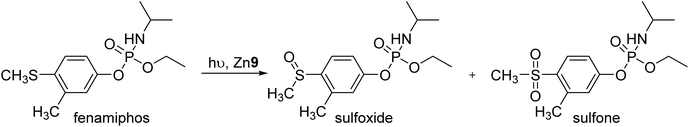
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in photocatalytic degradation of organic pollutants in wastewaters: harnessing the power of phthalocyanines and phthalocyanine-containing materials
Sara R. D. Gamelas a,
João P. C. Tomé
a,
João P. C. Tomé *b,
Augusto C. Tomé
*b,
Augusto C. Tomé *a and
Leandro M. O. Lourenço
*a and
Leandro M. O. Lourenço *a
*a
aLAQV–REQUIMTE, Department of Chemistry, University of Aveiro, 3810–193 Aveiro, Portugal. E-mail: actome@ua.pt; leandrolourenco@ua.pt
bCentro de Química Estrutural, Institute of Molecular Sciences, Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, 1049–001 Lisboa, Portugal. E-mail: jtome@tecnico.ulisboa.pt
First published on 20th November 2023
Abstract
Access to clean water is increasingly challenging worldwide due to human activities and climate change. Wastewater treatment and utilization offer a promising solution by reducing the reliance on pure underground water. However, it is crucial to develop efficient and sustainable methods for wastewater purification. Among the emerging wastewater treatment strategies, photocatalysis has gained significant attention for decomposing organic pollutants in water, especially when combined with sunlight and a recoverable photocatalyst. Heterogeneous photocatalysts have distinct advantages, as they can be recovered and reused without significant loss of activity over multiple cycles. Phthalocyanine dyes, with their exceptional photophysical properties, are particularly valuable for homogeneous and heterogeneous photocatalysis. By immobilizing these photosensitizers in various supports, hybrid materials extend their light absorption into the visible spectrum, complementing most supports' limited UV light absorption. The novelty and research importance of this review stems from its discussion of the multifaceted approach to treating contaminated wastewater with phthalocyanines and materials containing phthalocyanines. It highlights key aspects of each study, including photocatalytic efficiency, recyclability characteristics, investigation of the generation of oxygen species responsible for degradation, identification of the major degradation byproducts for each pollutant, and others. Moreover, the review includes tables that illustrate and compare the various phthalocyanines and supporting materials employed in each study for pollutant degradation. Additionally, almost all photocatalysts mentioned in this review could degrade at least 5% of the pollutant, and more than 50 photocatalysts showed photocatalytic rates above 50%. When immobilized in some support, the synergistic effect of the phthalocyanine was visible in the photocatalytic rate of the studied pollutant. However, when performing these types of works, it is necessary to understand the degradation products of each pollutant and their relative toxicities. Along with this, recyclability and stability studies are also necessary. Despite the good results presented in this review, some of the works lack those studies. Moreover, none of the works mentions any study in wastewater.
1. Introduction
Clean water is an essential requirement for human health. The principal drinking water sources include groundwater, lakes, canals, rainwater, and sea water.1,2 Recently, there has been a concern worldwide about providing sustainable, pure water due to the continuous increase in consumption, population growth, and industrial activity.3,4 The demand for freshwater resources for domestic or industrial use has led to scarcity.3,5 This demand is expected to increase by nearly one-third in 2050, according to the United Nations' World Water Development Report 2018.6 Therefore, to meet the increased water requirement, the scientific community is developing efficient wastewater treatment methods.7 Conventional wastewater treatments consist of a combination of physical, chemical, and biological processes to remove organic matter and, in some cases, inorganic nutrients.8 The methods for water purification are divided into six processes: adsorption, biotechnological, magnetic, membrane, and (photo)catalytic.1 Concerning the photocatalytic processes, the disadvantages of the ‘traditional’ homogenous photocatalysts, namely their recovery and reuse, were overcome by the advent of heterogeneous photocatalysts and allowed the implementation of large-scale photocatalytic transformations. However, homogenous photocatalysts are still useful for finding potentially promising molecules for heterogeneous photocatalysis.9,10In the past decade, nanomaterials such as SiO2, ZnWO4, ZnO, fibrous materials, ferritic nanomaterials, and carbon-based and TiO2 nanomaterials have been reported in UV-visible light photocatalysis.7,11 Among them, the most studied and used are the TiO2 nanomaterials, discovered over three decades ago. They showed great photocatalytic activity, hydrophobicity, long-term stability, lower toxicity and costs (compared with other nanomaterials), and self-cleaning ability.9,10 To apply heterogeneous photocatalysis to wastewater treatment, the cost of the process should be minimal. Thus, recyclability presents a vital feature for a photocatalyst.10,12
There is an urge to find alternatives to improve the photocatalytic performance of materials frequently used as photocatalysts. For example, some materials only absorb UV light, representing ca. 5% of the solar spectrum. For example, modifying these materials with dyes can improve their photocatalytic efficiency under solar light.10,13 In fact, the sensitization of the photocatalyst induces faster destruction of the organic pollutants during the photocatalytic activity.7,10,14
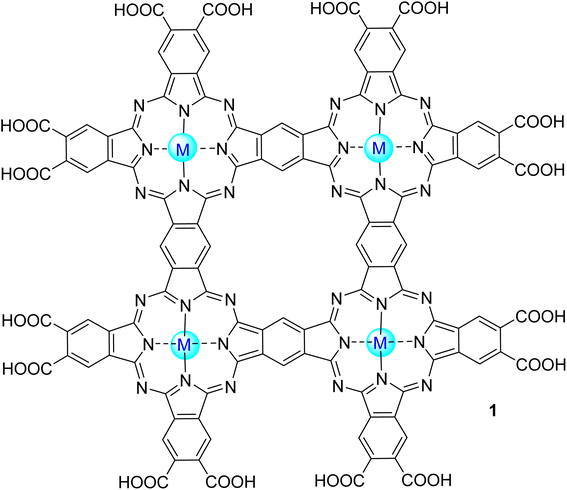
Phthalocyanines (Pcs) are dyes that can be photoactivated by visible light to promote the degradation of organic materials.15 Regarding chemical structure, Pcs are synthetic aromatic compounds consisting of four iminoisoindoline units with 18 delocalized π-electrons and display excellent absorption in the visible and near-infrared region of the electronic spectrum.15–19 Usually, the synthesis of a phthalocyanine from a mono-substituted phthalonitrile affords a mixture of regioisomers due to the variation of the peripheral position of the R groups (Fig. 1). Similarly, the tetramerization of a non-symmetrical disubstituted phthalonitrile (R1 ≠ R2) also gives a mixture of regioisomeric phthalocyanines. Given that, in this paper there are symmetrical and non-symmetrical tetra- and octasubstituted phthalocyanines with various regioisomers that are represented by the condensed structural formulae notation “Formula type I′′ and “Formula type II” (Fig. 1). So, the type I notation, despite not being formal, is often preferred to type II due to its high level of clarity, and it will be used in this review when needed.
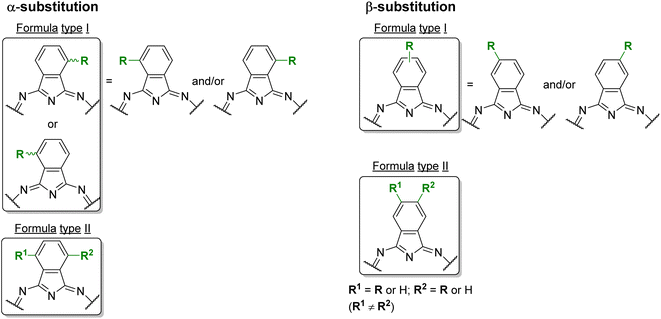 | ||
| Fig. 1 Condensed and simplified structural chemical formulae used for phthalocyanine having an α- or β-substitution patterns. | ||
Moreover, Pcs present great structural flexibility and particular properties that can be explored in several applications, including catalysts,20,21 sensors,22–24 solar cells,18,25–28 photosensitizers in biological targets,16,17,29,30 and, more importantly, as photocatalysts.15,24,31,32 When supported in a semiconductor, under visible light irradiation, Pcs are excited and inject electrons into the semiconductor's conduction band to initiate the photocatalytic process.
This review focuses on the photocatalytic degradation of organic pollutants typically found in wastewater by using several phthalocyanines as catalysts. There are several highly cited reviews in photocatalysis,15,32,33 but an up-to-date review is needed. A comprehensive review of the published works in this field during the last 17 years is presented herein. The pollutants mentioned in this review are divided into five main sections: phenol and phenol derivatives, organic dyes, agrochemicals, pharmaceuticals, and other pollutants. The novelty and research significance of this review are based on mentioning the multidisciplinary treatment of pollutant wastewater using phthalocyanines and phthalocyanine-containing materials, evidencing the main details of each work when these parameters are determined and provided in the multidisciplinary reports, such as the: (i) photocatalytic rates, (ii) recyclability properties, (iii) study of the main generated oxygen species responsible for the degradation, (iv) the main degradation products of each pollutant, and (v) among others. However, there is a lack of some of these parameters in several reports. In this review, it is also provided some tables to show and compare the different phthalocyanines and supports used in each work for pollutant degradation. At the end of the review, some key insights and future perspectives will be presented to inspire future research.
2. Mechanism of the photocatalytic degradation of organic pollutants
Among the various methods to remove the organic pollutants in wastewater, photooxidation is the most used. It involves the in situ generation of reactive oxygen species (ROS) that react with the pollutants, leading to their oxidation and, preferably, decomposition.31Under visible light irradiation, (metallo)phthalocyanines (MPc, M = 2H or metal ion) in a support system are excited and transfer electrons to the conduction band of the support (Fig. 2). The conduction band mediates the electron flow from the MPc to the electron acceptors on the support. It is important to highlight that the electron transfer between MPc and the conduction band of the support must be faster than the relaxation to the ground state.7,34 On the other hand, the excited phthalocyanine (MPc*) can act as a sensitizing oxidant (reacting directly with the pollutant) or transfer its energy to molecular oxygen to form ROS. Moreover, in the presence of water, which can act as an electron donor, the oxidized MPc can be regenerated quickly and, after a series of charge transfer reactions, superoxide radical anions (O2˙−), hydroperoxyl radicals 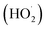 , and hydroxyl radicals (˙OH) are produced as powerful oxidizing species.35–37 In some studies, H2O2 is used to increase the amount of OH˙ and, thus, enhance the photocatalytic activity.14,31,38 The final step in the photocatalytic reaction is the relaxation of the photocatalyst to the ground state; a new catalytic cycle is then started.7,9,10,14,33 In the case of solar irradiation, UV light can also excite the support, increasing the formation of ROS.7
, and hydroxyl radicals (˙OH) are produced as powerful oxidizing species.35–37 In some studies, H2O2 is used to increase the amount of OH˙ and, thus, enhance the photocatalytic activity.14,31,38 The final step in the photocatalytic reaction is the relaxation of the photocatalyst to the ground state; a new catalytic cycle is then started.7,9,10,14,33 In the case of solar irradiation, UV light can also excite the support, increasing the formation of ROS.7
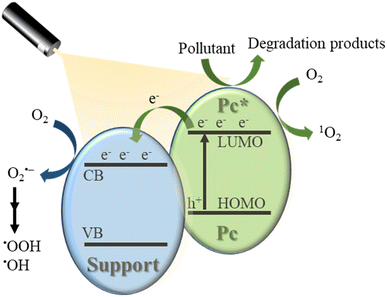 | ||
| Fig. 2 Proposed mechanism for the Pc mediated photodegradation of wastewater pollutants under visible light irradiation. | ||
3. Photocatalytic degradation of organic pollutants
Water contaminants such as phenolic compounds, dyes, agrochemicals, pharmaceuticals, etc., are hazardous to humans and harmful to the environment. Many of these substances are resistant to natural degradation processes. These contaminants reach natural waters through domestic and industrial activities in a continuous way, and that is attracting global concern. Even in localised contaminant sources, like industrial effluents, their elimination by conventional methods (chemical precipitation, filtration, electro-deposition, ion-exchange adsorption, and membrane systems) can be either slow or difficult.39 For the organic contaminants, photooxidation is a promising alternative to those methods.Due to structural differences, each contaminant type raises specific degradation problems. Therefore, the results concerning the photooxidation/degradation of organic pollutants are discussed here by contaminant families, aiming to highlight the successes and drawbacks of the method for each contaminant type. It is important to mention that complete mineralization of the pollutants should be achieved in an appropriate period. The conversion of a pollutant into another compound, which could also be toxic, should be avoided.40 Therefore, each pollutant's degradation should be studied to evaluate if complete mineralization was achieved. Unfortunately, the degradation products of the pollutants are not mentioned in many of the studies of published articles. Moreover, many published works also do not mention recyclability studies. The recyclability of the composites used as photocatalysts is also essential when considering their viability in water and wastewater treatment.41
3.1. Phenol and phenol derivatives
The first family of contaminants to be discussed is the phenolic compounds. These are aromatic compounds with one or more hydroxyl groups linked to the aromatic ring(s). These compounds are found in the wastewater of several industries like petroleum refineries, chemical synthesis, plastics, dyes, detergents, and textiles.42 The appearance of phenolic compounds could also arise in the aquatic environment from natural sources, namely through algal secretion, hydrolysable tannins, and flavonoids. However, the most hazardous ones are phenol derivatives like chlorophenols, nitrophenols, bisphenol A (BPA), naphthol, catechol, etc.39 The presence of phenolic pollutants and their metabolites in living cells can cause mutagenicity, carcinogenicity, and endocrine-disrupting chemicals.For this reason, they are considered human health and environmental hazards.43 The elimination of these derivatives is sometimes incomplete, so finding promising alternatives for its removal from wastewater becomes essential. The following sections will mention the degradation of these pollutants using several phthalocyanines. Also, in the following sections, studies regarding the degradation of chlorophenols using several phthalocyanines will be reported.
| MPc | Support | Light | Irradiation time (min) | Half-life time (r, min−1) | Efficiency (rate or %) | Recycle | Ref. |
|---|---|---|---|---|---|---|---|
| a pH 11.b pH 5, and.c pH 7.d With H2O2.e mol CO2 per mol substrate, light intensity at 38 mW cm−2. | |||||||
| Zn1 | — | Visible light (λ > 400 nm) | — | 152.44 | — | — | 45 |
| Zn2 | 1324.30 | ||||||
| Al1 | 39.21 | ||||||
| Al2 | 17.13 | ||||||
| Co1 | 0.12 | ||||||
| Pd3 | FDU-15 | 420 | — | 61%a,d | 46 | ||
| 69%b,d | |||||||
| 360 | 98%c,d | ||||||
| 56%a | |||||||
| Al3 | TiO2 | 590 | 90% | 47 | |||
| H24 | TiO2 | 600 | 1.5e | 48 | |||
| Fe4 | Graphene | 180 | 70% | 4 cycles (10% loss) | 49 | ||
| AlO3 | — | — | |||||
| CNTs | |||||||
| Cu4 | Surfactant modified bentonite | 240 | 20% | 50 | |||
| Co4 | 30% | ||||||
| AlCl4 | 70% | ||||||
Under the same conditions, the phenol degradation rate was lower using the mononuclear Zn2 (Fig. 4) (r = 27.0 min−1).45 When compared with Al2Cl (Fig. 4) (r = 17.13 min−1), the Al1Cl and Co1 (Fig. 3) exhibited higher photocatalytic activities (r = 39.21 and 0.12 min−1, respectively). The authors confirmed that 1O2 is the main ROS involved in the degradation process of phenol. The oxidation product, 1,4-benzoquinone, could be further degraded into fumarate and maleate at an alkaline pH (pH = 13, Fig. 5). No assays regarding the recyclability and photostability of the photocatalyst were performed in this study.
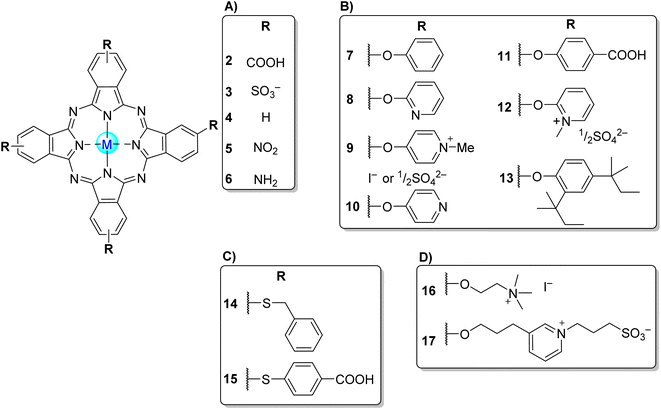 | ||
| Fig. 4 Symmetrical tetra-β-substituted phthalocyanines (A) 2–6,34,45–96 (B) 7–13,84,85,97–100 (C) 14,50,101 1535,101 and (D) 16,56,85 17.102 | ||
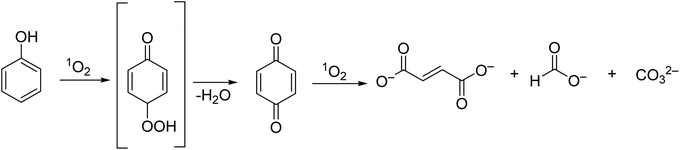 | ||
| Fig. 5 Degradation products of phenol, reported by Iliev and co-workers.45 | ||
Wu, Xing, and co-workers46 studied the photodegradation efficiency of phenol in water (at different pH) under visible light irradiation in the presence of H2O2, using Pd3 (Fig. 4) immobilized on a mesopolymer (Table 1). The photodegradation rate could achieve 61% and 69% at acidic and neutral pH values within 420 min. On the other hand, there was 98% phenol degradation at alkaline pH after 360 min. Also, H2O2 was essential for the photocatalytic process as it increased from 56% to 98% under the same conditions when H2O2 was added. After four cycles, the photodegradation of phenol remains unchanged using Pd1–FDU-15. The degradation products are the same as reported before.45
Xu and co-workers47 and Iliev and co-workers48 studied the photooxidation of phenol using TiO2 modified with Al3 and H24 (Fig. 4) in aqueous media under visible light irradiation (λ > 400 nm). The first authors compared the photooxidation rates with that obtained with H24 immobilized on Al2O3. This rate was obtained by measuring the amount of CO2 produced at the end of the catalytic process. When using H24–TiO2 (1.50 mol CO2/mol substrate), the photooxidation rate is much higher than H24–Al2O3 (0.95 mol CO2 per mol substrate). The second authors could efficiently degrade phenol (∼90%) within 590 min with an optimum amount of Al1 (Table 1) loaded on a TiO2 (1.0 wt%). Concerning the main ROS involved in the photodegradation process, Iliev48 reported O2˙− and HOO˙, which could lead to the same products reported before by these authors.45 Xu and co-workers47 identified the same ROS as Iliev48 but did not report the degradation products. None of these works mentioned the photostability studies of photocatalysts.
Yang and co-workers49 studied the photodegradation of phenol in aqueous media under visible light irradiation (λ ≥ 420 nm) in the presence of H2O2 (Table 1) and using Fe4 (Fig. 4) immobilized on graphene nanosheets (Fe4/GR) as the photocatalyst. According to the authors, the π–π stacking interaction between Fe4 and graphene (GR) forms a donor–acceptor system. The loading of Fe4 promotes the exfoliation of the graphene sheets and enables the dispersion of Fe4 on graphene. As expected, the introduction of Fe4 into the GR greatly enhanced the photocatalytic activity of the composites since the π–π interactions between the planar aromatic GR and Fe4 enable the electron transfer from the donor (Pc) to the acceptor (support). Within 180 min of irradiation, the GR/Fe4-0.25 (25 wt%) could achieve a photocatalytic rate of 77%, compared with Fe4/Al2O3 and Fe4/CNT, which were unable to degrade phenol. For the other materials used, such as Al2O3, the donor–acceptor system does not occur. The Fe4/GR could be reused up to 4 times without significant loss of activity (10%). In this study, the authors analysed the mechanism involved in forming the ROS (O2˙−, HOO˙, and ˙OH) but did not identify the main degradation products nor the stability and photostability of the used materials.
Xu and co-workers50 studied the photodegradation of phenol under visible light irradiation (λ > 450 nm) using AlCl4, Cu4, and Co4 (Fig. 4) immobilized into modified bentonite (with the surfactant cetyltrimethylammonium bromide). The best photooxidation rate was achieved using AlCl4 as a catalyst (complete degradation after 240 min of irradiation). The authors also performed studies involving other phenols, such as 4-chlorophenol (4-CP), 4-nitrophenol (4-NP), 2,4-dichlorophenol (2,4-DCP), and 2,4,6-trichlorophenol (2,4,6-TCP), which will be discussed later. Moreover, the photooxidation rate of phenol suffers a gradual loss of activity after 4 cycles of the experiment using AlCl4. This loss of activity might be due to the degradation of the surfactant by the generated singlet oxygen or by the reduction of the sorption process that seems essential for the degradation process.
FDU is a hexagonal mesoporous material, and this type of mesopolymer material has high physicochemical stability (despite the pH variation of the solution) and can adsorb phenolic pollutants from wastewater through π–π interactions and hydrogen bonding.114,115 So, Xing and co-workers51 studied the photocatalytic degradation of 4-methylphenol under visible light irradiation by using a palladium phthalocyanine Pd3 (Fig. 4) graphed through π–π interactions onto the FDU-14 mesopolymer (FDU-14-Pd3). After 180 min in basic conditions and with H2O2, it was possible to achieve 97% degradation of 4-methylphenol. Only 76% and 79% degradation rates were achieved in acidic and neutral conditions, respectively. This can be explained by the fact that 4-methylphenol must be deprotonated to 4-methylphenolate, which is more susceptible to oxidizing agents. Adding H2O2 to the photocatalytic system improved the degradation rate from ca. 40% to 97% after 180 min of light irradiation. In the dark, there is no degradation of 4-methylphenol. The degradation rate of 4-methylphenol remained unchanged after the experiment four times using the FDU-14-Pd3 photocatalyst. Similar photooxidation rates of 4-methylphenol were obtained using Pd3-FDU-15.
Nyokong and co-workers52,84,97,118 reported the photodegradation of 4-NP in aqueous or organic media in the presence of a large diversity of phthalocyanines. In a first study,52 the water-soluble octacarboxy Zn18 (Fig. 6) and a mixture of mono-, di-, tri-, and tetra-sulfonated zinc(II) phthalocyanines (ZnPcSmix) were used as photocatalysts, and the experiments were carried out in aqueous solutions at pH = 8.2. The authors concluded that the quantum yields of 4-NP degradation were closely correlated with the singlet oxygen quantum yields (ΦΔ) and the phthalocyanines' aggregation (Table 2). Zn18 showed the highest ΦΔ (Φ4NP = 6.5 × 10−3 and 29% aggregation) followed by ZnPcSmix (Φ4NP = 7.9 × 10−4 and 49% aggregation) and the Zn3 (Φ4NP = 1.5 × 10−4 and 78% aggregation).52 However, in this study, the most effective catalyst was ZnPcSmix since Zn18 degrades readily during catalysis. The products of the photodegradation of 4-NP were identified as hydroquinone and 4-nitrobenzene-1,2-diol.
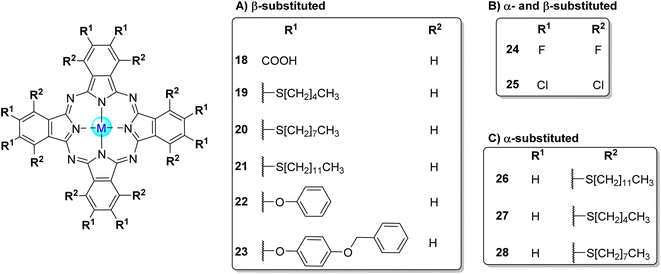 | ||
| Fig. 6 Symmetrical octa-substituted phthalocyanines in the (A) β-position 18,52,54,103–106 19–23,97,107 (B) β- and α-positions 24,53,108 2553,86,109 and (C) α-position 26,97,110 2797 and 28.97 | ||
| MPc | Support | Light | ΦΔ | t1/2 (min) | Efficiency (Φ, % or rate) | Recycle | Ref. |
|---|---|---|---|---|---|---|---|
| a Light intensity (photons per s per cm2): 4.1 × 1016.b After 240 min of irradiation.c Light intensity (photons per s per cm−2): 3.5 × 1020.d 5.0 × 1016.e After 60 min of irradiation. | |||||||
| Zn3 | — | Visible light (λ = 600 nm) | <0.01 | — | 1.5 × 10−4a | — | 52 |
| Zn18 | 0.52 | 6.5 × 10−3a | |||||
| ZnPcmix | 0.48 | 7.9 × 10−4a | |||||
| Al4Cl | Surfactant modified bentonite | Visible light (λ > 450 nm) | — | 73%b | 50 | ||
| Lu7OAc | PS | Visible light (λ > 400 nm) | 61.9 | — | 97 | ||
| Lu8OAc | 72.8 | ||||||
| Zn4 | 93.7 | ||||||
| Lu29OAc | 0.22 | 63.9 | |||||
| Lu7OAc | 0.28 | 50.2 | |||||
| Lu12OAc | 0.17 | 35.9 | |||||
| Lu8OAc | 0.15 | 37.9 | 84 | ||||
| PSU | — | 0 | |||||
| Zn10 | 0.24 | 57.3 | |||||
| Pd19 | SWCNTs | Visible light (λ > 400 nm) | 0.22 | — | 1.52 × 10−3 | 3 cycles (7% loss) | 118 |
| Pd20 | 0.22 | 1.32 × 10−3 | |||||
| Pd21 | 0.27 | 1.74 × 10−3 | |||||
| Pd22 | 0.19 | 0.92 × 10−3 | |||||
| Pd23 | 0.2 | 1.27 × 10−3 | |||||
| Pd26 | 0.20 | 1.86 × 10−3 | |||||
| Pd27 | 0.23 | 1.67 × 10−3 | |||||
| Pd28 | 0.21 | 1.33 × 10−3 | |||||
| Pd19 | — | Visible light (λ = 600 nm) | 0.38 | 14 × 10−4c | — | 107 | |
| Pd20 | 0.36 | 15 × 10−4c | |||||
| Pd21 | 0.39 | 18 × 10−4c | |||||
| Pd22 | 0.30 | 9.0 × 10−4c | |||||
| Pd23 | 0.32 | 16 × 10−4c | |||||
| Pt22 | 0.29 | 0.3 × 10−4c | |||||
| Pt23 | 0.26 | 0.7 × 10−4c | |||||
| Mg4 | 0.40 | 25%d | 53 | ||||
| Al4Cl | 0.29 | 89%d | |||||
| Zn4 | 0.67 | 53%d | |||||
| Zn5 | 0.11 | 45%d | |||||
| Zn6 | 0.11 | 54%d | |||||
| Zn24 | 0.13 | 75%d | |||||
| Zn25 | — | 23%d | |||||
| Cu13 | TiO2 | UV | 100%e | 120 | |||
| H213 | 50%e | ||||||
| No | 86%e | ||||||
In another study,107 a set of water-insoluble palladium(II) and platinum(II) phthalocyanines (Pd19–Pd23 and Pt22, Pt23, Fig. 6 and Table 2) were used as photocatalysts. In terms of quantum yield, the Pd(II) complexes exhibited better results than the Pt(II) ones being Pd21 (Φ4NP = 18 × 10−4) the best photocatalyst followed by Pd23 (Φ4NP = 16 × 10−4), Pd20 (Φ4NP = 15 × 10−4), Pd19 (Φ4NP = 14 × 10−4), Pd22 (Φ4NP = 9 × 10−4), Pt23 (Φ4NP = 0.7 × 10−4), and Pt22 (Φ4NP = 0.3 × 10−4). The experiments were carried out in dichloromethane in the presence of triethylamine. The degradation products were identified as hydroquinone and 1,4-benzoquinone. Concerning the (photo)stability of the catalysts, the authors only mentioned that Pd7 was stable during the photocatalytic studies.
The degradation of 4-NP in aqueous media under visible light irradiation (λ = 600 nm) using the water-insoluble MPc complexes Mg4, Zn4, Al4Cl (Fig. 4), Zn5, Zn6 (Fig. 4), Zn24, and Zn25 (Fig. 6) as photocatalysts was also accessed (Table 2).53 The best photocatalytic rate was obtained using Al4Cl (with 89 ± 8% degradation of 4-NP after 100 min), followed by Zn24 (75%), Zn6 (54%), Zn4 (53%), Zn5 (45%), Mg4 (25%), and Zn25 (23%). The photocatalytic activity of these MPcs involved Type I (radicals) and Type II (singlet oxygen) mechanisms. The major products of the photodegradation of 4-NP were identified as 4-nitrobenzene-1,2-diol, hydroquinone, 1,4-benzoquinone, and fumaric acid. The authors did not report any studies regarding the stability of the photocatalysts during the assay.
The same authors97 studied the photooxidation of 4-NP under visible light irradiation (λ > 400 nm) in aqueous media (pH = 8.2) when using tetra-substituted phthalocyanines bearing phenoxy and 2-pyridyloxy groups (7 and 8, Fig. 4) that were immobilized in polystyrene (PS) polymer fibre (Table 2). There was no photocatalytic degradation of 4-NP when using the non-immobilized PS fibres. However, the photodegradation of 4-NP was observed using supported Lu7OAc and Lu8OAc, being slightly higher when using Lu7OAc/PS fibre (t1/2 = 61.9 min−1) than using Lu8OAc/PS fibre (t1/2 = 72.8 min−1) or Zn4/PS fibre (t1/2 = 93.7 min−1). Table 2 compares the photoactivity of Lu7OAc (Fig. 4) with that of the isomeric α-substituted Lu29OAc (Fig. 7), indicating that substituents at β-positions improve photocatalysis. In this study, the major degradation products of 4-NP were hydroquinone and 4-nitrobenzene-1,2-diol, with a slight formation of 1,4-benzoquinone. Regarding the (photo)stability of the catalysts, it is only mentioned that a slight photodecomposition of Lu8OAc was observed after 12 h of irradiation.
 | ||
| Fig. 7 Lu(III)29OAc α-substituted phthalocyanine complex used by Nyokong and co-workers.84,97,121 | ||
Nyokong and co-workers84 studied the photodegradation of 4-NP, 4-CP, and methyl orange (MO) under aqueous media (pH = 12) using a series of peripherally substituted zinc(II) and Lu(III)OAc phthalocyanine complexes 6–12 (Fig. 4) and 29 (Fig. 7) immobilized on PS, PSU, PAA, and PUR (Tables 2, 3 and 7). Different phthalocyanine complexes were immobilized in several polymers: (i) Zn7, Zn8, Zn12, Lu29OAc–7, 8, and Lu12OAc in PS polymer fibres; (ii) Zn9, Zn10, and Lu8OAc in PSU polymer fibres; and (iii) Zn6, Lu6OAc, and Lu10OAc in the PAA and PUR fibres. Complex Lu11OAc was covalently linked to PUR by amide bonds. Similarly, Zn6 and Lu6OAc were covalently linked to PAA through amide bonds. The three phthalocyanines were mixed with PAA and PUR to assess the difference in the photocatalytic activity between the hybrids with or without a chemical bond.
| MPc | Support | ΦΔ | Irradiation time (min) | t1/2 (min) | Efficiency (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|---|
| a Not determined due to the solubility of the polymer in water.b With H2O2. | |||||||
| Al4Cl | Bentonite | — | 300 | — | 75 | — | 50 |
| Lu29OAc | PS | — | 68.6 | — | 121 | ||
| Zn4 | 1824.1 | ||||||
| Lu29OAc | 0.22 | — | 63.6 | — | 84 | ||
| Lu7Ac | 0.28 | 50.2 | |||||
| Lu8OAc | 0.17 | 64.8 | |||||
| Lu12OAc | 0.15 | 70.7 | |||||
| Zn6 | a | — | |||||
| Lu6OAc | |||||||
| Zn9 | PSU | 0.21 | 63.6 | ||||
| Zn10 | 0.24 | 57.3 | |||||
| Lu8OAc | 0.26 | — | |||||
| Lu11OAc | PUR | 0.11 | |||||
| In30Cl | PS | — | 866.0 | 98 | |||
| In9Cl | 217 | ||||||
| In31Cl | 182.0 | ||||||
| Zn14 | AuNPs- PS | 17.8 | 101 | ||||
| PS | 52.1 | ||||||
| Al3 | Amberlite | 30 | — | 70 | 54 | ||
| Pd26 | SWCNTs | — | 1440 | 42 | 110 | ||
| — | — | 91 | |||||
| Zn2 | g-C3N4 | 90 | 98 | 10 cycles (<5% loss) | 55 | ||
| g-C3N4/PAN | 270 | 5 cycles (<5% loss) | 56 | ||||
| Al3 | TiO2 | 360 | 100 | — | 47 | ||
| Co3 | 90 | 90 | 57 | ||||
| 100b | |||||||
| 95 | 58 | ||||||
| Co15 | 30 | 100 | 5 cycles (9% loss) | 35 | |||
| Zn15 | 5 cycles (12% loss) | ||||||
| Co15 | 30 | 99 | — | 99 | |||
| Zn15 | 98 | ||||||
| Co32 | 120 | 88 | 36 | ||||
| Zn32 | 90 | ||||||
| Co33 | 85 | 5 cycles (37% loss) | |||||
| Zn33 | 86 | 5 cycles (34% loss) | |||||
| Without Pc | 3 | — | |||||
According to the authors, the PAA polymer fibres were unsuitable for application in aqueous media due to their extensive solubility that induces a gelatinous solution. No photocatalytic degradation of any pollutants occurred even after 720 min of irradiation using the covalently linked Lu11OAc–PUR fibres. The same occurred for the fibres where Lu11OAc and PUR were mixed. These results contrast those reported for Lu29OAc and Lu7Oac, which are very photoactive materials.97,122 The absence of photocatalytic activity can be related to the morphology of the fibres because they are not porous enough for sufficient interaction between the reactive species and the photoactive phthalocyanines.
The PS and PSU polymer fibres are insoluble in water and suitable for photocatalytic degradation assays. There was a 40% degradation of 4-NP under visible light irradiation (λ > 400 nm) after 150 min of irradiation when using Lu7OAc/PS, Lu8OAc/PS, Lu12OAc/PS, and Lu29OAc/PS (Table 2). It was possible to obtain a better photocatalytic degradation rate when using the unquaternized Zn8/PS fibres compared to the quaternized Zn12/PS fibres. The 4-NP could be degraded more efficiently using the peripherally substituted Zn7/PS fibres compared to their non-peripheral analogue (Lu29/PS). The photooxidation rate of 4-NP could be maintained even after the photocatalyst was reused up to three times. Regarding the stability of these catalysts, the author only mentioned that the PSU functionalized with metal phthalocyanines was relatively stable under the same light intensity as in the photocatalytic studies. It is important to mention that after the first cycle of the reusability studies when dried, these fibres folded up, forming a hard lump, and could not be applied again. The 1,4-benzoquinone and hydroquinone were identified as the major products of the 4-NP degradation.
In another study,118 they studied the degradation of 4-NP in aqueous media under visible light irradiation (λ = 400–600 nm) using composites based on the adsorption of several palladium(II) phthalocyanines 19–23 (Fig. 6) and 26–28 (Fig. 6) in single-walled carbon nanotubes (SWCNTs, Table 2). In basic media (pH = 8.5), the photocatalytic rate using all composites follows the order: 26/SWCNTs (kobs = 1.86 × 10−3 min−1) > 21/SWCNTs (kobs = 1.74 × 10−3 min−1) > 27/SWCNTs (kobs = 1.67 × 10−3 min−1) > 19/SWCNTs (kobs = 1.52 × 10−3 min−1) > 28/SWCNTs (kobs = 1.33 × 10−3 min−1) > 20/SWCNTs (kobs = 1.32 × 10−3 min−1) > 23/SWCNTs (kobs = 1.27 × 10−3 min−1) > 22/SWCNTs (kobs = 0.92 × 10−3 min−1). It was possible to obtain a better photodegradation of 4-NP for the composites bearing long alkyl chains. There was no significant loss of degradation rate of 4-NP (∼7%) after three cycles of the experiment. This study identified 1,4-benzoquinone, hydroquinone, and 4-nitrobenzene-1,2-diol as the major products of the degradation process. All photocatalysts proved to be stable for 400 min.
Palmisano and co-workers120 studied the degradation of 4-NP in aqueous media using H213 and Cu13 (Fig. 4) immobilized in polycrystalline TiO2 samples and investigated their photocatalytic behaviour in the degradation of (Table 2). The photocatalysts remained stable for 420 min under irradiation. Complete degradation of 4-NP was observed after 60 min irradiation with UV light and using 1% or 1.5% Cu13/TiO2. On the other hand, there was only 50% degradation after 60 min irradiation (but complete degradation after 5 h) when using H213/TiO2. Compared with bare TiO2 (86% degradation), only Cu13/TiO2 showed enhanced photocatalytic activity.
Xu and co-workers50 accessed the degradation of 4-NP in an aqueous medium at pH 11.2 under visible light irradiation (λ > 450 nm) using Al4Cl (Fig. 4) inserted into modified bentonite (with the surfactant cetyltrimethylammonium bromide) (Table 2). It was possible to achieve 73% degradation of 4-NP after 290 min of irradiation. For this pollutant, no degradation products were identified.
Nyokong and co-workers84,98,121 studied the degradation of 4-CP under visible light irradiation (λ > 400 nm) in aqueous media (pH = 12) using different phthalocyanine complexes immobilized in several polymers: (i) Zn7, 8, Lu7OAc, Lu8OAc (Fig. 4) and Lu21OAc (Fig. 6), In9 and Lu12OAc (Fig. 4), 30 and 31 (Fig. 8) in PS polymer fibres; (ii) Zn9 (Fig. 4), Zn10 (Fig. 4), and Lu8OAc (Fig. 4) in PSU polymer fibres; and (iii) Zn6, Lu6OAc (Fig. 4), and Lu10OAc (Fig. 4) in the PAA and PUR fibres (Table 3). Regarding PS fibres, these authors101 also prepared Zn(II) phthalocyanine 14 (Fig. 4 and Table 3)–gold nanoparticles (AuNPs) conjugates immobilized on PS fibres. Besides polymer fibres, Nyokong and co-workers54 immobilized Al1 (Fig. 3) on Amberlite® and supported the Pd(II) phthalocyanine Pd26 (Fig. 6) on SWCNTs functionalized with carboxylic acid moieties.110
 | ||
| Fig. 8 Non-symmetrical tetra-β-substituted phthalocyanines 30, 31 used by Nyokong and co-workers,98 and 32, 33 used by Sevim and co-workers.36 | ||
Overall, in the studies performed by Nyokong and co-workers,54,84,98,101,110,121 despite the catalyst used, the degradation products were hydroquinone and 1,4-benzoquinone. In the first study,121 upon visible light irradiation (λ > 400 nm) and during 720 min, the prepared composite could degrade 4-CP, showing a half-life of 30 min for the smallest concentration of 4-CP (0.156 mM). Even at the highest concentration of 4-CP (0.506 mM), the half-life is only 68.6 min (Table 4). Moreover, the Zn4 (Fig. 4)/PS fibre was also used for comparison and showed half-times much higher (1155.3 min for 0.156 mM and 1824.1 min for 0.506 mM, respectively) than the prepared Lu29OAc/PS fibre. However, after 720 min of continuous light irradiation, a photodegradation of the Lu29OAc (Fig. 7) could explain the incomplete degradation of 4-CP. The Zn4/PS fibres were degraded entirely after 720 min of irradiation. In the second study,84 it was possible to observe a degradation of 4-CP after 150 min under visible light irradiation (λ > 400 nm) using photoactive materials of Lu7OAc/PS, Lu8OAc/PS, Lu12OAc/PS (Fig. 4), and Lu29OAc/PS (Fig. 7) (Table 3). The hybrids can degrade 4-CP faster (complete degradation after 150 min) when compared with 4-NP (∼40% of degradation after 150 min). There was a faster degradation of 4-NP when using the peripherally substituted Zn7/PS fibres rather than the non-peripheral analogue (Lu29/PS).
| Catalyst | Photocatalyst activity (% of degradation) | ||||
|---|---|---|---|---|---|
| 1st cycle | 2nd cycle | 3rd cycle | 4th cycle | 5th cycle | |
| Co11–TiO2 | 99 | 97 | 95 | 92 | 91 |
| Zn11–TiO2 | 98 | 95 | 93 | 90 | 88 |
In another study, the authors98 observed that a higher degradation rate constant of 4-NP was obtained using In31Cl/PS (t1/2 = 182.0 min) fibre followed by In9Cl/PS (t1/2 = 217.0 min) and In30Cl/PS (t1/2 = 866.0 min) – Table 3. The high activity of In31Cl/PS could be due to the high singlet oxygen generation values (ΦΔ = 0.50) and the high surface area of their fibres. The number of charges did not seem to influence the photocatalytic activity of the conjugates since In9Cl/PS has more charges than In31Cl/PS and lower photocatalytic activity.
Regarding gold nanoparticles,101 high degradation rate constants and low lifetimes were observed for the Zn14–AuNPs/PS fibres (Fig. 4 and Table 3). Also, it was possible to achieve higher degradation rates when coupling AuNPs. In a study with Amberlite, Nyokong and co-workers54 observed that the composite degraded ∼70% of 4-CP after 30 min of irradiation. Regarding this pollutant, no degradation products were identified. The authors extended the photocatalytic study to 2,4-CP, 2,4,6-TCP, and pentachlorophenol (PCP). Moreover, the study was extended to other metallated phthalocyanines for this last organic pollutant. Regarding carbon materials, Nyokong and co-workers110 compared the degradation efficiencies of 4-CP when using Pd19/SWCNTs and Pd26 in aqueous media under visible light irradiation (Fig. 6 and Table 3). Pure Pd26 exhibited higher photocatalytic activity (91% for 4-CP) than the hybrid material (42% for 4-CP). The Pd26/SWCNTs catalyst cannot be reused without significant activity loss. Despite the low photocatalytic rates of the Pd26/SWCNTs hybrid, Pd26 had excellent photocatalytic activity and may be applied in homogenous photocatalysis. Chen, Lu and co-workers55 studied the photooxidation of 4-CP in aqueous media under visible light (λ > 400 nm) using visible light-responsive photocatalysts based on g-C3N4 and polyacrylonitrile (PAN)-supported g-C3N4 coupled with zinc(II) phthalocyanine 2 (g-C3N4/Zn2and g-C3N4/Zn2/PAN, Fig. 4. Zn2 was covalently bonded to g-C3N4 through the carboxy group of the MPc and an amine of g-C3N4 (to form an amide). After 90 min of visible light irradiation, ∼98% of 4-CP was degraded by this photocatalyst, which is higher when compared with bare g-C3N4 and Zn2 (Table 3). These results are due to the synergistic effect between g-C3N4 and Zn2. More importantly, the photocatalytic degradation of 4-CP was as high as 99% after ten cycles, with no decrease, indicating that the catalyst has broad application for removing organics in polluted waters. The authors did not report the degradation products nor the stability of the photosensitizers under irradiation.55
In a new report,56 the authors studied the photooxidation of 4-CP using g-C3N4/Zn2 (Fig. 4) as the catalytic entity, and PAN nanofibers were employed as support to overcome the shortcomings of easy aggregation and to enable an easy recycling process. In the visible light studies, the removal rate of 4-CP using g-C3N4/Zn2/PAN as photocatalyst was higher than with g-C3N4/PAN, reaching a maximum of ∼98% after 270 min of irradiation (Table 3). The g-C3N4/Zn2/PAN was reused up to five times without significant loss of activity. In both cases, the authors did not refer to the stability of the catalysts (effect of photobleaching) under irradiation nor the degradation products of 4-CP.
Several studies were performed regarding the degradation of 4-CP with MPcs supported on TiO2. For example, Xu and co-workers47 evaluated the photocatalytic ability of Al2 (Fig. 4 adsorbed on TiO2 and could achieve a complete degradation after 420 min of visible light irradiation with an optimum amount of Al2 loaded on TiO2 of 1.0 wt% (Table 3). Also, the authors could perform four photocatalytic cycles with a decrease of 40% in the photodegradation rate, which can be due to the photobleaching of Al2 during the photocatalytic assay. 1,4-Benzoquinone, hydroquinone, formic and acetic acid were identified as the degradation products of 4-CP.
Pirbazari and co-workers57,58 studied the photocatalytic activity, in aqueous media, of 4-CP by using Co(II)3 immobilized on TiO2 nanoparticles. In both studies,57,58 after 90 min under visible light irradiation (λ > 400 nm), it was possible to degrade 50% of 4-CP with Co3–TiO2, which could be increased to ∼100% in the presence of H2O2 (Table 3). These results are auspicious; however, recyclability studies are crucial to determine the potential use of this photocatalyst in wastewater treatment. In this sense, in the second study,58 a decrease in the efficiency of the degradation process of 95% to 60% (in the presence of H2O2) after four photocatalytic studies was observed. In this study, the photocatalytic experiments were extended to 2,4-DCP. In both studies,57,58 the authors reported carboxylic acids and CO2 as the degradation products of 4-CP.
Two studies were developed by Gül and co-workers35,99 where they studied the degradation of 4-CP after the incorporation of Co(II) and Zn(II) Pcs Co/Zn11 and Co/Zn15 (Fig. 4) into TiO2 semiconductors. The results showed that under visible light (λ > 400 nm), it was possible to achieve complete degradation of 4-CP in aqueous media after 30 min with Zn11, Co11, Zn15, and Co15 (Table 3). Moreover, they observed that the MPcs were anchored into the surface of TiO2 through CO–O–TiO2 bonds. In the first study,99 recyclability studies showed a loss of 16% in the degradation of the pollutant using Zn11 and Co11 after 5 cycles (Table 4). In the second study,35 after 5 cycles of the experiment, the majority of the photodegradation rate of 4-CP could be maintained (∼9% decrease) using Co15. On the other hand, there was a decrease of ∼16% in the degradation of 4-CP with Zn15. Herein, the authors extended the photocatalytic assays to chlorobenzene (PhCl) and 1,2,4-trichlorobenzene (TCB). None of these studies revealed the stability of the compounds under irradiation nor the degradation products of 4-CP.
Sevim36 developed a study regarding the photooxidation of 4-CP under visible light irradiation (λ > 400 nm) using non-symmetrical tetra-substituted phthalocyanine bearing one carboxy group (either 4-sulfanylbenzoic acid or 4-hydroxybenzoic acid) and three 4-tert-butylphenoxy substituents and their zinc(II) and cobalt(II) complexes (32 and 33, Fig. 8) immobilized on TiO2 as catalysts. The MPcs were anchored on the surface of TiO2 through CO–O–TiO2 bonds (Table 3). It was possible to achieve ≥85% of 4-CP degradation within 120 min of irradiation using all photocatalysts compared with bare TiO2 (3%). The best photocatalytic rate was obtained with Co33 (90%), followed by Co32 (88%), Zn33 (86%), and Zn32 (85%). The reusability studies showed that, after five cycles, there was a decrease in the photooxidation rate from 90% to 66% when using Co33 and from 85% to 63% when using Zn32. The degradation products of 4-CP were not revealed.
| MPc | Support | Light | Irradiation time (min) | Efficiency (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| a With H2O2. | ||||||
| Al4Cl | Surfactant modified bentonite | Visible light (λ > 450 nm) | 60 | 100 | — | 50 |
| Al3 | Amberlite | Visible light (λ > 400 nm) | 30 | 40 | 54 | |
| Co3 | MCM-41 | UV-A (λ = 280–400 nm) | 180 | 45 | 59 | |
| 93a | 4 cycles (35% loss) | |||||
| Al3 | TiO2 | Visible (λ > 400 nm) | 360 | 100 | — | 47 |
| Co3 | 180 | 95 | ||||
| 50 | 4 cycles (40% loss) | 58 | ||||
| 100a | ||||||
| Co4 | N-GR | 135 | 80 | 3 cycles (25% loss) | 60 | |
| Zn4 | g-C3N4 | 60 | 85 | – | 61 | |
Nyokong and co-workers,54 on the other hand, could achieve ∼40% degradation of 2,4-DCP in aqueous media after 30 min of visible light irradiation (λ > 400 nm) by using Al3 immobilized on Amberlite® (Fig. 4 and Table 5). 2-Chloro-1,4-benzoquinone and formic acid were identified as the degradation products of 2,4-DCP. No stability or recyclability studies were reported for the catalyst.
The photodecomposition of 2,4-DCP in aqueous media was also assessed using sulfonated cobalt(II) phthalocyanine Co3 (Fig. 4) immobilized onto MCM-41.59 The immobilization of Co3 onto MCM-41 was performed through the ionic interactions between the cationic groups of the 3-(aminopropyl)triethoxysilane and the sulfonato groups of the phthalocyanine. The authors stated that both light and catalyst are essential for the degradation of 2,4-DCP since it could only be observed in the presence of the catalyst and light (visible or UV-A). When irradiated with UV-A light for 180 min, Co3 (0.6 g L−1) exhibited a higher decrease of 2,4-DCP degradation (93%) than when irradiated with visible light (55%) in the presence of H2O2. As indicated in Table 5, the presence of H2O2 is essential when using the UV-A light – the sample containing H2O2 + Co3 could achieve 93% degradation after 180 min of irradiation, while only 45% degradation was observed in the sample containing only Co3. After 4 cycles under UV-A irradiation, the photocatalytic degradation of 2,4-DCP could maintain up to 70%. The authors identified methyl pyruvate, dimethyl oxalate, dimethyl malonate, methyl levulinate, and methyl benzoate as the primary intermediates of 2,4-DCP degradation. However, the final degradation products were simple acids like oxalic acid and acetic acid.
Xu and co-workers47 and Pirbazari and co-workers58 accessed the degradation of 2,4-DCPusing Al3 and Co3, respectively, adsorbed on TiO2 as photocatalysts. The first authors,47 could achieve a complete degradation in aqueous media after 420 min of visible light irradiation (λ > 400 nm) with 1.0 wt% of Al3 loaded on TiO2 (Table 5). There were no studies regarding the stability of the catalyst and the degradation products of 2,4-DCP. In the second study, they achieved 50% degradation of 2,4-DCP after 180 min of visible light irradiation (λ > 400 nm), which could be increased to 100% in the presence of H2O2. Moreover, after four cycles, there was a loss of 40% in the photocatalytic oxidation of 2,4-DCP using Co3–TiO2. Regarding the degradation products of 2,4-DCP, the authors identified carboxylic acids and CO2.
Recently, Frajood and co-workers60 could degrade 80% of 2,4-DCP within 135 min of visible light irradiation (λ > 400 nm) by using Co4/nitrogen-doped graphene (N-GR). According to the authors, after 3 cycles, there was a loss of 25% of activity. The authors identified carboxylic acids and CO2 as the degradation products of 2,4-DCP. Zada, Dong, Fu, and co-workers61 prepare a Zn4/g-C3N4 nanocomposite for the photodegradation of 2,4-DCP. The authors could achieve a degradation of 85% after 60 min of visible light irradiation (λ > 420 nm). The enhanced catalytic activity of this nanocomposite is due to its high visible light absorption and effective generation of super oxide anions and holes. However, no studies were performed regarding the recyclability of the catalysts or degradation products of 2,4-DCP.
Xu and co-workers47,50 reported two works regarding the degradation58 of 2,4,6-TCP. In the first study,50 a complete degradation was achieved in less than 60 min using visible light irradiation (λ > 450 nm) and Al4Cl–bentonite (modified with cetyltrimethylammonium bromide) in aqueous media (pH = 12). However, a gradual loss of activity was observed after each catalytic cycle, and it might be due to the degradation of the surfactant by the generated singlet oxygen or by reducing the sorption process. In the second work,47 the authors could achieve a 90% degradation in aqueous media after 420 min of visible light irradiation (λ > 400 nm) with 1.0 wt% of Al3 loaded on TiO2. No photostability studies of the catalysts nor the 2,4,6-TCP degradation products were reported. Nyokong and co-workers,54 on the other hand, could achieve 30% degradation in aqueous media after 30 min of visible light irradiation (λ > 400 nm) by using Al3 immobilized on Amberlite® (Fig. 4). The 2,5-dichloro-1,4-benzoquinone was identified as the main degradation product of 2,4,6-TCP. Zanjanchi and co-workers126 assessed the photodegradation of 2,4,6-TCP in aqueous media using visible light irradiation (λ > 400 nm) and BiVO4–Silica composites grafted with sulfonated cobalt phthalocyanine Co3 (Fig. 9). Different catalytic activities were observed depending on the silica and loaded phthalocyanine amount. The best results (∼100% degradation after 240 min of irradiation) were obtained using the sample containing 15% silica grafted with Co3; materials without the Co3 could only degrade 50% of 2,4,6-TCP. Regarding the stability of the composite, there was a leak of 15.2%, and the composite could be recovered and reused up to 4 times without significant activity loss (3%). The degradation products were not identified, but the pH decrease during the assays could indicate the formation of carboxylic acids.
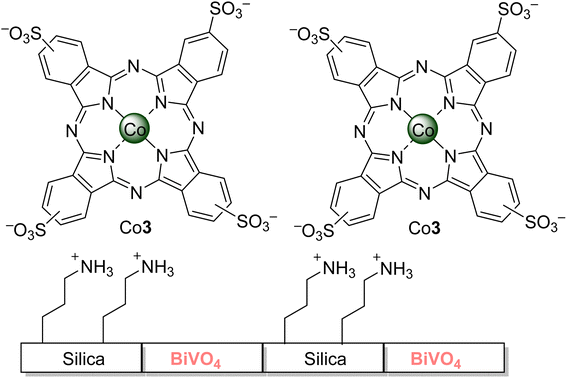 | ||
| Fig. 9 Silica–BiVO4 composites grafted with cobalt phthalocyanine Co3 used by Zanjanchi and co-workers126 for the photodegradation of 2,4,6-TCP. | ||
Nyokong and co-workers54,110 developed various materials for the degradation of PCP. In their first work,54 Al3, Zn3 (Fig. 4 and Table 6), Al18, Zn18 (Fig. 6 and Table 6), and a mixture of mono-, di-, tri-, and tetra-substituted sulfonated metalled phthalocyanines MPcSmix (M = Al(III), Zn(II), Ge(IV), Si(IV), or Sn(IV)) were immobilized on Amberlite®. These materials were used as photocatalysts in the degradation of PCP in aqueous media (pH = 10) under visible light irradiation (λ > 400 nm). The composites showed the following activity order after 5 min of irradiation: Zn18 > SiPcSmix > SnPcSmix > ZnPcSmix > GePcSmix > Zn3 > AlPcSmix ≈ Al18 > Al3. The Zn18/Amberlite was selected to perform the recyclability studies, where it was observed that there was only a 10% loss of activity after three cycles. However, when performing homogenous photocatalysis, there was a 51% degradation of the catalyst (Zn2) after 3 min of irradiation, which means that this phthalocyanine could not be applied to other pollutants as PCP since it needed more irradiation time. The tetrachloro-1,4-benzoquinone was identified as the major degradation product of PCP.
| Pc | % degradation |
|---|---|
| Zn18 | 30 |
| SiPcSmix | 26 |
| SnPcSmix | 24 |
| ZnPcSmix | 21 |
| GePcSmix | 17 |
| Zn3 | 13 |
| AlPcSmix | 11 |
| Al18 | 11 |
| Al3 | 6 |
Later,110 they degraded PCP in a homogenous catalytic process in dichloromethane (DCM) with Pd26 (Fig. 6) and in a heterogeneous photocatalytic process in water with Pd26/SWCNTs, both under visible light irradiation (λ > 400 nm). It was possible to achieve a degradation rate of 70% and 30% in the homogenous and heterogenous processes, respectively. Again, tetrachloro-1,4-benzoquinone was identified as the major degradation product of PCP. The catalyst cannot be reused without a significant loss of activity. Regarding the recyclability studies using 4-CP, the activity loss after each cycle was less drastic than PCP. This can be due to the permanent adsorption of intermediates or products on the surface of the catalyst, thereby reducing the adsorption activity of the composite. Despite the low photocatalytic rates of Pd26/SWCNTs hybrid, Pd26 had excellent photocatalytic activity by itself and, thus, may be applied in homogenous photocatalysis.
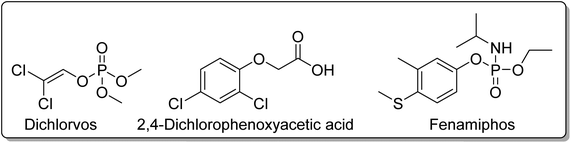
Regarding silica materials, Pereira, Azenha, and co-workers85 studied the photooxidation of PCP with three zinc(II) phthalocyanines Zn5, Zn7, and Zn16 (Fig. 4) immobilized into Al-MCM-41 in aqueous media. The best photocatalysts were Zn5/Al-MCM-41 (kobs = 7.3 × 10−4 min−1) and the cationic derivative Zn16 (kobs = 1.1 × 10−3 min−1) after 300 min of UV-visible light irradiation (λ = 320–460 nm). The authors identified tetrachloro-1,4-benzoquinone as the major degradation product. The photocatalytic studies were extended to fenamiphos, where the recyclability studies were performed.
Xu and co-workers47 reported the degradation of hydroquinone, catechol, salicylic acid, and 4-sulfosalicylic acid in aqueous media using 1.0 wt% of Al2 (Fig. 4) loaded on TiO2 and visible light irradiation (λ > 400 nm). Here, hydroquinone and catechol were degraded at 90% and 50%, respectively, after irradiation for 420 min. However, only 20% and 10% degradation of salicylic acid and 4-sulfosalicylic acid were observed under similar conditions. Raducan and co-workers86 reported the photodegradation of naphthol yellow S using Cu3 (Fig. 4), Cu4 (Fig. 4), and Cu25 (Fig. 6) immobilized on TiO2. Notably, with Cu4, a maximum degradation of 18% was achieved after 30 min under visible light irradiation (λ > 400 nm). Jiang and co-workers132 studied the photocatalytic degradation of BPA in aqueous media using polynuclear phthalocyanines M1 (Fig. 3) under visible light irradiation (λ > 400 nm) for 40 min. Among the four complexes (Fe1, Cu1, Zn1, and Al1), the highest photocatalytic activity was obtained for Zn1. For this reason, only this photocatalyst was used to study the optimized conditions to degrade BPA. It is essential to highlight that Zn1 evidenced their decomposition during the photocatalytic process, and it is still possible to achieve a total degradation of the pollutant after 20 min using a tungsten lamp. With solar irradiation, the same result was achieved after 40 min. Regarding the degradation products, the authors could identify oxalic acid, hydroquinone, and 4-isopropenyl phenol.
Wu, Xing, and co-workers46 also studied the photodegradation of BPA but using Pd3 (Fig. 4) immobilized on FDU-15 mesopolymer. Herein, the photodegradation efficiency of BPA was also observed in aqueous media at different pH values, in the presence of H2O2, and under visible light irradiation. The pollutant was degraded within 60 min and 180 min when using 0.04 and 1.0 mmol L−1, respectively. The degradation products were identified as dimethyl malonate, dimethyl oxalate, dimethyl D-malate, and CO2. The stability and photostability of the catalyst were not evaluated. In another study, Nyokong and co-workers98 studied the photooxidation of BPA under visible light irradiation using indium(III) using the phthalocyanines In9 (Fig. 4), 30, 31Cl/PS fibres (Fig. 8). The In31Cl/PS (t1/2 = 178.0 min) fibre was the best photocatalyst followed by In9Cl/PS (t1/2 = 267.0 min) and In30Cl/PS (t1/2 = 385.0 min).
The photodegradation of BPA under UV light irradiation (λ = 365 nm) in the presence of a ZnWO4/Mn17Cl material with 1 wt% Mn17Cl (Fig. 4) was investigated by Anucha and co-workers.102 It is highlighted that 60% of BPA was degraded after 4 h of light exposure, but the degradation could be increased to 80% by adding H2O2. However, a complete degradation of BPA could be achieved after 30 min only under visible light irradiation (λ > 450 nm) and adding H2O2.
3.2. Organic dyes
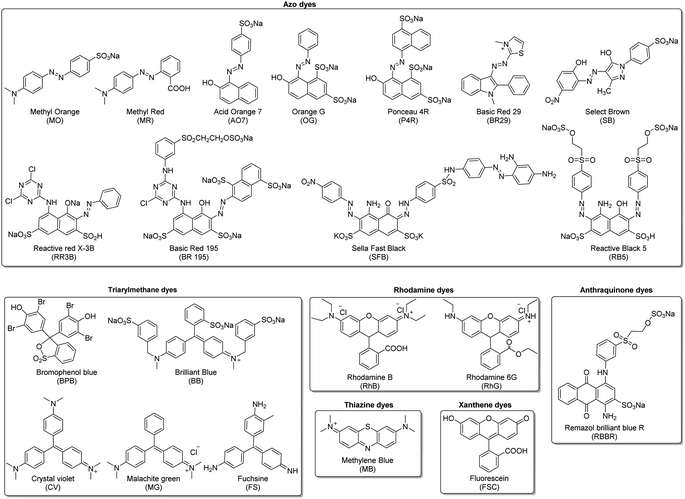
Organic dyes are another group of water pollutants of significant concern because many of the dyes used in industry are toxic to aquatic organisms. The wastewater from the dye industry has received attention in recent studies due to the toxicity of some raw materials (aromatic amines) used to produce the dyes. It has been shown that their disposal into the environment has led to severe contamination of significant areas in some countries. Decolourizing these dyes is challenging due to their stability and complex structures. Current technologies used to eliminate these compounds are considered ineffective.9,13In this review, the organic dyes were divided into six groups (Fig. 10): azo, triarylmethane, rhodamine, thiazine, xanthene, and anthraquinone dyes.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) functional group that can be found with other groups as aromatic rings. Due to their high production, their toxicity has been studied extensively. Their manufacturing was related to several cancer appearances and later proved that some azo dyes were carcinogenic.133 For this reason, the appearance of these dyes in wastewater could be considered a considerable health problem. In this review, we reported the studies of the degradation of nine azo dyes (Fig. 10): methyl orange (MO), methyl red (MR), acid orange 7 (AO7), orange G (OG), ponceau 4R (P4R), select brown (SB), sella fast black (SFB), basic red 29 (BR29), and reactive red 195 (RR195).
N–) functional group that can be found with other groups as aromatic rings. Due to their high production, their toxicity has been studied extensively. Their manufacturing was related to several cancer appearances and later proved that some azo dyes were carcinogenic.133 For this reason, the appearance of these dyes in wastewater could be considered a considerable health problem. In this review, we reported the studies of the degradation of nine azo dyes (Fig. 10): methyl orange (MO), methyl red (MR), acid orange 7 (AO7), orange G (OG), ponceau 4R (P4R), select brown (SB), sella fast black (SFB), basic red 29 (BR29), and reactive red 195 (RR195).
3.2.1.1. Methyl orange. MO is an unmanageable dye present in Nature that is hard to degrade and, if released into the environment, could cause severe threats to human health.134
The photocatalytic degradation of MO was studied by Nyokong and co-workers100 in the presence of Zn9 (Fig. 4) immobilized into PSU fibres to avoid aggregation. According to the authors, upon visible light irradiation, the use of PSU fibre (t1/2 = 135.9 min) seems to reduce the ability of the degradation of MO when compared with the Zn9 (t1/2 = 81.6 min) by itself (Table 7). Despite having higher half-life times and a lower degradation rate constant, degrading MO with the Zn9/PSU fibres was possible. It is essential to highlight that with an increase in the MO concentration, the higher half-life time for Zn9. The same authors84 also used a series of peripherally substituted zinc(II) and Lu(III)OAc phthalocyanine complexes 6–12 (Fig. 4) and 29 (Fig. 7) immobilized on PAA and PUR. In aqueous media, it was possible to degrade MO with the photoactive materials Lu7OAc/PS, Lu8OAc/PS, Lu12OAc/PS, and Lu29OAc/PS (Table 7) after 150 min under visible light irradiation (λ > 400 nm). These hybrids can degrade the MO slower (t1/2 = 113.63–182.43 min) than 4-NP and 4-CP. The degradation of MO could be achieved faster with peripherally substituted Zn7/PS fibres (t1/2 = 113.63 min) when compared with their non-peripheral analogue (Lu29/PS) (t1/2 = 130.78 min).
| MPc | Support | Light | ΦΔ | Irradiation time (min) | t1/2 (min) | Efficiency (% or rate) | Recycle | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Performed at 11.0 mW cm−2.b Amorphous TiO2.c P25 TiO2; with.d 0.125.e 0.25, and.f 0.5 of (mPc/mZIF) ratio. | ||||||||
| Zn9 | PSU | Visible light (λ > 400 nm) | — | — | 135.9 | — | — | 100 |
| — | 81.6 | |||||||
| Lu29OAc | PS | 0.22 | 130.8 | 84 | ||||
| Lu7OAc | 0.28 | 113.6 | ||||||
| Lu8OAc | 0.17 | 182.4 | ||||||
| Lu12OAc | 0.15 | 182.4 | ||||||
| Zn34 | PS/Ag NPs | 0.28 | 693 | 135 | ||||
| PS | 0.26 | 336 | ||||||
| Zn35 | PS/Ag NPs | 0.25 | 336 | |||||
| PS | 0.28 | 1125 | ||||||
| Zn40 | CoFe PA-6 | — | 60 | 288.8 | 142 | |||
| Zn42 | 364.7 | |||||||
| Zn40 | PA-6 | 533.1 | ||||||
| Zn42 | 495.0 | |||||||
| Zn40 | α-Fe2O3 | 46.2 | 143 | |||||
| Zn41 | 42.8 | |||||||
| Without Pc | 53.3 | |||||||
| H22 | TiO2 | Visible (λ > 550a nm) | 180 | — | 80b | 87 | ||
| 50c | ||||||||
| Without Pc | 10b | |||||||
| Cu3 | Visible (λ > 400 nm) | 60 | 80 | 88 | ||||
| H24 | Fe–TiO2 | — | 19.8 | — | 89 | |||
| TiO2 | 23.1 | |||||||
| Without Pc | 138.6 | |||||||
| Zn4 | ZnO | Red light (λ = 620 nm) | 180 | — | 35 | 90 | ||
| Without Pc | 1 | |||||||
| Visible light (λ = 400–800 nm) | 60 | |||||||
| Zn4 | 30 | |||||||
| Zn36 | MWCNT | Visible (λ > 400 nm) | 240 | 86 | 136 | |||
| Co3 | MCM-41 | 120 | 98 | 4 cycles (10% loss) | 91 | |||
| Cu4 | N-ZIF | 90 | 0d | 7 cycles (<5% loss) | 144 | |||
| 0.063/mine | ||||||||
| 0.055/minf | ||||||||
| PACA HIOB | Visible (λ > 420 nm) | 20 | 100 (0.085/min) | – | 93 | |||
By conjugating silver nanoparticles and fibres, the same authors135 degrade MO using α- and β-substituted zinc(II) phthalocyanines bearing carbazole groups 34 and 35 (Fig. 11), respectively. They linked them to silver nanoparticles, which were further immobilized in PS fibres to be used in the photocatalysis of MO (Table 7). Under visible light irradiation (λ > 400 nm), lower half-life times for the degradation of MO were achieved using the α-substituted Zn34/PS relative to the β-substituted Zn35/PS. The photooxidation process increased in the presence of silver nanoparticles when compared with the phthalocyanines.
 | ||
| Fig. 11 Tetra-substituted phthalocyanines: (A) α-substituted 34,135–137 36,37,78,136,138 38,139 39,140 and (B) β-substituted 35,135 37.141 | ||
The photooxidation of MO and OG in aqueous media under visible light irradiation (λ > 400 nm) with an amine-functionalized cobalt ferrite (CoFe) magnetite magnetic nanoparticle (MNP) conjugated with zinc(II) phthalocyanines Zn40 and Zn42 (Fig. 12) was studied by Nyokong and co-workers.142 These MNPs were embedded into electrospun polyamide-6 (PA-6) fibres for support and catalyst regeneration after the photocatalytic process. The authors compared the photocatalytic activity of CoFe–Zn40/PA-6 and CoFe–Zn42/PA-6 with CoFe/PA-6, Zn40/PA-6, and Zn42/PA-6 (Table 7) for the MO degradation experiment. After 60 min of irradiation, it was possible to achieve better MO degradation rates with the CoFe–Zn40 and CoFe–Zn42 compared with the respective electrospun Pcs and MNPs.
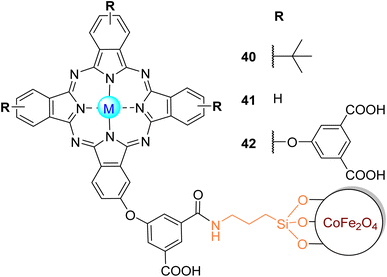 | ||
| Fig. 12 Zn40–Zn42 coupled to CoFe2O4 MNP used by Nyokong and co-workers142,143 for the degradation of MO. | ||
More recently, the same authors143 studied the photodegradation of MO under visible light irradiation (λ > 400 nm) with fabricated α-Fe2O3 nanofibres modified with the tetra-substituted phthalocyanine Zn40 (Fig. 12) or mono-substituted phthalocyanine Zn41 (Fig. 12). It was possible to achieve a 50% degradation more rapidly using Zn41–α-Fe2O3 (t1/2 = 42.78 min), followed by Zn40–α-Fe2O3 (t1/2 = 46.20 min) and α-Fe2O3 (t1/2 = 53.31 min) (Table 7). The results show that sensitising α-Fe2O3 with phthalocyanines reduced the half-life time, increasing the photocatalyst efficiency. By increasing the amount of MO, the half-life time was also increased. After reuse, there was a slight loss of activity as observed by an increase in the half-life times: Zn41–α-Fe2O3 (t1/2 = 45.00 min) > Zn40–α-Fe2O3 (t1/2 = 48.46 min) > α-Fe2O3 (t1/2 = 69.30 min). These authors reported in some of their studies 2-amino-5-(3-hydroxy-4-oxo-cyclohexa-2,5-dienylideneamino)benzene sulfonic acid and poly(catechol) as degradation products of MO.84,100
Chen, Zhang, and co-workers87 and Wang and co-workers88 studied the degradation of MO in aqueous media under visible light irradiation with H22/TiO2 amorphous hybrid (Fig. 4) and nanocrystalline anatase TiO2 with copper phthalocyanine Cu3 (Fig. 4), respectively (Table 7). In the first study,87 it was possible to achieve ∼80% of degradation with H22/TiO2 after 180 min of light irradiation. These results are much higher when compared with H24/P25 (50% within 180 min). At the same time, there was no degradation using pristine TiO2 and H22/SiO2. In the second study,88 80% of MO could be degraded with the hybrid Cu3/TiO2 within 60 min. The samples were heated at different temperatures, and the photocatalytic activity was again evaluated. After increasing the temperature, the dimeric form of Cu3 was disaggregated and vaporized or desorbed, and the amount of immobilized monomer was much higher when compared with the dimer. The excited state of the monomer has a much higher half-life than the aggregates, favouring the electron injection process. Gharagozlou and co-workers89 evaluated the photooxidation of MO after doping TiO2 with Fe and preparing H24 (Fig. 4)–Fe-doped TiO2 (H24/Fe–TiO2) with different Fe doping content (0, 0.05, 0.5, and 3.0 mol% Fe). As expected, the Fe amount curiously influences the activity of the TiO2 composites. Under visible light irradiation (λ > 400 nm) and in the presence of H24/Fe–TiO2 with different doping amounts of Fe, it was possible to observe the photocatalytic activity on MO (Table 7). The results showed that the degradation of MO is more effective using H24/Fe–TiO2 (t1/2 = 19.8 min) than using undoped H24/TiO2 (t1/2 = 23.1 min) and bare TiO2 (t1/2 = 138.6 min). These results can be due to electron–hole pairs' formation and electrons' transference from H24 to TiO2. Also, according to the field theory, Fe2+ is unstable compared to Fe3+, and a release of a trapped electron occurs, returning to the Fe3+ form. Initially, the photocatalytic degradation of MO increased with the increase of the doping amount of Fe on the composite, reaching a maximum activity of 0.5% and then decreasing with higher amounts of Fe. This activity can be explained by the fact that above 0.5 mol% of Fe, the Fe3+ ions act as recombination centres for photo-generated electrons and holes, decreasing photocatalytic activity.
Regarding zinc oxide, Ahmed, Pal, and co-workers90 developed a study of the degradation of MO using a hybrid containing zinc oxide nanorods and Zn4 (Fig. 4) (Zn4–ZnO) functionalized with two carboxyl groups of a tartrate molecule by interaction with the metallic zinc. According to Table 7, the authors performed the photocatalytic studies under red light (λ = 620 nm) and white light irradiation (λ > 365 nm). It was possible to degrade MO more efficiently with the hybrid material (35%) compared to bare ZnO (1%) after 180 min of red light irradiation. The best results were obtained under white light irradiation (λ > 365 nm), where the nanohybrid material Zn4–ZnO could achieve about 60% photocatalytic efficiency.
Xu, Li, and co-workers136 studied the degradation of MO in an aqueous solution under visible light irradiation (λ > 400 nm) by using substituted zinc(II) aminophthalocyanine Zn36 (Fig. 11) supported by functionalized multi-walled carbon nanotubes (MWCNTs). The authors proved by Raman spectroscopy that the covalent attachment of Zn36 to MWCNTs and the π–π interactions between these two entities induce the disaggregation of the Zn36. After 4 h, 86% of MO was degraded (Table 7). The authors also assessed these photocatalysts' ability to degrade Rhodamine B (RhB).
Liu, Zhao, and co-workers91 studied the degradation of MO with Co4 (Fig. 4) immobilized onto MCM-41 under visible light irradiation. The authors established that either less or an excess amount of photocatalyst in the reaction solution impacted the degradation of MO. So, a low photocatalyst amount was insufficient to interact with all the MO molecules. However, an excess photocatalyst amount of Co3 caused a shielding effect in light penetration. It was possible to achieve 98% MO degradation after 120 min of light irradiation with an optimal catalyst amount of 0.2 mg mL−1 (Table 7). After 4 cycles, it was possible to maintain a degradation rate of 90%.
Dabiri and co-workers144 developed a study where they could degrade MO with nitrogen-doped carbon photocatalyst based on the carbonization of Cu4 (Fig. 4) on/in zeolitic imidazolate framework-8 (ZIF-8) hybrid–Cu4/N-PC. The authors could obtain Cu40.125/N-PC, Cu40.25/N-PC, and Cu40.5/N-PC with different weight ratios were obtained. After 90 min of visible light irradiation (λ > 400 nm), there was no degradation of MO with Cu40.125/N-PC (Table 7). On the other hand, in the presence of H2O2, the authors could degrade MO when using Cu40.25/N-PC (0.0627 min−1) followed by Cu40.5/N-PC (0.0551 min−1). Also, after seven cycles, there was a loss of 4% in the degradation rate of MO. These studies were extended to RhB.
More recently, Zang, Sun, Zhang, and co-workers93 studied the degradation of MO in an aqueous solution under visible light irradiation (λ > 420 nm) by using the Cu4 (Fig. 4) supported by poly(acrylamide-acrylic acid copolymer) hydrogel inverse opal beads (PACA HIOB). The authors assembled silica microspheres as sacrificial templates and infused monomers within the pores for UV polymerisation to form the PACA HIOBs. After 20 min of light irradiation, there was a complete degradation of MO with a degradation rate of 0.0850 min−1 (Table 7). The authors also assessed these photocatalysts' ability to degrade other anionic dyes as Reactive red X-3B (RR3B), reactive black 5 (RB5), Remazol brilliant blue R (RBBR) and cationic dyes as RhB and Malachite green (MG). According to the authors, the degradation kinetics of the anionic dyes, except for RBBR, were faster than those of cationic dyes. The work of Zang, Sun, Zhang, and co-workers93 was better than other hydrogel-based photocatalysts because the hydrogel-base inverse opal scaffold can increase light absorption, catalytically active sites, reduce charge transfer resistance and inhibit the recombination of photogenerated electron–hole pairs. Regarding MO, the authors did not study the degradation products or the recyclability of the photocatalyst.
3.2.1.2. Methyl red. MR is an azo dye that appears in textiles and other commercial products. This dye can cause eye and skin sensitization and digestive tract irritation when swallowed, and thus, it is important to remove it from wastewater.145
Nyokong and co-workers103 studied the degradation of MR in aqueous media (pH = 7.4) with a covalently linked In18Cl to MNP already functionalized with (3-aminopropyl)triethoxysilane (Fig. 13). The use of MNP allows the catalyst to be recovered several times with magnetic separation. Furthermore, In18Cl and its conjugate with MNP have been embedded in PAN electrospun fibres. Under visible light irradiation (λ > 400 nm), it was possible to degrade more efficiently the MR with the MNP–In18Cl/PAN, given the lower time of half-life (t1/2 = 29.5 min) and higher degradation rate (kobs = 0.0235 min−1). After the reuse of the catalyst, there was a slight decrease in the MR degradation rate and an increase in the half-life time. In this study, the authors did not perform stability studies, but they mentioned that the conjugates are stable because, during the singlet oxygen generation assays, the Q bands of the compounds remained intact on their absorption spectra.
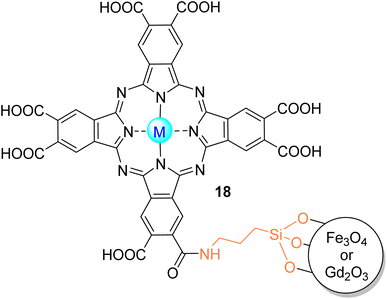 | ||
| Fig. 13 In(III)18Cl or Zn(II)18 covalently bonded to MNP of Fe3O4 or Gd2O3 used by Nyokong and co-workers103 for the degradation of MR. | ||
3.2.1.3. Acid orange 7 (or orange II). Acid orange 7 (AO7), also known as orange II, is an azo dye extensively used for dyeing wool. This dye is non-biodegradable, and for that reason, its removal from wastewater is needed.146 Nowakowska and co-workers147 studied its degradation in aqueous media under visible light irradiation (λ = 400–550 nm) using the fluorinated phthalocyanine Zn43 (Fig. 14) immobilized into bentonite as the photocatalyst. A degradation of 80% was observed after 200 min of light irradiation. Reusability studies showed that the catalyst could be reused four times without significant activity loss (15%).
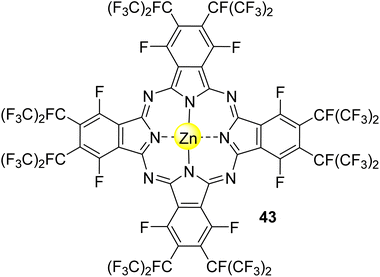 | ||
| Fig. 14 Zinc(II) perfluoroalkyl perfluorophthalocyanine 43 used by Nowakowska and co-workers147 for the degradation of AO7. | ||
The degradation of AO7 in aqueous media using visible light irradiation was also evaluated by Schneider and co-workers104 in the presence of Cu18 (Fig. 6) immobilized on g-C3N4. A maximum degradation rate of 95% was achieved after 180 min of light irradiation with 0.5 wt% of Cu18. Recyclability studies of the catalyst revealed a slight decrease in activity (5% loss) after five cycles. Qu and co-worker141 selected AO7 as a target pollutant for their decomposition, evaluating the use of cobalt(II) phthalocyanine Co37 (Fig. 11) immobilized on activated carbon fibres. The AO7 removal was enhanced using Co37/carbon fibres as a catalyst and UV light irradiation (λ = 365 nm). In fact, within 120 min of irradiation, there was a 23% enhancement in the dye removal. The best photocatalytic rate (47%) was achieved using a 2 g L−1 catalyst concentration. The authors also assessed the influence of H2O2 addition, where it was possible to achieve 99% of the photodegradation rate within 60 min of irradiation with a concentration of H2O2 of 12.5 mmol L−1. After four cycles, the photocatalytic rate was still above 92%, meaning no significant activity loss occurred.
3.2.1.4. Orange G. Orange G (OG) is non-biodegradable, toxic, and potentially carcinogenic.148 The photooxidation of OG in aqueous media under visible light irradiation (λ > 400 nm) using Zn18 immobilized on the surface of magnetite magnetic nanoparticles (Fig. 13) was studied by Antunes and co-workers.105 According to the authors, it was possible to degrade OG more efficiently using the nanoparticle (lower half-life time (t1/2 = 131 min) and rate (kobs = 0.0053 min−1)) when compared with the phthalocyanine by itself (t1/2 = 157 min, kobs = 0.044 min−1). After one reuse, the catalyst remained stable towards OG photodegradation with a slight decrease in the degradation rates (kobs = 0.0049 min−1) and an increase in the half-life time (t1/2 = 141 min). Also, for the degradation of orange G, Nyokong and co-workers95,142 prepared conjugates of octa-substituted carboxylic zinc(II) phthalocyanine Zn18 with Gd2O3 MNPs with PA-6 fibres (Fig. 13) and an amine-functionalized CoFe MNP conjugated with zinc(II) phthalocyanines Zn40 and Zn42 (Fig. 12). In their first study,142 after 60 min under visible light irradiation (λ > 400 nm), the electrospun Pcs CoFe–Zn40 (t1/2 = 41.50 min) and CoFe–Zn42 (t1/2 = 44.42 min) were the most effective photocatalysts compared with the respective Pc-MNP conjugates (t1/2 = 51.33 and 52.11 min, respectively). In their second study,95 OG was easily degraded (∼90%) after 30 min of visible light irradiation (λ > 400 nm) in the presence of the composite Zn2–MNPs/PA-6 fibres. More recently, Yilmaz and co-workers140 could degrade OG in aqueous media with a tetra-substituted thiocarboxylic zinc(II) phthalocyanine 39 (Fig. 11) immobilized onto Au@SiO2. After 25 min of visible light irradiation (λ > 400 nm), there was an 80% of OG degradation. After 3 cycles, half of the photocatalytic activity was lost.
3.2.1.5. Ponceau 4R (acid red 18), select brown (acid brown 98) and sella fast black (acid black 210). Textile dyes like P4R, SB and SFB are intended to resist degradation, chemically stable, non-biodegradable, toxic, and carcinogenic.62,86 Machado and co-workers62 studied the degradation of P4R under visible light irradiation with f Zn4 (Fig. 4) functionalized on TiO2 (Zn4/TiO2 1.6 wt%). According to the authors, the as-prepared composite showed a better performance when compared with bare TiO2 reaching 50% of P4R degradation within 120 min. For the photodegradation of P4R, SB and SFB, Raducan and co-workers86 used Cu3 (Fig. 4)–TiO2, Cu4–TiO2 (Fig. 4), and Cu25–TiO2 (Fig. 6). The different photocatalytic activities in the aqueous media of these nanocomposites are presented in Table 8. Regarding the recyclability studies, the authors selected Cu3–TiO2 and brilliant blue as a dye.
| Pollutant/MPc | No | Cu3 | Cu4 | Cu25 |
|---|---|---|---|---|
| P4R (%) | 20 | 20 | 20 | 20 |
| SB (%) | 42 | 60 | 42 | 60 |
| SFB (%) | 8 | 0 | 5 | 18 |
3.2.1.6. Reactive black 5. RB5 is most widely used for dying cotton and other cellulose fibres. There is a huge consumption of this dye in industry.149 Zang, Sun, Zhang, and co-workers studied93 the degradation of RB5 in an aqueous solution under visible light irradiation (λ > 420 nm) by using the Cu4 (Fig. 4) supported by PACA HIOB. After 20 min of light irradiation, there was a complete degradation of RB5 with a degradation rate of 0.0772/min. The authors also assessed these photocatalysts' ability to degrade other anionic dyes such as Reactive red X-3B (RR3B), RBBR and cationic dyes such as RhB and Malachite green (MG). Regarding RB5, the authors did not study the products of degradation or recyclability.
3.2.1.7. Basic red 29 and reactive red 195. Azo dyes like BR29 and RR195 are resistant to biodegradation under aerobic conditions, whereas anaerobic treatment could be applied successfully. However, anaerobic processes cannot be used in wastewater treatments because the breakdown of these dyes can form aromatic amines, which are considered more toxic than the dyes themselves.150,151
The degradation of BR29 and RR195 using Fe4/AT-PAN and Fe4/PAN (Fig. 6) was studied in aqueous media under visible light irradiation by Han, Zhao, and co-workers.63 In the presence of H2O2, it was possible to have a complete degradation of RR195 with and without light irradiation with the AT-PAN as a catalyst. On the other hand, only 20% of RR195 could be degraded either in the presence or absence of light, showing no difference in using an irradiation system for both dyes. Regarding the PAN fibres catalyst, the influence of visible light is noticeable, with an increase in the degradation rate of 50–90% for BR29 and 40–80% for RR195, respectively. The degradation products of these dyes were not identified, and the authors did not perform stability and photodecomposition studies of the catalysts.
3.2.1.8. Reactive red X-3B. RR3B is an azo dye used in fabric dyeing and represents almost 60% of the total dyes used in the dyeing industry. Due to their toxicity, its discharge into the water will threaten aquatic and human organisms.152 Zang, Sun, Zhang, and co-workers93 studied the degradation of RR3B in an aqueous solution under visible light irradiation (λ > 420 nm) by using the Cu4 (Fig. 4) supported by PACA HIOBs. After 15 min of light irradiation, there was a complete degradation of RR3B with a degradation rate of 0.1218 min−1. After five cycles, the degradation efficiency was still 98.1%. The authors also assessed these photocatalysts' ability to degrade other anionic dyes, such as RBBR and cationic dyes, such as RhB and Malachite green (MG). Regarding RR3B, the authors did not study the degradation products.
3.2.2.1. Crystal violet. CV is considered carcinogenic with acute cytotoxicity to some animals and plants. The inadequate discharge into the environment can cause some serious health problems.154 For the degradation of CV, Mohamed and co-workers155 used a composite based on ZnO and Cu44 (Fig. 15) and a solar simulator as an irradiation source. A complete degradation was achieved with the composite Cu44/ZnO in the presence of H2O2 (80% in the absence of H2O2) after 40 min of irradiation in aqueous conditions (pH = 5). These results showed an enhancement of the photocatalytic activity compared with bare ZnO and Cu44. There was a decrease of 10% in the photocatalytic degradation of CV after three cycles of the experiment.
 | ||
| Fig. 15 Phthalocyanine 44 used by Mohamed, Youssef and co-workers155,157 for the degradation of CV and BPB. | ||
3.2.2.2. Bromophenol blue. BPB is a triarylmethane dye considered hazardous for all living beings, negatively affecting photosynthesis in aquatic organisms. It is considered carcinogenic, mutagenic, and allergenic.156 For the BPB degradation in aqueous media, Mohamed and Youssef157 used a nickel(II) phthalocyanine Ni44 (Fig. 15) immobilized on TiO2 nanoparticles and visible light irradiation (λ > 400 nm). The characterization of the resulting material showed that Ni44 is anchored to the surface of TiO2 through SO2–O–TiO2 bonds. It was possible to achieve complete degradation of BPB within 50 min. No significant loss of activity was observed after 3 cycles.
3.2.2.3. Brilliant blue (acid blue 9) and fuchsine (basic violet 14). BB was a popular colorant in the textile industry and used as a common food additive. Due to its toxic effects on humans and animals, it was banned. It is reported to be carcinogenic, causing reproductive and neurological disorders, allergies, and trouble breathing.158 FS is also carcinogenic and mutagenic and has a very slow degradation in Nature.159
Raducan and co-workers86 studied the degradation of BB and FS in aqueous media using Cu3–TiO2 (Fig. 4), Cu4–TiO2 (Fig. 4), and Cu25–TiO2 (Table 9). Regarding the recyclability studies, the authors selected Cu3–TiO2 as the catalyst and BB as the pollutant. The results showed that after 10 cycles a photodegradation rate of 20% was achieved for the selected dye.
| Pollutant/MPc | No | Cu3 | Cu4 | Cu |
|---|---|---|---|---|
| BB (%) | 10 | 10 | 22 | 22 |
| FS(%) | 38 | 38 | 58 | 40 |
3.2.2.4. Malachite green. MG is an extensively used biocide in aquaculture with genotoxic and carcinogenic properties.160 So, Zang, Sun, Zhang and co-workers93 studied the degradation of MG in an aqueous solution under visible light irradiation (λ > 420 nm) by using the Cu4 (Fig. 4) supported by PACA HIOBs. MG was degraded entirely after 40 min of light irradiation with a degradation rate of 0.0601 min−1. The authors also assessed these photocatalysts' ability to degrade other anionic dyes, such as RBBR and cationic dyes, such as RhB. Regarding MG, the authors did not study the degradation products or the recyclability of the photocatalyst.
3.2.3.1. Rhodamine B. RhB is a dye used in the textile industry and in food processing. However, since it has been classified as carcinogenic, it has been forbidden from being used in food processing for decades. Several strategies exist to obtain MPcs with high floating properties, like making phthalocyanine super-hydrophobic. So, for the degradation of RhB in aqueous media under visible light irradiation (λ > 400 nm), Shao, Chen, and co-workers64 used a hierarchical nanostructure with a hollow interior space composed of zinc(II) phthalocyanine Zn4 (Fig. 4). Within 660 min, 89% of the RhB was photodegraded in the presence of the hierarchical tubular structure (Table 10).
| MPc | Support | Light | Efficiency (%) | Irradiation time (min) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| a With 0.25 mg of photocatalyst amount. | ||||||
| Zn4 | — | Visible (λ > 400 nm) | 89 | 660 | — | 64 |
| Cu45–CMP | 50 | 150 | 65 | |||
| Zn45–CMP | 50 | |||||
| Co5 | 80 | |||||
| Co45–CMP | 100 | 30 | 4 cycles (10% loss) | |||
| Co46 | Chitosan | UV | 99 | 60 | 5 cycles (40% loss) | 164 |
| Fe4 | PAN | Visible (λ > 400 nm) | 100 | 60 | 5 cycles (12% loss) | 63 |
| AT-PAN | ||||||
| Zn4 | TiO2 | 70 | — | 7 cycles (10% loss) | 66 | |
| — | P-25 | 10 | — | |||
| Zn4 | TiO2 | Sunlight | 90 | |||
| — | P-25 | 80 | ||||
| Co4 | TiO2 | Solar | 73 | 90 | 34 | |
| Fe4 | 88 | |||||
| No | 78 | |||||
| Zn52 | TiO2 | Visible (λ > 400 nm) | 90 | 180 | 165 | |
| Cu5 | 87 | 240 | 67 | |||
| Fe5 | TiO2/carbon fibres | Visible (λ = 400–700 nm) | 91 | 180 | 3 cycles (<5% loss) | 68 |
| No | 35 | |||||
| Without Pc | P25 | 27 | ||||
| Without Pc | Carbon fibres | 46 | ||||
| Cu4 | rGO | Visible (λ > 400 nm) | 96 | 210 | — | 69 |
| Zn4 | MWCNTs | 93 | 60 | 70 | ||
| No | 54 | |||||
| No | MWCNTs | 4 | ||||
| Zn36 | 88 | 240 | 3 cycles (<5% loss) | 136 | ||
| No | 19 | |||||
| 14 | 120 | — | 139 | |||
| Zn38 | 93 | |||||
| No | 54 | |||||
| Cu47 | Perylene diimide | 80 | 180 | 166 | ||
| Sn4 | NiWO4 | UV | 71 (1 wt%) | 120 | 71 | |
| 48 (3 wt%) | ||||||
| 49 (2 wt%) | ||||||
| No | 37 | |||||
| No | NiWO4 | 30 | ||||
| Cu5 | BiOCl/PAN | UV-visible | 80 | 180 | 3 cycles (0% loss) | 167 |
| Fe4 | AT-PAN | Visible (λ > 400 nm) | 100 | 80 | 5 cycles (0% loss) | 73 |
| Zn16 | g-C3N4/PAN | 98 | 120 | — | 56 | |
| Solar | 100 | 180 | 5 cycles (<5% loss) | |||
| Co4 | GR–ZnO | 150 | — | 74 | ||
| No | 140 | |||||
| Cu4a | N-ZIF | Visible (λ > 400 nm) | 0.0627 min−1 | 90 | 7 cycles (<5% loss) | 144 |
| Mn3 | TiO2–SiO2 | 100 | 240 | — | 168 | |
| Fe18 | AT/Fe3O4 | 96 | 300 | 106 | ||
| Cu4 | PACA HIOBs | Visible (λ > 420 nm) | 40 | 100 0.0685 min−1 | 93 | |
Conjugated microporous polymers (CMP) are crosslinked polymers that merge porosity and extended π-conjugated systems. Their building blocks are spatially segregated to suppress the π–π interaction. They have a high surface area, rigid backbone, and high thermal stability.163 So, Duan and co-workers65 studied in aqueous media the degradation of RhB in the presence of H2O2 with a series of metallophthalocyanine-based microporous polymers M45–CMP (M = Co(II), Cu(II), or Zn(II), Fig. 16) and visible light irradiation (λ > 400 nm, Table 10).
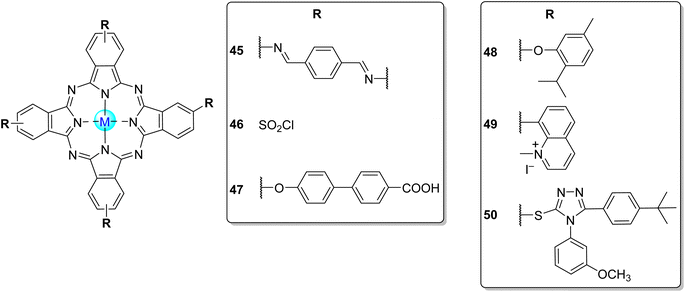 | ||
| Fig. 16 Tetra-β-substituted phthalocyanines 45–50.65,164,166,169–171 | ||
The authors discovered that the efficiency of the photocatalytic activity on RhB was improved when the amount of catalyst increased, but an excessive amount of catalyst proved useless for the system. Also, when comparing the photocatalytic activity of Co45–CMP with the monomer Co5, the polymer Co45–CMP showed an enhanced photocatalytic activity (∼100% after 30 min of irradiation vs. 80% after 150 min of irradiation). After discovering the ideal conditions, the three photocatalysts' ability to degrade RhB was accessed under the same conditions (15 mg of M45, in H2O2). It was possible to complete the degradation of RhB after 30 min with Co45–CMP (∼100% of degradation after 30 min of irradiation). On the other hand, it was only possible to degrade 50% of RhB with Cu45–CMP and Zn45–CMP after 180 min of light irradiation. The best photocatalytic rate with Co45–CMP could be maintained without a significant decrease after four experiments. The authors mentioned that these results indicate that the MPc-CMPs can potentially apply to the environmental purification of organic pollutants in industrial wastewater.
Another study of the RhB degradation in an aqueous media was performed by Wang and co-workers164 using a chitosan-supported cobalt(II) phthalocyanine Co46 (Fig. 16) membrane under UV light irradiation. According to the authors, chitosan is linked to the phthalocyanine Co46 through a sulfonamide bond established between the chlorosulfonyl groups and the amino groups of the chitosan. There was a 99% degradation of RhB with the polymer after 60 min (Table 10). After five experiments, retaining 60% of the degradation rate was possible. Han, Zhao, and co-workers63 studied the photooxidation for RhB in aqueous media using iron(II) phthalocyanine Fe4 (Fig. 4) immobilized onto copper(II)–amidoximated polyacrylonitrile fibre (AT-PAN), visible light, and H2O2. The amidoxime groups aided in the anchoring process of Fe4 to the PAN fibre through coordination interaction. The authors prepared two different catalysts: one containing unmodified PAN fibres and the other containing the AT-PAN. When using either catalyst, complete degradation of RhB was achieved in the presence of H2O2 within 60 min (Table 10). After 5 cycles, it was only observed a 12% decrease in the photodegradation rate. Regarding the degradation products, they reported the ones presented in Fig. 17. At the end of the photocatalytic assay, the authors could find carboxylic acids and alcohol as the final products of RhB.
 | ||
| Fig. 17 Degradation products of RhB reported by Han, Zhao, and co-workers.63 | ||
For the degradation of the same pollutant in aqueous media, Xin and co-workers66 and Varghese and co-workers34 used TiO2 sensitized with Zn4 (Zn4–TiO2, Fig. 4), Fe4 or Co4 and visible (λ > 400 nm) or simulated solar light. In the first study,66 the authors prepared TiO2 nanoparticles through a hydrothermal method with posterior impregnation of the Zn4. According to the results, the Zn4–TiO2 has an extended absorption band into the visible region compared to bare TiO2 (Degussa P25). This photophysical feature induces a higher photocatalytic activity under the simulated solar light and visible light (λ > 400 nm) when compared with bare TiO2. As expected, this difference is more pronounced under visible light than simulated solar light (Table 10). In the second study,34 under solar irradiation and using Fe4/TiO2, Co4/TiO2, and bare TiO2, it was possible to degrade 88%, 73%, and 78%, respectively (Table 10). Despite promising photocatalytic activities, evaluating the stability of compounds within several cycles would be essential.
Still studying the degradation of RhB in aqueous media, Sosa-Sánchez and co-worker165 used a hybrid photocatalyst with the non-symmetrical zinc(II) phthalocyanine Zn51 (Fig. 18) and visible light. This hybrid was compared to the individual molecules (TiO2 and Zn51) to evaluate the sensitization effect on their photocatalytic efficiency. It was observed that an improvement in the photocatalytic degradation of RhB occurred after sensitization of the TiO2. After 180 min, there was a decrease of 90% in the concentration of RhB (Table 10).
 | ||
| Fig. 18 β,β′-Dichlorinated zinc(II) phthalocyanine 51 used by Sosa-Sánchez and co-workers165 for the degradation of RhB. | ||
Shao, Mu, and co-workers67,68 accessed the photooxidation of RhB using photocatalysts based on hierarchical nanostructures with Cu5 (Fig. 4) immobilized on electrospun TiO2 nanofibers and the loading of Fe5 (Fig. 4) nanosheets on one-dimensional carbon nanofibers. In the first study,67 the photocatalytic degradation of RhB under visible light irradiation (λ > 400 nm) showed that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (molar ration) Cu5/TiO2 nanofibers could achieve a degradation rate of 38% after 4 h, much higher than bare TiO2 nanofibers (Table 10). After increasing the molar ratio to 1
50 (molar ration) Cu5/TiO2 nanofibers could achieve a degradation rate of 38% after 4 h, much higher than bare TiO2 nanofibers (Table 10). After increasing the molar ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 87% degradation was achieved within 240 min. In the second study,68 Fe5 nanosheets were uniformly distributed on the surface of each fibre without enhancing an aggregation phenomenon, offering a high surface area of the nanosheets. In the synthetic approach, the Fe5 nanosheets were grown onto the surface of the carbon fibres, which was proved by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In the photocatalytic studies, after 180 min under visible light irradiation (λ = 400 – 700 nm), the Fe5/carbon fibres could achieve a 91% degradation rate. It is important to highlight that pure Degussa P25 (TiO2 used as a comparison), Fe5, and carbon fibres did not exhibit high photocatalytic rates, reaching 27%, 35%, and 46% within the same irradiation time. The Fe5/carbon fibres could be reused (after separation by sedimentation process) for up to three cycles, maintaining the same photocatalytic activity. The authors also prepared two other materials by increasing the amount of the Pc starting materials to 2 times (2-Fe5/carbon fibres) and 4 times (4-Fe5/carbon fibres) higher than the initial one to find the ideal amount of Fe5 nanostructures. They observed that it was possible to degrade RhB more efficiently with 2-Fe5/carbon fibres, followed by Fe5/carbon fibres and 4-Fe5/carbon fibres.
20, 87% degradation was achieved within 240 min. In the second study,68 Fe5 nanosheets were uniformly distributed on the surface of each fibre without enhancing an aggregation phenomenon, offering a high surface area of the nanosheets. In the synthetic approach, the Fe5 nanosheets were grown onto the surface of the carbon fibres, which was proved by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In the photocatalytic studies, after 180 min under visible light irradiation (λ = 400 – 700 nm), the Fe5/carbon fibres could achieve a 91% degradation rate. It is important to highlight that pure Degussa P25 (TiO2 used as a comparison), Fe5, and carbon fibres did not exhibit high photocatalytic rates, reaching 27%, 35%, and 46% within the same irradiation time. The Fe5/carbon fibres could be reused (after separation by sedimentation process) for up to three cycles, maintaining the same photocatalytic activity. The authors also prepared two other materials by increasing the amount of the Pc starting materials to 2 times (2-Fe5/carbon fibres) and 4 times (4-Fe5/carbon fibres) higher than the initial one to find the ideal amount of Fe5 nanostructures. They observed that it was possible to degrade RhB more efficiently with 2-Fe5/carbon fibres, followed by Fe5/carbon fibres and 4-Fe5/carbon fibres.
Still using visible light (λ > 400 nm), Das, Chattopadhyay, and co-workers69 studied the photooxidation of RhB in aqueous by using a copper(II) phthalocyanine Cu4 (Fig. 4) functionalized with reduced graphene oxide (rGO) nanocomposite. Compared with Cu4, the degradation efficiency using the rGO/Cu4 nanocomposite was higher due to the redshift in the absorption spectrum that increases the catalytic activity under visible light (Table 10). The degradation efficiency reached 96% after 210 min of light irradiation when rGO was twice bigger than the Cu4 in the nanocomposite. The rGO played an essential role in enhancing the degradation efficiency due to the formed donor–acceptor system.
Li, Xu, and co-workers70,136,139 evaluated the degradation of RhB in aqueous media with visible light (λ > 400 nm) and composites of zinc(II) phthalocyanine Zn4 (Fig. 4), Zn36, and Zn38 (Fig. 11) with MWCNTs. In the first study,136 there was almost no degradation rate (∼19%) using MWCNTs due to the high specific surface area of these CNTs. After immobilization with Zn36 and after 240 min, it was possible to degrade 88% of RhB with the hybrid Zn36–MWCNT. More importantly, after three cycles, the degradation efficiency for RhB did not suffer any significant change (just a slight decrease of 4%). Regarding the second study,70 of the pure Zn4, MWCNTs, and Zn4/MWCNT hybrids exhibited a photodegradation efficiency of 54%, 4%, and 93%, respectively, within 60 min of irradiation (Table 10). The hybrid material reveals a superior photocatalytic activity compared with pure Zn4 and MWCNTs due to the MWCNTs that prevented the Zn4 aggregation and increased the catalytic active sites. According to the authors, the photocatalytic rate did not significantly change (89%) after reusing the Zn4/MWCNT for three consecutive experiments. In the later study,139 after 120 min of irradiation, it was possible to achieve 93% of RhB degradation with hybrid Zn38–MWCNTs when compared with bare MWCNT (14%) and Zn38 (54%).
Zhang and co-workers166 photodegraded RhB in aqueous media using Cu47/perylene diimide (PDIC12) (Fig. 19) p–n heterojunction under visible light irradiation (λ > 400 nm). This composite was based on the intramolecular hydrogen bond between the pyridyl moiety of the perylene and the carboxy group of Cu47. This type of composite shows an increase in photocatalytic activity compared with the precursors (PDIC12 and Cu47). Also, a higher molar fraction of the PDIC12 derivative on the composite allowed a higher photocatalytic degradation rate of RhB, reaching 80% after 180 min (Table 10).
 | ||
| Fig. 19 Perylene diimide derivative used by Zhang and co-workers166 for conjugation with Cu47 and used for the degradation of RhB. | ||
Varghese and co-workers71 investigated the ability to degrade RhB under UV light irradiation in aqueous media. The authors prepared three different inorganic transition metal tungstate (NiWO4)/Sn(IV)4 (Fig. 4) nanocomposites based on the Sn4 content: C1 (1 wt%), C2 (2 wt%), and C3 (3 wt%). The best photocatalytic rate was obtained in the following order: C1 (71%) > C2 (49%) > C3 (48%) > Sn4 (37%) > NiWO4 (30%) – Table 10. The use of UV-visible light for the degradation of RhB in aqueous media was studied by Li, Shao, and co-workers.167 The authors use PAN nanofibers functionalized with Cu5 (Fig. 4 and bismuth oxychloride (BiOCl) nanosheets. The degradation rate of RhB using BiOCl/Cu5/PAN nanofibers is ∼6 times higher when compared with Cu5/PAN and BiOCl/PAN nanofibers. After 180 min, it was possible to degrade ∼80% of RhB. After three experiments, there was no decrease in the degradation rate of RhB (Table 10). On the other hand, using visible light irradiation (λ > 400 nm), Zhao, Han, and co-worker73 used as catalysts amidoximated PAN fibres anchored onto iron phthalocyanine Fe4 (Fig. 4)/TiO2 through coordination bonds. The best photocatalytic activity was achieved with an optimal amount of 10.2 wt%. After 80 min, it was possible to complete RhB degradation (Table 10). After 5 cycles, the photocatalytic activity rate remained intact.
Still using PAN fibres and RhB as a pollutant under visible light and solar irradiation, Lu, Chen, and co-workers56 studied its degradation in aqueous media using a photocatalyst based on PAN-supported g-C3N4 coupled with Zn(II) phthalocyanine 2 (Fig. 4) nanofibers. In the visible light studies, the removal rate of RhB using g-C3N4/Zn2/PAN as photocatalyst was higher than g-C3N4/PAN, reaching a maximum of ∼98% after 120 min of light irradiation (Table 10). Under solar irradiation, almost a complete degradation of RhB was achieved after 180 min with the g-C3N4/Zn2/PAN. The degradation rate could be maintained after three cycles of experiment with g-C3N4/Zn2/PAN. The first degradation products are presented in Fig. 20. After this, these intermediates are degraded into the ones mentioned by Han, Zhao, and co-workers63 (Fig. 17).
By also using solar irradiation, Oki and co-workers74 studied the photooxidation of RhB using graphene–ZnO composite with a Co4 (Fig. 4). There was a complete degradation using GR–ZnO and GR–ZnO–Co4 after 140 and 150 min under sunlight irradiation, respectively (Table 10). On the other hand, there was only a 20–60% degradation when using the Co4, ZnO, and GR alone as catalysts. Under the same experimental conditions, the degradation efficiency of GR–ZnO–Co4 was found to be higher than GR–ZnO catalyst. The interactions between the GR nanosheets and ZnO particles enhanced the photocatalytic activity of the GR–ZnO composite when compared with bare GR and ZnO. The GR nanosheets were the main ones responsible for the absorption of RhB and its posterior degradation. The sensitization with Co4 enhances the composite's absorption ability, increasing the photocatalytic efficiency. Still regarding the degradation of RhB and using the same phthalocyanine 8 but with N-PC (Cu4/N-PC), Dabiri and co-workers144 prepared a series of hybrids with different weight ratios: Cu40.125/N-PC, Cu40.25/N-PC, and Cu40.5/N-PC. After 90 min of visible light irradiation (λ > 400 nm), there was no degradation for RhB with Cu40.125/N-PC. However, it was possible to observe a more efficient photocatalytic degradation with Cu40.25/N-PC (0.0627 min−1) than with Cu40.5/N-PC (0.0551 min−1) in the presence of H2O2. Also, after performing seven experiments with the best photocatalyst, it was possible to maintain most of the activity (4% loss) –Table 10.
Huang and co-workers168 studied the photooxidation for RhB in aqueous media under visible light irradiation (λ > 420 nm) with a tetra-substituted manganese(II) phthalocyanine bearing sulfonic acid groups Mn3 (Fig. 4) immobilized on a TiO2–SiO2 hybrid support. After 240 min of irradiation, complete degradation of RhB was achieved (Table 10). Under visible light irradiation (λ > 400 nm), Chen and co-workers106 studied the degradation of RhB using a magnetically recyclable composite AT/Fe3O4–Fe18 (Fig. 6). This composite was prepared using Fe18 and magnetic palygorskite nanoparticles (AT/Fe3O4). The remarkable superparamagnetic properties of this composite allow their recuperation by simply applying an external magnetic field. Different composites were prepared based on the amounts of phthalocyanine. The photodegradation rate was 96% after 300 min of light irradiation (Table 10) using Fe18 (0.6 nmol). However, the best photocatalytic activity was obtained with higher amounts of Fe2 (0.6 nmol). More recently, Zang, Sun, Zhang, and co-workers93 studied the degradation of RhB in an aqueous solution under visible light irradiation (λ > 420 nm) by using the Cu4 (Fig. 4) supported by PACA HIOBs. After 40 min of light irradiation, there was a complete degradation of RR3B with a degradation rate of 0.1218/min. After five cycles, the degradation efficiency was still 97.3%. The authors also assessed these photocatalysts' ability to degrade other anionic dyes, such as RBBR. Regarding RhB, the authors did not study the degradation products.
3.2.3.2. Rhodamine 6G. As RhB, RhG is also a chemically stable dye that is difficult to degrade.75 Nyokong and co-workers75 evaluated the photocatalytic degradation of RhG in aqueous media using bare zinc(II) tetraaminophthalocyanine Zn6 (Fig. 4) or conjugated with Ag nanoparticles. Both were incorporated into chitosan beads to facilitate the recovery after photocatalysis. In the presence of Ag nanoparticles, the photocatalytic degradation under visible light irradiation (λ > 400 nm) of RhG was enhanced (t1/2 = 41 min) when compared with Zn6 immobilized onto chitosan beads (t1/2 = 46 min, Table 10). Besides Ag nanoparticles, the previous authors137 also used the peripherally substituted phthalocyanine 35 (Fig. 11) but conjugated with ZnO affording Zn35–ZnO/PS fibres. The authors compared this composite's photocatalytic activity against RhB with Zn35–AgNPs/PS fibres. ZnO and AgNPs significantly improved the photocatalytic activity of the composites, being Zn35–AgNPs/Ps a better photocatalyst (shorter half-life times and higher degradation rate constant) compared to Zn35–ZnO/PS fibres (Table 10). The structure of the products was not identified, but the authors mentioned some peaks in the HPLC analysis that could be attributed to the removal of the diethyl group from the nitrogen atom, as presented in Fig. 20.
3.2.4.1. Methylene blue. Methylene blue (MB) has been shown to cause central nervous system toxicity. When released into wastewaters without treatment, it can cause a serious threat.173 So, Vallejo and co-workers174 studied the degradation of MB in aqueous media under visible light irradiation using Cu(II)2 and Zn(II)2 (Fig. 4) immobilized on TiO2. After 140 min, it was possible to achieve 47% and 30% of RhB degradation, respectively, which were 2.8 times and 3.6 times better than bare TiO2 (7%) (Table 11).
| MPc | Support | Light | Efficiency (%) | Time (min) | kobs (min−1) | Recycle | Ref. |
|---|---|---|---|---|---|---|---|
| a Amount of silicate polymer network. | |||||||
| No | TiO2 | Visible light (λ > 400 nm) | 7 | 140 | — | — | 174 |
| Zn2 | 30 | ||||||
| Cu2 | 47 | ||||||
| Co4 | Solar | 91 | 90 | 175 | |||
| Fe4 | 97 | ||||||
| No | 81 | ||||||
| Zn4 | Visible (λ > 400 nm) | 85 (3.7 nm) | 76 | ||||
| 90 (3.9 nm) | |||||||
| 70 (3.6 nm) | |||||||
| 67 (3.8 nm) | |||||||
| 42 (P25) | |||||||
| Cu49 | 100 | 169 | |||||
| Zn52 | 100 | 5 cycles (∼16% loss) | 38 | ||||
| Co52 | |||||||
| Ni52 | |||||||
| Cu52 | |||||||
| Cu18 | g-C3N4 | 98 | 270 | — | 104 | ||
| Cu4 | ZnO | 120 | 77 | ||||
| UV (λ = 365 nm) | 150 | ||||||
| Zn53 | UIO-66 (NH2) | Visible (λ > 400 nm) | 90 | 120 | 4 cycles (∼20% loss) | 176 | |
| Zn49 | P2W17 | 100 | 450 | — | 170 | ||
| P2W18 | 20 | ||||||
| PW12 | 100 | 240 | |||||
| Zn36 | S-g-C3N4 | 97 | 100 | 78 | |||
| Zn4 | 39 | ||||||
| Zn53 | 82 | ||||||
| Zn36 | g-C3N4 | 90 | 120 | 138 | |||
| S-g-C3N4 | 82 | 3 cycles (∼30% loss) | |||||
| Co3 | Fe3O4@SiO2@TiO2 | 100 | 30 | 3 cycles (0% loss) | 177 | ||
| UV-vis | 60 | — | |||||
| Zn37 | SiO2–TiO2 | Visible (λ > 400 nm) | 300 | 37 | |||
| Cu4 | PS-b-PAA + TiO2 | — | — | 2.9 × 10−3 (PS241-b-PAA51) | 79 | ||
| 1.8 × 10−3 (PS330-b-PAA34) | |||||||
| 1.6 × 10−3 (PS834-b-PAA162) | |||||||
| Cu50 | silicate–TiO2 | 40 (10%a) | 180 | — | 171 | ||
| 33 (25%a) | |||||||
| 30 (10%a) | |||||||
| Zn50 | 35 (50%a) | ||||||
| 28 (25%a) | |||||||
| 24 (10%a) | |||||||
| No | 29 (50%a) | ||||||
| 25 (25%a) | |||||||
| Zn54 | TiO2 | Visible (λ > 420 nm) | 100 | 110 | 3 cycles (18% loss) | 178 | |
| Co54 | |||||||
| Cu54 | 130 | ||||||
| Ti55(O) | |||||||
Using TiO2 and Pc 4, Kwak, Chung, and co-workers76 and Varghese and co-workers34 both studied the degradation of MB in aqueous media under visible light irradiation with TiO2/Zn4 hybrids with several pore sizes and or sensitized TiO2 with M4 (M = Co(II) or Fe(II)) to form M4/TiO2 composites. In the first study,76 as expected, no MB degradation was observed within 90 min in the presence of all hybrids and the absence of light. The hybrids incorporating Zn4 showed higher photocatalytic activity when compared with the unmodified samples. The photocatalytic degradation efficiencies against MB were 85%, 90%, 70%, 67%, and 42% for L121–TiO2/Zn4 (3.7 nm of pore size), P123–TiO2/Zn4 (3.9 nm of pore size), F68–TiO2/Zn4 (3.6 nm of pore size), F127–TiO2/Zn4 (3.8 nm of pore size), and Degussa P-25/Zn4, respectively (Table 11). Herein, it was shown that the size of the pores did not influence the photocatalytic activity. The high photocatalytic activity is due to a cascade of Mie light scattering. The pore size of P123–TiO2/Zn4, which is comparable with the wavelength of the irradiation source, strongly generated the Mie scattering, followed by a considerable enhancement of the photocatalytic activity. In the second study, it was possible to achieve higher photocatalytic rates using Fe4/TiO2 (97%) when compared with Co4/TiO2 (91%) and bare TiO2 (81%) after 90 min under solar irradiation (Table 11). Despite having promising photocatalytic activities, it would be essential to evaluate the stability of the compounds within several cycles in future work.
For the degradation of MB in aqueous media, Zahmakiran, Agirtas, and co-workers169 also studied their photooxidation using a copper(II) phthalocyanine Cu48 (Fig. 16) immobilized in TiO2. MB was degraded entirely within 90 min under visible light irradiation (Table 11). After five cycles, the photocatalyst could retain > 80% of its initial activity.
The degradation of MB in aqueous media under visible light irradiation (λ > 400 nm) was performed using free-base and metallophthalocyanine M52 (M = Zn(II), Co(II), Ni(II), or Cu(II), Fig. 21) immobilized on TiO2 by Gorduk, Avciata, and co-workers.38 According to the authors, within 100 min, it was possible to achieve complete degradation of MB using all the composites. After being reused up to five times, they could maintain their activity above 76%.
 | ||
| Fig. 21 Tetra-α-substituted phthalocyanines 5238,78 and 53176 developed by Gorduk and Avciata,38 Li and Liang78,176 and co-workers. | ||
Schneider and co-workers104 could degrade 98% of MB in aqueous media within 270 min under visible light irradiation using Cu18 (Fig. 6) immobilized in g-C3N4 (Table 11). Under UV (λ = 365 nm) or visible light (λ = 400–700 nm) irradiation, Maya-Treviño and co-workers77 studied the photodegradation for MB using Cu4 (Fig. 4) sensitized on ZnO through a sol–gel method. The authors prepared two materials varying the percentage containing Cu4. The first material contains 0.1% wt, and the second comprises 0.5% wt. According to the authors, after 120 min under visible light irradiation, it was possible to achieve 98% degradation for MB (Table 11). Compared with TiO2 (a widely known photocatalyst), it could only achieve similar percentages after 180 min of light irradiation. Under UV light irradiation, the same activity could be achieved after 150 min under light exposure. Li, Liang, and co-workers176 achieved a 90% degradation for MB in aqueous media after 120 min under visible light irradiation (λ > 420 nm) using a zirconium-based MOF, UIO-66 (NH2), covalently linked to Zn53 (Fig. 21). This photocatalytic rate was much higher when compared with a mixture of Zn53 and UIO-66(NH2) (Table 11). Also, after four cycles, the photocatalytic activity decreased to 20%.
Wang, Liu, and co-workers170 studied the degradation of MB in aqueous media using Zn49 (Fig. 16) immobilized in three different polyoxometalates (POMs): P2W17, P2W18, and PW12. Under visible light irradiation (λ > 400 nm), achieving complete degradation after 240 min and 450 min was possible using Z49/PW12 and Zn49/P2W17, respectively. However, after 450 min, only 20% of MB degradation was achieved using Zn49/P2W18. A degradation rate of 100% was achieved with Zn49/P2W17 with a small photocatalyst dosage (Table 11). Still using visible light irradiation (λ > 400 nm), Liang, Li, and co-workers78,138 studied the photooxidation of MB in aqueous media with sulfur-doped g-C3N4 (CNS) coupled with zinc(II) phthalocyanines Zn36 (Fig. 11), Zn4 (Fig. 4), and Zn52 (Fig. 21). In the first study,78 composites exhibited higher photocatalytic degradation (39–97%) than bare CNS (30%). Zn36/CNS degrade ∼97% of MB after 100 min under irradiation. On the other hand, with Zn52/CNS and Zn4/CNS, the photocatalytic rate decreased to 82% and 39%, respectively (Table 11). In the second study,138 the pure g-C3N4 and Zn36 showed degradation efficiencies of 36% and 42%, respectively, within 120 min of irradiation. Incorporating sulfur in the g-C3N4 enhanced the photocatalytic activity of the catalysts. The activities of the composites followed the order: g-C3N4 < Zn36 < g-CNS < Zn36/g-C3N4 < Zn36/g-CNS. After three cycles, there is a decrease of 30% in the photocatalytic efficiency of the Zn36/g-CNS. Liu and co-workers177 studied the degradation of MB in aqueous media under visible light (λ > 400 nm) with cobalt(II) phthalocyanine Co3–sensitized hollow Fe3O4@SiO2@TiO2 hierarchical nanostructures (Fig. 22). According to the authors, the composite showed better photocatalytic activity, reaching ∼100% degradation of MB after 30 min of irradiation compared with the UV-visible light (60 min). The photocatalyst could be separated by an external magnetic field and reused for three cycles without significant activity loss.
 | ||
| Fig. 22 Hollow Fe3O4@SiO2@TiO2 nanostructure sensitized with Co3 used by Liu and co-workers177 for the degradation of MB. | ||
The degradation of MB in aqueous media under visible light irradiation (λ > 400 nm) using Zn37 (Fig. 11) bearing cyanuric chloride immobilized on SiO2–TiO2 microparticles (Zn37–SiO2–TiO2) through covalent bonds was studied by Yao and co-workers.37 After 300 min, it was possible to complete MB degradation in a saturated-O2 environment using the Zn37–SiO2–TiO2 hybrid. Despite the remarkable activity, there are no studies regarding the stability of the material for 300 min under light irradiation. Under the same light and media conditions, Nakatani and co-workers79 studied the degradation of MB with Cu4 incorporated in a poly(styrene-block-acrylic acid) (PS-b-PAA, Fig. 23) containing TiO2 gel. The authors prepared different lengths in the copolymers that afforded different photocatalytic activities. The photocatalytic activity under visible light irradiation was greatly improved (three times higher) when loading Cu4 into the system. It was possible to achieve better degradation rates with Cu4-PS241-b-PAA51 containing TiO2 gel (kobs = 2.9 × 10−3 min−1) followed by Cu4-PS330-b-PAA34 (kobs = 1.8 × 10−3 min−1) containing TiO2 gel and Cu4-PS834-b-PAA162 (kobs = 1.6 × 10−3 min−1) containing TiO2 gel.
 | ||
| Fig. 23 Cu4 and PS-b-PAA were used to incorporate TiO2 gel developed by Nakatani and co-workers.79 | ||
Saka and co-workers171 accessed the photodegradation of MB under visible light irradiation (λ > 400 nm) in aqueous media using hybrid organic–inorganic nanocomposite containing Cu(II) and Zn(II) phthalocyanines bearing 1,2,4-triazole groups modified TiO2 nanoparticles (Zn50–TiO2 and Cu50–TiO2). The hybrid polymer network used tetraethyl orthosilicate, 3-(glycidyloxypropyl)triethoxysilane. These Zn50 and Cu50 (Fig. 16) modified nanoparticles were added to the hybrid polymer network at 10% (M50–T10), 25% (M50–T25), and 50% (M50–T50). These coating solutions were then put in glass substrates through a spray methodology. After 180 min of irradiation, the best photocatalytic degradation was more efficient with Cu50–T10 with a 40% degradation rate, followed by Zn50–T50 (35%), Cu50–T25 (33%), Cu50–T10 (30%), bare T50(29%), Zn50–T25 (28%), bare T25 (25%), Zn50–T10 (24%), and bare T10 (20%).
Karaoğlan and co-workers178 could completely degrade MB using M54 (M = Zn(II), Co(II), Cu(II), and TiO(IV)(OPr)) (Fig. 24) and visible light irradiation (λ > 420 nm) in aqueous media. After 110 min, it was possible to completely degrade MB using Zn54 and Co54, whereas it took Cu54 and Ti54O 130 min to achieve the same degradation. After 3 reusability cycles, there was only a loss of 18% for all the photocatalysts. The authors did not study the degradation products of MB.
 | ||
| Fig. 24 Tetra-β-substituted phthalocyanine 54 used for the photodegradation of MB by Karaoğlan and co-workers.178 | ||
3.3. Agrochemicals
Agrochemical and pharmaceutical compounds play very important roles in modern society, providing greater quantity and quality of food as well as health and well-being for the entire population. However, due to the worldwide massive amounts of used agrochemicals (mainly insecticides, fungicides, and herbicides), their negative impact on the environment is unquestionable. When these compounds reach humans, they can cause neurotoxic, endocrine-disruptor, and DNA-damaging effects.181 The photodegradation of dichlorvos, 2,4-dichlorophenoxyacetic acid, and fenamiphos (Fig. 25), catalysed by phthalocyanines, is discussed in the following sections. | ||
| Fig. 26 Cu24 functionalized with 3-aminopropyltrimethoxysilane developed by Serra and co-workers.108 | ||
3.4. Pharmaceuticals
Antibiotics, anti-inflammatory, and anti-depressive drugs are among the thousands of human pharmaceuticals produced and consumed yearly. These drugs reach aquatic habitats via the disposal of domestic sewage. When they reach the wastewater treatment plants, after some degradation process, they can be converted into other potentially more harmful and long-lasting chemicals.186 Thus, it seems evident that finding innovative and sustainable treatment processes to remove these pollutants from wastewater is essential. In the following sections, the degradation of ibuprofen, naproxen, sulfathiazole, carbamazepine, erythromycin, and tetracycline (Fig. 27) using several phthalocyanines will be mentioned.More recently, Yang, Yu, and co-workers82 studied the degradation of TC in an aqueous solution under visible light irradiation (λ > 420 nm) by using the Fe4 (Fig. 4)/perylene diimide heterojunctions. After 60 min of light irradiation, there was degradation of 79% of TC (degradation rate of 0.0264/min). This degradation rate was higher when compared with perylene diimide (41%, 0.0088/min) and Fe4 (2%, 0.0003/min) by itself. After five cycles, the degradation efficiency was almost unchanged. The authors did not study the degradation products of TC.
In another study, Anucha, Altin, and co-workers193 studied the photooxidation of carbamazepine in aqueous media under UV-irradiation using boron/sodium fluorine co-doped titanium dioxide sensitized with an axial substituted phthalocyanine Si55 (Fig. 28) (B/NaF–SiHTiO2). After 240 min, there was complete degradation using the B/NaF–Si55TiO2, whereas the unsensitized B/Na–TiO2 could only achieve 70% of carbamazepine degradation. Both composites showed higher photocatalytic activity when compared with the bare TiO2 (40%). In this study, the authors identified similar degradation products to the ones reported by Lu, Chen, and co-workers.56,109
 | ||
| Fig. 28 Silicon phthalocyanine Si55 used by Anucha, Altin and co-workers193 for the degradation of carbamazepine. | ||
3.5. Other pollutants
Other pollutants such as trichlorobenzene (TCB), aniline, and dimethyl phthalate were found in wastewater. These aromatic compounds are considered toxic and should be eliminated from wastewater.83,99,196 In the following three studies, the authors analysed the degradation of these pollutants by using two different phthalocyanines.| Catalyst | Pollutant | Photocatalyst activity (% of degradation) | ||||
|---|---|---|---|---|---|---|
| 1st cycle | 2nd cycle | 3rd cycle | 4th cycle | 5th cycle | ||
| Co15–TiO2 | 4-CP | 99 | 97 | 95 | 92 | 91 |
| Zn15–TiO2 | 98 | 95 | 93 | 90 | 88 | |
| Co15–TiO2 | PhCl | 99 | 96 | 94 | 91 | 90 |
| Zn15–TiO2 | 97 | 94 | 90 | 87 | 86 | |
| Co15–TiO2 | TCB | 98 | 93 | 92 | 88 | 87 |
| Zn15–TiO2 | 95 | 91 | 89 | 86 | 84 | |
4. Summary and outlook
This review shows the significant advances for the photocatalytic degradation of several water pollutants using simple Pc derivatives or Pcs immobilized on organic or inorganic materials, such as polymers, fibres, carbon nanostructures, ZnO, SiO2, TiO2, or mixtures of these supports, when exposed to UV, visible, UV-visible, and/or solar light irradiation. It is also shown that the photocatalytic activities of the phthalocyanine dyes are strongly correlated with their substituents, the presence/absence of positive charges, and the metal ions on their macrocycle structure.The light source (UV, UV-visible, visible, or solar) is also a key element for the photodegradation approach. When solar/visible light is used, the Pc can be excited along with the support, but only a small or no excitation of the Pcs is observed using UV light. The use of solar light is considered an enormous advantage for photocatalysis since it reduces costs and takes advantage of the solar spectrum. It is also important to mention that most photocatalytic studies lack reusability studies, an essential parameter for the potential industry application. However, for most of the reused catalysts, no significant loss of activity was observed, and, in some cases, the photocatalyst is specific for a selected pollutant.
This review highlights the efforts of the scientific community to find new alternative methodologies for the degradation of pollutants, which are usually difficult to remove from water. The most critical issue is the prevention of Pcs leaching, which was revealed to be the leading cause of activity loss of the photocatalysts after several cycles of their use. Almost all the works mentioned in this review referred to the recyclability of the photocatalysts. Fortunately, the tendency to carry out those studies has been increasing over time. Moreover, for a scientist who wants to reproduce the reported work, it is important to know the exact conditions of the photocatalytic assay, namely the irradiance of the lamp used. However, most of the cited papers in this review did not mention the irradiance of the lamp used (nor the lamp's brand), making it difficult to compare results and even reproduce them. Nevertheless, the application of these photocatalysts in wastewater is (urgently) needed to offer an important insight for addressing future environmental challenges.
Literature review information
The search for research papers for this review was conducted in 2021, but it was updated in April 2023 before the submission of the manuscript. This review has 103 papers on photocatalytic studies and a total of 196 references dated from 2007 to 2023.Abbreviations
| 2,4-DCP | 2,4-Dichlorophenol |
| 2,4,6-TCP | 2,4,6-Trichlorophenol |
| 4-CP | 4-Chlorophenol |
| 4-NP | 4-Nitrophenol |
| AO7 | Acid orange 7 |
| BB | Brilliant blue |
| BPA | Bisphenol A |
| BPB | Bromophenol blue |
| BR29 | Basic Red 29 |
| BR195 | Basic Red 195 |
| CMP | Conjugated microporous polymers |
| CV | Crystal violet |
| DCM | Dichloromethane |
| FS | Fuchsine |
| FSC | Fluorescein |
| GR | Graphene |
| MB | Methylene blue |
| MNP | Magnetic nanoparticle |
| MO | Methyl orange |
| MPc | Metallophthalocyanine |
| MR | Methyl red |
| MWCNT | Multi-walled carbon nanotubes |
| N-GR | Nitrogen-doped graphene |
| NPS | Nanoparticles |
| OG | Orange G |
| PA-6 | Polyamide-6 |
| PAA | Polyacrylic acid |
| PAN | Polyacrylonitrile |
| PCP | Pentachlorophenol |
| Pc | Phthalocyanine |
| PDIC12 | Perylene diimide C12 derivative |
| P4R | Ponceau 4R |
| PS | Polystyrene |
| PSU | Polysulfone |
| PUR | Polyurethane |
| RDB | Reactive dark blue |
| rGO | Reduced graphene oxide |
| RBBR | Remazol brilliant blue R |
| RhB | Rhodamine B |
| RhG | Rhodamine G |
| ROS | Reactive oxygen species |
| SB | Select brown |
| SFB | Sella fast black |
| SWCNT | Single-walled carbon nanotubes |
| TCB | 1,2,4-trichlorobenzene |
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e a Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects UIDB/50006/2020, UIDP/50006/2020, and P2020-PTDC/QUI-QOR/31770/2017. S. Gamelas thanks FCT for her PhD grant (ref. SFRH/BD/143549/2019). Thanks are due to the University of Aveiro and FCT/MCTES (Fundação para a Ciência e a Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) for the financial support to LAQV-REQUIMTE (UIDB/50006/2020 and UIDP/50006/2020), Centro de Química Estrutural (UIDB/00100/2020 and UIDP/00100/2020), and Institute of Molecular Sciences (LA/P/0056/2020) funded by FCT/MCTEs through national funds. Authors acknowledge FCT/MCTES for the national funds received through the project P2020-PTDC/QUI-QOR/31770/2017. S. Gamelas PhD grant (ref. SFRH/BD/143549/2019).References
- R. D. Ambashta and M. Sillanpää, J. Hazard. Mater., 2010, 180, 38–49 CrossRef CAS PubMed.
- A. Y. Hoekstra, Nat. Clim. Change, 2014, 4, 318–320 CrossRef.
- N. Mohammed, P. Palaniandy and F. Shaik, Int. J. Environ. Anal. Chem., 2021, 1–12 CAS.
- J. Liu, H. Yang, S. N. Gosling, M. Kummu, M. Flörke, S. Pfister, N. Hanasaki, Y. Wada, X. Zhang, C. Zheng, J. Alcamo and T. Oki, Earths Future, 2017, 5, 545–559 CrossRef PubMed.
- L. Rosa, D. D. Chiarelli, M. C. Rulli, J. Dell'Angelo and P. D'Odorico, Sci. Adv., 2020, 6, 1–11 Search PubMed.
- WWAP, UNESCO 2018 The United Nat`ions World Water Development Report 2018: Nature-based Solutions for Water, 2018 Search PubMed.
- S. N. Ahmed and W. Haider, Nanotechnology, 2018, 29, 342001 CrossRef PubMed.
- A. Sonune and R. Ghate, Desalination, 2004, 167, 55–63 CrossRef CAS.
- F. Parrino and G. Palmisano, Molecules, 2021, 26, 5–7 Search PubMed.
- A. O. Ibhadon and P. Fitzpatrick, Catalysts, 2013, 3, 189–218 CrossRef CAS.
- G. He, Y. Mi, D. Wang, B. Zhang, D. Zheng, Y. Bai and Z. Shi, Energy Technol., 2021, 9, 2100733 CrossRef CAS.
- Y. Hong, Y. Jiang, C. Li, W. Fan, X. Yan, M. Yan and W. Shi, Appl. Catal., B, 2016, 180, 663–673 CrossRef CAS.
- S. Malato, P. Fernández-Ibáñez, M. I. Maldonado, J. Blanco and W. Gernjak, Catal. Today, 2009, 147, 1–59 CrossRef CAS.
- J. Byrne, P. Dunlop, J. Hamilton, P. Fernández-Ibáñez, I. Polo-López, P. Sharma and A. Vennard, Molecules, 2015, 20, 5574–5615 CrossRef CAS PubMed.
- L. Fernández, V. I. Esteves, Â. Cunha, R. J. Schneider and J. P. C. Tomé, J. Porphyrins Phthalocyanines, 2016, 20, 150–166 CrossRef.
- S. R. D. Gamelas, A. T. P. C. Gomes, M. A. F. Faustino, A. C. Tomé, J. P. C. Tomé, A. Almeida and L. M. O. Lourenço, ACS Appl. Bio Mater., 2020, 3, 4044–4051 CrossRef CAS PubMed.
- C. P. S. Ribeiro and L. M. O. Lourenço, J. Photochem. Photobiol., C, 2021, 48, 100422 CrossRef CAS.
- J. M. D. Calmeiro, J. P. C. Tomé and L. M. O. Lourenço, J. Mater. Chem. C, 2020, 8, 8344–8361 RSC.
- Y. Rio, M. S. Rodríguez-Morgade and T. Torres, Org. Biomol. Chem., 2008, 6, 1877–1894 RSC.
- Z. Weng, Y. Wu, M. Wang, J. Jiang, K. Yang, S. Huo, X. F. Wang, Q. Ma, G. W. Brudvig, V. S. Batista, Y. Liang, Z. Feng and H. Wang, Nat. Commun., 2018, 9, 1–9 CrossRef CAS PubMed.
- E. Anaya-Plaza, A. Aljarilla, G. Beaune, Nonappa, J. V. I. Timonen, A. de la Escosura, T. Torres and M. A. Kostiainen, Adv. Mater., 2019, 31, 1–6 CrossRef PubMed.
- H. Zheng, H. Yi, H. Dai, D. Fang, Z. Hong, D. Lin, X. Zheng and Y. Lin, Sens. Actuators, B, 2018, 269, 27–35 CrossRef CAS.
- J. Prasongkit, S. Tangsukworakhun, R. Jaisutti and T. Osotchan, Appl. Surf. Sci., 2020, 532, 147314 CrossRef CAS.
- K. Mitra and M. C. T. Hartman, Org. Biomol. Chem., 2021, 19, 1168–1190 RSC.
- L. Wibmer, L. M. O. Lourenço, A. Roth, G. Katsukis, M. G. P. M. S. Neves, J. A. S. Cavaleiro, J. P. C. Tomé, T. Torres and D. M. Guldi, Nanoscale, 2015, 7, 5674–5682 RSC.
- J. Guo, M. Sun, X. Meng, H. Zhu, C. Ma, S. Hu, J. Shen, Q. Wang and J. Gao, Dyes Pigm., 2020, 177, 108301 CrossRef CAS.
- M. Urbani, G. de La Torre, M. K. Nazeeruddin and T. Torres, Chem. Soc. Rev., 2019, 48, 2738–2766 RSC.
- Y. Zhang, S. Paek, M. Urbani, M. Medel, I. Zimmermann, K. T. Cho, M. Ince, M. K. Nazeeruddin and T. Torres, ACS Appl. Energy Mater., 2018, 1, 2399–2404 CrossRef CAS.
- L. M. O. Lourenço, P. M. R. Pereira, E. MacIel, M. Válega, F. M. J. Domingues, M. R. M. Domingues, M. G. P. M. S. Neves, J. A. S. Cavaleiro, R. Fernandes and J. P. C. Tomé, Chem. Commun., 2014, 50, 8363–8366 RSC.
- L. M. O. Lourenço, A. Sousa, M. C. Gomes, M. A. F. Faustino, A. Almeida, A. M. S. Silva, M. G. P. M. S. Neves, J. A. S. Cavaleiro, Â. Cunha and J. P. C. Tomé, Photochem. Photobiol. Sci., 2015, 14, 1853–1863 CrossRef PubMed.
- A. M. Schmidt and M. J. F. Calvete, Molecules, 2021, 26, 1–22 Search PubMed.
- Z. Youssef, L. Colombeau, N. Yesmurzayeva, F. Baros, R. Vanderesse, T. Hamieh, J. Toufaily, C. Frochot and T. Roques-Carmes, Dyes Pigm., 2018, 159, 49–71 CrossRef CAS.
- B. Cecconi, N. Manfredi, T. Montini, P. Fornasiero and A. Abbotto, Eur. J. Org. Chem., 2016, 2016, 5194–5215 CrossRef CAS.
- K. P. Priyanka, S. Sankararaman, K. M. Balakrishna and T. Varghese, J. Alloys Compd., 2017, 720, 541–549 CrossRef CAS.
- Y. Mahmiani, A. M. Sevim and A. Gül, J. Photochem. Photobiol., A, 2016, 321, 24–32 CrossRef CAS.
- A. M. Sevim, J. Organomet. Chem., 2017, 832, 18–26 CrossRef CAS.
- Z. Huang, B. Zheng, S. Zhu, Y. Yao, Y. Ye, W. Lu and W. Chen, Mater. Sci. Semicond. Process., 2014, 25, 148–152 CrossRef CAS.
- S. Gorduk, O. Avciata and U. Avciata, Inorg. Chim. Acta, 2018, 471, 137–147 CrossRef CAS.
- C. M. Teh and A. R. Mohamed, J. Alloys Compd., 2011, 509, 1648–1660 CrossRef CAS.
- M. J. Muñoz-Batista and R. Luque, ChemEngineering, 2021, 5, 26 CrossRef.
- S. N. Ahmed and W. Haider, Nanotechnology, 2018, 29, 342001 CrossRef PubMed.
- G. Yadav and M. Ahmaruzzaman, Chemosphere, 2022, 304, 135297 CrossRef CAS PubMed.
- N. Panigrahy, A. Priyadarshini, M. M. Sahoo, A. K. Verma, A. Daverey and N. K. Sahoo, Environ. Technol. Innovation, 2022, 27, 102423 CrossRef CAS.
- A. A. Gami, M. Y. Shukor, K. A. Khalil, F. A. Dahalan, A. Khalid and S. A. Ahmad, J. Environ. Microbiol. Toxicol., 2014, 2, 11–23 CrossRef.
- V. Iliev, A. Mihaylova and L. Bilyarska, J. Mol. Catal. A: Chem., 2002, 184, 121–130 CrossRef CAS.
- R. Xing, P. Wu, L. Wu and Z. Fei, J. Environ. Sci., 2013, 25, 1687–1695 CrossRef CAS PubMed.
- Q. Sun and Y. Xu, J. Phys. Chem. C, 2009, 113, 12387–12394 CrossRef CAS.
- V. Iliev, J. Photochem. Photobiol., A, 2002, 151, 195–199 CrossRef CAS.
- Q. Wang, H. Li, J.-H. Yang, Q. Sun, Q. Li and J. Yang, Appl. Catal., B, 2016, 192, 182–192 CrossRef CAS.
- Z. Xiong, Y. Xu, L. Zhu and J. Zhao, Environ. Sci. Technol., 2005, 39, 651–657 CrossRef CAS PubMed.
- R. Xing, L. Wu and Z. Fei, Acta Chim. Slov., 2014, 61, 406–413 CAS.
- E. Marais, R. Klein, E. Antunes and T. Nyokong, J. Mol. Catal. A: Chem., 2007, 261, 36–42 CrossRef CAS.
- E. Marais, E. Antunes and T. Nyokong, J. Coord. Chem., 2008, 61, 3727–3739 CrossRef CAS.
- B. Agboola, K. I. Ozoemena and T. Nyokong, J. Mol. Catal. A: Chem., 2006, 248, 84–92 CrossRef CAS.
- W. Lu, T. Xu, Y. Wang, H. Hu, N. Li, X. Jiang and W. Chen, Appl. Catal., B, 2016, 180, 20–28 CrossRef CAS.
- T. Xu, D. Ni, X. Chen, F. Wu, P. Ge, W. Lu, H. Hu, Z. X. Zhu and W. Chen, J. Hazard. Mater., 2016, 317, 17–26 CrossRef CAS PubMed.
- A. E. Pirbazari, Procedia Mater. Sci., 2015, 11, 622–627 CrossRef CAS.
- A. E. Pirbazari, Chem. Eng. Technol., 2017, 08, 10000333 Search PubMed.
- M. A. Zanjanchi, A. Ebrahimian and M. Arvand, J. Hazard. Mater., 2010, 175, 992–1000 CrossRef CAS PubMed.
- M. Ghaeini, M. A. Zanjanchi and M. Farjood, J. Organomet. Chem., 2023, 994, 122721 CrossRef CAS.
- S. U. Hasnain Bakhtiar, A. Zada, F. Raziq, S. Ali, M. I. Ali Shah, M. Ateeq, M. Khan, D. Alei, P. Fazil, M. Naeem, W. Khan, J. A. Khan, R. Nazir, W. Dong and Q. Fu, Int. J. Hydrogen Energy, 2023, 48, 16320–16329 CrossRef CAS.
- D. F. M. Oliveira, P. S. Batista, P. S. Muller, V. Velani, M. D. Frana, D. R. De Souza and A. E. H. Machado, Dyes Pigm., 2012, 92, 563–572 CrossRef CAS.
- X. Han, Z. Han, J. Li, J. Zhao and X. Zhao, J. Colloid Interface Sci., 2019, 533, 333–343 CrossRef CAS PubMed.
- Z. Guo, B. Chen, M. Zhang, J. Mu, C. Shao and Y. Liu, J. Colloid Interface Sci., 2010, 348, 37–42 CrossRef CAS PubMed.
- Q. Li, H. Wang, Y. Li, Y. Li and Q. Duan, Dyes Pigm., 2018, 149, 261–267 CrossRef CAS.
- L. Li and B. Xin, J. Cent. South Univ., 2010, 17, 218–222 CrossRef CAS.
- M. Zhang, C. Shao, Z. Guo, Z. Zhang, J. Mu, T. Cao and Y. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 369–377 CrossRef CAS PubMed.
- Z. Guo, J. Mu, Y. Lian and Z. Li, J. Alloys Compd., 2017, 690, 160–168 CrossRef CAS.
- M. Mukherjee, U. K. Ghorai, M. Samanta, A. Santra, G. P. Das and K. K. Chattopadhyay, Appl. Surf. Sci., 2017, 418, 156–162 CrossRef CAS.
- Y. Wan, T. Cong, Q. Liang, Z. Li, S. Xu, Y. Peng and D. Lu, Ceram. Int., 2016, 42, 2425–2430 CrossRef CAS.
- H. Hitha, A. Jose, M. John and T. Varghese, Mater. Chem. Phys., 2020, 239, 122080 CrossRef CAS.
- X. Guo, X. Zhou, X. X. X. Li, C. Shao, C. Han, X. X. X. Li and Y. Liu, J. Colloid Interface Sci., 2018, 525, 187–195 CrossRef CAS PubMed.
- J. Fei, Z. Han, Y. Deng, T. Wang, J. Zhao, C. Wang and X. Zhao, Colloids Surf., A, 2021, 625, 126901 CrossRef CAS.
- G. M. Neelgund, A. Oki and Z. Luo, J. Colloid Interface Sci., 2014, 430, 257–264 CrossRef CAS PubMed.
- P. Khoza and T. Nyokong, J. Mol. Catal. A: Chem., 2015, 399, 25–32 CrossRef CAS.
- H. J. Kim, J. D. Jeon, J. W. Chung and S. Y. Kwak, Microporous Mesoporous Mater., 2016, 227, 169–175 CrossRef CAS.
- M. L. Maya-Treviño, J. L. Guzmán-Mar, L. Hinojosa-Reyes and A. Hernández-Ramírez, Mater. Sci. Semicond. Process., 2018, 77, 74–82 CrossRef.
- M. Zhang, Q. Liang, S. Xu and Z. Li, Optik, 2016, 127, 7993–8001 CrossRef CAS.
- H. Nakatani, R. Hamachi, K. Fukui and S. Motokucho, J. Colloid Interface Sci., 2018, 532, 210–217 CrossRef CAS PubMed.
- K. Li, Y. Pang and Q. Lu, Inorg. Chem. Front., 2019, 6, 3215–3224 RSC.
- M. Pei, K. Li, X. Li, C. Song and X. Guo, Ind. Eng. Chem. Res., 2023, 62, 2698–2709 CrossRef CAS.
- K. Shi, M. Zhou, F. Wang, X. Li, W. Huang, K. Lu, K. Yang and C. Yu, Chemosphere, 2023, 329, 138617 CrossRef CAS PubMed.
- C. F. Chang and C. Y. Man, Colloids Surf., A, 2014, 441, 255–261 CrossRef CAS.
- R. Zugle and T. Nyokong, J. Appl. Polym. Sci., 2013, 128, 1131–1142 CrossRef CAS.
- M. Silva, M. J. F. Calvete, N. P. F. Gonçalves, H. D. Burrows, M. Sarakha, A. Fernandes, M. F. Ribeiro, M. E. Azenha and M. M. Pereira, J. Hazard. Mater., 2012, 233–234, 79–88 CrossRef CAS PubMed.
- C. Colbea, P. Oancea, M. Puiu, T. Galaon and A. Raducan, Mater. Today Commun., 2022, 30, 103091 CrossRef CAS.
- F. Chen, Z. Deng, X. Li, J. Zhang and J. Zhao, Chem. Phys. Lett., 2005, 415, 85–88 CrossRef CAS.
- Z. Wang, W. Mao, H. Chen, F. Zhang, X. Fan and G. Qian, Catal. Commun., 2006, 7, 518–522 CrossRef CAS.
- Z. Mesgari, M. Gharagozlou, A. Khosravi and K. Gharanjig, Spectrochim. Acta, Part A, 2012, 92, 148–153 CrossRef CAS PubMed.
- Z. S. Seddigi, S. A. Ahmed, S. Sardar and S. K. Pal, Energy Mater. Sol., 2015, 143, 63–71 CrossRef CAS.
- D. Wang, R. Guo, S. Wang, F. Liu, Y. Wang and C. Zhao, Desalin. Water Treat., 2016, 57, 25226–25234 CrossRef CAS.
- S. K. Movahed, Z. Piraman and M. Dabiri, J. Photochem. Photobiol., A, 2018, 351, 208–224 CrossRef CAS.
- F. Shen, J. Wang, L. Wang, L. Zang, Q. Xu, L. Sun and Y. Zhang, J. Mater. Chem. A, 2023, 11, 10195 RSC.
- K. Vignesh, M. Rajarajan and A. Suganthi, Mater. Sci. Semicond. Process., 2014, 23, 98–103 CrossRef CAS.
- P. Modisha and T. Nyokong, J. Mol. Catal. A: Chem., 2014, 381, 132–137 CrossRef CAS.
- Z. Gao, H. Yang, J. Mao and J. Wu, J. Cleaner Prod., 2019, 220, 668–676 CrossRef CAS.
- R. Zugle and T. Nyokong, J. Mol. Catal. A: Chem., 2012, 358, 49–57 CrossRef CAS.
- O. L. Osifeko and T. Nyokong, Inorg. Chim. Acta, 2017, 458, 50–57 CrossRef CAS.
- Y. Mahmiani, A. M. Sevim and A. Gül, J. Porphyrins Phthalocyanines, 2016, 20, 1190–1199 CrossRef CAS.
- R. Zugle and T. Nyokong, J. Mol. Catal. A: Chem., 2013, 366, 247–253 CrossRef CAS.
- S. Tombe, E. Antunes and T. Nyokong, J. Mol. Catal. A: Chem., 2013, 371, 125–134 CrossRef CAS.
- C. B. Anucha, I. Altin, Z. Biyiklioglu, E. Bacaksiz, I. Polat and V. N. Stathopoulos, Nanomaterials, 2020, 10, 2139 CrossRef CAS PubMed.
- A. Sindelo and T. Nyokong, Heliyon, 2019, 5, e02352 CrossRef PubMed.
- S. Ouedraogo, B. Chouchene, C. Desmarets, T. Gries, L. Balan, R. Fournet, G. Medjahdi, K. Bayo and R. Schneider, Appl. Catal., A, 2018, 563, 127–136 CrossRef CAS.
- P. Modisha, T. Nyokong and E. Antunes, J. Mol. Catal. A: Chem., 2013, 380, 131–138 CrossRef CAS.
- Z. Yuan, Y. Lan, S. Chen and D. Chen, Appl. Clay Sci., 2017, 147, 153–159 CrossRef CAS.
- T. B. Ogunbayo, E. Antunes and T. Nyokong, J. Mol. Catal. A: Chem., 2011, 334, 123–129 CrossRef CAS.
- E. DeOliveira, C. R. Neri, A. O. Ribeiro, V. S. Garcia, L. L. Costa, A. O. Moura, A. G. S. Prado, O. A. Serra and Y. Iamamoto, J. Colloid Interface Sci., 2008, 323, 98–104 CrossRef CAS PubMed.
- F. Wu, H. Huang, T. Xu, W. Lu, N. Li and W. Chen, Appl. Catal., B, 2017, 218, 230–239 CrossRef CAS.
- T. B. Ogunbayo and T. Nyokong, J. Mol. Catal. A: Chem., 2011, 350, 49–55 CrossRef CAS.
- R. de Smet, J. van Kaer, B. van Vlem, A. de Cubber, P. Brunet, N. Lameire and R. Vanholder, Clin. Chem., 2003, 49, 470–478 CrossRef CAS PubMed.
- S. Zhu, Y. Rong and T. K. L. Kiang, Pharmaceutics, 2021, 13, 857 CrossRef CAS PubMed.
- Y. Zhou and M. Nemati, Water, Air, Soil Pollut., 2022, 233, 1–17 CrossRef.
- I. Muylaert, A. Verberckmoes, J. de Decker and P. van der Voort, Adv. Colloid Interface Sci., 2012, 175, 39–51 CrossRef CAS PubMed.
- W. Libbrecht, A. Verberckmoes, J. W. Thybaut, P. van der Voort and J. de Clercq, Carbon, 2017, 116, 528–546 CrossRef CAS.
- H. Zhang, Q. Ji, L. Lai, G. Yao and B. Lai, Chin. Chem. Lett., 2019, 30, 1129–1132 CrossRef CAS.
- S. R. Subashchandrabose, M. Megharaj, K. Venkateswarlu and R. Naidu, Environ. Toxicol. Chem., 2012, 31, 1980–1988 CrossRef CAS PubMed.
- T. B. Ogunbayo and T. Nyokong, J. Mol. Catal. A: Chem., 2011, 337, 68–76 CrossRef CAS.
- G. Mele, E. Garcìa-Lòpez, L. Palmisano, G. Dyrda and R. Słota, J. Phys. Chem. C, 2007, 111, 6581–6588 CrossRef CAS.
- G. Mele, R. del Sole, G. Vasapollo, E. García-López, L. Palmisano and M. Schiavello, J. Catal., 2003, 217, 334–342 CrossRef CAS.
- R. Zugle, E. Antunes, S. Khene and T. Nyokong, Polyhedron, 2012, 33, 74–81 CrossRef CAS.
- R. Zugle, C. Litwinski and T. Nyokong, Polyhedron, 2011, 30, 1612–1619 CrossRef CAS.
- K. Elghniji, O. Hentati, N. Mlaik, A. Mahfoudh and M. Ksibi, J. Environ. Sci., 2012, 24, 479–487 CrossRef CAS PubMed.
- P. van Aken, R. van den Broeck, J. Degrève and R. Dewil, J. Chem. Eng., 2015, 280, 728–736 CrossRef CAS.
- F. F. Ventura, L. F. Mendes, A. G. Oliveira, R. C. Bazito, E. J. H. Bechara, R. S. Freire and C. v. Stevani, Environ. Toxicol. Chem., 2020, 39, 1558–1565 CrossRef CAS PubMed.
- H. Golmojdeh, M. A. Zanjanchi and M. Arvand, Photochem. Photobiol., 2013, 89, 1029–1037 CrossRef CAS PubMed.
- M. L. Hitchman, R. A. Spackman, N. C. Ross and C. Agra, Chem. Soc. Rev., 1995, 24, 423 RSC.
- M. L. Robertson, D. A. Eastmond and M. T. Smith, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1991, 249, 201–209 CrossRef CAS PubMed.
- R. K. Madan and J. Levitt, J. Am. Acad. Dermatol., 2014, 70, 788–792 CrossRef CAS PubMed.
- F. J. Enguita and A. L. Leitão, BioMed Res. Int., 2013, 2013, 542168 Search PubMed.
- B. Bukowska and S. Kowalska, Toxicol. Lett., 2004, 152, 73–84 CrossRef CAS PubMed.
- C. Tai, G. Jiang, J. Liu, Q. Zhou and J. Liu, J. Photochem. Photobiol., A, 2005, 172, 275–282 CrossRef CAS.
- M. Clark, Handbook of Textile and Industrial Dyeing, Woodhead, 2011 Search PubMed.
- R. Kishor, D. Purchase, G. D. Saratale, L. F. Romanholo Ferreira, C. M. Hussain, S. I. Mulla and R. N. Bharagava, J. Water Process. Eng., 2021, 43, 102300 CrossRef.
- P. Khoza and T. Nyokong, J. Mol. Catal. A: Chem., 2014, 395, 34–41 CrossRef CAS.
- Y. Wan, Q. Liang, T. Cong, X. Wang, Y. Tao, M. Sun, Z. Li and S. Xu, RSC Adv., 2015, 5, 66286–66293 RSC.
- P. Khoza and T. Nyokong, J. Coord. Chem., 2015, 68, 1117–1131 CrossRef CAS.
- Q. Liang, M. Zhang, C. Liu, S. Xu and Z. Li, Appl. Catal., A, 2016, 519, 107–115 CrossRef CAS.
- Y. Wan, S. Chen, G. Wang, Q. Liang, Z. Li and S. Xu, Acta Phys. Pol., A, 2016, 130, 785–790 CrossRef CAS.
- Y. Yılmaz, ChemistrySelect, 2021, 6, 7223–7231 CrossRef.
- T. Wang, Y. Li, G. Qu, D. Liang and S. Hu, Water, Air, Soil Pollut., 2016, 227, 464 CrossRef.
- S. Mapukata, N. Kobayashi, M. Kimura and T. Nyokong, J. Photochem. Photobiol., A, 2019, 379, 112–122 CrossRef CAS.
- S. Mapukata, J. Britton, N. Nwahara and T. Nyokong, J. Photochem. Photobiol., A, 2022, 424, 113637 CrossRef CAS.
- S. K. Movahed, Z. Piraman and M. Dabiri, J. Photochem. Photobiol., A, 2018, 351, 208–224 CrossRef CAS.
- B. M. Vinoda, M. Vinuth, D. B. Yaadav and J. Manjanna, J. Environ. Anal. Toxicol., 2015, 05, 2–6 Search PubMed.
- Y. Xia, G. Wang, L. Guo, Q. Dai and X. Ma, Chemosphere, 2020, 241, 125010 CrossRef CAS PubMed.
- D. Drozd, K. Szczubiałka, Ł. Łapok, M. Skiba, H. Patel, S. M. Gorun and M. Nowakowska, Appl. Catal., B, 2012, 125, 35–40 CrossRef CAS.
- I. A. Ike, S. L. Foster, S. R. Shinn, S. T. Watson, J. D. Orbell, L. F. Greenlee and M. C. Duke, J. Environ. Chem. Eng., 2017, 5, 4014–4023 CrossRef CAS.
- A. Srivastava, L. K. Dangi, S. Kumar and R. Rani, Heliyon, 2022, 8, e08834 CrossRef CAS PubMed.
- V. Belessi, G. Romanos, N. Boukos, D. Lambropoulou and C. Trapalis, J. Hazard. Mater., 2009, 170, 836–844 CrossRef CAS PubMed.
- Y. Yavuz, G. Canan and O. Bakır, J. Hazard. Mater., 2007, 145, 100–108 CrossRef PubMed.
- G. Cheng, J. Wan, Q. Li, L. Sun, Y. Zhang, Z. Li, C. Dang and J. Fu, Water, 2022, 14, 380 CrossRef CAS.
- C. J. Ogugbue and T. Sawidis, Biotechnol. Res. Int., 2011, 2011, 1–11 CrossRef PubMed.
- S. Mani and R. N. Bharagava, Rev. Environ. Contam. Toxicol., 2016, 237, 71–104 CAS.
- H. H. Mohamed, I. Hammami, S. Akhtar and T. E. Youssef, Composites, Part B, 2019, 176, 107314 CrossRef CAS.
- K. G. Akpomie and J. Conradie, Sci. Rep., 2020, 10, 1–18 CrossRef PubMed.
- H. H. Mohamed and T. E. Youssef, Mol. Catal., 2017, 433, 68–76 CrossRef CAS.
- A. Mittal, J. Hazard. Mater., 2006, 128, 233–239 CrossRef CAS PubMed.
- A. A. Renita, P. S. Kumar and S. A. Jabasingh, Bioresour. Technol. Rep., 2019, 7, 100300 CrossRef.
- S. Srivastava, R. Sinha and D. Roy, Aquat. Toxicol., 2004, 66, 319–329 CrossRef CAS PubMed.
- A. A. Al-Gheethi, Q. M. Azhar, P. Senthil Kumar, A. A. Yusuf, A. K. Al-Buriahi, R. M. S. Radin Mohamed and M. M. Al-shaibani, Chemosphere, 2022, 287, 132080 CrossRef CAS PubMed.
- T. Aarthi and G. Madras, Ind. Eng. Chem. Res., 2007, 46, 7–14 CrossRef CAS.
- J. X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef CAS PubMed.
- R. M. Wang, H. Wang, Y. Wang, H. R. Li, Y. F. He and E. X. Hao, Color. Technol., 2014, 130, 32–36 CAS.
- A. Luna-Flores, M. A. Valenzuela, J. A. Luna-López, A. D. Hernández De La Luz, L. C. Muñoz-Arenas, M. Méndez-Hernández and J. L. Sosa-Sánchez, Int. J. Photoenergy, 2017, 2017, 1604753 CrossRef.
- H. Wang, L. Zhao, X. Liu, J. Xu, W. Hou, J. Wang, E. He, R. Zhang and H. Zhang, Dyes Pigm., 2017, 137, 322–328 CrossRef CAS.
- X. Guo, X. Zhou, X. X. X. Li, C. Shao, C. Han, X. X. X. Li and Y. Liu, J. Colloid Interface Sci., 2018, 525, 187–195 CrossRef CAS PubMed.
- S. L. Wang, Y. F. Fang, Y. Yang, J. Z. Liu, A. P. Deng, X. R. Zhao and Y. P. Huang, Chin. Sci. Bull., 2011, 56, 969–976 CrossRef CAS.
- B. Cabir, M. Yurderi, N. Caner, M. S. Agirtas, M. Zahmakiran and M. Kaya, Mater. Sci. Eng. B: Solid-State Mater. Adv. Technol., 2017, 224, 9–17 CrossRef CAS.
- R. Wang, Y. Liu, P. Zuo, Z. Zhang, N. Lei and Y. Liu, Environ. Sci. Pollut. Res., 2020, 27, 18831–18842 CrossRef CAS PubMed.
- K. Tekintas, Ö. Kesmez, O. Bekircan and E. T. Saka, J. Mol. Struct., 2022, 1248, 131405 CrossRef CAS.
- H. ben Slama, A. C. Bouket, Z. Pourhassan, F. N. Alenezi, A. Silini, H. Cherif-Silini, T. Oszako, L. Luptakova, P. Golińska and L. Belbahri, Appl. Sci., 2021, 11, 1–21 Search PubMed.
- P. K. Gillman, Psychopharmacol, 2011, 25, 429–436 CrossRef CAS PubMed.
- W. Vallejo, C. Diaz-Uribe and Á. Cantillo, J. Photochem. Photobiol., A, 2015, 299, 80–86 CrossRef CAS.
- K. P. Priyanka, S. Sankararaman, K. M. Balakrishna and T. Varghese, J. Alloys Compd., 2017, 720, 541–549 CrossRef CAS.
- Q. Liang, M. Zhang, Z. Zhang, C. Liu, S. Xu and Z. Li, J. Alloys Compd., 2017, 690, 123–130 CrossRef CAS.
- S. H. Wu, J. L. Wu, S. Y. Jia, Q. W. Chang, H. T. Ren and Y. Liu, Appl. Surf. Sci., 2013, 287, 389–396 CrossRef CAS.
- A. Hışır, G. K. Karaoğlan and O. Avcıata, J. Mol. Struct., 2022, 1266, 133498 CrossRef.
- M. Berradi, R. Hsissou, M. Khudhair, M. Assouag, O. Cherkaoui, A. el Bachiri and A. el Harfi, Heliyon, 2019, 5, e02711 CrossRef PubMed.
- G. M. Ziarani, R. Moradi, N. Lashgari and H. G. Kruger, in Metal-Free Synthetic Organic Dyes, Elsevier, 2018, pp. 9–17 Search PubMed.
- E. Yabalak, J. Environ. Chem. Eng., 2021, 9, 105201 CrossRef CAS.
- H. U. Okoroiwu and I. A. Iwara, Interdiscip. Toxicol., 2018, 11, 129–137 CrossRef PubMed.
- E. Vargas, R. Vargas and O. Núñez, Appl. Catal., B, 2014, 156–157, 8–14 CrossRef CAS.
- D. H. Garabrant and M. A. Philbert, Crit. Rev. Toxicol., 2002, 32, 233–257 CrossRef CAS PubMed.
- T. Cáceres, M. Megharaj, K. Venkateswarlu, N. Sethunathan and R. Naidu, in Reviews of Environmental Contamination and Toxicology, ed. D. M. Whitacre, Springer New York, New York, NY, 2010, vol. 205, pp. 117–162 Search PubMed.
- A. K. Priya, L. Gnanasekaran, S. Rajendran, J. Qin and Y. Vasseghian, Environ. Res., 2022, 204, 112298 CrossRef CAS PubMed.
- A. Nikolaou, S. Meric and D. Fatta, Anal. Bioanal. Chem., 2007, 387, 1225–1234 CrossRef CAS PubMed.
- E. Brillas, Chemosphere, 2022, 286, 131849 CrossRef CAS PubMed.
- J. Musial, R. Krakowiak, R. Frankowski, M. Spychala, J. Dlugaszewska, B. Dobosz, W. Bendzinska-Berus, R. Krzyminiewski, E. Tykarska, A. Zgoła-Grześkowiak, T. Goslinski, D. T. Mlynarczyk and B. J. Stanisz, Mater. Sci. Eng. B: Solid-State Mater. Adv. Technol., 2022, 276, 115559 CrossRef CAS.
- B. H. Schafhauser, L. A. Kristofco, C. M. R. de Oliveira and B. W. Brooks, Environ. Pollut., 2018, 238, 440–451 CrossRef CAS PubMed.
- X. He, T. Kai and P. Ding, Heterojunction photocatalysts for degradation of the tetracycline antibiotic: a review, Springer International Publishing, 2021, vol. 19 Search PubMed.
- X. S. Miao, J. J. Yang and C. D. Metcalfe, Environ. Sci. Technol., 2005, 39, 7469–7475 CrossRef CAS PubMed.
- C. B. Anucha, I. Altin, D. Fabbri, I. Degirmencioglu, P. Calza, G. Magnacca, V. N. Stathopoulos and E. Bacaksiz, Separations, 2020, 7, 1–20 CrossRef.
- A. C. Velosa and C. A. O. Nascimento, Environ. Sci. Pollut. Res., 2017, 24, 6270–6277 CrossRef CAS PubMed.
- Z. Gao, H. Yang, J. Mao and J. Wu, J. Cleaner Prod., 2019, 220, 668–676 CrossRef CAS.
- A. Hamdia, S. Boufib and S. Bouattour, Appl. Surf. Sci., 2015, 339, 128–136 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |