricated MD hollow fiber membranes using TEP and compared their phase inversion kinetics with the conventional NMP/PVDF polymer solution system. The TEP/PVDF system presented a less rapid phase inversion rate and resulted in a more porous sponge-like membrane structure than NMP/PVDF system. Moreover, PVDF/TEP solution produced fibers with robust mechanical properties, high LEP and great porosity over 83% for all conditions studied, proving that green solvent TEP is able to replace commonly used NMP in membrane manufacture. Liu et al.39 used trioctyl trimellitate (TOTM) as an environmentally friendly solvent to prepare porous poly(ethylene-chlorotrifluoroethylene) (ECTFE) membranes by the TIPS method. They analyzed the effect of ECTFE content on the membrane morphology and structure. Importantly, the 15 wt% ECTFE membranes presented a continuous structure without the production of a dense layer; however, the further increase in the content of polymer resulted in the spherulite structure and denser surface. Xu et al.40 introduced the green diluent acetyl tributyl citrate (ATBC) for the preparation of ECTFE membrane and investigated the effects of polymer concentration and quenching temperature on the membrane properties. Here, together with the polymer concentration growth, the membrane became more integral, which resulted in a reduction of pore size, porosity and pure water flux but improved mechanical strength and hydrophobic properties.
| Solvent | Boiling point (°C) | Flash point (°C) | Water solubility (g L−1) | Molecule structure | Cost | Ref. |
|---|---|---|---|---|---|---|
| DMF | 153 | 58 | Miscible |
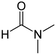
|
2–4 USD per kg | 30 and 50 |
| DMAc | 166 | 64 | Miscible |
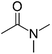
|
2–4 USD per kg | 30 |
| NMP | 202 | 91 | Miscible |
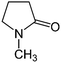
|
1–3 USD per kg | 30 and 50 |
| PolarClean | 280 | 145 | Miscible |
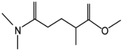
|
7.3 USD per kg | 5 |
| TEP | 215 | 112 | Miscible |
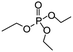
|
2.6 USD per kg for | 35 and 51 |
| ATBC | 343 | 204 | 0.0045 |
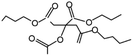
|
1.9 USD per kg | 51 |
| TamiSolve NxG | 241 | 108 | Miscible |
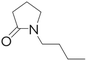
|
34 USD per kg | 49 |
| DMSO | 189 | 95 | Miscible |
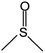
|
1.6 USD per kg for | 5 and 51 |
| TOTM | 414 | >240 | <0.00001 |
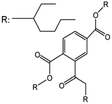
|
69.5 USD per kg | 39 |
Importantly, several studies have proved that membranes obtained with the use of green solvents are not only a “current hot topic”, but are also real competition for materials obtained in a traditional way. Very recently, Meringolo et al.48 prepared PVDF membranes with dimethyl sulfoxide (DMSO) as the solvent via a combined VIPS and NIPS method without using any chemical additive for pore-forming. The resulting membrane exhibited MD performance comparable to that presented by commercial PVDF membranes. Importantly, the cost of DMSO is almost at the same level as traditional solvents and is lower compared with other green solvents (see Table 3), which makes it a good candidate for large-scale membrane preparation. In a different approach, Marino et al.49 employed TamiSolve NxG to prepare poly-(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) membranes. Preliminary MD tests showed pore size comparable to commercial PP membranes and promising performance during desalination.49 The list of green solvents used in MD membrane fabrication together with membrane performance in MD process is given in Table 4.
| Solvent | Features | Polymer | Fabrication method | Membrane type | Configuration, performance | Ref. |
|---|---|---|---|---|---|---|
| Ethanol + diacetone alcohol | - Preferred solvents according to Pfizer solvent selection guide | PU | Electrospinning | Electrospun fiber | DCMD | 43 |
| 44 L m−2 h−1 | ||||||
| 99.96% | ||||||
| TOTM | - Better high-temperature resistance and less volatility than other solvents for ECTFE | ECTFE | TIPS | Flat sheet | VMD | 39 |
| 23.09 L m−2 h−1 | ||||||
| 99.9% | ||||||
| PolarClean | - An ecofriendly biodegradable solvent with no reported health hazards | PVDF | NIPS/N-TIPS | Flat sheet | DCMD | 44 |
| - Miscible with water | ∼37 L m−2 h−1 | |||||
| - Nonflammable with very low vapor pressure | 99.9% | |||||
| TEP | - Only harmful when being swallowed | PVDF | NIPS | Flat sheet | VMD | 45 |
| - Highly resistant to many organic and inorganic acids | 36 L m−2 h−1 | |||||
| - Good thermal stability | 100% | |||||
| PVDF | Wet-spinning | Hollow fiber | DCMD | 47 | ||
| 20 L m−2 h−1 | ||||||
| 99.99% | ||||||
| PVDF-HFP | NIPS | Flat sheet | DCMD | 35 | ||
| 16.1 L m−2 h−1 | ||||||
| 99.3% | ||||||
| PVDF | TIPS | Hollow fiber | VMD | 36 | ||
| 30.6 L m−2 h−1 | ||||||
| PVDF | TIPS | Hollow fiber | DCMD | 37 | ||
| 61.6 L m−2 h−1 | ||||||
| 99.99% | ||||||
| ATBC | - Non-toxic and eco-friendly | ECTFE | TIPS | Flat sheet | VMD | 40 |
| - High boiling point | 22.3 L m−2 h−1 99.9% | |||||
| TamiSolve NxG | - Nonreprotoxic and biodegradable solvent | PVDF-HFP | NIPS | Flat sheet | DCMD | 49 |
| - Similar properties with NMP | ∼25 L m−2 h−1 | |||||
| 99.5% | ||||||
| DMSO | - Polarity similar to those of DMF, DMA and NMP | PVDF | VIPS/NIPS | Flat sheet | DCMD | 48 |
| - Nonhazardous, biodegradable, and recyclable | 12.1 L m−2 h−1 | |||||
| - Good solvent power for many polymers | 99.9% | |||||
| Soybean oil | - Edible oil | PP | TIPS | Flat sheet and hollow fiber | VMD | 41 |
| - Limited solubility, thus it is used as a one component of binary solvents | 18.4 L m−2 h−1 | |||||
| 99.99% | ||||||
| PP | TIPS | Flat sheet | VMD | 42 | ||
| 41.2 L m−2 h−1 | ||||||
| 99.95% |
Recently, green plant-based binary diluents have been applied to prepare PP microporous membranes, such as carnauba wax and soybean oil42 and soybean oil and castor oil.41 The binary diluents ratio significantly influenced the phase separation behavior and membrane morphology. Adding a so-called poor diluent into the polymer/good diluent system induced liquid–liquid phase separation, which favors the membrane formation with a continuous sponge-like pore structure and increases the membrane elongation at break. The PP membranes fabricated using non-toxic binary diluents show the potentiality in VMD desalination of 10 g L−1 NaCl leading to a water flux up to 18.4 kg m−2 h−1,41 and 41.2 kg m−2 h−1.42
Remark. Based on recent studies, it can be concluded that the substitution of traditional solvents with greener ones is possible. However, there are two aspects to consider before implementing them in large-scale production. First, the membrane performance must be maintained or improved if possible. The second aspect is the economic estimation, as the price of green solvents is usually higher than that of traditional ones (see Table 3); however, researchers need to focus on the cost–benefit ratio, as today there is a greater need to avoid the negative impact of conventional solvents on the environment. Although some research has proved the feasibility of achieving both goals simultaneously, this is only a small part of the overall research conducted on membrane fabrication using green solvents. Finally, some of the novel green solvents have not yet been investigated as dissolving systems to prepare MD membranes, for instance, deep eutectic solvents, which, depending on their precursors, are considered low toxicity, biodegradable, eco-friendly, and cheap.52 It is worth exploring other possible solvents, like deep eutectic solvents, but always considering the physicochemical properties of the precursors (e.g., volatility)53 during the membrane preparation.
The melt-spinning and stretching (MS-S) technique is a green and simple membrane preparation process, given that it does not require the use of any diluents and solvents, and does not involve any phase separation. To fabricate the membrane via this process, the polymer melt is spun at a temperature close to its melting point, while the pores are formed through the stretching force acting on the material in a further cold-stretching step.54 In general, the formation mechanism of the microporous structure by the MS-S method is possible due to the presence of stacked crystalline lamellar in the crystalline polymers. During the stretching step, the crystalline lamellar is gradually separated, a large number of voids are formed, which are subsequently interconnected, and the micropores are then created. Therefore, MS-S is suitable for the preparation of microporous membranes from highly crystalline polymer membranes, such as PE,55 PP,56 and semi-crystalline ones, for example, PVDF.57 The PVDF membranes fabricated by MS-S exhibited excellent mechanical properties, much better than those made by the phase inversion method. Unfortunately, the porosity of these membranes was poor, as a consequence of PVDF semi-crystallinity, which led to low pure water flux.58 To increase the porosity of the membranes formed from semi-crystalline polymers, Hu et al.59,60 proposed interfacial pore theory during the membrane preparation process via MS-S, which states that the blend of polymers with poor compatibility would form an interface layer between the polymer matrices. During the stretching step, this interfacial layer with low adherent strength facilitates the formation of the pores between matrix phases. Following this hypothesis, the authors prepared a polyurethane/PVDF blend hollow fiber membrane, and observed improved pure water flux of 2174 L m−2 h−1 under the pressure of 0.1 MPa.
Although recent studies on MS-S membranes refer to membrane fabrication for oil/water separation,61 microfiltration,62 and membrane emulsification,63 there are several studies showing the possibility of applying MS-S for MD membrane fabrication.64,65 Chen et al.,65 for instance, fabricated poly(tetrafluoroethylene-co-hexafluoropropylene) (FEP) hollow fiber membranes through a melt spinning and stretching method. Similar to PTFE, FEP poses excellent thermal and chemical resistance and has a strongly hydrophobic nature. The authors investigated the effect of different stretching ratios on membrane structures and thus performances. Together with the stretching ratios, the membrane's porosity increased, while the membrane stretching had a negative effect on the mechanical properties and LEP value. The VMD tests proved that the FEP hollow fiber membranes exhibited satisfactory salt rejection (99%) and stable but relatively low permeate flux (ca. 8.4 LMH). In another study, Shao et al.64 fabricated PP hollow fiber membranes made by the MS-S method. On observing the membrane microstructure, the results indicated that the slit-like micropore size between 0.05–0.3 μm was formed during the MS-S. During the VMD test, the water flux reached 7.8 L m−2 h−1 while hindering the salt passage (rejection over 99.9%).
The innovative solvent-free methods were found to be useful in the fabrication of PTFE porous membranes. Besides its superior properties, this polymer is difficult to process and it is challenging to prepare membranes by the common phase inversion or melt spinning methods, due to its ultrahigh melt viscosity and its insolubility. Therefore, PTFE hollow fiber membranes are today fabricated via emulsion spinning, paste extrusion and stretching technology.66 Interestingly, Zhu et al.67 fabricated PTFE hydrophobic hollow fiber membranes through a cold pressing method, including paste extrusion, stretching and sintering, and investigated the effect of stretching ratios on membrane performance. An increase in the stretching ratio significantly improved the permeation flux in VMD desalination. The salt rejections for all the fabricated PTFE hollow fiber membranes achieved 99.9%. In the same research group, Wang et al.68 observed that the increasing of stretching ratio endowed the membrane with higher porosity and larger pore size. Their PTFE membranes were tested in VMD during the treatment of seawater reverse osmosis brines. The results showed that the increase in stretching ratio and heating temperature significantly improved the permeation flux. Although the permeate flux of as-prepared PTFE hollow fiber membrane was much lower than that of other hollow fiber membranes, the authors stated that membranes fabricated through cold pressing method are promising for practical application.
Remark. Compared with TIPS and NIPS, the MS-S process is more difficult to control in terms of pore formation and its size. It also requires crystalline polymers as the precursor materials for preparing the microporous membrane. However, the use of solvents and diluents in the phase separation processes results in the generation of a huge amount of waste solvents. Thus, the solvent-free processes are more environmentally friendly. Recent studies investigated the green and sustainable preparation of polymeric membranes using the MS-S method; unfortunately, none of them have been tested in the MD process.69–71
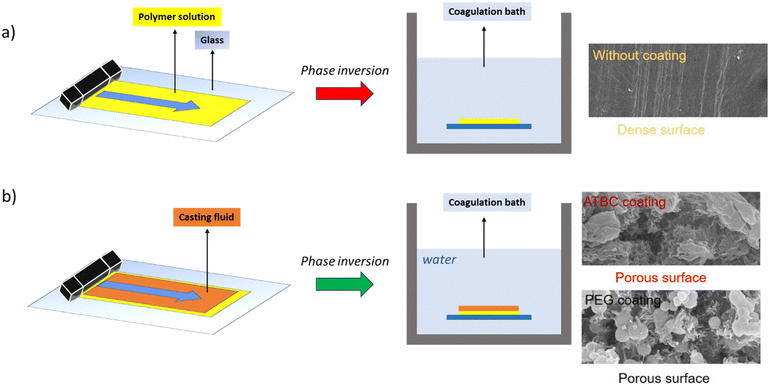 | ||
| Fig. 5 Membrane fabrication method using (a) traditional phase inversion, (b) co-casting method, and their morphology (SEM images reproduced from ref. 44 with permission from Elsevier, copyright 2022). | ||
Another solution to preventing the formation of dense membranes is the addition of pore-forming agents in the polymer dope solution, such as (PVP),77–79 polyethylene glycol (PEG),80,81 Pluronic,77 and LiCl.80 The addition of pore-forming agents induces a sponge-like membrane structure, prevents finger-like macrovoid formation, and enhances both pore formation and interconnectivity. For example, Jung et al.,77 aiming to prepare porous membranes from PVDF/PolarClean solution, investigated the effect of three different sets of casting solution additives (Pluronic F-127, PVP, and LiCl & glycerol). To some extent, the pore-forming additives improved the water permeability but greatly decreased the mechanical strength of the resulting PVDF membranes. Among the tested additives, Pluronic was found to be the most effective pore-former. The membrane prepared with Pluronic showed narrow pore size distribution and water permeability up to 2800 L m−2 h−1 bar−1.
Accordingly, it is highly important from environmental and practical perspectives to utilize a simple and green polymer system with only a water bath at room temperature to fabricate porous membranes without any dense layer for MD. Recently, an interesting method was proposed by Tian et al.,82 who produced a porous PVDF membrane by using a co-casting method. During the fabrication process, they first cast a PVDF layer on the glass plate and then immediately cast another isolation polyethersulfone (PES) layer onto the PVDF layer. After being immersed in the water coagulation bath the PES and PVDF layer separated automatically, and as a result, a porous PVDF membrane surface was formed without adding any pore-forming agents. Thus, the PES layer acted as an isolation layer which decreased the exchange rate of solvent and non-solvent, resulting in the formation of porous membrane surface. The porous PVDF membrane exhibited steady fluxes during a long-term DCMD test for 48 h using a 3.5 wt% NaCl solution as the feed, along with continuous high salt rejection of above 99.95%. Zou et al.44 fabricated a high-flux and stable PVDF membrane that was produced using a co-casting method, as illustrated in Fig. 5b. They applied two different green casting fluids including acetyl tributyl citrate (ATBC) and polyethylene glycol 400 (PEG 400). The as-prepared PVDF membrane top surfaces were highly porous and presented good performance during the concentration of saline water and ginseng extract in the MD process (99.99% rejection and ∼37 kg m−2 h−1 flux). Therefore, the co-casting method is a green approach for producing porous membranes, using water as a coagulation bath, without the need for the addition of any pore-forming agents.
Delayed phase inversion not only prevents dense structure formation but significantly enhances the membrane roughness and water contact angle. At this point, Zhang et al.83 introduced saturated NaCl·H2O solution to the PVDF casting solution, which was supposed to serve as a green additive for controlling the phase inversion process and hydrophobicity enhancement. The results showed that salt crystals acted as nuclei for the pre-gelation and crystallization of polymer chains. An optimum amount of NaCl·H2O solution led to the formation of a superhydrophobic membrane with micro–nano spherical structure. The membrane exhibited great DCMD performance with a permeate flux 1.6 times higher compared with the one without NaCl·H2O addition.
Lu et al.84 proposed a facile and green method to fabricate superhydrophobic PVDF membranes via pure rheological spray-assisted nonsolvent-induced phase separation (SANIPS). Unlike conventional NIPS, compressed air or a designated solution was sprayed on the glass cast membrane, prior to immersion in water. This method did not require any further modification, as spraying was adopted to manipulate the morphology of the membranes by controlling the phase inversion speed. The exceptional anti-wetting and self-cleaning properties of the SANIPS membranes have been demonstrated in DCMD tests, while treating hypersaline wastewater, comprising 10% sodium chloride and Rose Bengal dye. The high and stable vapor flux of 36.0 kg m−2 h−1, and a salt rejection over 99.9% during the long-term test of 100 h proved the SANIPS method to be effective for the fabrication of highly porous, hydrophobic and rough structures.
Remark. When the nascent membrane is transferred into a water coagulant, the exchange rate between the polymer solvent and water is fast and the undesirable dense layer is formed. Different studies have proposed green approaches for delaying the demixing during phase separation when water is used as a nonsolvent, thus producing a porous and rough structure. Some of them require the use of a multi-stage procedure or the use of additional professional equipment. The most preferred methods demonstrate one-step in situ construction of superhydrophobic membranes using only non-toxic and environmentally friendly additives.
3. Membrane modification – substitution of FAS
Fluoroalkyl silanes (FASs) are a group of substances commonly applied in the fabrication of MD membranes in order to create a superhydrophobic or omniphobic surface.85 FASs are composed of long hydrophobic carbon and fluorine chains and functional groups that may form strong covalent bonds with the membrane material.86 Since the omniphobic surface can repel almost any liquid that it comes into contact with, membrane modification has been studied extensively in order to achieve this property,87 as reported in Table 5. It is believed that combination of the multi-scale re-entrant topography and low surface energy material is the best solution for the creation of omniphobicity.88 In recent years, several studies have been carried out on omniphobic surfaces for MD application. For instance, various studies reported the use of FASs in order to lower the surface energy of membrane material.89,90 Lee et al.88 have fabricated omniphobic electrospun PVDF-HFP membrane for DCMD operation. To create multi-scale re-entrant structures, silica nanoparticles were attached to the polymer via electrostatic interaction. Then, low surface energy (heptadecafluoro-1,1,2,2-tetrahydrodecyl)trichlorosilane (FDTS) was grafted on the silica-modified PVDF-HFP nanofibers, which provided excellent omniphobic properties. Recently, Deng et al.91 fabricated a nanofibrous PVDF membrane with a self-roughened fluorosilane omniphobic coating, without the use of any auxiliary nanoparticles. The preparation method included the electrospinning of PVDF nanofibers, followed by fiber functionalization with long-chained fluorododecyltrichlorosilane (FTCS) via simple solution immersion. The morphologies of these materials revealed the presence of hierarchically re-entrant structures that exhibited the characteristics of high omniphobicity.| Membrane base | Modification method | FASs | Ref. |
|---|---|---|---|
| PVDF-HFP | Attaching silica nanoparticles (SiNPs) followed by FAS grafting | (Heptadecafluoro-1,1,2,2-tetrahydrodecyl)trichlorosilane (FDTS) | 88 |
| PVDF | Electrospinning followed by solution immersion treatment | 1H,1H,2H,2H-Perfluorododecyltrichlorosilane (FTCS) | 91 |
| PVDF | Spray coating of the nano/microspheres onto a commercial PVDF porous substrate | 1H,1H,2H,2H-Perfluorodecyltrimethoxysilane | 89 |
| PVDF-HFP | Attaching silica nanoparticles (SiNPs) to the fibers, followed by surface fluorination | 1H,1H,2H,2H-Perfluorodecyltriethoxysilane | 90 |
| PDA/PEI/PI | Electrospinning technique, electrostatic attraction, and FAS fluorination | (Heptadecafluorotetrahydrodecyl)-triethoxysilane | 92 |
| Alumina | Dip-coating on porous ceramic support followed by post-grafting process with PFOTES | 1H,1H,2H,2H-Perfluorooctyltriethoxysilane | 93 |
| PVDF | Indirect fluorination over CNT intermediate layer previously coated on PVDF substrate | 1H,1H,2H,2H-Perfluorodecyltriethoxysilane | 94 |
| PA6 (polyamide 6) | Surface fluorination followed by PVDF grafting | Trichloro(1H,1H,2H,2H-perfluorooctyl)silane | 95 |
| Cellulose | The attachment of SiNPs and chemical vapor deposition of fluoroalkylsilane | (Heptadecafluorotetrahydrodecyl)-triethoxysilane | 96 |
| PET | Graft polymerization of triethoxyvinylsilane (TEVS) and fluorination | 1H,1H,2H,2H-Perfuorododecyltrichlorosilane (PFDTS) | 97 |
| GO | Fluorination through the thiol–ene click reaction | 1H,1H,2H,2H-Perfluorodecanethiol (PFDT) | 98 |
An important aspect in the application of FASs is their cost; these chemical reagents are typically expensive feedstock. For example, 1 g of commonly used 1H,1H,2H,2H-perfluorodecyltriethoxysilane can cost 28–40$.99 Furthermore, FASs may pose a negative environmental impact. Because of the difficulty of breaking the carbon–fluorine bond, per- and polyfluoroalkyl substances are persistent in the environment. Based on their chemical properties and various studies suggesting potential human toxicity and emerging water pollutant concerns,100,101 researchers began to look for replacements for long-chain per-fluorinated additives in lowering the membrane surface energy.
A CF4 plasma treatment has been proven to be an effective method to enhance membrane hydrophobicity (Yang et al.,102,103). It provides a suitable membrane etching that increases surface roughness, and the formation of CF2–CF2 and CF3 bonds that decrease the membrane surface energy. While this treatment imparted the omniphobic characteristic to the membrane and significant improvement in transport properties,104 it still required the use of a fluorinated additive, that is CF4 gas, with the potential negative impact on the environment and the human body. Lee et al.105 proposed a two-step electrospinning–electrospray method to fabricate a superhydrophobic surface without fluorinated additives. They created a polymeric microsphere coating through electrospraying a polymer mixture of PVDF and poly(dimethylsiloxane). Although the pure microsphere-coated membrane showed superhydrophobic properties with a 156.7° contact angle, the investigators needed to add silica-based aerogel particles to strive toward omniphobicity. The 30% aerogel-assisted microsphere-coated membrane presented a high and stable resistance to wetting by a relatively low surface tension solution of 3.5% NaCl containing 0.1 mM sodium dodecyl sulfate. Nevertheless, such a surface coating method requires a more complicated two-step process, which leaves internal pores unmodified and poses a risk of inhomogeneous surface coverage. Guo et al.106 adopted a one-step biaxial electrospinning and electrospray method to fabricate a hybrid monolayer nanofiber and nanosphere superhydrophobic PVDF-HFP membrane without the use of any long-chain perfluorinated additive. The surface roughness of the nanosphere–nanofiber membrane increased 3.58 times, while good surface stability was achieved thanks to its single-layer structure. The fabricated membrane exhibited excellent resistance to wetting and fouling when operated with saline feed water, which consisted of dissolved low surface tension surfactants and organic matters, confirmed with a 7-day MD test (rejection rate of 99.8% and 30 LHM flux). Zheng et al.107 fabricated a hierarchically structured membrane by grafting octavinyl-polyhedral oligomeric silsesquioxane on PVDF membrane via UV-induced thiol–ene click reaction. Although this method requires chemical preparation of PVDF support (the hydroxylation and sulfhydrylation pretreatment), it offers a fluoride-free, green and cost-effective strategy for superhydrophobic membrane preparation. The fouling and anti-wetting properties of the as-prepared membranes were found to be superior compared with the peer membrane reported in the previous literature. In the study of Zhang et al.,108 fatty acids, as naturally occurring compounds, have been proposed as a cost-effective and more environmentally friendly alternative for hydrophobic modification. The carboxylic groups of the fatty acids can be involved in the creation of chemical bonds with the membrane surface, while the long carbon chains are supposed to contribute to the hydrophobicity enhancement. The authors studied two different fatty acid chlorides of palmitoyl chloride and stearoyl chloride with different carbon chain lengths. Due to the presence of an acyl chloride group, these chemicals are supposed to easily react with functional groups on ceramic membranes. Both fatty acid chlorides with different carbon chain lengths formed strong covalent bonds on the membranes with high water contact angle and LEP values, comparable to the hydrophobic membranes prepared through silanization. The comparison of as-reported FAS-free MD membranes is included in Table 6.
| Polymer | Modification | WCA0/WCA | LEP0/LEP | Performance | WSA | Ref. |
|---|---|---|---|---|---|---|
| WCA0 – initial water contact angle, WCA – water contact angle after modification, LEP0 – initial liquid entry pressure, LEP – liquid entry pressure after modification, WSA – water sliding angle. | ||||||
| PVDF | CF4 plasma treatment | 130°/162° | — | DCMD | — | 102 |
| 41.37 LMH | ||||||
| PVDF | CF4 plasma treatment | 130°/162° | 2.4/3.1 bar | DCMD | — | 103 |
| 32.8 LMH | ||||||
| 99.98% | ||||||
| PVDF | CF4 plasma treatment | 133°/160° | 142.7/186.7 kPa | AGMD | 51–52° | 104 |
| 15.3 LMH | ||||||
| ∼100% | ||||||
| PDMS/PVDF | Electrospraying of the polymer/aerogel solution | 128°/162° | 105.2/129.1 kPa | DCMD | 3.4° | 105 |
| 20 LMH | ||||||
| 97% | ||||||
| PVDF-HFP | Biaxial electrospinning | 139°/153° | 1.21/2.17 bar | DCMD | — | 106 |
| 32.7 LMH | ||||||
| 99.2% | ||||||
| PVDF | Octavinyl-polyhedral oligomeric silsesquioxane grafting | 136°/155° | — | DCMD | 7.5° | 107 |
| 10.5 LMH | ||||||
| 99.9% | ||||||
| PVDF | Solvent-thermal induced roughening | 132°/164° | 83/182k PA | DCMD | 8.1° | 109 |
| 20 LMH | ||||||
Remark. The elimination of fluoroalkyl silanes from the procedure for obtaining omniphobic membranes is in fact problematic due to their extremely low surface energy, which is unprecedented in other substances. The abovementioned studies on FAS-free synthesis of omniphobic membranes applied other fluorine materials instead, e.g., CF4 or fluoropolymers, to achieve low surface energy membranes. Therefore, these methods for obtaining omniphobic surfaces cannot be described as environmentally friendly. On the other hand, attempts have been made to replace FASs with naturally occurring compounds, e.g., fatty acids, giving promising results. However, in the field of MD membranes, this topic is still in its infancy.
4. Design for energy efficiency
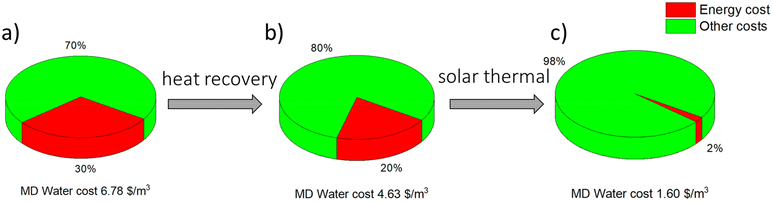
According to the 6th principle of green chemistry, the energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure. The MD process meets this regulation in two ways. First of all, it operates at low temperatures as compared with the conventional thermally driven desalination processes, namely multi-stage flash and multiple-effect distillation. Unlike conventional pressure-driven membrane processes, such as reverse osmosis, MD can operate at atmospheric pressure. On the other hand, since MD is a thermally driven process, it remains an energy-demanding technology. The overall estimations of the cost of energy in water production clearly emphasise the economic weakness of the standalone MD systems. Energy consumption accounts for 30% of the total cost of water price using MD (see Fig. 6), and at the same time, it is the highest contributing factor among others, such as the cost of heat exchangers, storage and membrane modules, and other devices. The high energy consumption in MD systems is due to the need to heat the feed to high temperatures (even up to 70 °C) to provide high driving force for vapor permeation through the membrane. Moreover, MD systems are prone to heat loss by the conduction that occurs in the membrane during the mass transport of vapor.110 However, based on the analysis and economic evaluation made by Usman et al.,111 MD systems may show better economic potential than reverse osmosis systems (Fig. 6). Due to relatively low operating temperatures, alternative energy sources can be applied to run the MD process. For instance, the integration of renewable energies into the MD process significantly reduces the contribution of energy consumption in the total cost of water price and makes the process environmentally friendly and sustainable. As shown in Fig. 6b, the implementation of waste heat recovery into MD systems decreases the water price from 6.78 $ per m3 to 4.63 $ per m3. In addition, as shown in Fig. 6c, the effect of using solar energy further reduces the price of the water to 1.60 $ per m3. The cost breakdown is a result of the reduction of energy impact on the total cost of water price to only 2%, which is a significant decrease when compared with conventional standalone MD systems (30%).The utilization of low-grade waste heat as a driving force has gained considerable attention, as it significantly reduces operating costs,112 giving MD promising potential as a viable technology for future desalination.113 Recent studies examined the suitability of the MD systems for integration with different types of low-grade heat sources,114 such as solar energy, geothermal energy115 and waste heat.116 Among all low-grade types of heat applied in MD systems, solar energy seems to be the most studied so far. However, the use of solar energy requires advanced control strategies.117,118 Furthermore, the instability of solar radiation and its limited supply time needs to be overcome using technically and economically feasible systems.119 Nevertheless, various studies confirm the possibility of implementing integrated modules in different MD configurations. For instance, Ma et al.120 recently built a small-scale VMD unit to provide drinking water in remote areas and provide electricity via direct solar heating. Concurrently, Soomro et al.121 investigated the performance and economic comparison of solar power plants integrated with DCMD. Interestingly, the water production cost was found to be 0.314 USD per m3 for the solar-powered plant integrated with the DCMD system. Moore et al.122 developed a comprehensive process model and designed an economically optimal system. Thermal energy for distillation was provided by solar thermal collectors and electricity was provided using a photovoltaic collector. Recently reported studies on MD systems using low-grade heat energy and their desalination performance are summarized in Table 7.
| Source of low-grade heat energy | MD configuration | Membrane module | Membrane material | Permeate flux | Salt rejection | Ref. |
|---|---|---|---|---|---|---|
| WGMD: water gap MD. | ||||||
| Ship engine | VMD | Flat sheet | PTFE | 13 L m−2 h−1 | 99.99% | 123 |
| Waste heat from ship engine | WGMD | Flat sheet | PTFE | 13.08 L m−2 h−1 | 99.99% | 116 |
| Waste heat from ship engine | AGMD | Flat sheet | PTFE | 6.73 L m−2 h−1 | 99.99% | 116 |
| Solar energy | VMD | Hollow fiber | PVDF | 17.68 L m−2 h−1 | Not available | 124 |
| Waste heat from diesel engine | Multi effect MD | Flat sheet | PTFE | 2.61 L m−2 h−1 | >99% | 125 |
| Effluent-waste heat | DCMD | Flat sheet | PTFE | 14 L m−2 h−1 | >99.89% | 126 |
Baghbanzadeh et al.127 have demonstrated the innovative concept of a zero-waste and energy efficient, thus sustainable MD strategy. Since the main energy concern of conventional MD desalination is its requirement for large quantities of thermal energy to heat up the feed, these authors have established a zero thermal energy input MD. The proposed process implies the use of the naturally occurring temperature difference between the surface of seawater (at 30 °C) and the bottom (at 10 °C) as the process driving force. The results show the feasibility of producing pure water with a flux reaching 11.3 L m−2 h−1 and a cost equal to $0.28 per m3, which is significantly lower than that offered by the currently dominating membrane desalination technology, reverse osmosis ($0.45–2.00 per m3).
Although the abovementioned studies are praised for the significant reduction of water cost production, the MD process still cannot withstand large production rates while maintaining energy efficiency. Various hybrid systems have already been thoroughly discussed in terms of water production and energy efficiency.128,129 For instance, MD integration with conventional desalination processes, such as reverse osmosis, multi-stage flash and multi-effect distillation may result in nearly zero liquid discharge and higher performance. The integration of a reverse osmosis system with MD led to a reported water recovery higher than 80%,130 which is twice as much as the value for the conventional reverse osmosis. Various integrated desalination systems have been evaluated by González-Bravo et al.131 It has been shown that MD desalination systems coupled with multi-stage flash or multi-effect distillation techniques achieved the maximum economic and environmental advantages among other MD integrated systems. Interest in integrated systems is also growing within emerging processes. The combination of forward osmosis with MD enables the simultaneous production of clean water and regeneration of the draw solution, ensuring process sustainability. The study of Wang et al.132 demonstrated the prospect of employing the forward osmosis–MD hybrid systems for seawater desalination with a high vapour flux of 6/32 LMH (FO/MD). Kim et al.133 used the low vacuum pressure naturally created by adsorbents during the adsorption desalination cycle to drive a VMD system without the need for a vacuum pump. The authors noted a 23% increase in the water recovery ratio and a 21% increase in water production compared with a standalone VMD. More recently, MD hybrids in which the electrodialysis133 or electrocoagulation134 modules were used for wastewater pretreatment have emerged. Pretreatment of the feed streams is essential when developing large-scale MD processes, as it significantly reduces the susceptibility of the membrane to fouling and wetting and removes chemicals potentially harmful to the membrane.
Remark. By using low-grade heat energy, it is possible to implement and improve MD for the water supply situation. The current literature suggests that inexpensive energy sources, such as solar energy, geothermal energy, and waste heat, play a crucial role in the water–energy nexus, making the MD process more sustainable and cheaper. Although the techno-economic analysis estimates that the utilization of energy from low-grade heat sources makes the MD process competitive with conventional desalination techniques, an important factor that has a significant impact on MD economics is its ability to create hybrid systems using the strengths of two or more processes. An extensive evaluation of each hybrid energy demand is needed in order to minimize their specific energy consumption.
5. Conclusions
In the future, the definition of the green MD membrane process should be expanded, including sustainability considerations. This transformation requires the innovation of science and technology coupled with new emerging systems design, resulting in a positive impact on a global scale. In the past decade, most research has mainly focused on treating wastewater using membrane technology but has ignored wastewater production during the membrane preparation process itself. According to the literature, the wastewater contributed to more than 95% of the total waste produced during the membrane fabrication process. To achieve a completely green and sustainable development of membrane technology, more attention should be paid to research on the green preparation process. Designing for a circular economy, which requires the establishment of a series of interconnected closed-loop processes, is crucial. This latter idea states that no waste streams are generated while being recycled or repurposed to retain or increase their value. This transition, applicable to membrane science and engineering as well as other domains, calls for innovative engineering solutions that enable new ways of designing. In general, some unsolved scientific and technical problems mentioned in the remark at the end of each section are pending and still need to be investigated.Abbreviations
| AGMD | Air gap membrane distillation |
| ATBC | Acetyl tributyl citrate |
| DCMD | Direct contact membrane distillation |
| DAMc | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| ECTFE | Poly(ethene-co-chlorotrifluoroethene) |
| EIPS | Evaporation induced phase separation |
| FASs | Fluoroalkyl silanes |
| FEP | Poly(tetrafluoroethylene-co-hexafluoropropylene) |
| FDTS | (Heptadecafluoro-1,1,2,2-tetrahydrodecyl)trichlorosilane |
| FTCS | Fluorododecyltrichlorosilane |
| GEMs | Gas-entrapping membranes |
| LEP | Liquid entry pressure |
| MD | Membrane distillation |
| MS-S | Melt-spinning and stretching |
| NCC | Nanocrystalline cellulose |
| NIPS | Non-solvent induced phase separation |
| NMP | N-Methyl-2-pyrrolidone |
| PE | Polyethylene |
| PEG | Polyethylene glycol |
| PES | Polyethersulfone |
| PMMA | Poly(methyl methacrylate) |
| PP | Polypropylene |
| PTFE | Polytetrafluorethylene |
| PVDF | Polyvinylidene difluoride |
| PVDF-HFP | Poly-(vinylidene fluoride-hexafluoropropylene) |
| SANIPS | Spray-assisted nonsolvent-induced phase separation |
| TEP | Triethyl phosphate |
| TIPS | Thermally induced phase separation |
| TOTM | Trioctyl trimellitate |
| VIPS | Vapor-induced phase separation |
| VMD | Vacuum membrane distillation |
| WGMD | Water gap membrane distillation |
Conflicts of interest
There are no conflicts to declare.References
- M. M. Pendergast and E. M. V. Hoek, A review of water treatment membrane nanotechnologies, Energy Environ. Sci., 2011, 4, 1946, 10.1039/c0ee00541j.
- H. B. Harandi, M. Rahnama, E. Jahanshahi Javaran and A. Asadi, Performance optimization of a multi stage flash desalination unit with thermal vapor compression using genetic algorithm, Appl. Therm. Eng., 2017, 123, 1106–1119, DOI:10.1016/j.applthermaleng.2017.05.170.
- A. Lee, J. W. Elam and S. B. Darling, Membrane materials for water purification: design, development, and application, Environ. Sci.: Water Res. Technol., 2016, 2, 17–42, 10.1039/C5EW00159E.
- N. C. Darre and G. S. Toor, Desalination of Water: a Review, Curr. Pollut. Rep., 2018, 4, 104–111, DOI:10.1007/s40726-018-0085-9.
- W. Xie, T. Li, A. Tiraferri, E. Drioli, A. Figoli, J. C. Crittenden and B. Liu, Toward the Next Generation of Sustainable Membranes from Green Chemistry Principles, ACS Sustainable Chem. Eng., 2021, 9, 50–75, DOI:10.1021/acssuschemeng.0c07119.
- S. A. Naziri Mehrabani, V. Vatanpour and I. Koyuncu, Green solvents in polymeric membrane fabrication: A review, Sep. Purif. Technol., 2022, 298, 121691, DOI:10.1016/j.seppur.2022.121691.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- United Nations General Assembly, Report of the world commission on environment and development: Our common future, Oslo, Norway, 1987.
- A. Samadi, T. Ni, E. Fontananova, G. Tang, H. Shon and S. Zhao, Engineering antiwetting hydrophobic surfaces for membrane distillation: A review, Desalination, 2023, 563, 116722, DOI:10.1016/j.desal.2023.116722.
- A. Deshmukh, C. Boo, V. Karanikola, S. Lin, A. P. Straub, T. Tong, D. M. Warsinger and M. Elimelech, Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges, Energy Environ. Sci., 2018, 11, 1177–1196, 10.1039/c8ee00291f.
- M. Rezaei, D. M. Warsinger, V. J. H. Lienhard, M. C. Duke, T. Matsuura and W. M. Samhaber, Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention, Elsevier B.V., 2018. DOI:10.1016/j.watres.2018.03.058.
- H. Mishra, A. M. Schrader, D. W. Lee, A. Gallo, S. Y. Chen, Y. Kaufman, S. Das and J. N. Israelachvili, Time-Dependent Wetting Behavior of PDMS Surfaces with Bioinspired, Hierarchical Structures, ACS Appl. Mater. Interfaces, 2016, 8, 8168–8174, DOI:10.1021/acsami.5b10721.
- H. Chang, B. Liu, Z. Zhang, R. Pawar, Z. Yan, J. C. Crittenden and R. D. Vidic, A Critical Review of Membrane Wettability in Membrane Distillation from the Perspective of Interfacial Interactions, Environ. Sci. Technol., 2021, 55, 1395–1418, DOI:10.1021/acs.est.0c05454.
- E. Drioli, A. Ali and F. Macedonio, Membrane distillation: Recent developments and perspectives, Desalination, 2015, 356, 56–84, DOI:10.1016/j.desal.2014.10.028.
- S. Bandehali, H. Sanaeepur, A. Ebadi Amooghin, S. Shirazian and S. Ramakrishna, Biodegradable polymers for membrane separation, Sep. Purif. Technol., 2021, 269, 118731, DOI:10.1016/j.seppur.2021.118731.
- A. K. Rana, V. K. Gupta, A. K. Saini, S. I. Voicu, M. H. Abdellattifaand and V. K. Thakur, Water desalination using nanocelluloses/cellulose derivatives based membranes for sustainable future, Desalination, 2021, 520, 115359, DOI:10.1016/j.desal.2021.115359.
- M. E. Leitch, C. Li, O. Ikkala, M. S. Mauter and G. V. Lowry, Bacterial Nanocellulose Aerogel Membranes: Novel High-Porosity Materials for Membrane Distillation, Environ. Sci. Technol. Lett., 2016, 3, 85–91, DOI:10.1021/acs.estlett.6b00030.
- X. Hu, X. Chen, M. Giagnorio, C. Wu, Y. Luo, C. Hélix-Nielsen, P. Yu and W. Zhang, Beaded electrospun polyvinylidene fluoride (PVDF) membranes for membrane distillation (MD), J. Membr. Sci., 2022, 661, 120850, DOI:10.1016/j.memsci.2022.120850.
- C. Y. Pan, G. R. Xu, K. Xu, H. L. Zhao, Y. Q. Wu, H. C. Su, J. M. Xu and R. Das, Electrospun nanofibrous membranes in membrane distillation: Recent developments and future perspectives, Sep. Purif. Technol., 2019, 221, 44–63, DOI:10.1016/j.seppur.2019.03.080.
- G. Siqueira, J. Bras and A. Dufresne, Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications, Polymers, 2010, 2, 728–765, DOI:10.3390/polym2040728.
- B. S. Lalia, E. Guillen, H. A. Arafat and R. Hashaikeh, Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application, Desalination, 2014, 332, 134–141, DOI:10.1016/j.desal.2013.10.030.
- D. Hou, T. Li, X. Chen, S. He, J. Dai, S. A. Mofid, D. Hou, A. Iddya, D. Jassby, R. Yang, L. Hu and Z. J. Ren, Hydrophobic nanostructured wood membrane for thermally efficient distillation, Sci. Adv., 2019, 5, 1–10, DOI:10.1126/sciadv.aaw3203.
- T. Arumugham, N. J. Kaleekkal, D. Rana and K. I. Sathiyanarayanan, PFOM fillers embedded PVDF/cellulose dual-layered membranes with hydrophobic-hydrophilic channels for desalination: Via direct contact membrane distillation process, RSC Adv., 2019, 9, 41462–41474, 10.1039/c9ra08945d.
- H. Nassrullah, O. Makanjuola, I. Janajreh, F. A. AlMarzooqi and R. Hashaikeh, Incorporation of nanosized LTL zeolites in dual-layered PVDF-HFP/cellulose membrane for enhanced membrane distillation performance, J. Membr. Sci., 2020, 611, 118298, DOI:10.1016/j.memsci.2020.118298.
- R. Joshi, J. Zheng, K. Chi, S. Zhang, X. Huang, P. Hadi, T. Lindstrom and B. S. Hsiao, Superhydrophobic Cellulosic Membranes for Membrane Distillation, ACS ES&T Water, 2022, 2, 1822–1833, DOI:10.1021/acsestwater.2c00343.
- N. Dizge, E. Shaulsky and V. Karanikola, Electrospun cellulose nanofibers for superhydrophobic and oleophobic membranes, J. Membr. Sci., 2019, 590, 117271, DOI:10.1016/j.memsci.2019.117271.
- M. Khayet, Membranes and theoretical modeling of membrane distillation: A review, Adv. Colloid Interface Sci., 2011, 164, 56–88, DOI:10.1016/j.cis.2010.09.005.
- B. S. Lalia, V. Kochkodan, R. Hashaikeh and N. Hilal, A review on membrane fabrication: Structure, properties and performance relationship, Desalination, 2013, 326, 77–95, DOI:10.1016/j.desal.2013.06.016.
- M. Khayet, T. Matsuura, J. I. Mengual and M. Qtaishat, Design of novel direct contact membrane distillation membranes, Desalination, 2006, 192, 105–111, DOI:10.1016/j.desal.2005.06.047.
- D. Zou, S. P. Nunes, I. F. J. Vankelecom, A. Figoli and Y. M. Lee, Recent advances in polymer membranes employing non-toxic solvents and materials, Green Chem., 2021, 23, 9815–9843, 10.1039/d1gc03318b.
- M. Chastrette, M. Rajzmann, M. Chanon and K. F. Purcell, Approach to a general classification of solvents using a multivariate statistical treatment of quantitative solvent parameters, J. Am. Chem. Soc., 1985, 107, 1–11, DOI:10.1021/ja00287a001.
- D. R. Joshi and N. Adhikari, An Overview on Common Organic Solvents and Their Toxicity, J. Pharm. Res. Int., 2019, 1–18, DOI:10.9734/jpri/2019/v28i330203.
- D. Prat, J. Hayler and A. Wells, A survey of solvent selection guides, Green Chem., 2014, 16, 4546–4551, 10.1039/c4gc01149j.
- C. M. Hansen, Hansen Solubility Parameters: A User's book, CRC Press, Taylor & Francis Group, New York, 2nd edn, 2007 Search PubMed.
- S. Fadhil, T. Marino, H. F. Makki, Q. F. Alsalhy, S. Blefari, F. Macedonio, E. Di Nicolò, L. Giorno, E. Drioli and A. Figoli, Novel PVDF-HFP flat sheet membranes prepared by triethyl phosphate (TEP) solvent for direct contact membrane distillation, Chem. Eng. Process., 2016, 102, 16–26, DOI:10.1016/j.cep.2016.01.007.
- H. Zhang, X. Lu, Z. Liu, Z. Ma, S. Wu, Z. Li, X. Kong, J. Liu and C. Wu, The unidirectional regulatory role of coagulation bath temperature on cross-section radius of the PVDF hollow-fiber membrane, J. Membr. Sci., 2018, 550, 9–17, DOI:10.1016/j.memsci.2017.12.059.
- Y. Li, C. Jin, Y. Peng, Q. An, Z. Chen, J. Zhang, L. Ge and S. Wang, Fabrication of PVDF hollow fiber membranes via integrated phase separation for membrane distillation, J. Taiwan Inst. Chem. Eng., 2019, 95, 487–494, DOI:10.1016/j.jtice.2018.08.036.
- S. Nejati, C. Boo, C. O. Osuji and M. Elimelech, Engineering flat sheet microporous PVDF films for membrane distillation, J. Membr. Sci., 2015, 492, 355–363, DOI:10.1016/j.memsci.2015.05.033.
- G. Liu, J. Pan, X. Xu, Z. Wang and Z. Cui, Preparation of ECTFE porous membrane with a green diluent TOTM and performance in VMD process, J. Membr. Sci., 2020, 612, 118375, DOI:10.1016/j.memsci.2020.118375.
- K. Xu, Y. Cai, N. T. Hassankiadeh, Y. Cheng, X. Li, X. Wang, Z. Wang, E. Drioli and Z. Cui, ECTFE membrane fabrication via TIPS method using ATBC diluent for vacuum membrane distillation, Desalination, 2019, 456, 13–22, DOI:10.1016/j.desal.2019.01.004.
- Y. J. Wang, Y. Liu, X. Wei, L. M. Ding, Y. N. Feng and Z. P. Zhao, Polypropylene hollow fiber membrane prepared with green binary diluents via TIPS: Effects of spinning process parameters and VMD desalination performance, Desalination, 2022, 541, 116026, DOI:10.1016/j.desal.2022.116026.
- Y. J. Wang, Z. P. Zhao, Z. Y. Xi and S. Y. Yan, Microporous polypropylene membrane prepared via TIPS using environment-friendly binary diluents and its VMD performance, J. Membr. Sci., 2018, 548, 332–344, DOI:10.1016/j.memsci.2017.11.023.
- R. Ding, S. Chen, H. Xuan, B. Li and Y. Rui, Green-solvent-processed amphiphobic polyurethane nanofiber membranes with mechanically stable hierarchical structures for seawater desalination by membrane distillation, Desalination, 2021, 516, 115223, DOI:10.1016/j.desal.2021.115223.
- D. Zou, C. Hu, E. Drioli and Z. Zhong, Engineering green and high-flux poly(vinylidene fluoride) membranes for membrane distillation via a facile co-casting process, J. Membr. Sci., 2022, 655, 120577, DOI:10.1016/j.memsci.2022.120577.
- M. Pagliero, A. Comite, C. Costa, I. Rizzardi and O. Soda, A Single Step Preparation of Photothermally Active Polyvinylidene Fluoride Membranes Using Triethyl Phosphate as a Green Solvent for Distillation Applications, Membranes, 2021, 11, 896, DOI:10.3390/membranes11110896.
- T. Marino, S. Blefari, E. Di Nicolò and A. Figoli, A more sustainable membrane preparation using triethyl phosphate as solvent, Green Process. Synth., 2017, 6, 295–300, DOI:10.1515/gps-2016-0165.
- J. Chang, J. Zuo, L. Zhang, G. S. O'Brien and T. S. Chung, Using green solvent, triethyl phosphate (TEP), to fabricate highly porous PVDF hollow fiber membranes for membrane distillation, J. Membr. Sci., 2017, 539, 295–304, DOI:10.1016/j.memsci.2017.06.002.
- C. Meringolo, T. F. Mastropietro, T. Poerio, E. Fontananova, G. De Filpo, E. Curcio and G. Di Profio, Tailoring PVDF Membranes Surface Topography and Hydrophobicity by a Sustainable Two-Steps Phase Separation Process, ACS Sustainable Chem. Eng., 2018, 6, 10069–10077, DOI:10.1021/acssuschemeng.8b01407.
- T. Marino, F. Russo, A. Criscuoli and A. Figoli, TamiSolve® NxG as novel solvent for polymeric membrane preparation, J. Membr. Sci., 2017, 542, 418–429, DOI:10.1016/j.memsci.2017.08.038.
- C. Ursino, F. Russo, R. M. Ferrari, M. P. De Santo, E. Di Nicolò, T. He, F. Galiano and A. Figoli, Polyethersulfone hollow fiber membranes prepared with Polarclean® as a more sustainable solvent, J. Membr. Sci., 2020, 608, 118216, DOI:10.1016/j.memsci.2020.118216.
- W. Xie, T. Li, C. Chen, H. Wu, S. Liang, H. Chang, B. Liu, E. Drioli, Q. Wang and J. C. Crittenden, Using the Green Solvent Dimethyl Sulfoxide to Replace Traditional Solvents Partly and Fabricating PVC/PVC- g-PEGMA Blended Ultrafiltration Membranes with High Permeability and Rejection, Ind. Eng. Chem. Res., 2019, 58, 6413–6423, DOI:10.1021/acs.iecr.9b00370.
- B. Tang, H. Zhang and K. H. Row, Application of deep eutectic solvents in the extraction and separation of target compounds from various samples, J. Sep. Sci., 2015, 38, 1053–1064, DOI:10.1002/jssc.201401347.
- F. A. Janjhi, R. Castro-Muñoz and G. Boczkaj, Deep eutectic solvents – Ideal solution for clean air or hidden danger?, Sep. Purif. Technol., 2023, 314, 123590, DOI:10.1016/j.seppur.2023.123590.
- Y. Huang, C. Xiao, Q. Huang, H. Liu and J. Zhao, Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development, Chem. Eng. J., 2021, 403, 126295, DOI:10.1016/j.cej.2020.126295.
- S. Mosadegh-Sedghi, D. Rodrigue, J. Brisson and M. C. Iliuta, Highly hydrophobic microporous low-density polyethylene hollow fiber membranes by melt-extrusion coupled with salt-leaching technique, Polym. Adv. Technol., 2013, 24, 584–592, DOI:10.1002/pat.3122.
- B. T. Kim, K. Song and S. S. Kim, Effects of nucleating agents on preparation of polypropylene hollow fiber membranes by melt spinning process, Macromol. Res., 2002, 10, 127–134, DOI:10.1007/BF03218302.
- C.-H. Du, Y.-Y. Xu and B.-K. Zhu, Structure formation and characterization of PVDF hollow fiber membranes by melt-spinning and stretching method, J. Appl. Polym. Sci., 2007, 106, 1793–1799, DOI:10.1002/app.26867.
- C.-H. Du, B.-K. Zhu and Y.-Y. Xu, A Study on the Relationship Between the Crystal Structure and Hard Elasticity of PVDF Fibers, Macromol. Mater. Eng., 2005, 290, 786–791, DOI:10.1002/mame.200400327.
- X. Hu, C. Xiao, S. An and G. Jia, Study on the interfacial micro-voids of poly(vinylidene difluoride)/polyurethane blend membrane, J. Mater. Sci., 2007, 42, 6234–6239, DOI:10.1007/s10853-006-1107-3.
- X. Y. Hu, Y. B. Chen, H. X. Liang and C. F. Xiao, Preparation of polyurethane/poly(vinylidene fluoride) blend hollow fibre membrane using melt spinning and stretching, Mater. Sci. Technol., 2011, 27, 661–665, DOI:10.1179/026708309X12526555493233.
- D. Ji, Y. Gao, W. Wang, H. Feng, K. Chen and C. Xiao, Green preparation of PVDF hollow fiber membranes with multiple pore structure via melt spinning method for oil/water separation, J. Environ. Chem. Eng., 2022, 10, 108337, DOI:10.1016/j.jece.2022.108337.
- D. Ji, C. Xiao, K. Chen, F. Zhou, Y. Gao, T. Zhang and H. Ling, Solvent-free green fabrication of PVDF hollow fiber MF membranes with controlled pore structure via melt-spinning and stretching, J. Membr. Sci., 2021, 621, 118953, DOI:10.1016/j.memsci.2020.118953.
- Y. Huang, Q. Huang, H. Liu, C. Xiao and K. Sun, A facile and environmental-friendly strategy for preparation of poly (tetrafluoroethylene-co-hexafluoropropylene) hollow fiber membrane and its membrane emulsification performance, Chem. Eng. J., 2020, 384, 123345, DOI:10.1016/j.cej.2019.123345.
- F. Shao, L. Ni, Y. Zhang, Y. Chen, Z. Liu and Z. Cao, Study on vacuum membrane distillation of PP hollow fiber membranes used in concentrated seawater from low-pressure reverse osmosis, Desalin. Water Treat., 2013, 51, 3925–3929, DOI:10.1080/19443994.2013.795003.
- K. Chen, C. Xiao, Q. Huang, H. Liu, H. Liu, Y. Wu and Z. Liu, Study on vacuum membrane distillation (VMD) using FEP hollow fiber membrane, Desalination, 2015, 375, 24–32, DOI:10.1016/j.desal.2015.07.021.
- G. Liu, C. Gao, X. Li, C. Guo, Y. Chen and J. Lv, Preparation and properties of porous polytetrafluoroethylene hollow fiber membrane through mechanical operations, J. Appl. Polym. Sci., 2015, 132, 1–10, DOI:10.1002/app.42696.
- H. Zhu, H. Wang, F. Wang, Y. Guo, H. Zhang and J. Chen, Preparation and properties of PTFE hollow fiber membranes for desalination through vacuum membrane distillation, J. Membr. Sci., 2013, 446, 145–153, DOI:10.1016/j.memsci.2013.06.037.
- H. Wang, S. Ding, H. Zhu, F. Wang, Y. Guo, H. Zhang and J. Chen, Effect of stretching ratio and heating temperature on structure and performance of PTFE hollow fiber membrane in VMD for RO brine, Sep. Purif. Technol., 2014, 126, 82–94, DOI:10.1016/j.seppur.2014.02.027.
- D. Luo, G. Xie and S. Qin, The hydrophilic polypropylene/poly(ethylene-co-vinyl alcohol) hollow fiber membrane with bimodal microporous structure prepared by melt-spinning and stretching, Sep. Purif. Technol., 2021, 274, 118890, DOI:10.1016/j.seppur.2021.118890.
- D. Ji, C. Xiao, S. An, K. Chen, Y. Gao, F. Zhou and T. Zhang, Completely green and sustainable preparation of PVDF hollow fiber membranes via melt-spinning and stretching method, J. Hazard. Mater., 2020, 398, 122823, DOI:10.1016/j.jhazmat.2020.122823.
- Y. Huang, H. Liu, C. Xiao, C. Wang, D. Chen and K. Sun, Robust preparation and multiple pore structure design of poly (tetrafluoroethylene-co-hexafluoropropylene) hollow fiber membrane by melt spinning and post-treatment, J. Taiwan Inst. Chem. Eng., 2019, 97, 441–449, DOI:10.1016/j.jtice.2019.02.010.
- G. R. Guillen, Y. Pan, M. Li and E. M. V. Hoek, Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review, Ind. Eng. Chem. Res., 2011, 50, 3798–3817, DOI:10.1021/ie101928r.
- S. Munirasu, F. Banat, A. A. Durrani and M. A. Haija, Intrinsically superhydrophobic PVDF membrane by phase inversion for membrane distillation, Desalination, 2017, 417, 77–86, DOI:10.1016/j.desal.2017.05.019.
- M. Pagliero, A. Bottino, A. Comite and C. Costa, Novel hydrophobic PVDF membranes prepared by nonsolvent induced phase separation for membrane distillation, J. Membr. Sci., 2020, 596, 117575, DOI:10.1016/j.memsci.2019.117575.
- A. K. Ghosh and E. M. V. Hoek, Impacts of support membrane structure and chemistry on polyamide-polysulfone interfacial composite membranes, J. Membr. Sci., 2009, 336, 140–148, DOI:10.1016/j.memsci.2009.03.024.
- M. Razali, J. F. Kim, M. Attfield, P. M. Budd, E. Drioli, Y. M. Lee and G. Szekely, Sustainable wastewater treatment and recycling in membrane manufacturing, Green Chem., 2015, 17, 5196–5205, 10.1039/c5gc01937k.
- J. T. Jung, J. F. Kim, H. H. Wang, E. di Nicolo, E. Drioli and Y. M. Lee, Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS), J. Membr. Sci., 2016, 514, 250–263, DOI:10.1016/j.memsci.2016.04.069.
- S. Simone, A. Figoli, A. Criscuoli, M. C. Carnevale, A. Rosselli and E. Drioli, Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD, J. Membr. Sci., 2010, 364, 219–232, DOI:10.1016/j.memsci.2010.08.013.
- N. T. Hassankiadeh, Z. Cui, J. H. Kim, D. W. Shin, S. Y. Lee, A. Sanguineti, V. Arcella, Y. M. Lee and E. Drioli, Microporous poly(vinylidene fluoride) hollow fiber membranes fabricated with PolarClean as water-soluble green diluent and additives, J. Membr. Sci., 2015, 479, 204–212, DOI:10.1016/j.memsci.2015.01.031.
- D. Hou, J. Wang, D. Qu, Z. Luan and X. Ren, Fabrication and characterization of hydrophobic PVDF hollow fiber membranes for desalination through direct contact membrane distillation, Sep. Purif. Technol., 2009, 69, 78–86, DOI:10.1016/j.seppur.2009.06.026.
- M. Khayet, C. Cojocaru and M. C. García-Payo, Experimental design and optimization of asymmetric flat-sheet membranes prepared for direct contact membrane distillation, J. Membr. Sci., 2010, 351, 234–245, DOI:10.1016/j.memsci.2010.01.057.
- M. Tian, J. Zhu, S. Yuan, Y. Zhang and B. Van der Bruggen, A co-casting route enables the formation of skinless, hydrophobic poly(vinylidene fluoride) membranes for DCMD, J. Membr. Sci., 2021, 630, 119299, DOI:10.1016/j.memsci.2021.119299.
- R. Zhang, W. Tang, H. Gao, C. Wu, S. Gray and X. Lu, In situ construction of superhydrophobic PVDF membrane via NaCl-H2O induced polymer incipient gelation for membrane distillation, Sep. Purif. Technol., 2021, 274, 117762, DOI:10.1016/j.seppur.2020.117762.
- K. J. Lu, D. Zhao, Y. Chen, J. Chang and T.-S. Chung, Rheologically controlled design of nature-inspired superhydrophobic and self-cleaning membranes for clean water production, npj Clean Water, 2020, 3, 30, DOI:10.1038/s41545-020-0078-2.
- H. T. Nguyen, H. M. Bui, Y.-F. Wang and S.-J. You, Non-fluoroalkyl functionalized hydrophobic surface modifications used in membrane distillation for cheaper and more environmentally friendly applications: A mini-review, Sustainable Chem. Pharm., 2022, 28, 100714, DOI:10.1016/j.scp.2022.100714.
- S. Liu, X. Liu, S. S. Latthe, L. Gao, S. An, S. S. Yoon, B. Liu and R. Xing, Self-cleaning transparent superhydrophobic coatings through simple sol-gel processing of fluoroalkylsilane, Appl. Surf. Sci., 2015, 351, 897–903, DOI:10.1016/j.apsusc.2015.06.016.
- K. J. Lu, Y. Chen and T. S. Chung, Design of omniphobic interfaces for membrane distillation – A review, Water Res., 2019, 162, 64–77, DOI:10.1016/j.watres.2019.06.056.
- J. Lee, C. Boo, W. H. Ryu, A. D. Taylor and M. Elimelech, Development of Omniphobic Desalination Membranes Using a Charged Electrospun Nanofiber Scaffold, ACS Appl. Mater. Interfaces, 2016, 8, 11154–11161, DOI:10.1021/acsami.6b02419.
- R. Zheng, Y. Chen, J. Wang, J. Song, X. M. Li and T. He, Preparation of omniphobic PVDF membrane with hierarchical structure for treating saline oily wastewater using direct contact membrane distillation, J. Membr. Sci., 2018, 555, 197–205, DOI:10.1016/j.memsci.2018.03.041.
- C. Li, X. Li, X. Du, T. Tong, T. Y. Cath and J. Lee, Antiwetting and Antifouling Janus Membrane for Desalination of Saline Oily Wastewater by Membrane Distillation, ACS Appl. Mater. Interfaces, 2019, 11, 18456–18465, DOI:10.1021/acsami.9b04212.
- L. Deng, H. Ye, X. Li, P. Li, J. Zhang, X. Wang, M. Zhu and B. S. Hsiao, Self-roughened omniphobic coatings on nanofibrous membrane for membrane distillation, Sep. Purif. Technol., 2018, 206, 14–25, DOI:10.1016/j.seppur.2018.05.035.
- Z. Zhu, Y. Liu, H. Hou, W. Shi, F. Qu, F. Cui and W. Wang, Dual-Bioinspired Design for Constructing Membranes with Superhydrophobicity for Direct Contact Membrane Distillation, Environ. Sci. Technol., 2018, 52, 3027–3036, DOI:10.1021/acs.est.7b06227.
- K. Y. He, Q. Wei, Y. L. Wang, S. Wang, S. P. Cui, Q. Y. Li and Z. R. Nie, Hydrophobic mesoporous organosilica membranes: Preparation and application in the separation of volatile organic compounds from water, Microporous Mesoporous Mater., 2019, 288, 109606, DOI:10.1016/j.micromeso.2019.109606.
- Y. Wang, M. Han, L. Liu, J. Yao and L. Han, Beneficial CNT Intermediate Layer for Membrane Fluorination toward Robust Superhydrophobicity and Wetting Resistance in Membrane Distillation, ACS Appl. Mater. Interfaces, 2020, 12, 20942–20954, DOI:10.1021/acsami.0c03577.
- E. Koh and Y. T. Lee, Preparation of an omniphobic nanofiber membrane by the self-assembly of hydrophobic nanoparticles for membrane distillation, Sep. Purif. Technol., 2021, 259, 118134, DOI:10.1016/j.seppur.2020.118134.
- N. Dizge, E. Shaulsky and V. Karanikola, Electrospun cellulose nanofibers for superhydrophobic and oleophobic membranes, J. Membr. Sci., 2019, 590, 117271, DOI:10.1016/j.memsci.2019.117271.
- A. B. Yeszhanov, I. V. Korolkov, Y. G. Gorin, S. S. Dosmagambetova and M. V. Zdorovets, Membrane distillation of pesticide solutions using hydrophobic track-etched membranes, Chem. Pap., 2020, 74, 3445–3453, DOI:10.1007/s11696-020-01173-7.
- D. Gong, Y. Yin, H. Chen, B. Guo, P. Wu, Y. Wang, Y. Yang, Z. Li, Y. He and G. Zeng, Interfacial Ions Sieving for Ultrafast and Complete Desalination through 2D Nanochannel Defined Graphene Composite Membranes, ACS Nano, 2021, 15, 9871–9881, DOI:10.1021/acsnano.1c00987.
- Sigma-Aldrich, 1H,1H,2H,2H-perfluorodecyltriethoxysilane, 2022.
- C. McCarthy, W. Kappleman and W. DiGuiseppi, Ecological Considerations of Per- and Polyfluoroalkyl Substances (PFAS), Curr. Pollut. Rep., 2017, 3, 289–301, DOI:10.1007/s40726-017-0070-8.
- H. Mahoney, Y. Xie, M. Brinkmann and J. P. Giesy, Next generation per- and poly-fluoroalkyl substances: Status and trends, aquatic toxicity, and risk assessment, Eco-Environ. Heal, 2022, 1, 117–131, DOI:10.1016/j.eehl.2022.05.002.
- C. Yang, M. Tian, Y. Xie, X. M. Li, B. Zhao, T. He and J. Liu, Effective evaporation of CF4 plasma modified PVDF membranes in direct contact membrane distillation, Elsevier, 2015. DOI:10.1016/j.memsci.2015.01.059.
- C. Yang, X. M. Li, J. Gilron, D. Kong, Y. Yin, Y. Oren, C. Linder and T. He, CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation, J. Membr. Sci., 2014, 456, 155–161, DOI:10.1016/j.memsci.2014.01.013.
- Y. C. Woo, Y. Chen, L. D. Tijing, S. Phuntsho, T. He, J. S. Choi, S. H. Kim and H. K. Shon, CF4 plasma-modified omniphobic electrospun nanofiber membrane for produced water brine treatment by membrane distillation, J. Membr. Sci., 2017, 529, 234–242, DOI:10.1016/j.memsci.2017.01.063.
- E. J. Lee, B. J. Deka and A. K. An, Reinforced superhydrophobic membrane coated with aerogel-assisted polymeric microspheres for membrane distillation, J. Membr. Sci., 2019, 573, 570–578, DOI:10.1016/j.memsci.2018.12.019.
- J. Guo, B. J. Deka, P. W. Wong, J. Sun and A. K. An, Fabrication of robust green superhydrophobic hybrid nanofiber-nanosphere membrane for membrane distillation, Desalination, 2021, 520, 115314, DOI:10.1016/j.desal.2021.115314.
- L. Zheng, K. Wang, D. Hou, X. Jia and Z. Zhao, Hierarchically-structured superhydrophobic POSS/PVDF composite membrane for anti-fouling and anti-wetting membrane distillation, Desalination, 2022, 526, 115512, DOI:10.1016/j.desal.2021.115512.
- Y. Zhang, J. Y. Chong, R. Xu and R. Wang, Hydrophobic ceramic membranes fabricated via fatty acid chloride modification for solvent resistant membrane distillation (SR-MD), J. Membr. Sci., 2022, 658, 120715, DOI:10.1016/j.memsci.2022.120715.
- W. Qing, J. Wang, X. Ma, Z. Yao, Y. Feng, X. Shi, F. Liu, P. Wang and C. Y. Tang, One-step tailoring surface roughness and surface chemistry to prepare superhydrophobic polyvinylidene fluoride (PVDF) membranes for enhanced membrane distillation performances, J. Colloid Interface Sci., 2019, 553, 99–107, DOI:10.1016/j.jcis.2019.06.011.
- A. Alkhudhiri, N. Darwish and N. Hilal, Membrane distillation: A comprehensive review, Desalination, 2012, 287, 2–18, DOI:10.1016/j.desal.2011.08.027.
- H. S. Usman, K. Touati and M. S. Rahaman, An economic evaluation of renewable energy-powered membrane distillation for desalination of brackish water, Renewable Energy, 2021, 169, 1294–1304, DOI:10.1016/j.renene.2021.01.087.
- Z. Yuan, Y. Yu, L. Wei, X. Sui, Q. She and Y. Chen, Pressure-retarded membrane distillation for simultaneous hypersaline brine desalination and low-grade heat harvesting, J. Membr. Sci., 2020, 597, 117765, DOI:10.1016/j.memsci.2019.117765.
- G. Naidu, L. Tijing, M. A. H. Johir, H. Shon and S. Vigneswaran, Hybrid membrane distillation: Resource, nutrient and energy recovery, J. Membr. Sci., 2020, 599, 117832, DOI:10.1016/j.memsci.2020.117832.
- A. Yadav, P. K. Labhasetwar and V. K. Shahi, Membrane distillation using low-grade energy for desalination: A review, J. Environ. Chem. Eng., 2021, 9, 105818, DOI:10.1016/j.jece.2021.105818.
- R. Sarbatly and C. K. Chiam, Evaluation of geothermal energy in desalination by vacuum membrane distillation, Appl. Energy, 2013, 112, 737–746, DOI:10.1016/j.apenergy.2012.12.028.
- D. Amaya-Vías and J. A. López-Ramírez, Techno-Economic Assessment of Air and Water Gap Membrane Distillation for Seawater Desalination under Different Heat Source Scenarios, Water, 2019, 11, 2117, DOI:10.3390/w11102117.
- P. Bendevis, A. Karam and T. M. Laleg-Kirati, Optimal model-free control of solar thermal membrane distillation system, Comput. Chem. Eng., 2020, 133, 106622, DOI:10.1016/j.compchemeng.2019.106622.
- J. D. Gil, L. Roca, A. Ruiz-Aguirre, G. Zaragoza and M. Berenguel, Optimal operation of a Solar Membrane Distillation pilot plant via Nonlinear Model Predictive Control, Comput. Chem. Eng., 2018, 109, 151–165, DOI:10.1016/j.compchemeng.2017.11.012.
- Y. H. Chen, Y. W. Li and H. Chang, Optimal design and control of solar driven air gap membrane distillation desalination systems, Appl. Energy, 2012, 100, 193–204, DOI:10.1016/j.apenergy.2012.03.003.
- Q. Ma, A. Ahmadi and C. Cabassud, Optimization and design of a novel small-scale integrated vacuum membrane distillation - solar flat-plate collector module with heat recovery strategy through heat pumps, Desalination, 2020, 478, 114285, DOI:10.1016/j.desal.2019.114285.
- M. I. Soomro, W. S. Kim and Y. D. Kim, Performance and cost comparison of different concentrated solar power plants integrated with direct-contact membrane distillation system, Energy Convers. Manage., 2020, 221, 113193, DOI:10.1016/j.enconman.2020.113193.
- S. E. Moore, S. D. Mirchandani, V. Karanikola, T. M. Nenoff, R. G. Arnold and A. E. Sáez, Process modeling for economic optimization of a solar driven sweeping gas membrane distillation desalination system, Desalination, 2018, 437, 108–120, DOI:10.1016/j.desal.2018.03.005.
- D. Amaya-Vías, E. Nebot and J. A. López-Ramírez, Comparative studies of different membrane distillation configurations and membranes for potential use on board cruise vessels, Desalination, 2018, 429, 44–51, DOI:10.1016/j.desal.2017.12.008.
- R. Miladi, N. Frikha, A. Kheiri and S. Gabsi, Energetic performance analysis of seawater desalination with a solar membrane distillation, Energy Convers. Manage., 2019, 185, 143–154, DOI:10.1016/j.enconman.2019.02.011.
- M. Morciano, M. Fasano, L. Bergamasco, A. Albiero, M. Lo Curzio, P. Asinari and E. Chiavazzo, Sustainable freshwater production using passive membrane distillation and waste heat recovery from portable generator sets, Appl. Energy, 2020, 258, 114086, DOI:10.1016/j.apenergy.2019.114086.
- M. R. Silva, B. G. Reis, L. B. Grossi and M. C. S. Amaral, Improving the energetic efficiency of direct-contact membrane distillation in mining effluent by using the waste-heat-and-water process as the cooling fluid, J. Cleaner Prod., 2020, 260, 121035, DOI:10.1016/j.jclepro.2020.121035.
- M. Baghbanzadeh, D. Rana, C. Q. Lan and T. Matsuura, Zero thermal input membrane distillation, a zero-waste and sustainable solution for freshwater shortage, Appl. Energy, 2017, 187, 910–928, DOI:10.1016/j.apenergy.2016.10.142.
- N. Ghaffour, S. Soukane, J. G. Lee, Y. Kim and A. Alpatova, Membrane distillation hybrids for water production and energy efficiency enhancement: A critical review, Appl. Energy, 2019, 254, 113698, DOI:10.1016/j.apenergy.2019.113698.
- F. E. Ahmed, R. Hashaikeh and N. Hilal, Hybrid technologies: The future of energy efficient desalination – A review, Desalination, 2020, 495, 114659, DOI:10.1016/j.desal.2020.114659.
- H. C. Duong, A. R. Chivas, B. Nelemans, M. Duke, S. Gray, T. Y. Cath and L. D. Nghiem, Treatment of RO brine from CSG produced water by spiral-wound air gap membrane distillation - A pilot study, Desalination, 2015, 366, 121–129, DOI:10.1016/j.desal.2014.10.026.
- R. González-Bravo, J. M. Ponce-Ortega and M. M. El-Halwagi, Optimal Design of Water Desalination Systems Involving Waste Heat Recovery, Ind. Eng. Chem. Res., 2017, 56, 1834–1847, DOI:10.1021/acs.iecr.6b04725.
- P. Wang, Y. Cui, Q. Ge, T. Fern Tew and T. S. Chung, Evaluation of hydroacid complex in the forward osmosis-membrane distillation (FO-MD) system for desalination, J. Membr. Sci., 2015, 494, 1–7, DOI:10.1016/j.memsci.2015.07.022.
- Y. D. Kim, K. Thu, K. C. Ng, G. L. Amy and N. Ghaffour, A novel integrated thermal-/membrane-based solar energy-driven hybrid desalination system: Concept description and simulation results, Water Res., 2016, 100, 7–19, DOI:10.1016/j.watres.2016.05.002.
- K. Sardari, P. Fyfe, D. Lincicome and S. R. Wickramasinghe, Combined electrocoagulation and membrane distillation for treating high salinity produced waters, J. Membr. Sci., 2018, 564, 82–96, DOI:10.1016/j.memsci.2018.06.041.
| This journal is © The Royal Society of Chemistry 2024 |