Open Access Article
Open Access ArticleBorane-catalysed C2-selective indole reductive functionalisation†
Kieran
Nicholson
 a,
Sarah L.
McOnie
a,
Sarah L.
McOnie
 a,
Thomas
Langer
b,
Gary S.
Nichol
a,
Thomas
Langer
b,
Gary S.
Nichol
 a and
Stephen P.
Thomas
a and
Stephen P.
Thomas
 *a
*a
aEaStCHEM School of Chemistry, University of Edinburgh David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: stephen.thomas@ed.ac.uk
bPharmaceutical Technology & Development, Chemicals Development U.K., AstraZeneca, Macclesfield, SK10 2NA, UK
First published on 9th September 2024
Abstract
Indolines are common motifs within pharamceuticals and natural products. Boron catalysis enables the chemoselective allylation of indoles to give allylic indolines in excellent diastereoselectivity. Mechanistic studies revealed in situ formation of the allylic borane, allylation of the imine tautomer of the indole and B–N/B–H transborylation for catalytic turnover.
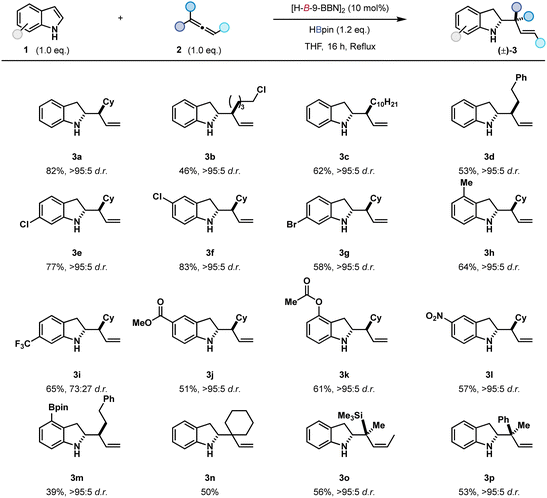
Indolines are useful building blocks in organic synthesis as their derivatives are widely found throughout pharmaceuticals and natural products, thus the development of facile functionalisation is a worthwhile pursuit.1 Indoles are commonly used as nucleophiles in C3 selective Friedel–Crafts-type acylation2 and alkylation reactions,3 although electrophilic functionalisation4 at other positions including C2,5 C4,6 C5,7 C68 and C79 has been reported.10 The reaction of an indole as the electrophile is less developed.11 Stoichiometric, reductive allylation to give allylic indolines has been reported, though is limited to pre-functionalised substrates, telescope reactivity, and often requires the use of indoles bearing electron-donating groups.12 A notable catalytic example is Szabó's use of enantioenriched diol catalysts and allylic boronic acids for C2 reductive allylation (Scheme 1a).12j
 | ||
| Scheme 1 (a) Existing functionalisations of indoles at C2 position. (b) This work: boron catalysed allylation of indoles. | ||
The electrophilic reactivity of indoles is proposed to proceed through an imine tautomer at the C2 position.12a The imine has been trapped using allylic boronic acids and esters in stoichiometric studies (Scheme 1a), with these pre-functionalised substrates typically prepared by transition metal catalysts.13 Allylic boranes have not been as widely used due to their reduced stability14 compared to boronic acids and esters, however, they can be readily prepared by allene hydroboration15 and are more reactive than the boronic ester equivalents. Stoichiometric reaction of an indole with an allylic borane gives a B–N bond of the indoline, which is cleaved upon work-up and the borane destroyed. Recently, transborylation (boron exchange) catalysis has been developed to achieve catalytic turnover at B–heteroatom bonds.16
Here we sought to develop a catalytic, reductive allylation of unprotected indoles using allenes and boron catalysis. The C2 allylation of indoles faces several challenges, firstly unwanted reductive dimerisation of indoles must be avoided.17 To achieve high diastereoselectivity in the product indoline, strict control of the diastereoselectivity in forming the allylic borane reagent and (E)/(Z)-isomerisation of the allylborane is required. This is particularly relevant for dialkyl allylic boranes as both (E) and (Z) diastereomers exists in equilibrium with rapid interchange.15d Chemoselectivity must also be considered to prevent (nucleophilic) C3 functionalisation. Finally, direct reduction of the indole to indoline,16c and hydroboration of the indole18 must be negated both of which have been reported using boron catalysis.
Inspired by our previous studies on borane-catalysed allylation reactions,16g H-B-9-borabicyclo[3.3.1]nonane, [H-B-9-BBN]2, was tested as a catalyst for the reductive allylation of indole with cyclohexylallene using HBpin as the turnover reagent. [H-B-9-BBN]2 (10 mol%) was found to be the optimal catalyst when the reaction was carried out under reflux in THF for 16 hours to give 2-(1-cyclohexylallyl)indoline 3a in excellent yield (81%), diastereoselectivity (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) and as a single (C2) regioisomer. Indole reductive dimerisation,17 hydroboration,18 reduction to the indoline16c or C3 functionalisation were not observed. The anticipated anti-indoline product was observed presumably due to reaction of the (E)-allylic borane – formed by syn hydroboration and isomerisation15a – through a Zimmerman–Traxler-type transition-state structure. The use of other solvents including hexane, toluene and CH2Cl2 gave reduced yields, likely due to the poor solubility of the indole substrate. Decreasing the reaction temperature and catalyst loading resulted in reduced yields, while increasing reaction time did not result in a significant yield enhancement with no effect on diastereoselectivity in each case. Application of this catalytic system to other N-heterocycles including pyrrole, quinolone, isoquinoline, benzoimidazole, benzoxazole, and benzothiazole was unsuccessful as was reaction of benzofuran and benzothiophene.
5 d.r.) and as a single (C2) regioisomer. Indole reductive dimerisation,17 hydroboration,18 reduction to the indoline16c or C3 functionalisation were not observed. The anticipated anti-indoline product was observed presumably due to reaction of the (E)-allylic borane – formed by syn hydroboration and isomerisation15a – through a Zimmerman–Traxler-type transition-state structure. The use of other solvents including hexane, toluene and CH2Cl2 gave reduced yields, likely due to the poor solubility of the indole substrate. Decreasing the reaction temperature and catalyst loading resulted in reduced yields, while increasing reaction time did not result in a significant yield enhancement with no effect on diastereoselectivity in each case. Application of this catalytic system to other N-heterocycles including pyrrole, quinolone, isoquinoline, benzoimidazole, benzoxazole, and benzothiazole was unsuccessful as was reaction of benzofuran and benzothiophene.
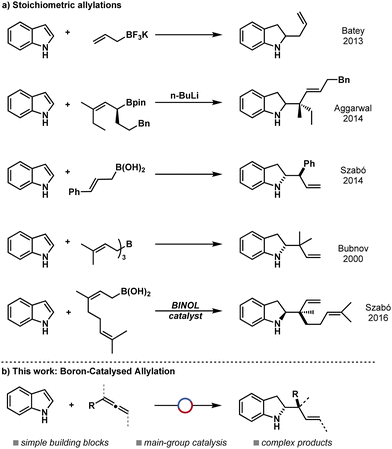
Having optimised the reaction conditions the catalytic protocol was applied to a diverse scope of indoles and allenes (Fig. 1). 2-(1-Cyclohexylallyl)indoline was isolated in good yield and excellent d.r. 3a (82% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.). Other allenes were tested to expand the scope of allylation with good yields and excellent diastereoselectivities including chloro-containing allene 3b (46% yield, >95
5 d.r.). Other allenes were tested to expand the scope of allylation with good yields and excellent diastereoselectivities including chloro-containing allene 3b (46% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.), alkyl allene 3c (62% yield, >95
5 d.r.), alkyl allene 3c (62% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) and reaction of penta-3,4-dienyl-benzene to give 3d (53% yield, >95
5 d.r.) and reaction of penta-3,4-dienyl-benzene to give 3d (53% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.). Previous stoichiometric studies showed limited substitution about the indole, often limited to only alkyl12a,12d or methoxy12c groups. The only stoichiometric example of a reductive allylation of halide substituted indole resulted in poor d.r.12e This catalytic protocol was applied to numerous halo-indoles including chloro-3e (77% yield, >95
5 d.r.). Previous stoichiometric studies showed limited substitution about the indole, often limited to only alkyl12a,12d or methoxy12c groups. The only stoichiometric example of a reductive allylation of halide substituted indole resulted in poor d.r.12e This catalytic protocol was applied to numerous halo-indoles including chloro-3e (77% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) 3f (83% yield, >95
5 d.r.) 3f (83% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) and bromo-3g (58% yield, >95
5 d.r.) and bromo-3g (58% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) exhibiting good yields and excellent diastereoselectivities in all cases. This clearly demonstrates the benefits of this protocol over the stoichiometric counterparts by enabling wider functional group tolerance and orthogonal reactivity. Other substituents on the indole including methyl 3h (64% yield, >95
5 d.r.) exhibiting good yields and excellent diastereoselectivities in all cases. This clearly demonstrates the benefits of this protocol over the stoichiometric counterparts by enabling wider functional group tolerance and orthogonal reactivity. Other substituents on the indole including methyl 3h (64% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) and trifluoromethyl 3i (65% yield, 73
5 d.r.) and trifluoromethyl 3i (65% yield, 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 d.r.) were reacted in good yields and selectivity, the latter being especially interesting as previous studies often required the use of electron-donating groups on the arene. Ester groups were tolerated under the standard reaction conditions 3j (51% yield, >95
27 d.r.) were reacted in good yields and selectivity, the latter being especially interesting as previous studies often required the use of electron-donating groups on the arene. Ester groups were tolerated under the standard reaction conditions 3j (51% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) 3k (61% yield, >95
5 d.r.) 3k (61% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.), in contrast to stoichiometric studies which gave only trace yield for these substrates.12e An indole bearing a nitro group underwent chemoselective allylation giving good yield and excellent diastereoselectivity 3l (56%, >95
5 d.r.), in contrast to stoichiometric studies which gave only trace yield for these substrates.12e An indole bearing a nitro group underwent chemoselective allylation giving good yield and excellent diastereoselectivity 3l (56%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.), again showing reactivity beyond stoichiometric allylations where this substrate resulted in only trace product.12e X-ray crystallography of this indoline product allowed assignment as the anti diastereomer (Scheme 2a). Bpin-bearing indole was reacted to give the allyl indoline in good yield and diastereoselectivtiy 3m (39% yield, >95
5 d.r.), again showing reactivity beyond stoichiometric allylations where this substrate resulted in only trace product.12e X-ray crystallography of this indoline product allowed assignment as the anti diastereomer (Scheme 2a). Bpin-bearing indole was reacted to give the allyl indoline in good yield and diastereoselectivtiy 3m (39% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) with no observed protodeboronation or boron exchange of the aryl Bpin. Vinylidenecyclohexane 3n (55% yield) was successfully reacted with indole to give the product in good yield. Trisubstituted allene gave indoline 3o (56% yield, >95
5 d.r.) with no observed protodeboronation or boron exchange of the aryl Bpin. Vinylidenecyclohexane 3n (55% yield) was successfully reacted with indole to give the product in good yield. Trisubstituted allene gave indoline 3o (56% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) in good yield and diastereoselectivity. 1-Methyl-1-phenylallene was also reacted with indole to provide a further example of allylation 3p (53% yield, >95
5 d.r.) in good yield and diastereoselectivity. 1-Methyl-1-phenylallene was also reacted with indole to provide a further example of allylation 3p (53% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.) and the generation of a quaternary stereocenter.
5 d.r.) and the generation of a quaternary stereocenter.
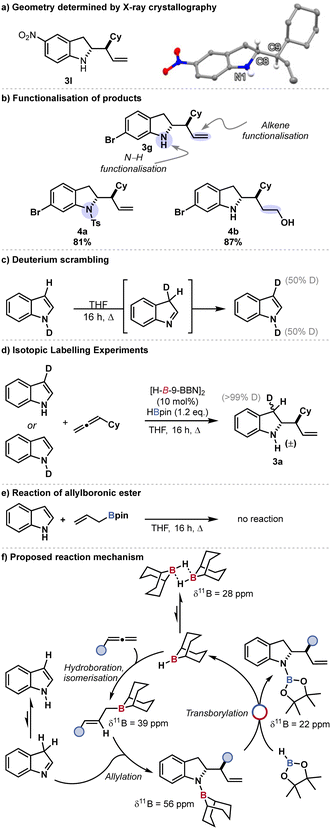 | ||
| Scheme 2 (a) Crystal structure of allyl indoline 3l. Grey = carbon, blue = nitrogen, white = hydrogen. All other H atoms omitted for clarity. (b) Derivatisation of allylindoline 3g. 4a synthesised by reaction of the indoline 3g with tosylchloride at room temperature in CH2Cl2/pyridine. 4b synthesised by hydroboration/oxidation of 3g with H-B-9-BBN then hydrogen peroxide and sodium hydroxide. (c) Deuterium scrambling under reaction conditions in the absence of catalyst and allene (d) isotopic labelling experiments. (e) Reaction of allylboronic ester (f) proposed catalytic cycle.21 | ||
The synthetic utility of the indoline products was demonstrated through onward reaction of bromoindoline 3g;. N-tosylation194a proceeded smoothly as did chemoselective alkene hydroboration–oxidation204b (Scheme 2b).
Indoles have been proposed to react through an aldimine intermediate for C2 C–C bond forming reactions where the indole acts as an electrophile.12a To investigate if such an intermediate was involved in this catalysis, N-D-indole-d1 was subject to catalytic conditions (reflux, THF, 16 h) in the absence of catalyst and allene. The 3-D-indole-d1 was observed indicating that indole tautomerisation was possible under reaction conditions. Catalytic reaction of N-D-indole-d1 or 3-D-indole-d1 similarly resulted in the same deuteroproduct 3-D-allylic indoline 3a-d1. Reaction of allyl pinacol bornic ester (allyl-Bpin) with indole under reaction conditions gave no observed indoline product, indicating the reaction proceeds through an allyllic-B-9-BBN intermediate and that transborylation (boron-boron exchange) occurs exclusively at nitrogen (N–B/B–H exchange).16a–d A catalytic cycle was thus proposed whereby: 1. The dialkylborane catalyst reacts with the allene to give an allylic borane which isomerises to the (E)-allylborane; 2. The allylic borane reacts with the imine tautomer of the indole at the C2 position to give an N-B-9-BBN-2-allylic indoline.12a 3. B–N/B–H transborylation of the N-B-9-BBN-allylic indoline with HBpin regenerates the catalyst (H-B-9-BBN) and gives a N-boronic ester, N-Bpin-2-allylic indoline.
In summary a catalytic protocol for the reductive allylation of indoles has been developed. The reaction was successfully applied to a broad substrate scope of indoles and allenes, and, significantly, greatly expanded on the reactivity and functional group tolerance of the (previously reported) stoichiometric reactions. The reaction proceeded in excellent diastereoselectivity to give allylic indoline products which could be derivatised through synthetic handles thus allowing onward reactivity. Mechanistic studies indicated that the catalysis proceeds through a key indole-imine tautomerisation and B–N/B–H transborylation for catalytic turnover.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. Dadashpour and S. Emami, Eur. J. Med. Chem., 2018, 150, 9–29 CrossRef CAS; (b) N. Chadha and O. Silakari, Eur. J. Med. Chem., 2017, 134, 159–184 CrossRef CAS PubMed; (c) S. Sugimoto, M. Naganuma and T. Kanai, J. Gastroenterol., 2016, 51, 853–861 CrossRef CAS; (d) T. V. Sravanthi and S. L. Manju, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS PubMed; (e) H. A. Hamid, A. N. M. Ramli and M. M. Yusoff, Front. Pharmacol., 2017, 8, 1–7 Search PubMed.
- O. Ottoni, A. d V. F. Neder, A. K. B. Dias, R. P. A. Cruz and L. B. Aquino, Org. Lett., 2001, 3, 1005–1007 CAS.
- M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550–556 CrossRef CAS PubMed.
- N. Cironis, K. Yuan, S. P. Thomas and M. J. Ingleson, Eur. J. Org. Chem., 2022, e202101394 CrossRef CAS.
- A. A. Toutov, W.-B. Liu, K. N. Betz, A. Fedorov, B. M. Stoltz and R. H. Grubbs, Nature, 2015, 518, 80–84 CrossRef CAS.
- J. Kalepu, P. Gandeepan, L. Ackermann and L. T. Pilarski, Chem. Sci., 2018, 9, 4203–4216 RSC.
- (a) M. Montesinos-Magraner, C. Vila, A. Rendón-Patiño, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, ACS Catal., 2016, 6, 2689–2693 CrossRef; (b) M. Montesinos-Magraner, C. Vila, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, Org. Lett., 2017, 19, 1546–1549 CrossRef PubMed; (c) Y. Yang, P. Gao, Y. Zhao and Z. Shi, Angew. Chem., Int. Ed., 2017, 56, 3966–3971 CrossRef PubMed.
- (a) H. Liu, C. Zheng and S.-L. You, J. Org. Chem., 2014, 79, 1047–1054 CrossRef PubMed; (b) Y. Yang, R. Li, Y. Zhao, D. Zhao and Z. Shi, J. Am. Chem. Soc., 2016, 138, 8734–8737 CrossRef PubMed.
- S. A. Iqbal, J. Cid, R. J. Procter, M. Uzelac, K. Yuan and M. J. Ingleson, Angew. Chem., Int. Ed., 2019, 58, 15381–15385 CrossRef PubMed.
- (a) J. Wen and Z. Shi, Acc. Chem. Res., 2021, 54, 1723–1736 CrossRef; (b) J. A. Leitch, Y. Bhonoah and C. G. Frost, ACS Catal., 2017, 7, 5618–5627 CrossRef CAS.
- (a) M. Bandini, Org. Biomol. Chem., 2013, 11, 5206–5212 RSC; (b) B. Deka, M. L. Deb and P. K. Baruah, Top. Curr. Chem., 2020, 378, 22 CrossRef CAS PubMed.
- (a) F. Nowrouzi and R. A. Batey, Angew. Chem., Int. Ed., 2013, 52, 892–895 CrossRef CAS; (b) J. L. Y. Chen and V. K. Aggarwal, Angew. Chem., Int. Ed., 2014, 53, 10992–10996 CrossRef CAS PubMed; (c) R. Alam, A. Das, G. Huang, L. Eriksson, F. Himo and K. J. Szabó, Chem. Sci., 2014, 5, 2732–2738 RSC; (d) Yury N. Bubnov, Ilya V. Zhun, Elena V. Klimkina, Anatoly V. Ignatenko and Zoya A. Starikova, Eur. J. Org. Chem., 2000, 3323–3327 CrossRef CAS; (e) P. Ullrich, J. Schmauck, M. Brauns, M. Mantel, M. Breugst and J. Pietruszka, J. Org. Chem., 2020, 85, 1894–1905 CrossRef CAS PubMed; (f) Y. N. Bubnov, E. V. Klimkina, I. V. Zhun, F. V. Pastukhov and I. V. Yampolsky, Pure Appl. Chem., 2000, 72, 1641–1644 CrossRef CAS; (g) Y. N. Bubnov, Russ. Chem. Bull., 1995, 44, 1156–1170 CrossRef; (h) I. V. Zhun and A. V. Ignatenko, Russ. Chem. Bull., 2004, 53, 2221–2223 CrossRef CAS; (i) J. A. Forni, S.-H. Lau, J.-S. Poh, C. Battilocchio, S. V. Ley and J. C. Pastre, Synlett, 2018, 825–829 CAS; (j) R. Alam, C. Diner, S. Jonker., L. Eriksson and K. Szabó, Angew. Chem., Int. Ed., 2016, 55, 14417–14421 CrossRef CAS.
- C. Diner and K. J. Szabó, J. Am. Chem. Soc., 2017, 139, 2–14 CrossRef CAS PubMed.
- R. W. Hoffmann, Angew. Chem., Int. Ed. Engl., 1982, 21, 555–566 CrossRef.
- (a) Y. Nagashima, K. Sasaki, T. Suto, T. Sato and N. Chida, Chem. – Asian J., 2018, 13, 1024–1028 CrossRef CAS PubMed; (b) R. H. Fish, J. Am. Chem. Soc., 1968, 90, 4435–4439 CrossRef CAS; (c) L. Chevolot, J. Soulié and P. Cadiot, Tetrahedron Lett., 1974, 15, 3435–3438 CrossRef; (d) G. W. Kramer and H. C. Brown, J. Organomet. Chem., 1977, 132, 9–27 CrossRef CAS.
- (a) K. Benn, K. Nicholson, T. Langer and S. P. Thomas, Chem. Commun., 2021, 57, 9406–9409 RSC; (b) E. Jeong, J. Heo, S. Park and S. Chang, Chem. – Eur. J., 2019, 25, 6320–6325 CrossRef CAS PubMed; (c) A. Jayaraman, H. Powell-Davies and F.-G. Fontaine, Tetrahedron, 2019, 75, 2118–2127 CrossRef CAS; (d) W. Zou, L. Gao, J. Cao, Z. Li, G. Li, G. Wang and S. Li, Chem. – Eur. J., 2022, 28, e202104004 CrossRef CAS PubMed; (e) K. Nicholson, J. Dunne, P. Dabell, A. Beaton Garcia, A. D. Bage, J. H. Docherty, T. A. Hunt, T. Langer and S. P. Thomas, ACS Catal., 2021, 11, 2034–2040 CrossRef CAS; (f) S. Pradham, R. Vijaya Sankar and C. Gunanathan, J. Org. Chem., 2022, 87, 12386–12396 CrossRef; (g) K. Nicholson, Y. Peng, N. Llopis, D. R. Willcox, G. S. Nichol, T. Langer, A. Baeza and S. P. Thomas, ACS Catal., 2022, 12, 10887–10893 CrossRef CAS; (h) K. Nicholson, T. Langer and S. P. Thomas, Org. Lett., 2021, 23, 2498–2504 CrossRef CAS PubMed; (i) D. R. Willcox, G. S. Nichol and S. P. Thomas, ACS Catal., 2021, 11, 3190–3197 CrossRef CAS; (j) A. Moreno González, K. Nicholson, N. Llopis, G. S. Nichol, T. Langer, A. Baeza and S. P. Thomas, Angew. Chem., Int. Ed., 2022, 61, e202209584 CrossRef; (k) J. L. Lavergne, H.-M. To and F.-G. Fontaine, RSC Adv., 2021, 11, 31941–31949 RSC; (l) D. R. Willcox and S. P. Thomas, Beilstein J. Org. Chem., 2023, 19, 325–348 CrossRef CAS; (m) E. Nieto-Sepulveda, A. D. Bage, L. A. Evans, T. A. Hunt, A. G. Leech, S. P. Thomas and G. C. Lloyd-Jones, J. Am. Chem. Soc., 2019, 141, 18600–18611 CrossRef CAS PubMed; (n) J. H. Docherty, K. Nicholson, A. P. Dominey and S. P. Thomas, ACS Catal., 2020, 10, 4686–4691 CrossRef CAS; (o) F. Meger, A. C. Kwok, F. Gilch, D. R. Willcox, A. J. Hendy, K. Nicholson, A. D. Bage, T. Langer, T. A. Hunt and S. P. Thomas, Beilstein J. Org. Chem., 2022, 18, 1332–1337 CrossRef CAS PubMed; (p) R. S. Phatake, A. Averdunk, C. Würtele and U. Gellich, ACS Catal., 2022, 12, 13961–13968 CrossRef CAS; (q) A. D. Bage, K. Nicholson, T. A. Hunt, T. Langer and S. P. Thomas, Synthesis, 2023, 62–74 CAS.
- (a) T. Guo, S.-L. Han, Y.-C. Liu, Y. Liu and H.-M. Liu, Tetrahedron Lett., 2016, 57, 1097–1099 CrossRef CAS; (b) N. Wahlström, J. Slätt, B. Stensland, A. Ertan, J. Bergman and T. Janosik, J. Org. Chem., 2007, 72, 5886–5889 CrossRef PubMed; (c) G. Quartarone, A. Pietropolli Charmet, L. Ronchin, C. Tortato and A. Vavasori, J. Phys. Org. Chem., 2014, 27, 680–689 CrossRef CAS; (d) X. Chen, H. Zhao, C. Chen, H. Jiang and M. Zhang, Org. Lett., 2018, 20, 1171–1174 CrossRef CAS; (e) X.-H. Xu, G.-K. Liu, A. Azuma, E. Tokunaga and N. Shibata, Org. Lett., 2011, 13, 4854–4857 CrossRef CAS.
- A. Jayaraman, L. C. Misal Castro, V. Desrosiers and F.-G. Fontaine, Chem. Sci., 2018, 9, 5057–5063 RSC.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- C. G. Frost, J. P. Hartley and D. Griffin, Synlett, 2002, 1928–1930 CrossRef CAS.
- Mechanism akin to that for ketone allylation, see 16g. 11B NMR shifts assigned by analogy to: (a) H-B-9-BBN R. Z. Contreras, Naturforsch. B., 1980, 3, 1229–1236 CrossRef; (b) Dialkylamino borane and amino boronic ester H. Nöth and H. Vahrenkamp, Chem. Ber., 1966, 99, 1049–1067 CrossRef; (c) Allylic-B-9-BBN G. W. Kramer and H. C. Brown, J. Organomet. Chem., 1977, 132, 9 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra and crystallography details available here. CCDC 2374825. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03880k |
| This journal is © The Royal Society of Chemistry 2024 |