 Open Access Article
Open Access ArticleReview of the application of Cu-containing SSZ-13 in NH3-SCR-DeNOx and NH3-SCO
Magdalena Jabłońska
 *
*
Institute of Chemical Technology, Universität Leipzig, Linnéstr. 3, 04103 Leipzig, Germany. E-mail: magdalena.jablonska@uni-leipzig.de
First published on 7th September 2022
Abstract
The reduction of NOx emissions has become one of the most important subjects in environmental protection. Cu-containing SSZ-13 is currently the state-of-the-art catalyst for the selective catalytic reduction of NOx with ammonia (NH3-SCR-DeNOx). Although the current-generation catalysts reveal enhanced activity and remarkable hydrothermal stability, still open challenges appear. Thus, this review focuses on the progress of Cu-containing SSZ-13 regarding preparation methods, hydrothermal resistance and poisoning as well as reaction mechanisms in NH3-SCR-DeNOx. Remarkably, the paper reviews also the progress of Cu-containing SSZ-13 in the selective ammonia oxidation into nitrogen and water vapor (NH3-SCO). The dynamics in the NH3-SCR-DeNOx and NH3-SCO fields make this review timely.
Introduction
Nitrogen oxides (NOx, consisting of >95% NO and <5% NO2) are one of the major atmospheric pollutant gasses emitted from vehicle engines (e.g., automobiles, ships, etc.) and industrial boiler processes. NOx are formed via different mechanisms such as thermal-NOx, prompt-NOx and fuel-NOx.1,2 Nitrogen oxides affect human health (e.g., lowering the body's resistance to bacterial infections, eye and respiratory system irritation, causing problems with breathing, allergic diseases, etc.). Furthermore, the hazards of NOx include the promotion of global warming and the formation of photochemical smog, acid rain, atmospheric haze (fine particle pollution) and ozone depletion, while the control of NOx emission remains a challenging task in the field of environmental catalysis.1–4 The upcoming EU emission legislation challenges researchers and engineers to keep the NOx emissions at a very low level under various boundary conditions. The selective catalytic reduction with ammonia (NH3-SCR-DeNOx: 4NH3 + 4NO + O2 → 4N2 + 6H2O) is one of the most effective technologies for removing NOx from diesel engine exhausts with an 80–95% removal efficiency.1,2 Catalysts – typically metal-oxides, noble metals, metal exchanged zeolites or hybrid systems – are an integral part of NH3-SCR-DeNOx.5–7 Much research has been done on Cu-containing ZSM-5 catalysts since its discovery in 1986 by Iwamoto et al.8 However, Cu-containing ZSM-5, beta or SAPO-34 suffer from poor hydrothermal stability.9,10 Cu-containing SSZ-13 (standard oil synthetic zeolite-thirteen, chabazite (CHA)-type zeolite) catalysts have been commercialized in the US and Europe since 2010 due to their efficient reduction of NOx and enhanced hydrothermal stability.11,12 The CHA framework is composed of four-, six-, and eight-membered rings arranged to form a tridimensional system of channels perpendicular to each other (0.38 × 0.38 nm; R3m (#166) space group).13,14 Examples of morphology of SSZ-13 are given in Fig. 1a. SSZ-13 was first synthesized by Zones in 198515 applying the very costly template N,N,N-trimethyl-1-adamantammonium hydroxide (TMAdaOH). Nowadays, SSZ-13 can be synthesized by applying a variety of cheaper templates, including choline chloride,16,17 N,N,N-dimethylethylcycloxexylammonium bromide (DMCHABr),18 etc. Cu-SSZ-13 prepared via ion-exchange showed improved activity and N2 selectivity (i.e., lower amounts of NOx by-products) compared to Cu-ZSM-5 and Cu-beta (Fig. 1b).11 The activity of catalysts was maintained even after severe hydrothermal treatment at 800 °C for 16 h.11,19–21 Still, some challenges remain for Cu-containing SSZ-13, including broadening the reaction temperature window together with improved (thermal) stability against chemical poisons. Although some review articles on the Cu-containing SSZ-13 were already published in 2015–2021,22–27 extensive studies on the Cu-containing SSZ-13 catalysts concerning preparations methods, hydrothermal stability and poisoning, the reaction mechanisms as well as the determination of active sites are of great interest. Thus, all these factors constitute the present review, which covers the thorough literature of the last ten years. However, this review did not include patents.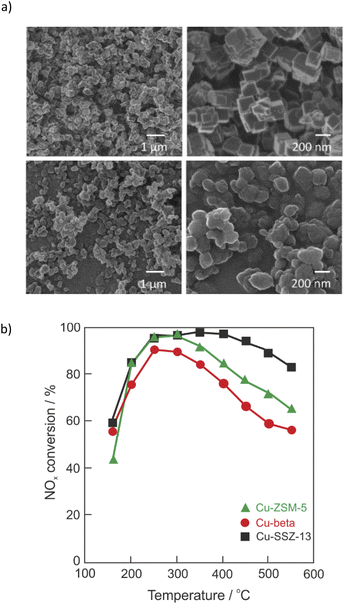 | ||
| Fig. 1 (a) Examples of helium ion microscopy (HIM) images of SSZ-13. Reproduced from ref. 14 with permission from Elsevier, copyright 2017; (b) NOx conversion over Cu-SSZ-13 (squares), Cu-beta (circles), and Cu-ZSM-5 (triangles). Reproduced from ref. 11 with permission from Elsevier, copyright 2010. | ||
Preparation methods of Cu-containing SSZ-13
Kwak et al.28 first suggested the presence of two different cationic Cu sites in Cu-SSZ-13, while Giordanino et al.29 first reported the presence of [Cu(OH)]+ species in Cu-SSZ-13. Cu-containing SSZ-13 contains two active sites for NH3-SCR-DeNOx, Cu2+ in double-six-membered ring (D6R) cages and [Cu(OH)]+ in CHA cages, which are balanced by a pair of negative charges (2Z) and a single framework negative charge (Z), respectively30–32 (Fig. 2a). The concentration of [Cu(OH)]+ ions within the eight-ring windows of Cu-SSZ-13 increases as the n(Cu)/n(Al) ratio rises, unlike that of Cu2+ ions on D6Rs.33 The dehydration of the [Cu(OH)]+ ions may lead to the formation of Cu+ species ([Cu(OH)]+ → Cu+ + HO˙),34 or [Cu–O–Cu]2+ oxocations (–Al–O–Cu2+–OH + –Al–O–Cu2+–OH → –Al–O–[Cu–O–Cu]2+–O–Al– + H2O).35 Furthermore, Verma et al.36,37 suggested that when the n(Cu)/n(Al) is above 0.2, CuxOy are also present. Such aggregated copper oxide species catalyze ammonia oxidation.38 Fickel and Lobo12 pioneered in the investigation over Cu species in Cu-SSZ-13 with low n(Si)/n(Al) and 4.39 wt% of Cu. They proposed that the Cu cations exist exclusively in the form of isolated Cu2+ ions and are located in the cages coordinated with three oxygen atoms of the six-membered rings (6MRs). Nowadays it is recognized that the location, as well as the nature of the Cu cations, varies significantly depending on the n(Si)/n(Al) ratio and also on the Cu loading. Thus, the copper species can occupy four types of cationic sites in CHA, such as site I – displaced from the six-membered-ring into the ellipsoidal cavity, site II – located near the center of the ellipsoidal cavity, site III – located in the center of the hexagonal prism, and site IV – located near the eight-membered-ring window.39,40 The copper species can be introduced into the CHA structure via different techniques, including ion-exchange, one-pot hydrothermal method, solid-state ion-exchange, etc. Table 1 summarizes the catalyst preparation, reaction conditions, and NOx conversion in NH3-SCR-DeNOx above 80% and related by-products in the range. The activity and selectivity of the Cu-containing systems depend on multiple factors, particularly catalyst composition (e.g., n(Si)/n(Al) ratio and copper species loading) and treatment history (e.g., degreening and hydrothermal ageing). It is important to note that the reaction conditions are generally different from each other, including the feed gas composition, total flow, catalyst dosage, and even measurement systems. Therefore, it is hard to compare the activity of the various catalysts in detail. However, a rough comparison is still feasible. Other transition metals have been introduced to improve the activity of catalysts (e.g., Fe,41–44 Mn,45,46 etc.). In the current review, the focus is given mainly on the Cu-containing SSZ-13.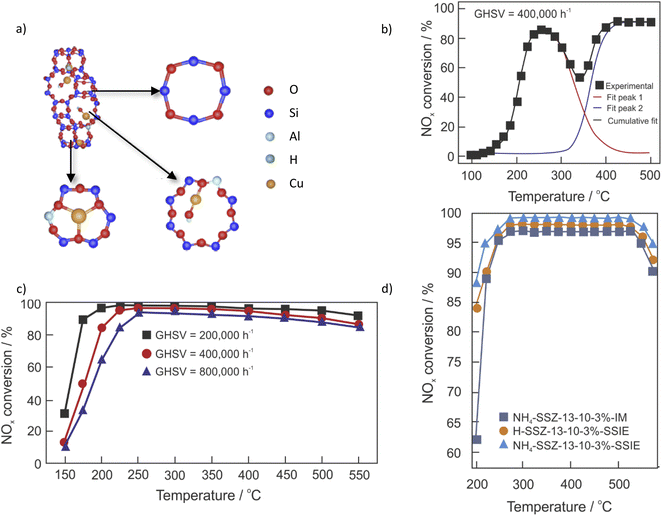 | ||
| Fig. 2 (a) The structure of Cu-SSZ-13, which contains four-membered, six-membered, and eight-membered rings. Reproduced from ref. 30 with permission from Elsevier, copyright 2022; (b) NOx conversion (■) over Cu-SSZ-13 with n(Si)/n(Al) = 12 and n(Cu)/n(Al) = 0.13. Also included are simulated curves assuming low- and high-temperature reaction routes. Reproduced from ref. 51 with permission from ACS Publications, copyright 2017; (c) NOx conversion over (3.8 wt%)Cu-SSZ-13 under different GHSVs. Reproduced from ref. 67 with permission from ACS Publications, copyright 2014; (d) NOx conversion over Cu-SSZ-13 prepared via solid-state ion-exchange (SSIE) and impregnation (IM). Reproduced from ref. 47 with permission from Elsevier, copyright 2022. | ||
| Pos. | Sample | Preparation method | Reaction conditions | Operation temperature for achieving > 80% NOx conversion/°C ((by-)product formation) | Ref. |
|---|---|---|---|---|---|
| 1 | (1.4 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.8) monolith catalyst | Commercial; degreening: 14 vol% O2, 5 vol% H2O, 5 vol% CO2, N2 balance, 600 °C, 4 h | 0.035 vol% NO, 0.035 vol% NH3, 14 vol% O2, 5 vol% CO2, 5 vol% H2O, N2 balance, GHSV 30,000 h−1 | 200–500 (<7% N2O selectivity) | 68 |
| 2 | (1.63 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 16) monolith catalyst | Commercial; degreening: 10 vol% O2, 7 vol% H2O, 8 vol% CO2, N2 balance, 650 °C, 4 h; *hydrothermal treatment: 10 vol% H2O, 18.9 vol% O2, N2 balance, 650 °C, 100 h | 0.05 vol% NO, 0.05 vol% NH3, 7 vol% H2O, 8 vol% CO2, 10 vol% O2, N2 balance, GHSV 60,000 h−1 | 200–500 (not shown), *200–500 (not shown) | 69 |
| 3 | (1.63 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 16) monolith catalyst | Commercial; degreening: 10 vol% O2, 7 vol% H2O, 8 vol% CO2, N2 balance, 650 °C, 50 h | 0.05 vol% NO, 0.05 vol% NH3, 7 vol% H2O, 8 vol% CO2, 10 vol% O2, N2 balance, GHSV 60,000 h−1 | 200–500 (<15 ppm N2O) | 70 |
| 4 | (2.2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 13.6) monolith catalyst | Commercial; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 12 h | 0.1 vol% NO, 0.11 vol% NH3, 5 vol% O2, 10 vol% H2O, N2 balance, GHSV 30,000 h−1 | 225–550 (not shown) *225–575 (not shown) | 71 |
| 5 | (2.4 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 15) monolith catalyst | Commercial; degreening: 10 vol% O2, 7 vol% H2O, 8 vol% CO2, Ar balance, 550 °C, 4 h | 0.02 vol% NO, 0.02 vol% NH3, 5 vol% O2, 5 vol% H2O, Ar balance, GHSV 40,000 h−1 | 200–450 (<6% NO2, N2O concentration) | 72 |
| 6 | (2.7 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Commercial; degreening: 10 vol% H2O, air balance, 650 °C, 12 h; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h; pre-treatment: 21 vol% O2, N2 balance, 600 °C, 20 min | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 200–600 (<10 ppm N2O), *200–500 (<10 ppm N2O) | 73 |
| 7 | (2.8 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 35) monolith catalyst | Commercial; calcination, 450 °C, 0.5 h; 12.5 vol% H2O, air balance, 800 °C, 16 h | 0.02 vol% NO, 0.02 vol% NH3, 10 vol% O2, 5 vol% H2O, 8 vol% CO2, N2 balance, GHSV 30,000 h−1 | 250–450 (not shown) | 21 |
| 8 | (3 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 7) monolith catalyst | Commercial; degreening: 10 vol% O2, 5 vol% H2O, N2 balance, 500 °C, 1 h | 0.1 vol% NO, 0.1 vol% NH3, 10 vol% O2, 5 vol% H2O, N2 balance, GHSV 120,000 h−1 | 200–500 (<15 ppm N2O) | 52 |
| 9 | Cu-SSZ-13 (Cu content, n(Si)/n(Al) not shown) monolith catalyst | Commercial; degreening: 10 vol% O2, 7 vol% H2O, 8 vol% CO2, N2 balance, 550–600 °C, 4 h | 0.02 vol% NO, 0.02 vol% NH3, 10 vol% O2, 5 vol% H2O, Ar balance, GHSV 40,000 h−1 | 200–500 (not shown) | 74 |
| 10 | Cu-SSZ-13 (Cu content, n(Si)/n(Al) not shown) monolith catalyst | Commercial; hydrothermal treatment: 10 vol% O2, 8 vol% CO2, 7 vol% H2O, N2 balance, 800 °C, 4 h | 0.02 vol% NO, 0.02 vol% NH3, 10 vol% O2, 8 vol% CO2, 7 vol% H2O, N2 balance, GHSV 40,000 h−1 | 200–500 (not shown) | 75 |
| 11 | Cu-SSZ-13 (Cu content, n(Si)/n(Al) not shown) monolith catalyst | Commercial; hydrothermal treatment: 14 vol% O2, 5 vol% CO2, 5 vol% H2O, N2 balance, 750 °C, 16 h | 0.035 vol% NO, 0.035 vol% NH3, 14 vol% O2, 2 vol% H2O, N2 balance, GHSV 140,000 h−1 | 250–500 (not shown) | 76 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Ion-exchange | |||||
| 12 | Cu-SSZ-13 (n(Si)/n(Al) = 6) (Cu content not shown) | Ion-exchange, calcination, 500 °C, 2 h, air | 0.035 vol% NO, 0.035 vol% NH3, 14 vol% O2, 2 vol% H2O, N2 balance, GHSV 30,000 h−1 | 200–550 (<10 ppm NO2 formation, <5 ppm N2O formation) | 11 |
| 13 | (0.87 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Ion-exchange, calcination, 550 °C, 8 h, air; **hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 12 h | 0.035 vol% NO, 0.035 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 100,000 h−1 | 200–500 (not shown) | 77 |
| 14 | (0.98 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | 200–500 (<5 ppm N2O), *200–500 (not shown) | |||
| 15 | (0.88 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 16) | Ion-exchange, calcination, 550 °C, 8 h, air; pre-treatment: 21 vol% O2, N2 balance, 500 °C, 2 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 10 vol% H2O, N2 balance, GHSV 100,000 h−1 | 200–600 (not shown) | 78 |
| 16 | (0.95–1.38 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 20) | Ion-exchange; pre-treatment: 5 vol% O2, He balance, 550 °C, 1 h | 0.1 vol% NO, 0.1 vol% NH3, 5 vol% O2, He balance, GHSV 100,000 h−1 | 300–450 (not shown) | 79 |
| 17 | (1.04 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 5.5) | Ion-exchange, calcination, 500 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 12 h | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 5 vol% H2O, N2 balance, GHSV 30,000 h−1 | 200–600 (not shown), *200–550 (not shown) | 80 |
| 18 | (1.00–1.17 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 23.6–23.8) | Ion-exchange, calcination, 550 °C, 6 h, air; pre-treatment: 500 °C, 0.5 h, 5 vol% O2, N2 balance | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 3 vol% H2O, N2 balance, GHSV 40,000 h−1 | 250–500 (<6 ppm N2O) | 81 |
| 19 | (2.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 15) | Ion-exchange, calcination, 500 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 5 h | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 5 vol% H2O, N2 balance, GHSV 80,000 h−1 | 175–550 (<15 ppm N2O), *175–600 (<15 ppm N2O) | 82 |
| 20 | (1.21 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Ion-exchange, calcination, 550 °C, 4 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 600 °C, 20 h | 0.036 vol% NO, 0.036 vol% NH3, 10 vol% O2, N2 balance, GHSV 400,000 h−1 | 225–550 (not shown), *250–550 (not shown) | 83 |
| 21 | (1.25–1.27 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 21–24) | Ion-exchange, calcination, 550 °C, 6 h, air; pre-treatment: 500 °C, 1 h, 14 vol% O2, N2 balance; *hydrothermal treatment: 5 vol% H2O, N2 balance, 800 °C, 16 h | 0.005 vol% NO, 0.005 vol% NH3, 5 vol% O2, N2 balance, GHSV 48,000 h−1 | 200–500 (>90% N2 selectivity), *200–500 (>90% N2 selectivity) | 84 |
| 22 | (1.3 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 17.6) | Ion-exchange, calcination, 550 °C, 4 h, air; pre-treatment: 5 vol% O2, He balance, 550 °C, 1 h | 0.01 vol% NO, 0.01 vol% NH3, 5 vol% O2, He balance, GHSV 100,000 h−1 | 250–450 (not shown) | 58 |
| 23 | (1.45–1.62 wt%)Cu-SSZ-13 (n(Si)/n(Al) not shown) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.035 vol% NO, 0.035 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 200,000 h−1 | 200–500 (not shown), *225–450 (not shown) | 14 |
| 24 | (1.73 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 9.5) | Ion-exchange, calcination, 550 °C, 4 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 400,000 h−1 | 225–600 (>95% N2 selectivity), *250–500 (>90% N2 selectivity) | 85 |
| 25 | (1.8–2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 7.8) | Ion-exchange, calcination, temperature and time not shown; *hydrothermal treatment: 5 vol% H2O, N2 balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 14 vol% O2, 5 vol% H2O, N2 balance, GHSV 48,000 h−1 | 200–600 (>97% N2 selectivity), *200–500 (>97% N2 selectivity) | 86 |
| 26 | (1.88–2.16 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 16.5–20.3) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h; pre-treatment: 21 vol% O2, N2 balance, 550 °C, 20 min | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 200–550 (<5 ppm N2O), *200–500 (<5 ppm N2O) | 87 |
| 27 | (2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 9) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h; **degreening: 10 vol% H2O, air balance, 700 °C, 4 h | 0.036 vol% NO, 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 100,000 h−1 | *200–550 (not shown), **200–550 (not shown) | 88 |
| 28 | (2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 11) | Ion-exchange, calcination conditions not shown; degreening: Reaction conditions, 600 °C, 4 h; pre-treatment: 10 vol% O2, 7 vol% H2O, N2 balance, 600 °C, 1 h | 0.035 vol% NO, 0.035 vol% NH3, 10 vol% O2, 7 vol% H2O, N2 balance, GHSV 300,000 h−1 | 225–550 (>97% N2 selectivity) | 89 |
| 29 | (2.08 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.5) | Ion-exchange, calcination, 550 °C, 4 h, air; hydrothermal treatment: 20 vol% O2, 10 vol% H2O, N2 balance, 550 °C, 0.5 h | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, N2 balance, *0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 5 vol% H2O, N2 balance, WHSV 240,000 ml g−1 h−1 | 250–300 (not shown), *250–300 (not shown) | 90 |
| 30 | (2.1 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Ion-exchange, calcination conditions not shown; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.036 vol% NO, 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 200,000 h−1 | 175–500 (not shown), *200–450 (not shown) | 91 |
| 31 | (2.2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 10.5) | Ion-exchange, calcination, 600 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 400,000 h−1 | 225–550 (<10 ppm N2O), *225–500 (<15 ppm N2O) | 92 |
| 32 | (2.23 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 8.8) | Ion-exchange, calcination condition not shown; hydrothermal treatment: *700 °C (**800 °C), 10 vol% H2O, air balance, 15 h | 0.03 vol% NO, 0.03 vol% NH3, 5 vol% O2, 3 vol% H2O, N2 balance, WHSV 60,000 ml g−1 h−1 | 250–500 (not shown), *250–500 (not shown), **300–350 (not shown) | 93 |
| 33 | (2.25 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Ion-exchange, calcination, 550 C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, 700 °C, 16 h; pre-treatment: 14 vol%, N2 balance, 500 °C, 1 h | 0.036 vol% NO, 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 200,000 h−1 | 175–500 (<7 ppm N2O), *200–500 (<25 ppm N2O) | 94 |
| 34 | (2.3 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 15) monolith catalyst | Ion-exchange, calcination, 600 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 12 h | 0.1 vol% NO, 0.11 vol% NH3, 5 vol% O2, 10 vol% H2O, N2 balance, GHSV 30,000 h−1 | 200–575 (not shown), *200–575 (not shown) | 95 |
| 35 | (2.35 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12.93) | Ion-exchange, calcination, 550 °C, 4 h, air; pre-treatment: 8 vol% O2, N2 balance, 1 h, 500 °C | 0.05 vol% NO, 0.05 vol% NH3, 6.5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 125–400 (>95% N2 selectivity) | 96 |
| 36 | (2.38 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Ion-exchange, calcination, 600 °C, time not shown, air; pre-treatment: 5 vol% O2, N2 balance, 200 °C, 1 h | 0.06 vol% NO, 0.06 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 450,000 h−1 | 250–550 (not shown) | 97 |
| 37 | (2.38 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Ion-exchange, calcination, 600 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h; pre-treatment: 5 vol% O2, N2 balance, 1 h, 600 °C | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV not shown | 250–550 (not shown), *300–400 (not shown) | 98 |
| 38 | (2.5–3 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12.5) | Ion-exchange, calcination, 500 °C, 6 h, air; *hydrothermal treatment: 5 vol% H2O, air balance, 800 °C, 12 h | 0.1 vol% NO, 0.1 vol% NH3, 5 vol% O2, N2 balance, GHSV 130,000 h−1 | 200–800 (100% N2 selectivity), *200–800 100% N2 selectivity) | 30 |
| 39 | (2.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 8.7) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 200,000 h−1 | 200–550 (100% N2 selectivity), *250–500 (100% N2 selectivity) | 99 |
| 40 | (2.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) not shown) | Ion-exchange, calcination, 550 °C, 6 h, air; *one-pot hydrothermal synthesis, calcination, 550 °C, 6 h, air | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, Ar balance, GHSV 180,000 h−1 | 250–600 (not shown), *200–600 (not shown) | 100 |
| 41 | (2.8 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 5) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 5 vol% H2O, N2 balance, GHSV 80,000 h−1 | 175–650 (<15 ppm N2O), *175–550 (<15 ppm N2O) | 101 |
| 42 | (3.0 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Ion-exchange, calcination, 550 °C, 8 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.036 vol% NO, 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 100,000 h−1 | 200–450 (not shown) | 102 |
| 43 | (3.4 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 9) | Ion-exchange, calcination, 700 °C, 5 h, air | 0.05 vol% NO, 0.05 vol% NH3, 4 vol% O2, N2 balance, GHSV 60,000 h−1 | 175–500 (<10 ppm N2O) | 103 |
| 44 | (3.43–5.15 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Ion-exchange, calcination, 550 °C, 8 h, air | 0.035 vol% NO, 0.035 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 800,000 h−1 | 250–550 (not shown) | 32 |
| 45 | (3.6 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6.5) monolith catalyst | Ion-exchange, calcination, 700 °C, 4 h, air | 0.02 vol% NO, 0.02 vol% NH3, 8 vol% O2, 10 vol% H2O, N2 balance, GHSV 60,000 h−1 | 200–500 (>95% N2 selectivity) | 104 |
| 46 | (3.97 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 13.26) | Ion-exchange, calcination, 550 °C, 6 h, air | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 5 vol% H2O, N2 balance, GHSV 100,000 h−1 | 200–450 (>95% N2 selectivity) | 64 |
| 47 | (4.0–4.7 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12.0–12.7) | Ion-exchange, calcination, 500 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 12 h | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, N2 balance, GHSV 30,000 h−1 | 200–500 (<25 ppm N2O), *200–500 (<25 ppm N2O) | 48 |
| 48 | (4.1 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) monolith catalyst | Ion-exchange, calcination, 600 °C, 4 h, air | 0.04 vol% NO, 0.04 vol% NH3, 8 vol% O2, 5 vol% H2O, Ar balance, GHSV 22,100 h−1 | 175–500 (<15 ppm NO2, <8 ppm N2O) | 105 |
| 49 | (4.93 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6.48) | Ion-exchange, calcination, 550 °C, 6 h, air | 0.1 vol% NO, 0.1 vol% NH3, 6 vol% O2, 5 vol% H2O, He balance, GHSV 50,000 h−1 | 150–450 (>70% N2 yield) | 16 |
| 50 | (4.93 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.03) | Ion-exchange, vacuum evaporator, calcination, 550 °C, 6 h, air | 0.1 vol% NO, 0.1 vol% NH3, 10 vol% O2, He balance, GHSV 30,000 h−1 | 150–450 (>70% N2 yield) | 17 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| One-pot hydrothermal synthesis | |||||
| 51 | Cu-SSZ-13 (n(Si)/n(Al) = 14.2) (Cu content not shown) | One-pot hydrothermal synthesis, calcination, 550 °C, time not shown, air; *hydrothermal treatment: 2.2 ml min−1 H2O, air balance, 750 °C, 13 h pre-treatment: 550 °C, 1 h, N2 | 0.05 vol% NO, 0.053 vol% NH3, 7 vol% O2, 5 vol% H2O, N2 balance, WHSV 450,000 ml g−1 h−1 | 250–500 (not shown), *300–450 (not shown) | 62 |
| 52 | (2.82 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 15) monolith catalyst | One-pot hydrothermal synthesis, calcination, 600 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 850 °C, 12 h | 0.1 vol% NO, 0.11 vol% NH3, 5 vol% O2, 10 vol% H2O, N2 balance, GHSV 30,000 h−1 | 200–550 (not shown), *250–550 (not shown) | 63 |
| 53 | (3.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6.5) | One-pot hydrothermal synthesis, calcination, 600 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, N2 balance, GHSV 400,000 h−1 | 200–600 (>90% N2 selectivity), *200–550 (>90% N2 selectivity) | 106 |
| 54 | (3.8 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 8.3) | One-pot hydrothermal synthesis, calcination, 600 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, N2 balance, GHSV 400,000 h−1 | 200–550 (100% N2 selectivity), *250–450 (not shown) | 67 |
| 55 | (4.06–4.11 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4) | One-pot hydrothermal synthesis, calcination, 600 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 12 h; pre-treatment: 5 vol% O2, N2 balance, 550 °C, 1 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, WHSV 300,000 ml g−1 h−1 | 175–550 (<10 ppm N2O), *175–450 (<20 ppm N2O) | 107 |
| 56 | (4.92 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.36) | One-pot hydrothermal synthesis, calcination, 550 °C, 4 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h; pre-treatment: Reaction conditions, 550 °C, 1 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 300,000 h−1 | 200–500 (not shown), *250–500 (not shown) | 108 |
| 57 | (6.31 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 13) | One-pot hydrothermal synthesis, calcination, 600 °C, 6 h, air | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, N2 balance, GHSV 100,000 h−1 | 150–550 (not shown) | 65 |
| 58 | (9.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4) | One-pot hydrothermal synthesis, calcination, 550 °C, 8 h, air | 0.1 vol% NO, 0.1 vol% NH3, 10 vol% O2, Ar balance, GHSV not shown | 150–450 (not shown) | 61 |
| 59 | (9.7 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 3.82 | One-pot hydrothermal synthesis, calcination, 550 °C, 6 h, air | 0.06 vol% NO, 0.06 vol% NH3, 6 vol% O2, 5 vol% H2O, He balance, GHSV 400,000 h−1 | 200–550 (>98% N2 selectivity) | 109 |
| 60 | (10.0 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.5) | One-pot hydrothermal synthesis, calcination, 600 °C, 6 h, air | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 400,000 h−1 | 225–400 (not shown) | 110 |
| 61 | (10.61 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.17) | One-pot hydrothermal synthesis, calcination, 550 °C, 6 h, air | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, N2 balance, GHSV 120,000 h−1 | 150–400 (>90% N2 selectivity) | 111 |
| 62 | (11.27 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.2) | One-pot hydrothermal synthesis, calcination, 600 °C, 5 h, air | 500 ml m−3 NO, 500 ml m−3 NH3, 10 vol% O2, N2 balance, GHSV 180,000 h−1 | 200–400 (>95% N2 selectivity) | 66 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Solid-state ion-exchange | |||||
| 63 | (1.79 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 10.8) | Solid-state ion-exchange, vacuum evaporator, calcination, 300 °C, 0.5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 200–550 (not shown), *200–550 (not shown) | 112 |
| 64 | (3.7 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 13) | Solid-state ion-exchange, calcination, 600 °C, 5 h, 800 °C 12 h, air | 0.04 vol% NO, 0.04 vol% NH3, 8 vol% O2, 5 vol% H2O, Ar balance, GHSV 205,000 h−1 | 250–500 (not shown) | 113 |
| 65 | (3.08 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 10.1) monolith catalyst | Solid-state ion-exchange, vacuum evaporator, calcination conditions not shown; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 12 h | 0.1 vol% NO, 0.11 vol% NH3, 10 vol%% O2 N2 balance, GHSV 80,000 h−1 | 200–550 (>90% N2 selectivity), *200–550 (not shown) | 47 |
| 66 | (3.9 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 5.2) | Solid-state ion-exchange, calcination, 600 °C, time not shown, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 400,000 h−1 | 200–550 (>97% N2 selectivity), *200–450 (not shown) | 114 |
| 67 | (4.10 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) | Solid-state ion-exchange, calcination, 700 °C, 16 h, dry air; *hydrothermal treatment: 10 vol% H2O, 10 vol% O2, N2 balance, 750 °C, 16 h | 0.05 vol% NO, 0.05 vol% NH3, 10 vol% O2, 10 vol% H2O, N2 balance, GHSV 240,000 h−1 | 225–550 (not shown), *225–500 (not shown) | 115 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Impregnation | |||||
| 68 | (1.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12.6) | Impregnation, calcination, 550 °C, 1 h, air | 0.02 vol% NO, 0.02 vol% NH3, 10 vol% O2, 3 vol% H2O, N2 balance, GHSV 60,000 h−1 | 200–500 (not shown) | 69 |
| 69 | (1.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 11.5) | Impregnation, calcination, 550 °C, 1 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 900 °C, 4 h, **8 h | 0.02 vol% NO, 0.02 vol% NH3, 10 vol% O2, 3 vol% H2O, N2 balance, GHSV 60,000 h−1 | 200–500 (not shown), *200–500 (not shown), **200–500 (not shown) | 116 |
| 70 | (2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 11.4) monolith catalyst | Impregnation, calcination, 600 °C, 8 h; 750 °C, 2 h, air; degreening: 0.04 vol% NO, 0.04 vol% NH3, 5 vol% H2O, Ar balance, 250 °C, 1 h; 10 vol% O2, 500 °C; pre-treatment: 10 vol% O2, 5 vol% H2O, Ar balance, 500 °C, 20 min | 0.04 vol% NO, 0.04 vol% NH3, 10 vol% O2, Ar balance, *0.04 vol% NO, 0.04 vol% NH3, 10 vol% O2, 5 vol% H2O, Ar balance, GHSV 20,400 h−1 | 250–500 (>3 ppm N2O), *200–500 (<5 ppm N2O) | 117 |
| 71 | (2.2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Impregnation, 550 °C, 5 h, air; degreening: 10 vol% H2O, air balance, 650 °C, 4 h; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.036 vol% NO, 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 100,000 h−1 | 175–500 (not shown), *175–400 (not shown) | 118 |
Ion-exchange
In the most widely applied preparation method reported in the literature – ion-exchange, the initial Na-SSZ-13 must also be ion-exchanged with NH4NO3 and Cu2+ salt solutions to obtain Cu-SSZ-13. The NH4+-form has been reported to improve the mobility of Cu2+ ions and promote the ion-exchange rates and levels.47 However, the ion-exchange properties (thus, the nature and location of copper species) are influenced by the synthesis conditions of SSZ-13, such as silica source, aluminium source, n(Si)/n(Al) ratio, organic structure-directing agent, water content, alkalinity, ageing time, crystallization temperature or time,48–50 etc. Furthermore, ion-exchange variables such as the copper precursor, temperature and time are also influencing factors. For example, Gao et al.49 prepared SSZ-13 with different n(Si/)n(Al) ratios (6, 12 and 35) by the traditional synthesis method with N,N,N-trimethyl-1-adamantammonium hydroxide as a structure-directing agent. They proposed that the location of Cu2+ ions and their redox properties can be adjusted through the variation of the n(Si)/n(Al) ratio of Cu-SSZ-13. Moreover, the isolated Cu2+ ions in Cu-SSZ-13 are adjusted by the application of different Cu precursors,30 i.e., in 6MRs for the catalysts prepared with CuCl2 and Cu(CH3COO)2 precursors and in 8MRs for the catalysts prepared with Cu(NO3)2 and CuSO4 precursors. The presence of catalytic centers with different activities can be visualized via the seagull profile, i.e., first, an increase in NO conversion with temperature, followed by a decrease in conversion at intermediate temperatures (ca. 250–350 °C), and finally an increase again at higher temperatures51,52 (Fig. 2b). The seagull profile is often seen for Cu-SSZ-13 with low Cu content (i.e., 0.5–3 wt%),53,54 NH3-SCR-DeNOx with high space velocity, low oxygen concentration (e.g., 2 vol%) as well as in the presence of hydrocarbons.52The ion-exchange properties can also be varied for the micro-/mesoporous materials.55–57 In the case of SSZ-13,58 mesopores with diameters of 2–10 nm were introduced into the zeolite H-SSZ-13 after treatment with NaOH. Cu-containing SSZ-13 with the support treated with an aqueous solution of 0.1 M NaOH showed enhanced activity in NH3-SCR-DeNOx. The use of higher concentrations of NaOH led to a drop in activity. Moreover, lower activity was also reported for the steamed Cu-SSZ-13. Further studies59 proved Cu-containing SSZ-13, with the support treated with 0.1 M aqueous solution to be the most active catalyst in the series (0–0.3 M concentration of NaOH solution). The copper species loading introduced via ion-exchange was comparable for the catalysts with unmodified and post-modified support; however, they varied significantly between both studies. As can be seen from Table 1, pos. 12–50, the amount of introduced copper species varies significantly among the samples, i.e., it is uncontrollable. Furthermore, as the ion-exchange requires successive washing, filtering, drying, calcining and solute recycling procedures, many researchers turn to alternative preparation procedures, e.g., the one-pot hydrothermal method.
One-pot hydrothermal method
Cu-containing SSZ-13 prepared by the one-pot hydrothermal method shows higher content and dispersion of copper species.60 Ren et al.61 designed a one-pot synthesis method for Cu-SSZ-13 (with a low n(Si)/n(Al) ratio of 4) using low-cost copper-tetraethylenepentamine (Cu-TEPA) as a template. The catalyst exhibited superior catalytic activity with more than 80% NO conversion at 150–450 °C. In addition, Martínez-Franco et al.62 used Cu-TEPA and N,N,N-trimethyl-1-adamantamonium (TMAdaOH) in one-pot prepared Cu-SSZ-13 with n(Si)/n(Al) of 14.2 and Cu loading of n(Cu)/n(Si + Al) = 0.059, i.e., controlled ratios and a Cu loading. Furthermore, in another study,63 a n(Si)/n(Al) ratio of 15 was reported to guarantee enhanced NO conversion among n(Si)/n(Al) ratios of 6, 15 and 30. In other studies, the authors mentioned n(Si)/n(Al) of 13.26 (among 6.54–33.12)64 or 13 (among 10.6, 13.0 and 16.0)65 for Cu-SSZ-13 prepared via the one-pot hydrothermal method, and investigated its activity for the NH3-SCR-DeNOx.One of the main drawbacks of the one-pot hydrothermal method appears to be a high loading of copper species (i.e., 6.31–11.27 wt% of Cu) as the result of a large amount of metal ions in the corresponding structure-directing agent61,65,66 (Table 1, pos. 57–62). Unfortunately, these copper ions cannot be completely removed, even after an ion-exchange (i.e., reverse ion-exchange) of Cu-SSZ-13 with an aqueous solution of 1 M NH4NO3 for several times,67 limiting its control. Following these studies, the synthesis was further optimized by decreasing the amount of template required and changing some post-treatment steps (e.g., with dilute HNO3 solution or with HNO3 followed by NH4NO3 solution)60,66,67,110,114,119 or even via the introduction of a second transition metal.111 As a result, materials with a significantly lower amount of copper species (e.g., <3.9 wt%) were achieved, thus, enhancing its activity in NH3-SCR-DeNOx (Fig. 2c). For example, Liu et al.66 reported an effective strategy to regulate the nature and distribution of Cu species of a one-pot synthesized Cu-SSZ-13 zeolite via ion-exchange with an aqueous 0.1 M HNO3 solution (for 4, 8, 12 and 16 h) before removing the template. An optimum time of 4 h guarantees enlarged specific surface area, pore volume, n(Si)/n(Al) ratio and isolated Cu2+ content, and thus enhanced activity and N2 selectivity over Cu-SSZ-13.
Solid-state ion-exchange
Relatively limited studies – compared to Cu-SAPO-34,120 were conducted on Cu-containing SSZ-13 prepared via solid-state ion-exchange (SSIE, Table 1, pos. 63–67). However, it has been reported that SSIE can be carried out either via the physical mixture of CuO and H-SSZ-13 at 700–800 °C (reduction of Cu2+ in CuO to Cu+/Cu0, and reoxidation at the ion-exchange sites of the zeolites)47,115 or ammonia-assisted SSIE (below 350 °C, formation of the [Cu(NH3)2]+ complex via the interaction of Cu2O(111) with H- or NH4+-SSZ-13).121 In the first approach, framework deteriorations and inadequately reacted CuOx was inevitable, while in the second approach the application of ammonia may limit the commercialization of the NH3-assisted SSIE. Moreover, the modification of HT-SSIE (e.g., after 150–500 °C NH3-SCR-DeNOx due to movement of external CuO to Cu+/2+ ions inside the zeolite channels under reaction conditions113), or LT-SSIE (e.g., application of the copper salts that decompose below 300 °C),122 as well as the use of NO and NH3 for the generation of the [Cu(NH3)x]+ (≥2),112 were reported. Furthermore, Zhang et al.47 claimed that the application of the NH4+-form (rather than the H-form) led to the presence of more Cu2+ ions and fewer CuOx species. The formation of [Cu(NH3)x]2+ intermediates (x = 2 or 4) promotes the mobility of Cu2+ species, facilitating its introduction into ion-exchange sites, which was favorable for NH3-SCR-DeNOx (Fig. 2d).Hydrothermal stability and poisoning
NH3-SCR-DeNOx catalysts used in diesel vehicles operate under high temperature and humid conditions alongside poisons derived from biodiesel and lubricant oil additives. The activity and stability of respective Cu-containing SSZ-13 vary in NH3-SCR-DeNOx depending on the applied hydrothermal aging treatment (Table 1). Both the dealumination of the Cu-containing SSZ-13 catalysts, as well as the transformation of ZCuOH sites to Z2Cu, coupled with the consumption of Brønsted acid sites, were reported during the rather mild hydrothermal aging treatment ≤ 700 °C (ZCuOH + ZH → Z2Cu + H2O;69,75,83,91,123 confirmed via DFT calculations;83 promoted by other transition metals44,99). Water molecules tend to attack the aluminum sites (i.e., Brønsted acid sites, –Si–(OH)–Al–), which causes dealumination and consequently structural amorphization (decomposed to SiO2 and Al(OH)3, Fig. 3a).77,124,125 Al(OH)3 possesses a kinetic diameter of 0.503 nm, which means that it cannot escape from the SSZ-13 pores during hydrothermal treatments. Thus, it can be inserted back into the framework during cooldown (i.e., reversible dealumination) to maintain the integrity of the zeolite structure.19 The aluminum sites can be protected by adjusting the n(Si)/n(Al) ratio accordingly, thus accommodating the highly hydrothermally stable Cu2+-2Z sites. The Cu-SSZ-13 catalysts (n(Si)/n(Al) = 6–11, <3 wt%) are reported to be resistant to hydrothermal aging with 10 vol% H2O at 800 °C for 16 h (i.e., comparable to the exposure of a 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-mile vehicle-aged catalyst20,21,92), which is influenced by the copper species loading (Fig. 3b).104 A high copper loading is attributed to the abundancy of [Cu(OH)]+-Z sites that tend to gradually transform to CuOx upon hydrothermal aging, which further destabilizes the zeolite framework.33,80,91 However, the onset of zeolite-framework occurs above 800–900 °C
000-mile vehicle-aged catalyst20,21,92), which is influenced by the copper species loading (Fig. 3b).104 A high copper loading is attributed to the abundancy of [Cu(OH)]+-Z sites that tend to gradually transform to CuOx upon hydrothermal aging, which further destabilizes the zeolite framework.33,80,91 However, the onset of zeolite-framework occurs above 800–900 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21,126 (Fig. 3c). During the hydrothermal aging (>700 °C) of Cu-SSZ-13, [Cu(OH)]+-Z (rather than Cu2+-2Z) convert to CuOx clusters via [Cu(OH)+]-Z → Cu(OH)2 → CuOx sequence, where [Cu(OH)]+-Z is first hydrolyzed to Cu(OH)2, and then the latter agglomerates to form CuOx clusters.91,127,128 Furthermore, CuOx can interact with Al species without the formation of defined structures.129 CuOx clusters lower NH3-SCR-DeNOx selectivity by catalyzing the NH3 oxidation side reactions, as well as actively promoting the degradation of SSZ-13 during aging. It was observed that hydrothermal aging at 800 °C for 16 h led to a significant increase in mesopores ≤ 4 nm, which is believed to result from increased CuOx formation.80,91,127 The hydrothermal treatment causes the zeolite structure to contract in the c direction which then leads to the collapse of the 4MRs126 (Fig. 3d). Despite this, some of the isolated copper species are still protected in the larger rings. Prodinger et al.14 reported higher hydrothermal stability over sub-micron Cu/SSZ-13 compared to Cu/SSZ-13 with particle sizes 10 times bigger.
21,126 (Fig. 3c). During the hydrothermal aging (>700 °C) of Cu-SSZ-13, [Cu(OH)]+-Z (rather than Cu2+-2Z) convert to CuOx clusters via [Cu(OH)+]-Z → Cu(OH)2 → CuOx sequence, where [Cu(OH)]+-Z is first hydrolyzed to Cu(OH)2, and then the latter agglomerates to form CuOx clusters.91,127,128 Furthermore, CuOx can interact with Al species without the formation of defined structures.129 CuOx clusters lower NH3-SCR-DeNOx selectivity by catalyzing the NH3 oxidation side reactions, as well as actively promoting the degradation of SSZ-13 during aging. It was observed that hydrothermal aging at 800 °C for 16 h led to a significant increase in mesopores ≤ 4 nm, which is believed to result from increased CuOx formation.80,91,127 The hydrothermal treatment causes the zeolite structure to contract in the c direction which then leads to the collapse of the 4MRs126 (Fig. 3d). Despite this, some of the isolated copper species are still protected in the larger rings. Prodinger et al.14 reported higher hydrothermal stability over sub-micron Cu/SSZ-13 compared to Cu/SSZ-13 with particle sizes 10 times bigger.
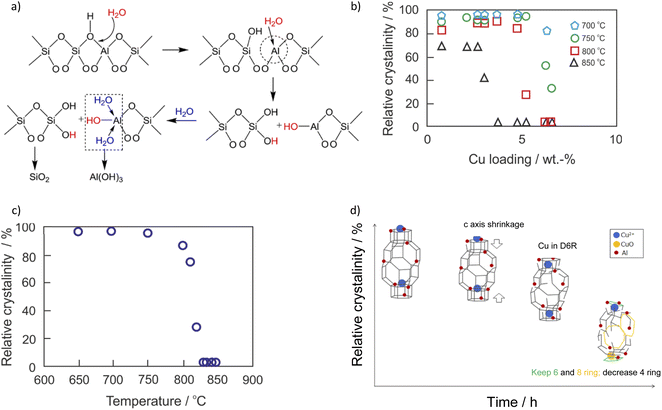 | ||
| Fig. 3 (a) Zeolite hydrolysis process. Reproduced from ref. 77 with permission from ACS Publications, copyright 2015; (b) effect of copper loading on the hydrothermal stability of Cu-SSZ-13. The hydrothermal stability test was conducted at several temperatures for 5 h in flowing air containing 10 vol% H2O. The crystallinity of the solid product was calculated based on the areas on the peaks in the 2θ range from 20° to 32° in its XRD pattern, and Cu-SSZ-13 before hydrothermal aging was chosen as the standard for the crystallinity calculation. Reproduced from ref. 104 with permission from ACS Publications, copyright 2018; (c) (3.6 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6.5) degradation curve by XRD. The hydrothermal stability test was conducted at several temperatures for 5 h in flowing air containing 10 vol% H2O; (d) proposed degradation scheme for Cu-SSZ-13. Reproduced from ref. 126 with permission from ACS Publications, copyright 2019. | ||
Co-cation modification
The introduction of second metal ions into the Cu-containing SSZ-13 catalyst is a facile way to improve its hydrothermal stability. Sodium is a common element found in Cu-SSZ-13 as a result of a strong basic environment provided by NaOH during SSZ-13 synthesis and incomplete exchange between Na-SSZ-13 and NH4NO3 solutions (before Cu-exchange). For example, Gao et al.77 demonstrated that the presence of certain amounts of Na+ (1.78 wt%) or Li+ (0.40 wt%) enhanced the hydrothermal stability of low-Cu loaded (0.87 wt%) Cu-SSZ-13 (n(Si)/n(Al) = 6). Further studies confirmed an optimal loading of 1–1.5 wt% of Na+ in the materials with preserved NOx activity (Fig. 4a).88 The optimized Al-rich (2.7 wt%)Cu–(1.7 wt%)Na-SSZ-13 (n(Si)/n(Al) = 4) catalyst exhibits >80% NO conversion between 200–650 °C in NH3-SCR-DeNOx after hydrothermal aging at 750 °C.82 The Na+ cations were reported to compete with Cu ions for catalytic exchange sites and provide NH3 adsorption function as Lewis acids, as well as neutralize Brønsted acid sites (–Si–(OH)–Al–; thus preventing dealumination during hydrothermal aging).77,88 The K+ cations show effects similar to those of Na+ and the optimal low-temperature activity for Cu, K-SSZ-13 is also found at n(K)/n(Cu) = 0.7.102 Cu2+ ions occupy windows of 6MRs and co-cations occupy windows of 8MRs.102 Furthermore, the hydrothermal stability of (3.4–4.1 wt%)Cu-SSZ-13 was remarkably enhanced through loading with a small amount of cerium (i.e., ca. 0.2–0.4 wt%, optimum ca. 0.35 wt%).104 This can be achieved either via ion-exchange or solid-state ion-exchange. Cerium ions can serve as an oxygen reservoir that can store and release oxygen via the redox shift between Ce4+ and Ce3+ under oxidizing and reducing conditions, which presumably stabilize the zeolite framework. Also, Ce3+ ions tend to fill defect sites of zeolites, where they attract water molecules, reducing the probability of them attacking the aluminum sites. Other cations, like Ca2+, are detrimental at any loading,102 according to the order of poisoning effect: Mg > Ca > Na > K.130 The hydrothermal stability of Cu-SSZ-13 can be further improved via its modification with Fe,94,131 Y,101 Sm132,133 or Zn,109 as well as by the passivation of its surface with Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 or ZrO2.118 Furthermore, the hydrothermal stability of the composite samples, e.g., Cu-SSZ-13 + Fe-SSZ-13,134 H-SAPO-34, Cu-SSZ-13,98 Cu-SSZ-13 and Cu-SAPO-34,135,136 was significantly improved compared to that of the single Cu-SSZ-13.
87 or ZrO2.118 Furthermore, the hydrothermal stability of the composite samples, e.g., Cu-SSZ-13 + Fe-SSZ-13,134 H-SAPO-34, Cu-SSZ-13,98 Cu-SSZ-13 and Cu-SAPO-34,135,136 was significantly improved compared to that of the single Cu-SSZ-13.
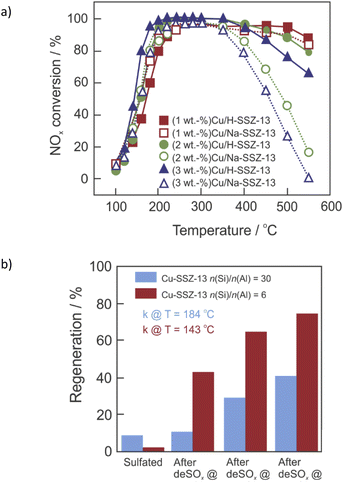 | ||
| Fig. 4 (a) NOx conversion over degreened Cu–H-SSZ-13 and Cu–Na-SSZ-13. Reproduced from ref. 88 with permission from Elsevier, copyright 2020; (b) NH3-SCR-DeNOx activity regeneration after stepwise desulfation at different temperatures based on rate constant measured at T = 143 °C for Cu-SSZ-13 (n(Si)/n(Al) = 6), and T = 184 °C for Cu-SSZ-13 (n(Si)/n(Al) = 30). Reproduced from ref. 137 with permission from ACS Publications, copyright 2018. | ||
Sulfur poisoning
Thus, in addition to their excellent catalytic activity, hydrothermal stability and high resistance against chemical poisons are also highly required properties for these catalysts. As it also happens with hydrothermal stability, [Cu(OH)]+-Z is more susceptible (than Cu2+-2Z) to SO2 poisoning (coming from the burning of sulfurous species in fuel). Sulfur species adsorb on the ZCuOH sites (in the presence and absence of NH3), and exist in bisulfate form (ZCuOH + SO2 → ZCuHSO3). Moreover, ammonium sulfate ((NH4)2SO4) blocks the Z2Cu sites along with Cu sulfate species – CuHSO4 (in the presence of NH3 and SO2) and Cu sulfate (in the presence of only SO2 in the feed).137 The lower mobility of sulfated Cu species compared to non-sulfated Cu species led to a drop in catalyst activity.69 Ammonium sulfate can be removed at 350 °C,138,139 while in the case of other species temperatures higher than 550 °C were needed (i.e., can be removed via regeneration of the diesel particle filter).137,140 Non-decomposed sulfate species block the pore of Cu-SSZ-13, thus decreasing its catalytic activity. However, many researchers showed that NOx conversion can reach its original level after the removal of SO2 and/or H2O from the feed gas, i.e., reversible inactivation.64,84 A combination of other metals (e.g., Zn,141 Ce,142–144 Fe,43,145,146 etc.) with Cu-containing SSZ-13 can adequately protect active sites from sulfur poisoning. Furthermore, Cu-SSZ-13 becomes more robust against sulfur as a result of hydrothermal aging.69Phosphorus poisoning
Besides poisoning by sulfur-containing species, Cu-containing SSZ-13 is also affected by phosphorus and zinc (coming from engine lubricating oil, e.g., zinc dialkyldithiophosphate (ZDDP) as an additive), alkali and alkaline earth metals (e.g., Na, K, Ca, and Mg, coming from engine lubricating oil), noble metals (coming from the upper stream emission abatement components, e.g., diesel oxidation catalysts (DOC), etc.147–149). Lezcano-Gonzalez et al.150 gave an overview of the key deactivation mechanism observed for Ca, Zn, (1–2 wt%) Pt and P. Doping of Cu-SSZ-13 with P (2.2 wt%) fully suppressed the catalytic activity as a result of site blocking of the zeolite framework, CuO formation coupled with the reduction in the number of isolated Cu2+ ions, between other phenomena. In other studies, the catalyst's activity with higher P loading of 0.4–1.0 wt%, was significantly lower than that without P between 100 to 300 °C.96 The N2 selectivity was not affected by phosphorus at low temperatures. However, the introduction of Ca and Zn (1.3–5.4 and 2.2–8.8 wt%, respectively) led mainly to pore-blocking/filling together with the formation of CuO species. It has been widely reported that Pt species promote N2O and NO2 formation.107,150,151 Regarding the P-poisoning studies, Dahlin et al.52 investigated the activity of Cu-SSZ-13 exposed to exhaust generated by a biodiesel burner. Cu-SSZ-13 was deactivated at low temperatures, and could not be regenerated until temperatures up to 550 °C when the phosphorous content in the catalysts was above 0.1 wt%. As the main approaches for introducing P into Cu-containing SSZ-13, impregnation with H3PO4, (NH4)2HPO4,105,108,130 or synthesis of SSZ-13 by using trimethyladamanthylammonium hydroxide (TMAdaOH) and tetramethylphosphonium hydroxide (TEPOH) as dual-template agents116,152 are reported. Further studies on the influence of phosphorus on the catalytic properties of Cu-SSZ-13 for NH3-SCR-DeNOx can be found in ref. 95, 108, 153 and 154. Several researchers suggest that phosphorus tends to poison ZCuOH more easily than Z2Cu.96,155 To regenerate the P-poisoned Cu-SSZ-13 catalysts, phosphorous species in the catalysts should be removed. As an example of that approach, Chen et al.71 successfully applied the combination of washing with hot water (90 °C) and hydrothermal treatment (800 °C for 12 h) to recover the activity of the phosphorous-poisoned Cu-SSZ-13.Hydrocarbons poisoning
The other aspect associated with the application of NH3-SCR-DeNOx is poisoning by hydrocarbons (HCs). The limited DOC activity leads to HCs slipping over the SCR component, which negatively affects its NOx reduction activity. Therefore, few works regarding the HCs poisoning (carbonaceous deposit, including formed aliphatic compounds: acrolein, acetate and acetone, and aromatic compounds: hydrogen-deficient) effect on Cu-SSZ-13 were reported mainly over C3H6 (a representative low-chain HC in the exhaust).76,94 For example, Ma et al.76 found that Cu-SSZ-13 deactivated in the presence of C3H6, due to the competitive adsorption of NH3 and C3H6 on the catalysts as well as blockage of coke in the pore channels. In the case of the aged materials, Wang et al.94 found that Cu-SSZ-13 (aged at 700 °C) displayed a more severe NOx conversion decline than the fresh sample in the presence of C3H6. On the other hand, Zhao et al.97 found that less carbonaceous deposit forms in the hydrothermally aged samples of Cu-SSZ-13 (due to the lower content of Brønsted acid sites). The presence of aromatic compounds increases with higher HTA temperatures (700, 750, and 800 °C) due to a greater generation of mesopores. Based on these studies, aged Cu-SSZ-13 can prevent the deactivation of Cu-containing SSZ-13 due to HCs poisoning, and thus, maintain its NO activity and stability. Other approaches like hybrid catalysts (e.g., Cu-SSZ-13 with CeO2–SnO2)103 were also reported.Reaction mechanisms
NH3-SCR-DeNOx (4NH3 + 4NO + O2 → 4N2 + 6H2O) over Cu-containing SSZ-13 can proceed through the adsorption stage of NH3 and/or NO, according to the Eley–Rideal (E–R; i.e., the gaseous NO reacts with pre-adsorbed NH3 to produce N2 and H2O) and/or Langmuir–Hinshelwood (L–H; both the adsorbed NO and NH3 simultaneously participate in the reaction) mechanisms.23,25,156 For example, the L–H mechanism was proposed over calcined Cu-SSZ-13, while the E–R mechanism was proposed over its aged form (based on in situ DRIFTS analysis). The amount of surface CuO species increased after hydrothermal aging treatment, while the Lewis sites and Brønsted sites (i.e., surface nitrates adsorbed sites and NH3 capacity) decreased, thus, limiting the formation of intermediate products NH4NO3/NH4NO2 (below 350 °C) or NO2(NH4+)2 (above 350 °C), which in turn led to the loss of NH3-SCR-DeNOx activity106 (Fig. 5a).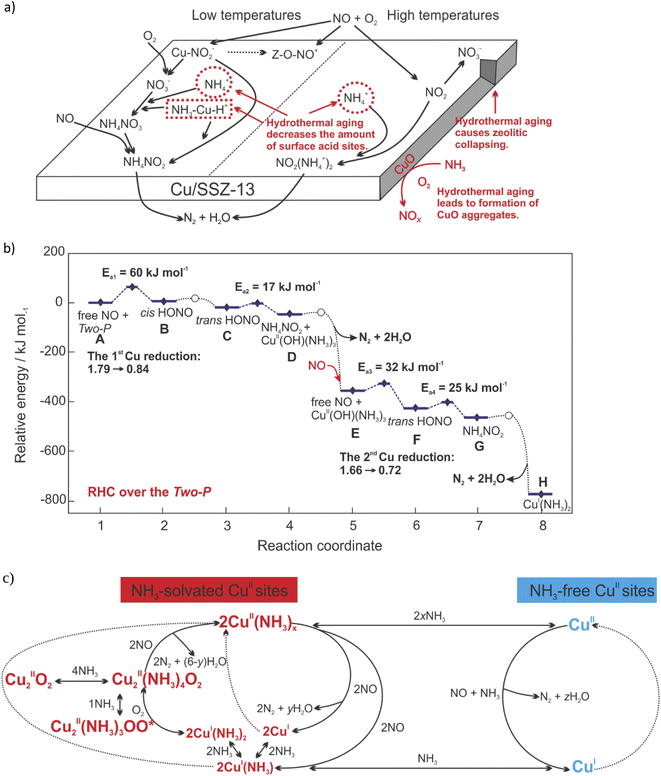 | ||
| Fig. 5 (a) The influence of hydrothermal aging on the NH3-SCR-DeNOx mechanism. Reproduced from ref. 106 with permission from Elsevier, copyright 2019; (b) DFT-computed (HSE06 + D3) energy landscape of LT-RHC over the Two-P. Activation energies and CuII reduction are reported. [CuII(OH)(NH3)3⋯CuII(OH)(NH3)3] stands for the Two-P configuration. Reproduced from ref. 163 with permission from ACS Publications, copyright 2021; (c) Schematic representing the redox of Cu sites during standard NH3-SCR-DeNOx. x represents temperature and CuII speciation-dependent NH3 solvation of CuII sites. x range: {1–2}. y represents CuII speciation-dependent H2O produced upon the reduction of two NH3-solvated CuII sites. y range: {2–4}. z represents CuII speciation-dependent H2O produced upon the reduction of one NH3-free CuII site. z range: {1–2}. Reproduced from ref. 185 with permission from ACS Publications, copyright 2022. | ||
Specifically, NH3-SCR-DeNOx on Cu-SSZ-13 follows a redox reaction mechanism, consisting of a reduction half-cycle (RHC; Cu2+ → Cu+) and an oxidation half-cycle (OHC; Cu+ → Cu2+). The detailed reaction mechanism is still under debate, indicating the complexity of both RHC and OHC.157
Reduction half-cycle
When NH3 is present in the feed, solvation of Z2Cu with NH3 is preferred rather than with H2O,158–160 leading to the formation of Cu–amine coordination complexes, i.e., the principal active sites in the redox cycle.24,161 The NH3 solvation of ZCuOH sites (or [Cu2O2]2+ in their O2 activated form162) may lead to the formation of dimeric or two-proximate Cu-amine complexes (i.e., [CuII(OH)(NH3)3]+ units).163 NH3-solvated Z2Cu sites can hydrolyze to NH3-solvated ZCuOH sites in the presence of H2O.164 Furthermore, the complexes containing a mixture of NH3 and NO, i.e., [CuII(OH)(NH3)n−1(NO)]+, were evidenced for the first time by the application of rapid-scan FT-IR spectroscopy and two-dimensional correlation spectroscopy (2D COS) analysis.165 These solvated Cu moieties were suggested to be active sites in low-temperature NH3-SCR-DeNOx (<250 °C).51,161 The neighboring NH3 and NO ligands rearrange to create N–N bonds via the formation of nitrosamide (NH2NO) or ammonium nitrite (NH4NO2) intermediates.51,161 Subsequently, NH2NO and NH4NO2 decompose into N2 and H2O to complete the reduction part of the NH3-SCR-DeNOx. As an alternative mechanism, Chen et al.166,167 proposed the formation of Cu(H2NNO)2+ and Cu(OHNO)2+ intermediates that decompose over neighboring Brønsted acid sites to complete the catalytic cycle. The formation of the HONO intermediates was reported also by Usberti et al.,168 who found that activated NO reacted with the NH3 adsorbed on the Lewis sites (verified also by DFT calculations). This mechanism consists of two sequential NO oxidative activation processes, consisting of NO oxidative activation to HONO catalyzed by [CuII(OH)(NH3)3]+, HNO reacting with one NH3 ligand to NH4NO2, and NH4NO2 decomposition to N2 and H2O163 (eqn (1)–(6)):| NO + [CuII(OH)(NH3)3⋯CuII(OH)(NH3)3] → HONO + CuI(NH3)3 + CuII(OH)(NH3)3; structure A, Fig. 5b | (1) |
| HONO + CuI(NH3)3 → CuI(NH3)2 + NH4NO2 | (2) |
| NH4NO2 → N2 + 2H2O | (3) |
| NO + CuII(OH)(NH3)3 → HONO + CuI(NH3)3; structure E, Fig. 5b | (4) |
| HONO + CuI(NH3)3 → CuI(NH3)2 + NH4NO2 | (5) |
| NH4NO2 → N2 + 2H2O | (6) |
Summing up the above steps results in a global reaction (eqn (7)):
| 2NO + [CuII(OH)(NH3)3⋯CuII(OH)(NH3)3] → 2CuI(NH3)3 + 2N2 + 4H2O | (7) |
Thus, Hu et al.163 and Gramigni et al.169 proved (based on kinetic experimental and DFT calculations) a second-order dependence, suggesting that binuclear reactions between two Cu species could occur during RHC.
The decomposition of NH4NO3 (NH4NO2 can be oxidized into NH4NO3) allows also the side reaction leading to undesired N2O (<300 °C).89 On the other hand, Feng et al.170 claimed that the formation of N2O resulted from the H2NNO decomposition over Cu–OOH–Cu complexes, which explains the enhanced N2O formation with increasing Cu loading. This was further supported by the studies of Xi et al.,70 who claimed that N2O formation occur over Cu-oxy species, such as [Cu–O2–Cu]2+. However, N2O formation has also been reported to mainly occur over [Cu(OH)+] located in CHA cages.89,171–173 The high-temperature N2O production (>300 °C), on the other hand, takes place because of unselective ammonia oxidation. The N2O production profiles of the different types of SCR catalysts differ considerably.117
Below 250 °C, a linear SCR rate versus (Cu loading)2 correlation was reported, suggesting that the reaction limiting step involves the participation of two Cu ions.54,161 The activation energy for NH3-SCR-DeNOx over Cu-containing SSZ-13 varies in the range of 32,106 40–41 kJ mol−1,54,174 to 43–57 kJ mol−1,106 also depending on the temperature, i.e., 175–250 °C – 130 kJ mol−1, and 250–300 °C – 60 kJ mol−1.32 Thus, at temperatures above 350 °C, the active sites of Cu-SSZ-13 change from mobile Cu ions coordinated by NH3 into immobilized Cu ions, thus, giving a seagull profile for NO conversion (based on the Gao et al.32 assumptions). Fahami et al.53 attributed the activity decrease at around 350 °C to a more localized structure of mono(μ-oxo)dicopper complexes. At high temperatures (above 350 °C), the activity has instead been measured to have a first-order dependence on copper loading, indicating that the reaction can proceed with other possible Cu sites.54
Oxidation half-cycle
The [Cu(NH3)2]+ complexes become the intermediate starting the oxidation half-cycle. The two [CuI(NH3)2]+ intermediates and an oxygen molecules then combine to form [CuI(NH3)2]+–O2–[CuI(NH3)2]+ intermediates (2[CuI(NH3)2]+ + O2 → [CuI(NH3)2]+–O2–[CuI(NH3)2]+).51,161,175 The mobility of the Cu ions (i.e., one [CuI(NH3)2]+ must migrate to the vicinity of another; enhanced in the presence of H2O176–178) guarantees the formation of these dimeric Cu species,90,166 thus improving the NH3-SCR-DeNOx activity. The 1Al–Cu species (located at isolated single Al sites, i.e., ZCuOH) can form dimeric Cu intermediates more easily than the 2Al–Cu (located at two adjacent Al sites, i.e., Z2Cu).179,180 The ratio of n(1Al–Cu)/n(2Al–Cu) can be controlled through the catalyst's hydrothermal aging.90 Krishna et al.181 suggested their perspective that spatial proximity of active Cu sites is required in the redox cycle of Cu2+/Cu+. Such proximity assists the oxidation of NH3-solvated Cu+ ions to form dimeric Cu intermediates [CuI(NH3)2]+–O2–[CuI(NH3)2]+.51 The structure of mobile dicopper(II) complexes vary depending on the n(Si)/n(Al) ratio of the zeolite host.182 Finally, the newly formed [CuII(NH3)2]2+–O–[CuII(NH3)2]2+ hydrolyzes in the presence of H2O, restoring two [CuI(OH)(NH3)2]+ adducts. O2 dissociation and NO2 formation occur on complexes in the presence of NO ([CuI(NH3)2]+–O2–[CuI(NH3)2]+ + NO → [CuII(NH3)2]2+–O2−–[CuII(NH3)2]2+ + NO2). Negri et al.183 proposed (based on combined spectroscopic and DFT studies) that these intermediates are CuII2(NH3)4O2 complexes with a side on μ-η2,η-peroxo diamino dicopper(II) structure. Besides that, the intermediates were approved via DFT calculations and their formation was spectroscopically approved via fiber-optic DR UV-Vis spectroscopy during NH3-SCR-DeNOx over Cu-containing zeolites.184 However, the mechanism associated with the reduction of Z2Cu2(NH3)4O2 complexes is still unclear. As mentioned above, Paolucci et al.180 claimed that the [CuII(NH3)2]2+–O–[CuII(NH3)2]2+ hydrolyzes in the presence of H2O, restoring two [CuI(OH)(NH3)2]+ adducts, thus completing the low-temperature SCR catalytic cycle. However, upon exposure of Z2Cu2(NH3)4O2 complexes to NH3, the formation of unreactive superoxo amino ZCu(NH3)3OO* complexes was observed183,185 (Fig. 5c). Furthermore, the reaction mechanism changed over field aged (including hydrothermal aging and sulfur aging) Cu-SSZ-13, i.e., lower mobility of the Cu ions coordinated to sulfur-related species led to decreased formation of Cu-dimers necessary for the OHC. The field aging has a minimal impact on RHC.72Selective ammonia oxidation (NH3-SCO)
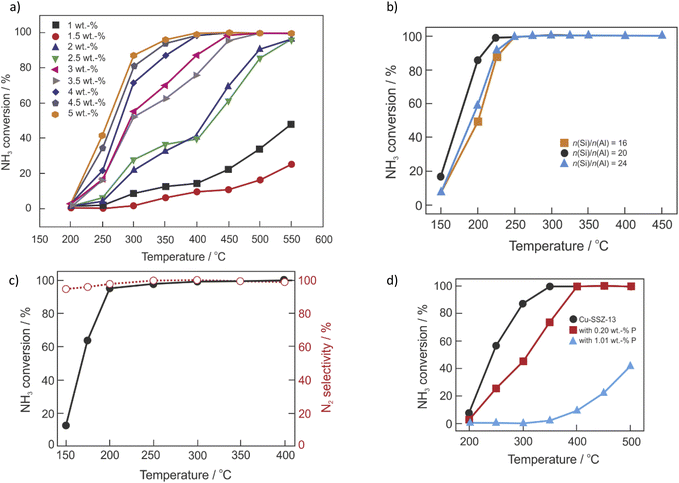
To achieve the desired NOx conversion, a stoichiometric or even an excess quantity of NH3 is required, which can result in unreacted NH3 (also known as NH3 slip). Thus, oxidation catalysts (ASC, guard catalysts, AMOX) are usually employed to selectively oxidize the unreacted NH3 (from NH3-SCR-DeNOx) into nitrogen and water vapor. Some researchers investigate NH3-SCO (4NH3 + 3O2 → 2N2 + 6H2O) as a side process of the NH3-SCR-DeNOx, while, there are not many publications dedicated separately to the ammonia oxidation over Cu-containing CHA.38,186,187 Similar to NH3-SCR-DeNOx, NH3-SCO is enhanced over Cu-containing catalysts, including zeolite-based catalysts (e.g., Cu-ZSM-5, Cu–Y, Cu-Beta, etc.).38,188 The aggregated CuOx species lead to higher NH3 oxidation,115 thus, across investigated catalytic systems, mainly 10 wt% of Cu loading was found as the optimal.188,189 Progress on selective catalytic ammonia oxidation over Cu-containing zeolite-based catalysts was already published by Jabłońska in 2020.38 Furthermore, NH3-SCO over different catalysts, including proposed reaction mechanisms were reviewed.188–190 Thus, in this chapter, there is a focus on the Cu-containing SSZ-13 based-catalysts prepared via different techniques i.e., containing different amounts of Cu species. Table 2 lists representative results of NH3-SCO over Cu-containing SSZ-13 reported in the literature. For example, Gao et al.32 found that NH3 conversion increases with higher Cu loading (exchange level 23–90%), which possibly arises from the weaker interactions between Cu2+ ions and the SSZ-13 framework (i.e., more facile Cu2+ ↔ Cu+ redox-cycling) or higher amount of Cu2+ ions placed closer to the pore openings (being more accessible to reactants). Similar to the NH3-SCR-DeNOx, the authors observed the seagull profile of NH3 conversion, which was later also approved by Olsson et al.191,192 These two regimes, below and above 250 °C (400 °C in ref. 191) with different values of activation energy over Cu-SSZ-13 were attributed to the change in the catalytic centers resulting in a different activity. Cui et al.88 reported a higher activity for Cu/SSZ-13 with increasing Cu loading up to 5 wt% (Fig. 6a). After hydrothermal aging (at 800 °C for 16 h) the full NH3 conversion is shifted about 50 °C higher (Table 2, pos. 10). In another example, NH3 oxidation activity decreases with increasing HTA temperature (550–900 °C). The activity decrease was assigned to the loss of active isolated Cu2+ species (active below 400 °C) during aging, and a decrease in NH3 storage capacity.91 Both Cu2+ ions and CuO coexist in Cu/SSZ-13 (n(Si)/n(Al) = 16–24) prepared by impregnation.193 Enhanced activity over Cu/SSZ-13 with n(Si)/n(Al) = 20 (activation energy of 68 kJ mol−1) resulted from the highest content of CuO species and acid sites (Fig. 6b). Contrary to that, Han et al.80 reported no significant changes for Cu-SSZ-13 with a n(Si)/n(Al) ratio of 5.5 (among 11, 21) in NH3 conversion for the whole temperature range. However, the NOx conversion slightly increased from 10 to 20 ppm at 625 °C after hydrothermal aging at 800 °C. Based on the in situ DRIFTS, the authors claimed that NH3-SCO over Cu/SSZ-13 follows the in situ/internal SCR (i-SCR) mechanism, consisting of two steps. In the first step, NH3 was oxidized to NOx by surface CuO. Subsequently, NOx was reduced to N2 and H2O by unreacted NH3 on isolated Cu2+ sites (i.e., NH3-SCR-DeNOx).193 Thus, Cu-SSZ-13 revealed higher NH3 oxidation than CuO/SSZ-13 (CuO mechanically mixed with SSZ-13).87 A similar conclusion about the reaction mechanism was given over Cu-SSZ-13 (prepared by one-pot hydrothermal synthesis) treated with dilute HNO3 solution.186 The reaction mechanism was assigned based on the in situ DRIFTS studies, excluding the hydrazine mechanism (as the N2H4 species were not observed in the spectra). Details on the reaction mechanisms can be found elsewhere.188,189,194 The one-pot synthesized Cu-SSZ-13 treated with dilute HNO3 possessed added Cu2+ ions that accelerated low-temperature NH3-SCR-DeNOx (second step of NH3-SCO). Thus, enhanced activity and N2 selectivity over this catalyst were reported (Fig. 6c) compared to non-modified zeolite-based catalysts. One-pot hydrothermally prepared Cu-SSZ-13 (n(Si)/n(Al) = 6.5–12.5, 1.8–3.5 wt% of Cu) did not show significant changes before and after the hydrothermal aging treatment (Table 2, pos. 12) in the whole temperature range.106 However, Luo et al.74 have shown that NH3 oxidation activity of commercial Cu-SSZ-13 (detail of composition or preparation not shown) decreases with increased temperature up to 800 °C. Cu-SSZ-13 – prepared via the solid-state ion-exchange method at 700–800 °C, did not reach full conversion.115 On the other hand, the enhanced activity of bifunctional catalysts was reported, with single examples given as mixed, dual-layer and hybrid layer designs composed of Pt/Al2O3 and Cu-SSZ-13,196–198 or Ru/Cu-SSZ-13.199 Similar to Cu-containing SSZ-13 applied in NH3-SCR-DeNOx, also the catalysts applied in NH3-SCO lost their activity after poisoning, e.g., with P96,105 (Fig. 6d). Catalyst activity decreased remarkably with P loading (0.26–1.21 wt%) and especially above 300 °C, because of the interaction between phosphorus with oligomeric CuxOy species inside the large cages. As these species are not active in NH3-SCR-DeNOx the effect is less pronounced, as reported before.105| Sample | Preparation method | Reaction conditions | Operation temperature for achieving 100% NH3 conversion/°C ((by-)product formation) | Ref. | |
|---|---|---|---|---|---|
| 1 | (2.4 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 15) monolith catalyst | Commercial; degreening: 10 vol% O2, 7 vol% H2O, 8 vol% CO2, Ar balance, 550 °C, 4 h | 0.02 vol% NH3, 5 vol% O2, 5 vol% H2O, Ar balance, GHSV 40,000 h−1 | 400–450 (not shown) | 72 |
| 2 | Cu-SSZ-13 (Cu loading, n(Si)/n(Al) not shown) monolith catalyst | Commercial; degreening: 10 vol% O2, 7 vol% H2O, 8 vol% CO2, N2 balance, 550–600 °C, 4 h | 0.02 vol% NH3, 10 vol% O2, 5 vol% H2O, Ar balance, GHSV 40,000 h−1 | 450–550 (<20 ppm NOx) | 74 |
| 3 | (2.7 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Commercial; degreening: 10 vol% H2O, air balance, 650 °C, 12 h; pre-treatment: 21 vol% O2, N2 balance, 600 °C, 20 min | 0.05 vol% NH3, 5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 500–600 (<20 ppm N2O) | 73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Ion-exchange | |||||
| 4 | Cu-SSZ-13 (n(Si)/n(Al) = 6) (Cu content not shown) | Ion-exchange, calcination, 500 °C, 2 h, air | 0.035 vol% NH3, 14 vol% O2, 2 vol% H2O, N2 balance, GHSV 30,000 h−1 | 500–550 (not shown) | 11 |
| 5 | (0.88 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 25) | Ion-exchange, calcination, 550 °C, 4 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.05 vol% NH3, 5 vol% O2, N2 balance, GHSV 400,000 h−1 | 500–600 (not shown) | 85 |
| 6 | (2.1 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Ion-exchange, calcination conditions not shown; *hydrothermal treatment: 10 vol% H2O, air balance, 550 °C, 16 h | 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 200,000 h−1 | 500–500 (not shown), *500–550 (not shown) | 91 |
| 7 | (2.35 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12.93) | Ion-exchange, calcination, 550 °C, 4 h, air; pre-treatment: 8 vol% O2, N2 balance, 1 h, 500 °C | 0.05 vol% NH3, 6.5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 350–500 (not shown) | 96 |
| 8 | (1.88–2.16 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 16.5–20.3) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h; pre-treatment: 21 vol% O2, N2 balance, 550 °C, 20 min | 0.05 vol% NH3, 5 vol% O2, 3 vol% H2O, N2 balance, GHSV 120,000 h−1 | 400–550 (<10 ppm NO), *500–550 (>20 ppm NO) | 87 |
| 9 | (4 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 11) | Ion-exchange, calcination conditions not shown; degreening: Reaction conditions, 600 °C, 4 h; pre-treatment: 10 vol% O2, 7 vol% H2O, N2 balance, 600 °C, 1 h | 0.035 vol% NH3, 10 vol% O2, 7 vol% H2O, N2 balance, GHSV 300,000 h−1 | 400–550 (<120 ppm NO2) | 89 |
| 10 | (4.5–5 wt%)SSZ-13 (n(Si)/n(Al) = 9) | Ion-exchange, calcination, 550 °C, 5 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h; **degreening: 10 vol% H2O, air balance, 700 °C, 4 h | 0.038 vol% NH3, 14 vol% O2, N2 balance, GHSV 100,000 h−1 | *450–550 (not shown), **400–550 (not shown) | 88 |
| 11 | (4.76 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 4.97) | Ion-exchange, vacuum evaporator, calcination, 550 °C, 6 h, air | 0.1 vol% NH3, 6 vol% O2, He balance, GHSV 300,000 h−1 | 400–550 (<3% NOx, N2O yield) | 195 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| One-pot hydrothermal synthesis | |||||
| 12 | (3.5 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6.5) | One-pot hydrothermal synthesis, HNO3 treatment, calcination, 600 °C, 6 h, air; *hydrothermal treatment: 10 vol% H2O, air balance, 750 °C, 16 h | 0.05 vol% NH3, 5 vol% O2, N2 balance, GHSV 400,000 h−1 | 550–600 (not shown), *550–600 (not shown) | 106 |
| 13 | (6.38 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 3.77) | One-pot hydrothermal synthesis, HNO3 treatment, calcination, 550 °C, 8 h, air | 0.05 vol% NH3, 5 vol% O2, N2 balance, *0.05 vol% NH3, 5 vol% O2, 5 vol% H2O, N2 balance, GHSV 160,000 h−1 | 250–400 (>90% N2 selectivity), *300–400 (>90% N2 selectivity) | 186 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Impregnation | |||||
| 14 | (2.2 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 12) | Impregnation, 550 °C, 5 h, air; degreening: 10 vol% H2O, air balance, 650 °C, 4 h; *hydrothermal treatment: 10 vol% H2O, air balance, 800 °C, 16 h | 0.036 vol% NH3, 14 vol% O2, 2.5 vol% H2O, N2 balance, GHSV 100,000 h−1 | 450–550 (not shown), *450–550 (not shown) | 118 |
| 15 | (4.1 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 6) monolith catalyst | Ion-exchange, calcination, 600 °C, 4 h, air | 0.04 vol% NH3, 8 vol% O2, 5 vol% H2O, Ar balance, GHSV 22,100 h−1 | 450–500 (<3 ppm N2O) | 105 |
| 16 | (10 wt%)Cu-SSZ-13 (n(Si)/n(Al) = 10) | Impregnation, calcination, 550 °C, 6 h, air | 0.04 vol% NH3, 10 vol% O2, N2 balance, WHSV 300,000 ml g−1 h−1 | 225–450 (>80% N2 selectivity) | 193 |
 | ||
| Fig. 6 (a) NH3 conversion over degreened Cu/H-SSZ-13. Reproduced from ref. 88 with permission from Elsevier, copyright 2020; (b) NH3 conversion over Cu/SSZ-13 with different n(Si)/n(Al) ratios. Reproduced from ref. 193 with permission from Elsevier, copyright 2020; (c) NH3 conversion over Cu-SSZ-13 post-modified with a solution of HNO3. Reproduced from ref. 186 with permission from ACS Publications, copyright 2018; (d) NH3 conversion over fresh and P-poisoned Cu-SSZ-13. Reproduced from ref. 96 with permission from ACS Publications, copyright 2021. | ||
Conclusions and perspectives
This review summarizes recent progress in the NH3-SCR-DeNOx and the NH3-SCO over Cu-containing SSZ-13 catalysts, concerning their preparation methods, hydrothermal stability and poisoning, as well as their reaction mechanisms. Various preparation methods have been applied to manipulate the nature and distribution of Cu species in the materials, with continuous efforts aiming at improving the (hydrothermal) stability of the Cu-CHA catalysts. Minimizing [Cu(OH)]+-Z sites, which are more susceptible to hydrothermal aging, sulfur or phosphorus poisoning than Cu2+-2Z, will be a challengeable task in the following years. Determining the effect of (new) dopants on catalyst stability is a promising research direction. Furthermore, more work should be spent on the application of the degreening and treatment/poisoning strategies closely related to the industrial applications (i.e., degreening at 700 °C in 10 vol% O2, 5 vol% of CO2 and H2O, followed by the aging at 800 °C for 50 h for diesel exhaust applications).200 Besides enhanced activity and stability, N2 selectivity also indicates the commercialization of catalysts. Despite recent findings, an accurate and experimentally established standard NH3-SCR-DeNOx reaction mechanisms at low temperatures including field aged materials is still missing. Thus, more detailed in situ and operando studies, transient kinetic investigations, as well as molecular-scale computational models, are required to further understand reaction mechanisms.Furthermore, compared to NH3-SCR-DeNOx, the studies of NH3-SCO are rather scarce. Mainly, ammonia oxidation is considered as the side process of NH3-SCR-DeNOx above 350–400 °C. The catalytic experiments have been conducted under conditions, which differ far from the ones existing in the diesel aftertreatment system, i.e., minor NH3 slip (O2 excess), presence of H2O, COx or SOx, etc. Besides, key requirements comprehend high activity and N2 selectivity up to 600–700 °C (in the cycle of diesel particulate filter regeneration) and stability under application-relevant reaction conditions. A limited amounts of studies focus on the investigation of the reaction mechanisms. Furthermore, there are no systematic studies on the development of bifunctional catalysts. As NH3-SCO proceeds mainly according to the i-SCR mechanism (i.e., with NH3-SCR-DeNOx as the second step), similar reaction paths can be potentially ascribed for a description of the activity of Cu-containing SSZ-13. Thus, more effort must be spent on studying NH3-SCO reaction mechanisms in the future. Systematic studies (i.e., careful design, appropriate coupling techniques, etc.) should also cover bifunctional catalysts composed of Cu-containing SSZ-13 (NH3-SCR-DeNOx function) and noble metal-based catalyst (NH3 oxidation function). A collective understanding of reaction and deactivation mechanisms of both components allows for the design of high-performing NH3-SCO catalysts with appropriate catalyst promotion strategies. Thus, the development of such hybrid catalysts (in monolith form) for diesel exhaust aftertreatment systems is a topic of interest.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. J. acknowledges a DFG Research Grant JA 2998/2-1 and support from Universität Leipzig for Open Access Publishing.References
- R. Chen, T. Zhang, Y. Guo, J. Wang, J. Wei and Q. Yu, Chem. Eng. J., 2021, 420, 127588–127631 CrossRef CAS.
- M. Jabłońska and L. Chmielarz, Zesz. Nauk., 2013, 7, 7–23 Search PubMed.
- T. Boningari and P. G. Smirniotis, Curr. Opin. Chem. Eng., 2016, 13, 133–141 CrossRef.
- C. Rusznak, S. Jenkins, P. R. Mills, R. J. Sapsford, J. L. Devalia and R. J. Davies, Rev. Fr. d'Allergologie d'Immunologie Clin., 1998, 38, S80–S90 CrossRef.
- X. Wang, Y. Xu, Z. Zhao, J. Liao, C. Chen and Q. Li, Fuel, 2021, 305, 121482–121501 CrossRef CAS.
- T. Andana, K. G. Rappé, F. Gao, J. Szanyi, X. Pereira-Hernandez and Y. Wang, Appl. Catal., B, 2021, 120054–120079 CrossRef CAS.
- Y. Zeng, K.-G. Haw, Y. Wang, S. Zhang, Z. Wang, Q. Zhong and S. Kawi, ChemCatChem, 2021, 13, 491–505 CrossRef CAS.
- M. Iwamoto, H. Furukawa, Y. Mine, F. Uemura, S. Mikuriya and S. Kagawa, J. Chem. Soc., Chem. Commun., 1986, 1272–1273 RSC.
- A. Wang, Y. Chen, E. D. Walter, N. M. Washton, D. Mei, T. Varga, Y. Wang, J. Szanyi, Y. Wang, C. H. F. Peden and F. Gao, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- Q. Ye, L. Wang and R. T. Yang, Appl. Catal., A, 2012, 427, 24–34 CrossRef.
- J. H. Kwak, R. G. Tonkyn, D. H. Kim, J. Szanyi and C. H. F. Peden, J. Catal., 2010, 275, 187–190 CrossRef CAS.
- D. W. Fickel and R. F. Lobo, J. Phys. Chem. C, 2010, 114, 1633–1640 CrossRef CAS.
- C. Baerlocher, L. B. McCusker and D. H. Olson, Atlas of zeolite framework types, Elsevier, 2007, pp. 96–97 Search PubMed.
- S. Prodinger, M. A. Derewinski, Y. Wang, N. M. Washton, E. D. Walter, J. Szanyi, F. Gao, Y. Wang and C. H. F. Peden, Appl. Catal., B, 2017, 201, 461–469 CrossRef CAS.
- S. I. Zones, US Pat. 4544538, 1985.
- B. Chen, R. Xu, R. Zhang and N. Liu, Environ. Sci. Technol., 2014, 48, 13909–13916 CrossRef CAS PubMed.
- R. Xu, R. Zhang, N. Liu, B. Chen and S. Zhang Qiao, ChemCatChem, 2015, 7, 3842–3847 CrossRef CAS.
- X. Wang, Q. Wu, C. Chen, S. Pan, W. Zhang, X. Meng, S. Maurer, M. Feyen, U. Müller and F.-S. Xiao, Chem. Commun., 2015, 51, 16920–16923 RSC.
- D. W. Fickel, E. D'Addio, J. A. Lauterbach and R. F. Lobo, Appl. Catal., B, 2011, 102, 441–448 CrossRef CAS.
- J. H. Kwak, D. Tran, S. D. Burton, J. Szanyi, J. H. Lee and C. H. F. Peden, J. Catal., 2012, 287, 203–209 CrossRef CAS.
- S. J. Schmieg, S. H. Oh, C. H. Kim, D. B. Brown, J. H. Lee, C. H. F. Peden and D. H. Kim, Catal. Today, 2012, 184, 252–261 CrossRef CAS.
- Y. Shan, J. Du, Y. Zhang, W. Shan, X. Shi, Y. Yu, R. Zhang, X. Meng, F.-S. Xiao and H. He, Natl. Sci. Rev., 2021, 8, nwab010 CrossRef CAS PubMed.
- J. Wang, H. Zhao, G. Haller and Y. Li, Appl. Catal., B, 2017, 202, 346–354 CrossRef CAS.
- F. Gao and F. Peden, Catalysts, 2018, 18, 140–163 CrossRef.
- A. M. Beale, F. Gao, I. Lezcano-Gonzalez, C. H. F. Peden and J. Szanyi, Chem. Soc. Rev., 2015, 44, 7371–7405 RSC.
- Y. Xin, Q. Li and Z. Zhang, ChemCatChem, 2018, 10, 29–41 CrossRef CAS.
- S. Zhang, L. Pang, Z. Chen, S. Ming, Y. Dong, Q. Liu, P. Liu, W. Cai and T. Li, Appl. Catal., A, 2020, 607, 117855–117876 CrossRef CAS.
- J. H. Kwak, H. Zhu, J. H. Lee, C. H. F. Peden and J. Szanyi, Chem. Commun., 2012, 48, 4758–4760 RSC.
- F. Giordanino, P. N. R. Vennestrøm, L. F. Lundegaard, F. N. Stappen, S. Mossin, P. Beato, S. Bordiga and C. Lamberti, Dalton Trans., 2013, 42, 12741–12761 RSC.
- X. Wang, Y. Xu, M. Qin, Z. Zhao, X. Fan and Q. Li, J. Colloid Interface Sci., 2022, 622, 1–10 CrossRef CAS PubMed.
- S. T. Korhonen, D. W. Fickel, R. F. Lobo, B. M. Weckhuysen and A. M. Beale, Chem. Commun., 2011, 47, 800–802 RSC.
- F. Gao, E. D. Walter, E. M. Karp, J. Luo, R. G. Tonkyn, J. H. Kwak, J. Szanyi and C. H. F. Peden, J. Catal., 2013, 300, 20–29 CrossRef CAS.
- Y. J. Kim, J. K. Lee, K. M. Min, S. B. Hong, I.-S. Nam and B. K. Cho, J. Catal., 2014, 311, 447–457 CrossRef CAS.
- A. Godiksen, F. N. Stappen, P. N. R. Vennestrøm, F. Giordanino, S. B. Rasmussen, L. F. Lundegaard and S. Mossin, J. Phys. Chem. C, 2014, 118, 23126–23138 CrossRef CAS.
- G. Centi and S. Perathoner, Appl. Catal., A, 1995, 132, 179–259 CrossRef CAS.
- S. A. Bates, A. A. Verma, C. Paolucci, A. A. Parekh, T. Anggara, A. Yezerets, W. F. Schneider, J. T. Miller, W. N. Delgass and F. H. Ribeiro, J. Catal., 2014, 312, 87–97 CrossRef CAS.
- A. A. Verma, S. A. Bates, T. Anggara, C. Paolucci, A. A. Parekh, K. Kamasamudram, A. Yezerets, J. T. Miller, W. N. Delgass, W. F. Schneider and F. H. Ribeiro, J. Catal., 2014, 312, 179–190 CrossRef CAS.
- M. Jabłońska, ChemCatChem, 2020, 12, 4490–4500 CrossRef.
- J. Dědeček, B. Wichterlova and P. Kubat, Microporous Mesoporous Mater., 1999, 32, 63–74 CrossRef.
- M. Zamadics, X. Chen and L. Kevan, J. Phys. Chem., 1992, 96, 2652–2657 CrossRef CAS.
- X. Yang, Z. Wu, M. Moses-Debusk, D. R. Mullins, S. M. Mahurin, R. A. Geiger, M. Kidder and C. K. Narula, J. Phys. Chem. C, 2012, 116, 23322–23331 CrossRef CAS.
- T. Zhang, J. Li, J. Liu, D. Wang, Z. Zhao, K. Cheng and J. Li, AIChE J., 2015, 61, 3825–3837 CrossRef CAS.
- Y. Wang, L. Xie, F. Liu and W. Ruan, J. Environ. Sci., 2019, 81, 195–204 CrossRef CAS PubMed.
- Y. Yue, B. Liu, P. Qin, N. Lv, T. Wang, X. Bi, H. Zhu, P. Yuan, Z. Bai, Q. Cui and X. Bao, Chem. Eng. J., 2020, 398, 125515–125527 CrossRef CAS.
- J. Du, J. Wang, X. Shi, Y. Shan, Y. Zhang and H. He, Catalysts, 2020, 10, 1375–1389 CrossRef CAS.
- Z. Wang, X. Xu, Y. Zhu, H. He, N. Wang, X. Yang and L. Liu, Microporous Mesoporous Mater., 2022, 333, 111720–111729 CrossRef CAS.
- S. Zhang, J. Chen, Y. Meng, L. Pang, Y. Guo, Z. Luo, Y. Fang, Y. Dong, W. Cai and T. Li, Appl. Surf. Sci., 2022, 571, 151328–151341 CrossRef CAS.
- D. He, Z. Wang, D. Deng, S. Deng, H. He and L. Liu, Mol. Catal., 2020, 484, 110738–110748 CrossRef CAS.
- F. Gao, N. M. Washton, Y. Wang, M. Kollár, J. Szanyi and C. H. F. Peden, J. Catal., 2015, 331, 25–38 CrossRef CAS.
- Z. Liu, T. Wakihara, K. Oshima, D. Nishioka, Y. Hotta, S. P. Elangovan, Y. Yanaba, T. Yoshikawa, W. Chaikittisilp, T. Matsuo, T. Takewaki and T. Okubo, Angew. Chem., 2015, 127, 5775–5779 CrossRef.
- F. Gao, D. Mei, Y. Wang, J. Szanyi and C. H. F. Peden, J. Am. Chem. Soc., 2017, 139, 4935–4942 CrossRef CAS PubMed.
- S. Dahlin, J. Englund, H. Malm, M. Feigel, B. Westerberg, F. Regali, M. Skoglundh and L. J. Pettersson, Catal. Today, 2021, 360, 326–339 CrossRef CAS.
- A. R. Fahami, T. Günter, D. E. Doronkin, M. Casapu, D. Zengel, T. H. Vuong, M. Simon, F. Breher, A. V Kucherov, A. Brückner and J.-D. Grunwaldt, React. Chem. Eng., 2019, 4, 1000–1018 RSC.
- F. Gao, E. D. Walter, M. Kollar, Y. Wang, J. Szanyi and C. H. F. Peden, J. Catal., 2014, 319, 1–14 CrossRef CAS.
- M. Rutkowska, I. Pacia, S. Basąg, A. Kowalczyk, Z. Piwowarska, K. A. Duda Michałand Tarach, K. Góra-Marek, M. Michalik, U. Díaz and L. Chmielarz, Microporous Mesoporous Mater., 2017, 246, 193–206 CrossRef CAS.
- R. S. R. Suharbiansah, K. Pyra, M. Liebau, D. Poppitz, K. Góra-Marek, R. Gläser and M. Jabłońska, Microporous Mesoporous Mater., 2022, 334, 111793–111804 CrossRef CAS.
- M. Jabłońska, K. Góra-Marek, M. Grilc, P. C. Bruzzese, D. Poppitz, K. Pyra, M. Liebau, A. Pöppl, B. Likozar and R. Gläser, Catalysts, 2021, 11, 843–870 CrossRef.
- R. Oord, I. C. Ten Have, J. M. Arends, F. C. Hendriks, J. Schmidt, I. Lezcano-Gonzalez and B. M. Weckhuysen, Catal. Sci. Technol., 2017, 7, 3851–3862 RSC.
- G. Wu, S. Liu, Z. Chen, Q. Yu, Y. Chu, H. Xiao, H. Peng, D. Fang, S. Deng and Y. Chen, J. Taiwan Inst. Chem. Eng., 2022, 134, 104355–104367 CrossRef CAS.
- H. Jiang, B. Guan, X. Peng, R. Zhan, H. Lin and Z. Huang, Chem. Eng. J., 2020, 379, 122358–122373 CrossRef.
- L. Ren, L. Zhu, C. Yang, Y. Chen, Q. Sun, H. Zhang, C. Li, F. Nawaz, X. Meng and F.-S. Xiao, Chem. Commun., 2011, 47, 9789–9791 RSC.
- R. Martínez-Franco, M. Moliner, J. R. Thogersen and A. Corma, ChemCatChem, 2013, 5, 3316–3323 CrossRef.
- C. Fan, Z. Chen, L. Pang, S. Ming, X. Zhang, K. B. Albert, P. Liu, H. Chen and T. Li, Appl. Catal., A, 2018, 550, 256–265 CrossRef CAS.
- J. Han, X. Jin, C. Song, Y. Bi, Q. Liu, C. Liu, N. Ji, X. Lu, D. Ma and Z. Li, Green Chem., 2020, 22, 219–229 RSC.
- J. Feng, D. Shi, Z. Xu, J. Wang, Y. Wang and X. Li, Russ. J. Phys. Chem. A, 2020, 94, 1797–1803 CrossRef.
- B. Liu, N. Lv, C. Wang, H. Zhang, Y. Yue, J. Xu, X. Bi and X. Bao, Chin. J. Chem. Eng., 2022, 41, 329–341 CrossRef.
- L. Xie, F. Liu, L. Ren, X. Shi, F.-S. Xiao and H. He, Environ. Sci. Technol., 2014, 48, 566–572 CrossRef CAS PubMed.
- L. Ma, Y. Cheng, G. Cavataio, R. W. McCabe, L. Fu and J. Li, Chem. Eng. J., 2013, 225, 323–330 CrossRef CAS.
- Y. Xi, C. Su, N. A. Ottinger and Z. G. Liu, Appl. Catal., B, 2021, 284, 119749–119759 CrossRef CAS.
- Y. Xi, N. A. Ottinger, C. J. Keturakis and Z. G. Liu, Appl. Catal., B, 2021, 294, 120245–120257 CrossRef CAS.
- Z. Chen, C. Bian, Y. Guo, L. Pang and T. Li, ACS Catal., 2021, 11, 12963–12976 CrossRef CAS.
- D. J. Deka, R. Daya, A. Ladshaw, D. Trandal, S. Y. Joshi and W. P. Partridge, Appl. Catal., B, 2022, 309, 121233–121248 CrossRef CAS.
- Y. Ma, Z. Shao, X. Wu, Y. Gao, B. Jin, R. Ran, Z. Si, Z. Li and D. Weng, React. Chem. Eng., 2022 10.1039/d2re00140c.
- J. Luo, H. An, K. Kamasamudram, N. Currier, A. Yezerets, T. Watkins and L. Allard, SAE Int. J. Engines, 2015, 8, 1181–1186 CrossRef.
- J. Luo, D. Wang, A. Kumar, J. Li, K. Kamasamudram, N. Currier and A. Yezerets, Catal. Today, 2016, 267, 3–9 CrossRef CAS.
- L. Ma, W. Su, Z. Li, J. Li, L. Fu and J. Hao, Catal. Today, 2015, 245, 16–21 CrossRef CAS.
- F. Gao, Y. Wang, N. M. Washton, M. Kollar, J. Szanyi and C. H. F. Peden, ACS Catal., 2015, 5, 6780–6791 CrossRef CAS.
- T. Ryu, H. Kim and S. B. Hong, Appl. Catal., B, 2019, 245, 513–521 CrossRef CAS.
- R. Oord, J. E. Schmidt and B. M. Weckhuysen, Catal. Sci. Technol., 2018, 8, 1028–1038 RSC.
- S. Han, J. Cheng, C. Zheng, Q. Ye, S. Cheng, T. Kang and H. Dai, Appl. Surf. Sci., 2017, 419, 382–392 CrossRef CAS.
- J. Wang, L. Shao, C. Wang, J. Wang, M. Shen and W. Li, J. Catal., 2018, 367, 221–228 CrossRef CAS.
- Z. Zhao, R. Yu, R. Zhao, C. Shi, H. Gies, F.-S. Xiao, D. De Vos, T. Yokoi, X. Bao, U. Kolb, M. Feyen, R. McGuire, S. Maurer, A. Moini, U. Müller and W. Zhang, Appl. Catal., B, 2017, 217, 421–428 CrossRef CAS.
- Y. Zhang, Y. Peng, J. Li, K. Groden, J.-S. McEwen, E. D. Walter, Y. Chen, Y. Wang and F. Gao, ACS Catal., 2020, 10, 9410–9419 CrossRef CAS.
- J. Liang, Y. Mi, G. Song, H. Peng, Y. Li, R. Yan, W. Liu, Z. Wang, P. Wu and F. Liu, J. Hazard. Mater., 2020, 398, 122986–122998 CrossRef CAS.
- Z. Liu, B. Guan, H. Jiang, Y. Wei, X. Wu, J. Zhou, H. Lin and Z. Huang, New J. Chem., 2022, 46, 13593–13608 RSC.
- J. Zhang, J. Liang, H. Peng, Y. Mi, P. Luo, H. Xu, M. He and P. Wu, Appl. Catal., B, 2021, 292, 120163–120175 CrossRef CAS.
- Y. Ma, S. Cheng, X. Wu, T. Ma, L. Liu, B. Jin, M. Liu, J. Liu, R. Ran, Z. Si and D. Weng, J. Catal., 2022, 405, 199–211 CrossRef CAS.
- Y. Cui, Y. Wang, E. D. Walter, J. Szanyi, Y. Wang and F. Gao, Catal. Today, 2020, 339, 233–240 CrossRef CAS.
- D. Yao, B. Liu, F. Wu, Y. Li, X. Hu, W. Jin and X. Wang, Ind. Eng. Chem. Res., 2021, 60, 10083–10093 CrossRef CAS.
- H. Lee, I. Song, S. W. Jeon and D. H. Kim, J. Phys. Chem. Lett., 2021, 12, 3210–3216 CrossRef CAS PubMed.
- J. Song, Y. Wang, E. D. Walter, N. M. Washton, D. Mei, L. Kovarik, M. H. Engelhard, S. Prodinger, Y. Wang, C. H. F. Peden and F. Gao, ACS Catal., 2017, 7, 8214–8227 CrossRef CAS.
- Y. Shan, X. Shi, J. Du, Z. Yan, Y. Yu and H. He, Ind. Eng. Chem. Res., 2019, 58, 5397–5403 CrossRef CAS.
- H. Al Jabri, K. Miyake, K. Ono, M. Nakai, R. Inoue, Y. Hirota, Y. Uchida, Y. Wang, T. Nishitoba, T. Yokoi, T. Ohnishi, M. Ogura and N. Nishiyama, Microporous Mesoporous Mater., 2020, 297, 109780–109786 CrossRef CAS.
- A. Wang, Y. Wang, E. D. Walter, N. M. Washton, Y. Guo, G. Lu, C. H. F. Peden and F. Gao, Catal. Today, 2019, 320, 91–99 CrossRef CAS.
- Z. Chen, C. Fan, L. Pang, S. Ming, P. Liu and T. Li, Appl. Catal., B, 2018, 237, 116–127 CrossRef CAS.
- A. Guo, K. Xie, H. Lei, V. Rizzotto, L. Chen, M. Fu, P. Chen, Y. Peng, D. Ye and U. Simon, Environ. Sci. Technol., 2021, 55, 12619–12629 CrossRef CAS.
- H. Zhao, X. Wu, Z. Huang, H. Shen, J. Dong, X. Li and G. Jing, Energy Fuels, 2022, 36, 2712–2721 CrossRef CAS.
- H. Zhao, M. Lin, Y. Wang and J. Zheng, RSC Adv., 2021, 11, 33334–33343 RSC.
- M. Chen, Y. Wei, J. Han, W. Yan and J. Yu, Mater. Chem. Front., 2021, 5, 7787–7795 RSC.
- M.-J. Han, Y.-L. Jiao, C.-H. Zhou, Y.-L. Guo, Y. Guo, G.-Z. Lu, L. Wang and W.-C. Zhan, Rare Met., 2019, 38, 210–220 CrossRef CAS.
- Z. Zhao, R. Yu, C. Shi, H. Gies, F.-S. Xiao, D. De Vos, T. Yokoi, X. Bao, U. Kolb, R. McGuire, A.-N. Parvulescu, S. Maurer, U. Müller and W. Zhang, Catal. Sci. Technol., 2019, 9, 241–251 RSC.
- Y. Cui, Y. Wang, D. Mei, E. D. Walter, N. M. Washton, J. D. Holladay, Y. Wang, J. Szanyi, C. H. F. Peden and F. Gao, J. Catal., 2019, 378, 363–375 CrossRef CAS.
- F. Martinovic, F. A. Deorsola, M. Armandi, B. Bonelli, R. Palkovits, S. Bensaid and R. Pirone, Appl. Catal., B, 2021, 282, 119536–119549 CrossRef CAS.
- T. Usui, Z. Liu, S. Ibe, J. Zhu, C. Anand, H. Igarashi, N. Onaya, Y. Sasaki, Y. Shiramata, T. Kusamoto and T. Wahikara, ACS Catal., 2018, 8, 9165–9173 CrossRef CAS.
- K. Xie, K. Leistner, K. Wijayanti, A. Kumar, K. Kamasamudram and L. Olsson, Catal. Today, 2017, 297, 46–52 CrossRef CAS.
- H. Jiang, B. Guan, H. Lin and Z. Huang, Fuel, 2019, 255, 115587–115604 CrossRef CAS.
- H. Zhao, L. Han, Y. Wang and J. Zheng, Catalysts, 2021, 11, 796–806 CrossRef CAS.
- H. Zhao, Y. Zhao, M. Liu, X. Li, Y. Ma, X. Yong, H. Chen and Y. Li, Appl. Catal., B, 2019, 252, 230–239 CrossRef CAS.
- R. Xu, Z. Wang, N. Liu, C. Dai, J. Zhang and B. Chen, ACS Catal., 2020, 10, 6197–6212 CrossRef CAS.
- Y. Shan, J. Du, Y. Yu, W. Shan, X. Shi and H. He, Appl. Catal., B, 2020, 266, 118655–118667 CrossRef CAS.
- X. Wang, Y. Sun, F. Han and Y. Zhao, J. Environ. Chem. Eng., 2022, 107888–107898 CrossRef CAS.
- S. Shwan, M. Skoglundh, L. F. Lundegaard, R. R. Tiruvalam, T. V. W. Janssens, A. Carlsson and P. N. R. Vennestrøm, ACS Catal., 2015, 5, 16–19 CrossRef CAS.
- A. K. S. Clemens, A. Shishkin, P.-A. Carlsson, M. Skoglundh, F. J. Martínez-Casado, Z. Matej, O. Balmes and H. Harelind, ACS Catal., 2015, 5, 6209–6218 CrossRef CAS.
- L. Xie, F. Liu, X. Shi, F.-S. Xiao and H. He, Appl. Catal., B, 2015, 179, 206–212 CrossRef CAS.
- D. Wang, F. Gao, C. H. F. Peden, J. Li, K. Kamasamudram and W. S. Epling, ChemCatChem, 2014, 6, 1579–1583 CrossRef.
- Y. Yamasaki, N. Tsunoji, Y. Takamitsu, M. Sadakane and T. Sano, Microporous Mesoporous Mater., 2016, 223, 129–139 CrossRef CAS.
- J. Han, A. Wang, G. Isapour, H. Härelind, M. Skoglundh, D. Creaser and L. Olsson, Ind. Eng. Chem. Res., 2021, 60, 17826–17839 CrossRef CAS.
- B. Peng, K. G. Rappé, Y. Cui, F. Gao, J. Szanyi, M. J. Olszta, E. D. Walter, Y. Wang, J. D. Holladay and R. A. Goffe, Appl. Catal., B, 2020, 263, 118359–118370 CrossRef CAS.
- L. Xie, F. Liu, K. Liu, X. Shi and H. He, Catal. Sci. Technol., 2014, 4, 1104–1110 RSC.
- M. Jabłońska, Mol. Catal., 2022, 518, 112111–112132 CrossRef.
- P. N. R. Vennestrøm, L. F. Lundegaard, C. Tyrsted, D. A. Bokarev, A. I. Mytareva, G. N. Baeva, A. Y. Stakheev and T. V. W. Janssens, Top. Catal., 2019, 62, 100–107 CrossRef.
- Y. Ma, S. Cheng, X. Wu, Y. Shi, L. Cao, L. Liu, R. Ran, Z. Si, J. Liu and D. Weng, ACS Catal., 2019, 9, 6962–6973 CrossRef CAS.
- J. Luo, F. Gao, K. Kamasamudram, N. Currier, C. H. F. Peden and A. Yezerets, J. Catal., 2017, 348, 291–299 CrossRef CAS.
- M. Nielsen, R. Y. Brogaard, H. Falsig, P. Beato, O. Swang and S. Svelle, ACS Catal., 2015, 5, 7131–7139 CrossRef CAS.
- K. Ehrhardt, M. Suckow and W. Lutz, in Studies in Surface Science and Catalysis, Elsevier, 1995, vol. 94, pp. 179–186 Search PubMed.
- T. Usui, Z. Liu, H. Igarashi, Y. Sasaki, Y. Shiramata, H. Yamada, K. Ohara, T. Kusamoto and T. Wakihara, ACS Omega, 2019, 4, 3653–3659 CrossRef CAS PubMed.
- F. Gao and J. Szanyi, Appl. Catal., A, 2018, 560, 185–194 CrossRef CAS.
- R. Zhang, J.-S. McEwen, M. Kollár, F. Gao, Y. Wang, J. Szanyi and C. H. F. Peden, ACS Catal., 2014, 4, 4093–4105 CrossRef CAS.
- J. E. Schmidt, R. Oord, W. Guo, J. D. Poplawsky and B. M. Weckhuysen, Nat. Commun., 2017, 8, 1–8 CrossRef CAS PubMed.
- C. Fan, Z. Chen, L. Pang, S. Ming, C. Dong, K. B. Albert, P. Liu, J. Wang, D. Zhu, H. Chen and T. Li, Chem. Eng. J., 2018, 334, 344–354 CrossRef CAS.
- R. Zhang, Y. Li and T. Zhen, RSC Adv., 2014, 4, 52130–52139 RSC.
- M. Chen, J. Li, W. Xue, S. Wang, J. Han, Y. Wei, D. Mei, Y. Li and J. Yu, J. Am. Chem. Soc., 2022, 144, 12816–12824 CrossRef CAS PubMed.
- Y. Wang, X. Shi, Y. Shan, J. Du, K. Liu and H. He, Ind. Eng. Chem. Res., 2020, 59, 6416–6423 CrossRef CAS.
- J. M. González and A. L. Villa, Catal. Lett., 2021, 151, 3011–3019 CrossRef.
- Y. Ma, H. Zhao, C. Zhang, Y. Zhao, H. Chen and Y. Li, Catal. Today, 2020, 355, 627–634 CrossRef CAS.
- L. Sun, M. Yang, L. Cao, Y. Cao, S. Xu, D. Zhu, P. Tian and Z. Liu, Microporous Mesoporous Mater., 2020, 309, 110585–110595 CrossRef CAS.
- Y. Jangjou, Q. Do, Y. Gu, L.-G. Lim, H. Sun, D. Wang, A. Kumar, J. Li, L. C. Grabow and W. S. Epling, ACS Catal., 2018, 8, 1325–1337 CrossRef CAS.
- L. Xu, C. Wang, H. Chang, Q. Wu, T. Zhang and J. Li, Environ. Sci. Technol., 2018, 52, 7064–7071 CrossRef CAS PubMed.
- K. Wijayanti, K. Leistner, S. Chand, A. Kumar, K. Kamasamudram, N. W. Currier, A. Yezerets and L. Olsson, Catal. Sci. Technol., 2016, 6, 2565–2579 RSC.
- Y. Jangjou, D. Wang, A. Kumar, J. Li and W. S. Epling, ACS Catal., 2016, 6, 6612–6622 CrossRef CAS.
- R. Yu, Z. Zhao, S. Huang and W. Zhang, Appl. Catal., B, 2020, 269, 118825–118833 CrossRef CAS.
- Q. Liu, Z. Fu, L. Ma, H. Niu, C. Liu, J. Li and Z. Zhang, Appl. Catal., A, 2017, 547, 146–154 CrossRef CAS.
- X. Li, J. Feng, Z. Xu, J. Wang, Y. Wang and W. Zhao, React. Kinet., Mech. Catal., 2019, 128, 163–174 CrossRef CAS.
- X. Liu, Y. Li and R. Zhang, RSC Adv., 2015, 5, 85453–85459 RSC.
- C. Yin, P. Cheng, X. Li and R. T. Yang, Catal. Sci. Technol., 2016, 6, 7561–7568 RSC.
- L. Xie, C. Liu, Y. Deng, F. Liu and W. Ruan, Ind. Eng. Chem. Res., 2022, 61, 8698–8707 CrossRef CAS.
- R. G. Silver, M. O. Stefanick and B. I. Todd, Catal. Today, 2008, 136, 28–33 CrossRef CAS.
- O. Kröcher and M. Elsener, Appl. Catal., B, 2008, 77, 215–227 CrossRef.
- H.-W. Jen, J. W. Girard, G. Cavataio and M. J. Jagner, SAE Int. J. Fuels Lubr., 2009, 1, 1553–1559 CrossRef CAS.
- I. Lezcano-Gonzalez, U. Deka, H. E. Van Der Bij, P. Paalanen, B. Arstad, B. M. Weckhuysen and A. M. Beale, Appl. Catal., B, 2014, 154, 339–349 CrossRef.
- G. Cavataio, J. R. Warner, J. W. Girard, J. Ura, D. Dobson and C. K. Lambert, SAE Int. J. Fuels Lubr., 2009, 2, 342–368 CrossRef CAS.
- Y. Kakiuchi, T. Tanigawa, N. Tsunoji, Y. Takamitsu, M. Sadakane and T. Sano, Appl. Catal., A, 2019, 575, 204–213 CrossRef CAS.
- V. Mesilov, S. Dahlin, S. L. Bergman, S. Xi, J. Englund, H. Malm, L. J. Pettersson and S. L. Bernasek, J. Phys. Chem. C, 2022, 126, 3385–3396 CrossRef CAS.
- K. Xie, J. Woo, D. Bernin, A. Kumar, K. Kamasamudram and L. Olsson, Appl. Catal., B, 2019, 241, 205–216 CrossRef CAS.
- A. Wang, K. Xie, D. Bernin, A. Kumar, K. Kamasamudram and L. Olsson, Appl. Catal., B, 2020, 269, 118781–118793 CrossRef CAS.
- N. Zhang, L. Li, Y. Guo, J. He, R. Wu, L. Song, G. Zhang, J. Zhao, D. Wang and H. He, Appl. Catal., B, 2020, 270, 118860–118876 CrossRef CAS.
- W. Hu, F. Gramigni, N. D. Nasello, N. Usberti, U. Iacobone, S. Liu, I. Nova, X. Gao and E. Tronconi, ACS Catal., 2022, 12, 5263–5274 CrossRef CAS.
- Y. Liu, W. Xue, S. Seo, X. Tan, D. Mei, C. Liu, I.-S. Nam and S. B. Hong, Appl. Catal., B, 2021, 294, 120244–120252 CrossRef CAS.
- H. Lee, I. Song, S. W. Jeon and D. H. Kim, Catal. Sci. Technol., 2021, 11, 4838–4848 RSC.
- H. Lee, R. J. G. Nuguid, S. W. Jeon, H. S. Kim, K. H. Hwang, O. Kröcher, D. Ferri and D. H. Kim, Chem. Commun., 2022, 58, 6610–6613 RSC.
- C. Paolucci, I. Khurana, A. A. Parekh, S. Li, A. J. Shih, H. Li, J. R. Di Iorio, J. D. Albarracin-Caballero, A. Yezerets, J. T. Miller, W. N. Delgass, F. H. Ribeiro, W. F. Schneider and R. Gounder, Science, 2017, 357, 898–903 CrossRef CAS PubMed.
- Y. Zhang, J. Zhang, H. Wang, W. Yang, C. Wang, Y. Peng, J. Chen, J. Li and F. Gao, J. Phys. Chem. C, 2022, 126, 8720–8733 CrossRef CAS.
- W. Hu, T. Selleri, F. Gramigni, E. Fenes, K. R. Rout, S. Liu, I. Nova, D. Chen, X. Gao and E. Tronconi, Angew. Chem., Int. Ed., 2021, 60, 7197–7204 CrossRef CAS.
- W. Hu, U. Iacobone, F. Gramigni, Y. Zhang, X. Wang, S. Liu, C. Zheng, I. Nova, X. Gao and E. Tronconi, ACS Catal., 2021, 11, 11616–11625 CrossRef CAS.
- K. A. Tarach, M. Jabłońska, K. Pyra, M. Liebau, B. Reiprich, R. Gläser and K. Góra-Marek, Appl. Catal., B, 2021, 284, 119752–119768 CrossRef CAS.
- L. Chen, T. V. W. Janssens, P. N. R. Vennestrøm, J. Jansson, M. Skoglundh and H. Gronbeck, ACS Catal., 2020, 10, 5646–5656 CrossRef CAS.
- L. Chen, H. Falsig, T. V. W. Janssens and H. Grönbeck, J. Catal., 2018, 358, 179–186 CrossRef CAS.
- N. Usberti, F. Gramigni, N. D. Nasello, U. Iacobone, T. Selleri, W. Hu, S. Liu, X. Gao, I. Nova and E. Tronconi, Appl. Catal., B, 2020, 279, 119397–119409 CrossRef CAS.
- F. Gramigni, N. D. Nasello, N. Usberti, U. Iacobone, T. Selleri, W. Hu, S. Liu, X. Gao, I. Nova and E. Tronconi, ACS Catal., 2021, 11, 4821–4831 CrossRef CAS.
- Y. Feng, T. V. W. Janssens, P. N. R. Vennestrøm, J. Jansson, M. Skoglundh and H. Gronbeck, J. Phys. Chem. C, 2021, 125, 4595–4601 CrossRef CAS.
- B. Liu, D. Yao, F. Wu, L. Wei, X. Li and X. Wang, Ind. Eng. Chem. Res., 2019, 58, 20516–20527 CrossRef CAS.
- L. Wei, D. Yao, F. Wu, B. Liu, X. Hu, X. Li and X. Wang, Ind. Eng. Chem. Res., 2019, 58, 3949–3958 CrossRef CAS.
- A. J. Shih, J. M. González, I. Khurana, L. P. Ramírez, A. Peña L, A. Kumar and A. L. Villa, ACS Catal., 2021, 11, 10362–10376 CrossRef CAS.
- J. H. Kwak, D. Tran, J. Szanyi, C. H. F. Peden and J. H. Lee, Catal. Lett., 2012, 142, 295–301 CrossRef CAS.
- N. Lv, Ch. Sun, X. Wang, Ch. Wang, Y. Yue and X. Bao, Inorg. Chem. Front., 2022, 9, 1300–1313 RSC.
- G. M. Psofogiannakis, J. F. McCleerey, E. Jaramillo and A. C. T. Van Duin, J. Phys. Chem. C, 2015, 119, 6678–6686 CrossRef CAS.
- F. Göltl, A. M. Love and I. Hermans, J. Phys. Chem. C, 2017, 121, 6160–6169 CrossRef.
- J.-S. McEwen, T. Anggara, W. F. Schneider, V. F. Kispersky, J. T. Miller, W. N. Delgass and F. H. Ribeiro, Catal. Today, 2012, 184, 129–144 CrossRef CAS.
- L. Chen, H. Falsig, T. V. W. Janssens, J. Jansson, M. Skoglundh and H. Grönbeck, Catal. Sci. Technol., 2018, 8, 2131–2136 RSC.
- C. Paolucci, A. A. Parekh, I. Khurana, J. R. Di Iorio, H. Li, J. D. Albarracin Caballero, A. J. Shih, T. Anggara, W. N. Delgass, J. T. Miller, F. H. Ribeiro, R. Gounder and W. F. Schneider, J. Am. Chem. Soc., 2016, 138, 6028–6048 CrossRef CAS PubMed.
- S. H. Krishna, C. B. Jones, J. T. Miller, F. H. Ribeiro and R. Gounder, J. Phys. Chem. Lett., 2020, 11, 5029–5036 CrossRef CAS PubMed.
- A. Martini, C. Negri, L. Bugarin, G. Deplano, R. K. Abasabadi, K. A. Lomachenko, T. V. W. Janssens, S. Bordiga, G. Berlier and E. Borfecchia, J. Phys. Chem. Lett., 2022, 13, 6164–6170 CrossRef CAS PubMed.
- C. Negri, T. Selleri, E. Borfecchia, A. Martini, K. A. Lomachenko, T. V. W. Janssens, M. Cutini, S. Bordiga and G. Berlier, J. Am. Chem. Soc., 2020, 142, 15884–15896 CrossRef CAS PubMed.
- A. Oda, H. Shionoya, Y. Hotta, T. Takewaki, K. Sawabe and A. Satsuma, ACS Catal., 2020, 10, 12333–12339 CrossRef CAS.
- R. Daya, D. Trandal, U. Menon, D. J. Deka, W. P. Partridge and S. Y. Joshi, ACS Catal., 2022, 12, 6418–6433 CrossRef CAS.
- T. Zhang, H. Chang, Y. You, C. Shi and J. Li, Environ. Sci. Technol., 2018, 52, 4802–4808 CrossRef CAS PubMed.
- T. Yu, J. Wang, Y. Huang, M. Shen, W. Li and J. Wang, ChemCatChem, 2014, 6, 2074–2083 CrossRef CAS.
- M. Jabłońska and R. Palkovits, Appl. Catal., B, 2016, 181, 332–351 CrossRef.
- L. Chmielarz and M. Jabłońska, RSC Adv., 2015, 5, 43408–43431 RSC.
- M. Jabłońska, Molecules, 2021, 26, 6461–6485 CrossRef PubMed.
- K. Leistner, O. Mihai, K. Wijayanti, A. Kumar, K. Kamasamudram, N. W. Currier, A. Yezerets and L. Olsson, Catal. Today, 2015, 258, 49–55 CrossRef CAS.
- L. Olsson, K. Wijayanti, K. Leistner, A. Kumar, S. Y. Joshi, K. Kamasamudram, N. W. Currier and A. Yezerets, Appl. Catal., B, 2015, 174, 212–224 CrossRef.
- J. Guo, W. Yang, Y. Zhang, L. Gan, C. Fan, J. Chen, Y. Peng and J. Li, Catal. Commun., 2020, 135, 105751–105756 CrossRef CAS.
- M. Jabłońska and A. Mollá Robles, Materials, 2022, 15, 4770–4794 CrossRef PubMed.
- H. Wang, R. Xu, Y. Jin and R. Zhang, Catal. Today, 2019, 295–307 CrossRef.
- S. Shrestha, M. P. Harold, K. Kamasamudram, A. Kumar, L. Olsson and K. Leistner, Catal. Today, 2016, 267, 130–144 CrossRef CAS.
- P. S. Dhillon, M. P. Harold, D. Wang, A. Kumar and S. Y. Joshi, Catal. Today, 2021, 360, 426–434 CrossRef CAS.
- P. S. Dhillon, M. P. Harold, D. Wang, A. Kumar and S. Y. Joshi, React. Chem. Eng., 2019, 4, 1103–1115 RSC.
- Y. Liao, H. Xu, Z. Li, L. Ji, L. Wang, G. Gao, W. Huang, Z. Qu and N. Yan, J. Phys. Chem. C, 2021, 125, 17031–17041 CrossRef CAS.
- A. K. Datye and M. Votsmeier, Nat. Mater., 2021, 20, 1049–1059 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |

