 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices
Alfredo Edoardo
Ongaro
 a,
Zibusiso
Ndlovu
a,
Zibusiso
Ndlovu
 b,
Elodie
Sollier
b,
Elodie
Sollier
 c,
Collins
Otieno
c,
Collins
Otieno
 d,
Pascale
Ondoa
d,
Pascale
Ondoa
 d,
Alice
Street
d,
Alice
Street
 e and
Maïwenn
Kersaudy-Kerhoas
e and
Maïwenn
Kersaudy-Kerhoas
 *fg
*fg
aThe Institute of Photonic Sciences (ICFO), Barcelona, Spain
bMedecins Sans Frontières (MSF), Southern Africa Medical Unit (SAMU), Cape Town, South Africa
cStratec Consumables, Anif, Austria
dAfrican Society for Laboratory Medicine (ASLM), Addis Ababa, Ethiopia
eSchool of Social and Political Sciences, University of Edinburgh, Edinburgh, UK
fSchool of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, UK. E-mail: m.kersaudy-kerhoas@hw.ac.uk
gInfection Medicine, College of Medicine and Veterinary Medicine University of Edinburgh, Edinburgh, UK
First published on 23rd June 2022
Abstract
Single-use, disposable, point-of-care diagnostic devices carry great promise for global health, including meeting urgent needs for testing and diagnosis in places with limited laboratory facilities. Unfortunately, the production and disposal of single-use devices, whether in lateral flow assay, cartridges, cassettes, or lab-on-chip microfluidic format, also poses significant challenges for environmental and human health. Point-of-care devices are commonly manufactured from unsustainable polymeric materials derived from fossil sources. Their disposal often necessitates incineration to reduce infection risk, thereby creating additional release of CO2. Many devices also contain toxic chemicals, such as cyanide derivatives, that are damaging to environmental and human health if not disposed of safely. Yet, in the absence of government regulatory frameworks, safe and sustainable waste management for these novel medical devices is often left unaddressed. There is an urgent need to find novel solutions to avert environmental and human harm from these devices, especially in low- and middle-income countries where waste management infrastructure is often weak and where the use of point-of-care tests is projected to rise in coming years. We review here common materials used in the manufacture of single-use point-of-care diagnostic tests, examine the risks they pose to environmental and human health, and investigate replacement materials that can potentially reduce the impact of microfluidic devices on the production of harmful waste. We propose solutions available to point-of-care test developers to start embedding sustainability at an early stage in their design, and to reduce their non-renewable plastic consumption in research and product development.
Introduction
Nowhere has the increased availability of diagnostic tests for medical practice and public health been more evident than in the global response to the recent coronavirus (COVID-19) pandemic.1,2 Millions of point-of-care tests (POCTs) for COVID-19 are used globally every day in hospitals, primary care facilities, workplaces, and people's homes, bringing projections for the global POCT market to $72B by 2024 from $43.3B in 2022, at an annual growth rate of 10%.3 Heightened awareness of the benefits of POCTs following the COVID-19 response is also widely viewed as an opportunity to galvanise diagnostic innovation for neglected diseases and improve access to a wider array of tests in resource-limited settings (Fig. 1A).4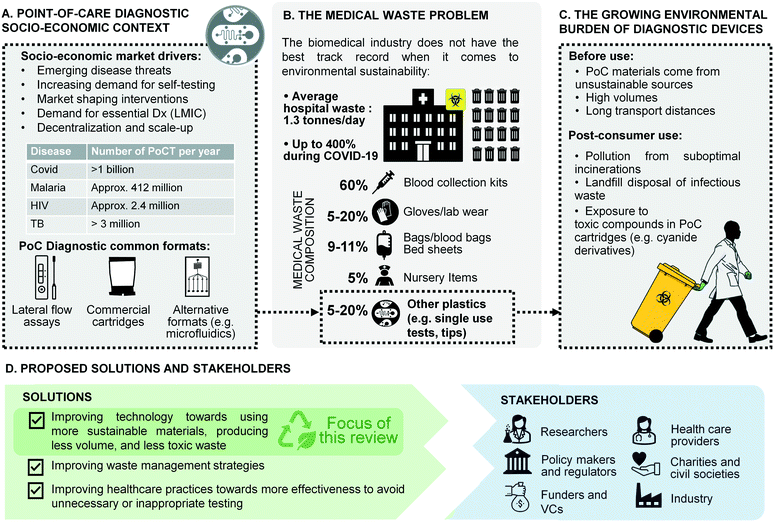 | ||
| Fig. 1 Overview of the challenges and solutions in single-use diagnostic devices. A) Point-of-care diagnostic socio-economic context.13–15 B) The medical waste problem.16,17 C) Growing burden of waste from diagnostic devices. D) Proposed solutions and stakeholders. | ||
Yet the mass deployment of POCT devices in health systems and communities across the globe comes with unforeseen costs for the environment and human health. Most single-use POCT devices are made from plastic materials issued from non-renewable sources, and contribute to the rising global tide of medical waste (Fig. 1B). A large proportion of POCT diagnostic waste falls into the category of infectious waste, which should be collected separately and treated in order to remove the infection risk.5 Infectious waste is most often incinerated, thus contributing to greenhouse gas (GHG) emissions. In low- and middle-income countries (LMICs), many health facilities either lack incinerators altogether, do not have the fuel to run them, or cannot operate them at required temperature thresholds.6–8 In such settings, used testing devices are often burned on open pits in health facility grounds or at municipal dump sites.5,9 In addition to causing the release of GHG, plastic waste burned at low temperatures emits toxic pollutants such as dioxins and furans.10 In addition, a lot of POC waste in LMICs ends up in landfills or in municipal water supplies, which increases the risk that health workers, waste workers and members of the public will come into contact with the hazardous reagents they contain, such as the cyanide derivatives used in PCR cartridges (Fig. 1C).11 A recent WHO report estimated that during the COVID-19 pandemic more than 140 million test kits have been shipped through the UN procurement portal alone, with the potential to generate 731![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 litres of chemical waste, the equivalent of a 25 m 8-lane swimming pool.12 So while single-use POCTs undoubtedly carry great potential for global health, they also contribute to growing global challenges related to plastic waste, GHG emissions, and human exposure to toxic pollutants (Fig. 1C). Concerns about contaminated medical waste resulting in infectious disease spill over into animal populations (reverse zoonoses) have also been reported previously, including recently in the 2022 monkey pox epidemic.18,19 Moreover, in LMICs, increased access to POCTs in the future will place significant additional pressures on already stretched waste management systems, undermining claims that such devices are ‘infrastructure-light’ and appropriately designed for such settings.20 To a lower but growing extent, such pressure is seen in high-income countries as well, where environmental impact and waste management have become a recurrent topic at funding levels and where limited access to plastic during the pandemics triggered strategic discussions on consumable recycling. This is exemplified for example, by the wording of the European Green Deal and a number of European Commission funds calling specifically for CO2 reduction, and reduced water and energy usages across the full product life cycle.21
000 litres of chemical waste, the equivalent of a 25 m 8-lane swimming pool.12 So while single-use POCTs undoubtedly carry great potential for global health, they also contribute to growing global challenges related to plastic waste, GHG emissions, and human exposure to toxic pollutants (Fig. 1C). Concerns about contaminated medical waste resulting in infectious disease spill over into animal populations (reverse zoonoses) have also been reported previously, including recently in the 2022 monkey pox epidemic.18,19 Moreover, in LMICs, increased access to POCTs in the future will place significant additional pressures on already stretched waste management systems, undermining claims that such devices are ‘infrastructure-light’ and appropriately designed for such settings.20 To a lower but growing extent, such pressure is seen in high-income countries as well, where environmental impact and waste management have become a recurrent topic at funding levels and where limited access to plastic during the pandemics triggered strategic discussions on consumable recycling. This is exemplified for example, by the wording of the European Green Deal and a number of European Commission funds calling specifically for CO2 reduction, and reduced water and energy usages across the full product life cycle.21
Meanwhile, advances in microfluidics, the technology of microscale fluid manipulation, are rapidly expanding the capabilities and reach of medical testing. A general trend in healthcare towards personalised, remote POCT procedures, and global concerns about emerging diseases are driving substantial investments in microfluidic innovation and rapid market growth in the diagnostics sector.22–26 As a consequence, point-of-care and microfluidic testing devices are now an essential component of disease control programmes, at the national and global level, from efforts to improve universal health care, to disease elimination campaigns and outbreak response.27
Until recently there has been little incentive for all actors (researchers, engineers, manufacturers) to develop and use more sustainable and less harmful materials in POCTs and single-use microfluidic devices. The Covid-19 pandemic had put a beneficial spotlight on medical waste issues, and solutions minimising PPE waste, as well as seminal frugal diagnostic solutions, have emerged recently.28–30 However, the considerations for safe and sustainable disposal have typically been excluded from design requirements so far. Accuracy, reliability, usability, and affordability are the main drivers in the industrial medical device sector. For example, the standard format for target product profiles (TPPs), which provide guidance to manufacturers on market needs and appropriate technical specifications, omits specifications for waste management. Global health policy efforts to address the rising challenge of healthcare waste in LMICs have tended to focus on improving country-level waste management regulation, monitoring and infrastructure rather than considering how the volume of healthcare waste might be reduced through improvements in design and manufacture earlier in the product life cycle. But improving waste management at the point of use can only take us so far, especially since the circular solutions in the medical diagnostic area will always be limited by the requirements for safe disposal of infectious waste.
However, change is on the horizon. Regulators around the world are now requiring more sustainability for single-use products, as laid out by public procurement approaches such as the United States' BioPreferred Programme31 and the EU's Green Public Procurement (GPP) framework.32 The Horizon EU grant scheme urges applicants to consider such life cycle, recycling and environmental impact. In the US, the Environmental Protection Agency (EPA) now requires that healthcare facilities under the umbrella of the federal government give preference to sustainable products. International financial institutions such as the Asian Development Bank and global health funding mechanisms such as the Global Fund make similar requirements for recipients to consider green procurement.33 But this pressure on health procurement agencies will not bear any positive outcomes, if there are no alternative sustainable products available. Furthermore, the focus on sustainability has not been accompanied by an equivalent regulatory pressure to reduce the risk of harm from toxic reagents in places where safe disposal is not possible. In this context, the design of POCT technologies for safe and sustainable disposal is both an ethical imperative for industry, and an opportunity for scientific innovation (Fig. 1D).
In this review, we use the term ‘POCT’ as an umbrella term to describe a range of single-use, portable tests and microfluidic-based or miniaturised devices. Devices like lateral flow assays and other devices meant to be used outside typical care settings should, strictly speaking, be referred to as ‘point of need’, rather than ‘point of care’. In addition ‘single-use’ devices can also be applied to the growing proliferation of purposes for such devices including food testing34 and environmental monitoring.35 In engineering contexts, microfluidic devices are also referred to as ‘micro-total analytical systems’, or ‘lab-on-a-chip’, and there are often blurry definitional boundaries between these different terms.
This review brings together perspectives from public health, social science, material science, microfluidic engineering and manufacturing to address the sustainability challenges posed by POCTs and explore technological solutions towards the improvement of POCT and single-use microfluidic sustainability (Fig. 1D). First, we give an overview of plastic materials currently used in the fabrication and manufacture of POCTs, and introduce more sustainable and less harmful alternatives to these. Then, we focus on the environmental and human health risks associated with many reagents used in POCTs, and provide a roadmap for all stakeholders in the sector.
Current plastics used in single-use diagnostic devices and sustainable alternatives
The requirements for materials in single-use POCT devices include, besides obviously performance and reproducibility: (i) compatibility with mass-manufacturing processes; (ii) chemical and mechanical resistance; (iii) impermeability; and (iv) low cost. These requirements have driven the use of glass and plastic in the prototyping and manufacturing of devices at R&D and the commercial level. An industry survey based on a sample of selected microfluidic companies revealed that 59% of all commercially available devices are made of plastics (mainly thermoplastics), 12% are of glass, 12% of papers, 6% of elastomers and 6% of epoxy resins.36 In academia, our own survey showed that 55% of published devices are made of polydimethylsiloxane (PDMS), 12% of silicon and glass, 20% of thermoplastic materials, and 13% of paper. Current plastics involved in the fabrication of POC devices are listed in Table 1, alongside their applications, pros and cons. The thermoplastics used in both settings include, but are not limited to: polymethylmethacrylate (PMMA), cyclic olefin copolymer (COC), cyclic olefin polymer (COP), polypropylene (PP), acrylonitrile butadiene styrene (ABS), and high impact polystyrene (HIPS). These materials answer the main requirements for single-use devices, but have a large CO2 footprint and are non-biodegradable.| Families of material | Associated prototyping and fabrication method | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Silicon and glass | Standard photolithography and soft lithography | • Thermal conductivity | • Higher cost of fabrication | Foret, 2013;36 Wlodarczyk, 201937 |
| • Stable electro-osmotic mobility | • Dangerous chemicals involved | |||
| • Resistance to organic solvent | ||||
| Thermoplastics (e.g. PMMA, PC, PS, PET, PVC, ABS, COC, COP) | Injection moulding; fusion deposition modelling; laser cutting | • Resistance to alcohols | • Unsustainable source of raw materials | Becker, 2002;16 Morgan, 2016;38 Liga, 2016;39 Attia, 200940 |
| • Mostly low cost | • Toxic fumes when incomplete combustion | |||
| • Rapid prototyping | ||||
| • Mechanical recycling | ||||
| Elastomers | Casting roll-to-roll | • Easy and low cost of microfabrication | • Incompatibility with organic solvents | Friend, 2010;41 Hiltunen, 201842 |
| • High elasticity | • Absorption of hydrophobic and small molecules | |||
| • Gas permeable | ||||
| Hybrids | Combination of the above methods | • Integration of functionalities | • High cost of fabrication | Sanjay, 201643 |
Further, when improperly incinerated, these plastics can generate toxic pollutants. Fig. 2 illustrates sub-optimal incineration observed in a public referral hospital in Sierra Leone. Incineration is a high-temperature, dry oxidation process that reduces organic and combustible waste to inorganic, incombustible matter and results in a significant reduction of waste volume and weight. Incineration is an environmentally damaging process that releases combustion by-products into the atmosphere and generates residual ash. Such by-products include nitrous oxide as well as known carcinogens, which include polychlorinated biphenyls, furans and dioxins.44 Persistent organic pollutants such as polychlorinated dioxins and furans from halogenated plastics (such as polyvinyl chloride, PVC) are toxic at extremely low concentrations. While highly sophisticated incineration systems fitted with filters are capable of removing dioxins and furans, rudimentary unfiltered, homemade systems, which are used in some low-resource settings, will not. In addition, when dumped in landfills (Fig. 2), plastics will persist for hundreds of years in the soil. The sustainability and safety of single-use POCTs could, to various degrees, be improved by technological solutions.
Recycled plastics
Recycling is a process that converts waste materials into new materials (primary to tertiary recycling) or energy (quaternary recycling).45 The adoption of recycled material for the production of POCTs could result in lower GHG emissions and reduce reliance on non-renewables such as petroleum. In particular, the most commonly used thermoplastics can be recycled by adopting one of three approaches: direct re-use, mechanical recycling or chemical recycling.- Direct re-use involves re-using devices following a wash. This solution is unsuitable for most POCT, due to the presence of intricate features such as nano or microchannels, valves and other complex geometries, which can easily trap debris or get clogged during the wash-out, leading to assay failure, cross-contamination, and erroneous results.
- Mechanical recycling involves the collection, sorting, washing and grinding of the material, followed by mechanical processing of the waste into a secondary product, for instance via melting, remoulding and extrusion.46 This approach is typically conducted during either post-industrial processing (where it is most effective) or the post-customer use stage. In this case, scraps of the materials are immediately collected after the polymer processing, for example from the sprue and runners (passage through the liquid material is introduced into the mould and from one part to another) at each cycle of injection moulding. These can be reprocessed as they can be blended with the virgin material, to produce fallout products or other functional parts. The recycled end-item does not require a sorting or cleaning process and its chemical composition and properties are known. On the other hand, post-customer use mechanical recycling is more challenging and has some limitations. Collection, sorting and washing are fundamental steps. In fact, polymer blends (mixtures of two or more different polymers) usually have reduced mechanical and thermal properties when compared with those of the single polymer. To overcome this issue, additives in the form of compatibilizing agents are often added.47–49 Some research studies have shown the possibility of using mechanically recycled PMMA for the production of optical fiber sensors and microfluidic devices.50,51 The waste generated and the demand for new raw materials are decreased, reducing energy usage, air pollution and water pollution. Wan et al. designed a novel method to recycle PMMA microfluidic devices in a laboratory setting, demonstrating that recycled plastics can be used for the production of microfluidic devices. They showed the possibility to thermo-mechanically recycle PMMA up to 4 iterations without losing optical properties and biocompatibility (Fig. 2).51 Despite optimum results in terms of final optical qualities and thermal properties, these processes require specific facilities, such as an extruder or a heated press. In the case of single-use medical devices, a sterilization step should be included prior to mechanical recycling of the device. Another emerging material of interest, that can easily be mechanically recycled, is the thermoplastic elastomer Flexdyme™.52,53 Flexdym™ is the first material to be created specifically for the microfluidic community. It combines the advantages of thermoplastics and elastomers and is free of additives, which makes it ideal for sensitive cell culture applications for example. Flexdym TM can be molded through hot embossing or injection moulding and easily remolded.
- Chemical recycling is a closed-loop recycling process. First, the used thermoplastic is depolymerized through a chemical process such as chemolysis or pyrolysis, to break the macromolecular chains. After a distillation process, all impurities are separated to obtain the recycled monomer with a purity up to 99.8%. The monomers are polymerized to obtain the recycled material with the same optical, mechanical and thermal properties as the virgin material. Chemically recycled thermoplastics are widely accessible. For example, chemically recycled PMMA is available from several suppliers and constitutes a sustainable and flexible material (it can be cut, engraved, milled and embossed). Plasticizers and compatibilizing agents can change the material's thermo-mechanical properties and solvent affinity, which can affect prototyping protocols, such as laser microstructuring or bonding. This is an issue encountered with recycled as well as non-recycled virgin materials. We demonstrated an ultra-fast bonding method on pristine PMMA sheets manufactured from different suppliers and showed significant bonding strength differences between manufacturers.46,54 We have also shown that chemically recycled PMMA yielded similar bonding strength to the pristine PMMA material.55 Carrying out a Differential Scanning Calorimetry (DSC) analysis can help to predict the presence of contaminants and establish if further optimization of the bonding parameters—in terms of temperature, pressure and time—is required.55
Finally, it is worth mentioning here that another thermoplastic polymer, which is widely employed, and for which there is already a recycling stream route, is polyethylene terephthalate (PET). PET is the same polymer used in the plastic bottle industry. Unlike PMMA, PET is not commonly used in the production of microfluidic devices, but a few examples have been demonstrated: Jackson et al. used PET to fabricate semi-automated DNA extraction on a centrifugal device using mixing via an external magnetic field.56,57 The reason why PET has still not been widely adopted by microfluidic researchers and manufacturers might be due to the fact that it is not easily available in sheet format. Fabrication technologies of microfluidic devices from hard plastic employed both in academia and industry rely on CNC milling and laser cutting, which require a sheet format substrate. Still, recycled PET is widely available and could be seriously considered as a substitute to thermoplastics.
Recycled plastics might offer the possibility of approaching net zero CO2 emissions. While commercially recycled plastics are more expensive at present (re-PMMA is about 20% more expensive than pristine PMMA, for instance), this could be offset by higher costs on pristine plastic products in the future, and further incentives on the use of recycled components in single-use device production. More research into the use of recycled plastics in microfluidic production is needed and manufacturers could help by supplying in-depth material information to accelerate the optimisation of manufacturing processes, such as moulding, engraving and bonding.
While reducing GHG emissions, recycled plastics do not entirely remove the reliance on non-renewable raw material sources, nor do they alleviate the problem of pollutants generated by incineration. The use of recycled plastics, such as re-PMMA, represents a suitable short-term solution for some of the environmental challenges highlighted but the need for specific infrastructure, the logistics required to collect and recover waste, and the cost involved in recycling PMMA (as opposed to other thermoplastic materials such as HDPE and PET, for which a recycling stream already exists) make this option challenging in many settings. Regrettably, recycling is not always an economically advantageous option, and up to 30–50% of plastic waste cannot be recycled. Recycled plastics' pros and cons are summarised in Table 2, alongside other sustainable alternatives to plastic non-renewable plastic sources.
| Families of material | Specific materials | Associated prototyping and fabrication method | Demonstrators | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Recycled plastics | Re-PMMA | Laser cutting, embossing, injection moulding | Simple microchannel structures; cell culture | • Readily available | • Non-renewable raw material | Ongaro, 2018;55 Wan, 201751 |
| • Compatible with conventional manufacturing | • Poor degradability | |||||
| • Good transparency | • Use of plasticizers aggravates incineration pollution | |||||
| • Low autofluorescence | ||||||
| • Easily recyclable | ||||||
| Bio-derived and biodegradable plastics | Shellac | Hot-embossing | Simple microchannel structures | • Bio-derived and biodegradable | • No transparency | Lausecker, 201658 |
| Zein | Hot-embossing | Microfluidic gradients | • Bio-derived and biodegradable | • No transparency | Hsiao 201159 | |
| PLA | 3D printing, laser cutting, injection moulding | Droplet, mixers, DNA melting, cell culture, protein analysis | • Good transparency | • No sheets available commercially | Tsuda, 2015;60 Tothill, 2017;61 Ongaro, 2018;62 Romanov, 2018;63 Ongaro, 202064 | |
| • Low autofluorescence | ||||||
| • Mechanical recycling | ||||||
| Natural fibrous materials | Paper | Wax printing | Lateral flow immunoassays; DNA-based assays; blood typing | • Low cost | • 2D microfluidic device | Martinez, 2010;65 Carrell, 2019;66 Reboud 201967 |
| • Light weight | • No transparency | |||||
| •Readily available | • Limited volume capacity | |||||
| •Easily recyclable | ||||||
| Wood | Laser cutting | Simple microfluidic structures; protein assay | • Low cost | •Material with inherent biological, chemical and mechanical variability | Andar 2019;68 Brigham 201869 | |
| • More rigid than paper | ||||||
| Cotton | Coating; laser writing | Immunoassay, colorimetry, wearable, blood microsampling | • Low cost | • Fragility | Wu, 2015;89 Ulum 2016;71 Xiao 2019;72 Stojanović, 202073 | |
| • High flexibility | • No transparency | |||||
| • Amenable to wearable applications |
Bio-based polymers
Bio-based plastics are polymers derived from organic biomass sources and may represent an alternative to recycled plastics.74–76 Biodegradable plastics are in line with the European Plastics Strategy, which aims to substitute polymers from fossil fuel-based resources with more environmentally friendly alternatives. The choice of bio-derived biodegradable material can strongly decrease the potential environmental footprint associated with raw material extraction, as well as reducing some of the environmental and human health concerns associated with incineration. In this section we review studies using bio-derived materials for microfluidic production.PLA, with its high biocompatibility and bioresorbability, has found applications in tissue engineering applications, food packaging and drug delivery. As a polyester alpha, it can be processed via injection moulding, extrusion, hot embossing, solvent casting and film blowing and has gained more and more attention after the development of fusion filament deposition 3D printing in desktop 3D printers. In addition to having medical grade properties, PLA is relatively cheap ($1.58 per kg as of January 2022), making this bio-based and biodegradable polymer attractive to the POCT market. A number of scientific providers have started proposing biodegradable consumables: for example, Sigma Aldrich sells plastic stirrers and spoons made of PLA.
PLA has been demonstrated in microfluidic applications using prototyping techniques such as 3D printing and laser cutting. A laser cutting and layer-by-layer lamination approach allows flexibility in the design, is user-friendly and low-cost, does not require the need for post-treatment and can be applied to an almost unlimited number of materials.80,81 This approach also enables surface and local treatments, and integration of various complex elements, such as membranes or electrodes.
We have pioneered techniques for the use of PLA in single-use microfluidic devices with well-controlled characteristics.62,64 Our work has shown the possibility to microstructure complex PLA-based microfluidic devices in a few minutes. The devices have shown better performance with respect to qPCR inhibition in comparison with PMMA; good transparency without the need to integrate optical windows; better biocompatibility than other typical thermoplastic materials employed for microfluidic cell culture or organ-on-a-chip;64 no absorption or adsorption of small molecules; and the possibility of integrating graphene water ink-printed electrodes to perform electrochemical analysis.62
Furthermore, advances in 3D printing technologies in the last five years have enabled fabrication of PLA-based devices on a scale compatible with microfluidic features. For example, Tothill et al. manufactured a 3D-printed device for a glucose assay.61 Despite very promising results, the fabrication of 3D-printed PLA microfluidic devices uses fused deposition modeling (FDM), which still has some limitations, such as printing time (resulting in low throughput), low resolution, poor surface finish and the need for post-treatment. Moreover, a good transparency has not yet been achieved, which can limit applications, thus necessitating imaging. Some research has been carried out in order to overcome this transparency issue.63,82,83
To conclude, PLA has been demonstrated as a new suitable substrate material for the production of environmentally sustainable microfluidic devices for POCT applications and shown to be compatible with prototyping techniques such as 3D printing and laser cutting. In addition, PLA can be injection moulded, and prototyped structures via FDM, or layer-by-layer assembly, should be easily adaptable to mass manufacture.
With incineration as the end-of-life scenario, the environmental benefit of biodegradable bioderived polymers lies in the use of renewable raw materials. In a landfill scenario, in addition to the raw materials, the benefits will also lie in fast degradation. However, unlike shellac or zein,84,85 PLA does not readily degrade in the natural environment.86 PLA biodegradation necessitates specific waste management, such as segregated collection and industrial composting facilities. While this is feasible and thus of interest for high-income countries, in an LMIC context, and considering landfills, PLA solutions would have no other positive environmental impact, beyond the use of renewable raw materials.
The transition from fossil-based to bio-based or recycled substrate materials represents, in the field of point-of-care and lab-on-chip, only a small step towards the goal of reaching truly sustainable solutions. A mere material substitution might not ease the environmental burden associated with the POCT, and water consumption and cradle-to-gate equivalent CO2 consumption per tonne of material produced should be taken into consideration when considering a material substitution. A recent review of bioplastics offers a comprehensive overview about the challenges and opportunities offered by these new materials.87 More research on the matter, outreach activities to raise awareness,88 clear regulations, and financial incentives will be pivotal to ensure the adoption of sustainable solutions that are adequate from both environmental and economical perspectives.
Natural fibres
Of interest to this review, paper is also being used to replace the casings and non-cellulose pads in traditional LFAs. LIA Diagnostics was the first commercial company to propose an FDA-approved, flushable LFA pregnancy test, based on a 100% cellulose structure.91 More recently, Okos diagnostics has introduced a 100% cellulose-based LFA, although not commercialised nor FDA approved yet.92 Overall, we hope to see paper microfluidic devices implemented on mass manufacturing lines in the near future, replacing plastic-based and bulkier LFAs when appropriate.
Reagents and sustainable alternatives
The problem of diagnostic waste goes well beyond the issue of how to replace or dispose of single-use plastics. Diagnostic tests often contain chemical reagents that need to be disposed of safely after use, dictated by local chemical waste disposal routes. Illegal or negligent disposal of chemical liquid waste in landfills and public sewage systems can lead to the contamination of groundwater and drinking water.6,11 Here we review some of the toxic waste associated with diagnostics, and possible alternatives.Alcohols represent the second major class of fixation agents adopted. They represent a safer and cleaner alternative solution to aldehydes, with ethanol, methanol, denatured alcohol and isopropyl alcohol being appropriate replacements.99 Natural alternatives such as honey, sugar syrup and jaggery have all been successfully proposed.100–103 Sabarinath et al. reported that honey can be effectively used as a fixative when compared with formalin.102 They were able to image nuclear details both in honey and formalin without observing differences in subsequent staining protocols and microscopic morphology. However, the intrinsic viscosity of honey can make it a complicated material to work with at the microfluidic scale. Culinary sugar has been proposed by Patil et al. as a fixative agent, together with jaggery, and sugar syrup.101 They observed that jaggery fixation performed as well as standard formaldehyde fixation methods. Another study investigated substitution of formalin for commercially available and ‘home-made’ fixative solutions. Zanini et al. demonstrated that formaldehyde can be replaced by commercially available alternatives (RCL2 and CellBlock); custom fixatives including PAGA (polyethylene glycol, ethyl alcohol, glycerol, acetic acid); and two zinc-based fixatives (ZBF, Z7) as tested on more than 200 specimens.104 We envisage that more advances in the green chemistry field and future regulatory requirements for safer and cleaner products will help to generate new potential candidates to replace hazardous fixative reagents.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 litres of effluent chemical waste have been generated for HIV testing alone. Such tests, and many others, such as tuberculosis diagnostics, involve solid-phase nucleic acid extraction. Most kits use chaotropic agents made of cyanide derivatives, such as guanidinium thiocyanate.105 Cyanide salts are highly toxic both to human and aquatic life, and should not be flushed into drains or buried. These issues are compounded by the fact that contact between cyanide salts and oxidising agents like bleach can lead to the release of toxic cyanide gas.
000 litres of effluent chemical waste have been generated for HIV testing alone. Such tests, and many others, such as tuberculosis diagnostics, involve solid-phase nucleic acid extraction. Most kits use chaotropic agents made of cyanide derivatives, such as guanidinium thiocyanate.105 Cyanide salts are highly toxic both to human and aquatic life, and should not be flushed into drains or buried. These issues are compounded by the fact that contact between cyanide salts and oxidising agents like bleach can lead to the release of toxic cyanide gas.
Manufacturers recommend guanidinium thiocyanate to be disposed of according to country-specific rules, but countries where these POCTs are mostly needed often have a lack of waste regulations. Furthermore, safe disposal of cyanide derivatives involves incineration at a high temperature (850 °C) under specific conditions.106 Incineration facilities with such capabilities are not readily available, especially in lower-income countries. In one anecdotal instance, practitioners have reported driving to a local cement factory to safely dispose of the materials.106 Such arrangements are cumbersome, and unlikely to be followed by many institutions, which are operating under time, staffing and financial pressures. A number of calls have already been made to identify waste management solutions to these problems.11 However, better waste management can only address part of the problem and safer reagents are needed.
In order to avoid the use of chaotropic reagents, non-chaotropic methods have been developed as alternative techniques. Recently Merck introduced GenElute™-E Single Spin DNA and RNA Purification Kits specifically to address environmental issues. With this product they claim waste prevention (55% reduction in plastic), sustainable packaging and safer disposal (no hazardous liquid waste). The kit works on the principle of negative chromatography using size exclusion, which has been implemented on-chip before. Rodrigues et al. reported the use of sodium dodecyl sulphate (SDS) and acetyl trimethyl ammonium bromide as extraction reagents for the isolation of DNA from two types of fungal materials.107 In this work they also reported using vortexing with glass beads inside a closed tube for mechanical disruption of conidia cell walls. Aminosilane-modified surfaces,108 magnetic particles coated with APTES,109 or chitosan-coated beads110 have been proposed and demonstrated for DNA extraction on whole blood or E. coli cells. The dimethyl adipimidate (DMA)-based solid-phase extraction method resulted in successful extraction of DNA in whole blood and urine, but yielded a significantly lower DNA amount than the commercial benchmark using standard chaotropes.111
A number of safer and more environmentally friendly alternatives to chaotropic agents have been proven to be efficient for DNA extraction but remain behind standard chaotropic methods in terms of DNA yield.
Roadmap to sustainable and safe POCT and LOC development
In order for the sustainability and safety challenges associated with the disposal of POCTs to be addressed, action is required across the product lifecycle. Significant investment is needed to strengthen waste management infrastructure in LMICs. More can also be done to strengthen regulatory requirements for green diagnostic products at a global and national level. However, researchers, product developers and manufacturers also need to play their part. Here, we discuss some guidelines to improve sustainability at multiple levels, from regulators to designers.Rather than place full responsibility for safe disposal of POCTs on users, there is a need for POC providers to contribute to solutions at the end of the product life-cycle. This includes improving the safety and sustainability of their products without decreasing the affordability of POCTs. To encourage such developments, TPPs published by the WHO and other international organizations, produced to guide technical specifications for future diagnostic tests, ought to provide dedicated guidance on acceptable features for waste disposal. Such guidance from TPPs can place emphasis on an expectation for the environmental footprint of such tests to be small, using either recyclable or compostable materials, without the need for high-temperature incineration. Advocacy work through civil society can also put pressure on POC providers to consider waste management at the design stage so as to minimize harm to human health and the environment.
POC test providers could also support initiatives to improve national waste management infrastructures and regulatory frameworks, including workforce training, in LMICs through either corporate social responsibility efforts or the adoption/enforcement of extended producer responsibility (EPR) mechanisms. The African Society for Laboratory Medicine (ASLM), through the Laboratory Systems Strengthening Community of Practice, could expand its regional influential role of monitoring national waste management systems in LMICs, coordinating and sharing resources to strengthen these whilst also linking POC providers, the WHO, etc., with countries that need support.
Commendable efforts from Europe's primary regulator for materials, ECHA, on banning certain types of plastics from the market will go a long way towards curbing plastic waste112 and such efforts should be replicated by other regulatory agencies. In 2017, the WHO published the global model regulatory framework for medical devices, including in vitro diagnostic medical devices,113 and this framework emphasises that waste management practices should follow the manufacturer's instructions. The revised version of this guidance, which is still in production, must contain explicit language regarding the need for POCTs to have a small environmental footprint.
The product life cycle starts with the extraction, processing and supply of the raw materials, to cover then the production of the product, its distribution, use (and possibly reuse and recycling), and its ultimate disposal. The life cycle of POCTs is illustrated in Fig. 3. Environmental impacts occur in different phases of the product lifecycle and should be accounted for in an integrated and holistic way. The DfS approach can be applied to medical microcomponents, taking into account the need to: (i) minimise non-renewable energy consumption; (ii) use environmentally preferable materials; (iii) protect and conserve water; (iv) minimise waste; and (v) lengthen the product shelf-life. In addition, in the context of sustainable POCT development, DfS principles should be expanded to minimise the potential for people to come into contact with harmful chemicals and take human risks associated with the disposal of POCTs into account.
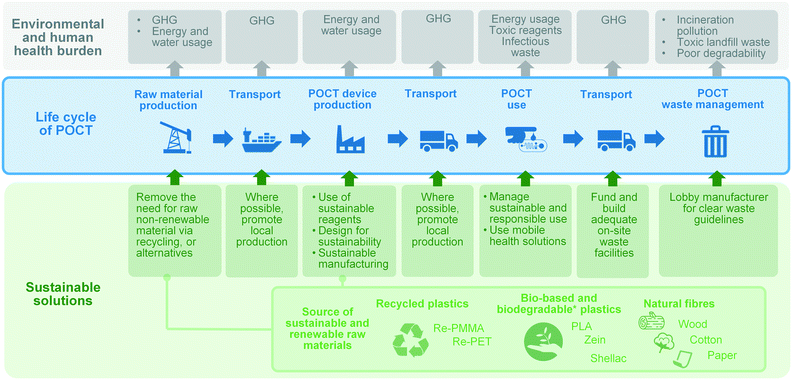 | ||
| Fig. 3 Overview of the life cycle of POCTs, POCT-associated environmental burden and sustainable solutions associated with each stage of the life-cycle. | ||
A simple approach to DfS for POC engineers starts by thinking about beginning of life (e.g., where does the raw material come from?) and end of life (e.g., what happens to the product once used?). Designing diagnostic methods that use lower reagent volumes, or do more multiplexing to be able to run several tests in one reaction would be advantageous in terms of environmental sustainability and economic considerations, both of which are already embedded in microfluidic concepts. Engineers may consider if the product could be recycled and, if so, how can the design improve the chance of it being recycled. The Sustainability Guide project117 has produced a number of freely accessible DfS resources.
Researchers may be able to reduce the environmental impact of their approaches by considering their substrate materials and reagents. When possible, natural fibres, which promise to cut the plastic burden, should be used. A pragmatic approach for researchers and designers is to create a list of materials and reagents used in the fabrication of prototypes, their pros and cons, and consider whether sustainable alternatives are available or not. One straightforward substitution is pristine PMMA for recycled PMMA. If a research lab cannot afford the replacement, a good practice to encourage sustainability might be to flag the possibility in the publication materials and methods section. Researchers should consider recycling prototypes and ancillary plastics when possible. Complete recycling and re-use cycles for microfluidic chips have been demonstrated.51 If such re-use cycles are not possible, specialist services may be used to collect plastic waste and get it recycled externally. Only plastics that have not been in contact with infectious waste should be recycled, however, and researchers should make sure that robust health and safety protocols are in place for recycling activities.
As illustrated in Fig. 3, one of the main GHG emission sources in the life cycle of a POCT is transportation. Where possible, local materials should be considered over materials shipped from overseas. In addition to the contribution that global supply chains make to GHG emissions, the shortage of tests during the COVID-19 pandemic has shown the importance of resilience and local manufacturing at a basic research and industrial level. Thus when possible, local production and adequate on-site waste facilities should be promoted. Furthermore, local facilities might serve as POCT production sites at the point-of-need by implementing state-of-the-art rapid and agile technologies, such as 3D printing. Finally, mobile health, the application of mobile devices and related technologies to healthcare, is playing a significant role in under-served communities where infrastructure around transport and healthcare is lacking.118–120 Automatic smartphone-based diagnostic systems have been the subject of numerous investigations as well as several commercial endeavours.118,121–123 While mobile health solutions are sought for providing convenient point-of-care solutions, they also effectively reduce associated infrastructure, transport and logistics around diagnostics, thus mitigating the GHG emission of single use diagnostics. Where possible, mobile health solutions should be considered by developers as another way to improve the environmental sustainability of point-of-care diagnostics.
Conclusion
There is a growing awareness of the challenge of diagnostic waste in the global health community, but the proposed solutions focus on the country level, i.e., by improving waste management infrastructure, or enhancing the quality of incinerators. Green procurement schemes focused on products' environmental sustainability (e.g., that of the Global Fund) are on the rise, but if there are no green products available, their impact will be limited. Meanwhile, due to the lack of economic incentives and regulatory hurdles, there has been little appetite among POC providers for investing in developing more sustainable and safer POCT devices.While this review focuses on the consumable element of POCTs, it is worth noting that the consumable is only one part of the POCT process. Packaging and instrumentation should also be considered as well. However, this review of sustainable POCTs is a first step, helping to highlight the importance of innovation for sustainable and safe diagnostics. The opportunities and challenges associated with mass manufacturing and high-volume deployment of POCTs reveal the intersection of environmental and human health crises, in a world increasingly vulnerable to the impacts of climate change and global pandemics and demonstrate the importance of a planetary health approach to biomedical innovation.
Design for sustainability and safety should be on everyone's mind and researchers, healthcare providers, policy makers and regulators, funders, industrials, charity and civil society all have a duty to create sustainable and safe innovations and a role to play. Importantly, sustainability should not be a ‘siloed’ academic activity, and interdisciplinarity is crucial.
Embedding sustainability and safety considerations at an early stage is key to reduce the impact that future technologies will have on the environment. In addition, it will help to reduce GHG emissions, reduce dependence on fossil resources and support the achievement of a net-zero CO2 emission by 2050, which is essential to deliver on post-Paris (COP 21) climate commitments. The rationale behind material choice for the manufacturing of a new microfluidic device has always been driven by physical and chemical properties and prototyping technologies. It should not perhaps come as a surprise that over 20 petrochemical-derived polymers are currently favoured by both academic laboratories and industries in the R&D of microfluidic techniques for point-of-care diagnostics because of their performance, reliability and well controlled manufacturing processes.
The adoption of recycled or bio-derived materials in the field of single-use POCT diagnostics will form strong foundations to pursue the reduction of CO2 emissions and plastic pollution in places of use. It is important to underline as well that the material choice cannot be decoupled from consideration of and actions around how the used material is manufactured and disposed of. The potential for circularity in the field of single-use medical consumable is very slowly emerging, but with adequate funding, technological advances and regulatory support could constitute a radical new approach to waste management in the sector.
All actors of the POCT market share responsibility for the waste generated from innovative microfluidic technologies and need to be a part of the solution. Moreover, some sustainable materials appropriate for POCT manufacture already exist and several academic and industry groups have sustainable POCT applications in the pipeline, even if such initiatives have received little visibility.
Although regulatory change is anticipated in the near future, POCT providers will remain reluctant to engage unless they can see tangible technical feasibility and economic benefits, without compromise on diagnostic performance. Therefore, we are in need of models to show that it is technically possible and commercially viable to develop single-use diagnostics using local and sustainable materials, and this initiative should be collaboratively supported by researchers within academia, the point-of-care diagnostic industry and end-users alike.
Author contributions
This work was conceptualised by A. E. O., A. S. and M. K. K., A. E. O., A. S. and M. K. K. wrote the original draft. Z. N., E. S., C. O. and P. O. reviewed and edited the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
MKK is funded by the UK Engineering and Physical Sciences Research Council (Healthcare Technology Challenge Award EP/R00398X/1). AS is funded by the European Research Council (DiaDev grant agreement No 715450).References
- P. Ondoa, Y. Kebede, M. Massinga Loembe, J. N. Bhiman, S. Kifle Tessema, A. Sow, A. Alpha Sall and J. Nkengasong, Lancet Microbe, 2020, 1(3), E103–E104 CrossRef CAS PubMed.
- S. Kleinert and R. Horton, Lancet, 2021, 398, 1945–1947 CrossRef CAS.
- Point of Care & Rapid Diagnostics Market - Global Forecast to 2027|MarketsandMarkets, https://www.marketsandmarkets.com/Market-Reports/point-of-care-diagnostic-market-106829185.html?gclid=Cj0KCQjwxtSSBhDYARIsAEn0thSq_WeoIJSYBUDc_RasH1oIbxcHQBjkcZlRi_96XlBe3oziTCKIYMQaApjCEALw_wcB, (accessed 12 April 2022).
- K. A. Fleming, S. Horton, M. L. Wilson, R. Atun, K. Destigter, J. Flanigan, S. Sayed, P. Adam, B. Aguilar, S. Andronikou, C. Boehme, W. Cherniak, A. N. Cheung, B. Dahn, L. Donoso-Bach, T. Douglas, P. Garcia, S. Hussain, H. S. Iyer, M. Kohli, A. B. Labrique, L.-M. Looi, J. G. Meara, J. Nkengasong, M. Pai, K.-L. Pool, K. Ramaiya, L. Schroeder, D. Shah, R. Sullivan, B.-S. Tan and K. Walia, Lancet, 2021, 398, 1997–2050 CrossRef.
- Y. Chartier, J. Emmanuel, U. Pieper, A. Prüss, P. Rushbrook, R. Stringer, W. Townend, S. Wilburn and R. Zghondi, Safe management of wastes from health-care activities, 2nd edn, 2014 Search PubMed.
- A. Street, E. Vernooij and M. H. Rogers, Glob. Health, 2022, 18, 1–7 CrossRef PubMed.
- J. M. Chisholm, R. Zamani, A. M. Negm, N. Said, M. M. Abdel Daiem, M. Dibaj and M. Akrami, Waste Manage. Res., 2021, 39, 1149–1163 CrossRef CAS PubMed.
- World Health Organization and The United Nations Children's Fund, WASH in health care facilities: Global Baseline Report, 2019 Search PubMed.
- S. F. Hayleeyesus and W. Cherinete, J. Health Pollut., 2016, 6, 64–73 CrossRef PubMed.
- Safe management of wastes from health-care activities, World Health Organization, 2nd edn, 2014 Search PubMed.
- C. O. Odhiambo, A. Mataka, G. Kassa and P. Ondoa, J. Hazard. Mater. Lett., 2021, 2, 100030 CrossRef PubMed.
- World Health Organization, Global Analysis of Health Care Waste in the Context of COVID-19: Status, Impacts and Recommendations, Geneva, 2022 Search PubMed.
- Centre for Disease Control and Prevention, CDC-Funded HIV Testing in the United States, Puerto Rico, and U.S. Virgin Islands, 2019 ANNUAL HIV TESTING REPORT, 2019 Search PubMed.
- M. Pai and J. Furin, eLife, 2017, 6 DOI:10.7554/ELIFE.25956.001.
- R. Wittenauer, S. Nowak and N. Luter, Malar. J., 2022, 21, 12 CrossRef PubMed.
- H. Becker and L. Locascio, Talanta, 2002, 56(2), 267–287 CrossRef CAS PubMed.
- E. S. Windfeld and M. S. L. Brooks, J. Environ. Manage., 2015, 163, 98–108 CrossRef CAS PubMed.
- A. M. Messenger, A. N. Barnes and G. C. Gray, PLoS One, 2014, 9, e89055 CrossRef PubMed.
- Developing world should reap benefits of new monkeypox research, experts urge|Reuters, https://www.reuters.com/business/healthcare-pharmaceuticals/concern-grows-that-human-medical-waste-implicated-virus-spillovers-2022-06-02/, (accessed 7 June 2022).
- U. Beisel, R. Umlauf, E. Hutchinson and C. I. R. Chandler, Malar. J., 2016, 15, 1–9 CrossRef PubMed.
- T. Källqvist, The Sustainable Development Goals in the EU budget, Briefing from the Policy Department for Budgetary Affairs, European Parliament, 2021, DOI:10.2861/504604.
- R. Peeling and D. Mabey, Clin. Microbiol. Infect., 2010, 16, 1062–1069 CrossRef CAS PubMed.
- G. L. Damhorst, M. Murtagh, W. R. Rodriguez and R. Bashir, Proc. IEEE, 2015, 103, 150–160 CAS.
- S. Nayak, N. R. Blumenfeld, T. Laksanasopin and S. K. Sia, Physiol. Behav., 2018, 176, 139–148 Search PubMed.
- W. C. Tian and E. Finehout, Microfluidic Diagnostic Systems for the Rapid Detection and Quantification of Pathogens, in Microfluidics for Biological Applications, Springer, Boston, MA, 2008, DOI:10.1007/978-0-387-09480-9_9.
- C. D. Chin, V. Linder and S. K. Sia, Lab Chip, 2007, 7, 41–57 RSC.
- World Health Oganization, The selection and use of essential in vitro diagnostics, Geneva, 2021 Search PubMed.
- X. Y. D. Soo, S. Wang, C. C. J. Yeo, J. Li, X. P. Ni, L. Jiang, K. Xue, Z. Li, X. Fei, Q. Zhu and X. J. Loh, Sci. Total Environ., 2022, 807, 151084 CrossRef CAS PubMed.
- P. Morone, G. Yilan, E. Imbert and L. Becchetti, Sci. Rep., 2022, 12, 1–11 CrossRef PubMed.
- A. H. Velders, M. Ossendrijver, B. J. F. Keijser, V. Saggiomo, A. H. Velders, V. Saggiomo, M. Ossendrijver and B. J. F. Keijser, Glob. Challenges, 2022, 6, 2100078 CrossRef PubMed.
- FACT SHEET: Overview of USDA's BioPreferred Program|USDA, https://www.usda.gov/media/press-releases/2016/02/18/fact-sheet-overview-usdas-biopreferred-program, (accessed 14 April 2022).
- Green Public Procurement - Environment - European Commission, https://ec.europa.eu/environment/gpp/index_en.htm, (accessed 14 April 2022).
- Sustainable Public Procurement | Asian Development Bank, https://www.adb.org/documents/sustainable-public-procurement, (accessed 14 April 2022).
- M. Ziyaina, B. Rasco and S. S. Sablani, Food Control, 2020, 110, 107008 CrossRef.
- J. Li, Y. Zhu, X. Wu and M. R. Hoffmann, Clin. Infect. Dis., 2020, 71, S84 CrossRef PubMed.
- F. Foret, P. Smejkal and M. Macka, Liq. Chromatogr.: Fundam. Instrum., 2013, 453–467 CAS.
- K. L. Wlodarczyk, D. P. Hand and M. M. Maroto-Valer, Sci. Rep., 2019, 9, 20215 CrossRef CAS PubMed.
- A. J. L. Morgan, L. Hidalgo San Jose, W. D. Jamieson, J. M. Wymant, S. Bing, P. Stephens, D. A. Barrow and O. K. Castell, PLoS One, 2016, 11, e0152023 CrossRef PubMed.
- A. Liga, J. A. S. Morton and M. Kersaudy-Kerhoas, Microfluid. Nanofluid., 2016, 20 DOI:10.1007/s10404-016-1823-1.
- U. M. Attia, S. Marson and J. R. Alcock, Microfluid. Nanofluid., 2009, 7(1) DOI:10.1007/s10404-009-0421-2.
- J. Friend and L. Yeo, Biomicrofluidics, 2010, 4, 026502 CrossRef PubMed.
- J. Hiltunen, C. Liedert, M. Hiltunen, O. Huttunen, J. Hiitola-Keinänen, S. Aikio, M. Harjanne, M. Kurkinen, L. Hakalahti and L. P. Lee, Lab Chip, 2018, 18, 1552–1559 RSC.
- S. T. Sanjay, M. Dou, J. Sun and X. Li, Sci. Rep., 2016, 6, 30474 CrossRef CAS PubMed.
- R. Lohmann and K. C. Jones, Sci. Total Environ., 1998, 219, 53–81 CrossRef CAS PubMed.
- Recycling of Plastics, Applied Plastics Engineering Handbook, ed. M. Kutz, 2011, ch. 11.1.1, Elsevier, ISBN 978-1-4377-3514-7 Search PubMed.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef CAS PubMed.
- J. W. Lee, S. R. Daly, A. Y. Huang-Saad, C. M. Seifert and J. Lutz, Microfluid. Nanofluid., 2018, 22, 1–22 CrossRef PubMed.
- L. A. Utracki, P. Mukhopadhyay and R. K. Gupta, Polym. Blends Handb., 2014, 3–170 Search PubMed.
- A. R. Prado, A. Frizera, A. G. Leal-Junior, C. Marques, G. L. de Sena, L. C. Machado, M. J. Pontes, M. R. N. Ribeiro and S. Leite, Opt. Express, 2017, 25(24), 30051–30060 CrossRef CAS PubMed.
- A. M. D. Wan, D. Devadas and E. W. K. Young, Sens. Actuators, B, 2017, 253, 738–744 CrossRef CAS.
- J. Lachaux, C. Alcaine, B. Gómez-Escoda, C. M. Perrault, D. O. Duplan, P. Y. J. Wu, I. Ochoa, L. Fernandez, O. Mercier, D. Coudreuse and E. Roy, Lab Chip, 2017, 17, 2581–2594 RSC.
- Flexdym Starter Pack - Alternative to PDMS - Eden Tech, https://eden-microfluidics.com/eden-materials/flexdym-pack-alternative-to-pdms/, (accessed 7 June 2022).
- A. E. Ongaro, G. Conoscenti, A. Liga, V. Brucato, M. P. Y. Desmulliez, N. Howarth, V. La Carrubba and M. Kersaudy-Kerhoas, Advances in Transdisciplinary Engineering, 2017, 6, 181–186 Search PubMed.
- A. E. Ongaro, N. Howarth, V. La Carrubba and M. Kersaudy-Kerhoas, Advances in Transdisciplinary Engineering, 2018, vol. 8, 107–112 Search PubMed.
- J. M. Li, C. Liu, H. C. Qiao, L. Y. Zhu, G. Chen and X. D. Dai, J. Micromech. Microeng., 2007, 18, 015008 CrossRef.
- K. R. Jackson, J. C. Borba, M. Meija, D. L. Mills, D. M. Haverstick, K. E. Olson, R. Aranda, G. T. Garner, E. Carrilho and J. P. Landers, Anal. Chim. Acta, 2016, 937, 1–10 CrossRef CAS PubMed.
- R. Lausecker, V. Badilita, U. Gleißner and U. Wallrabe, Biomicrofluidics, 2016, 10, 044101 CrossRef CAS PubMed.
- A. Hsiao, J. Luecha, J. Kokini and L. Liu, in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, 2011 Search PubMed.
- S. Tsuda, H. Jaffery, D. Doran, M. Hezwani, P. J. Robbins, M. Yoshida and L. Cronin, PLoS One, 2015, 10, e0141640 CrossRef PubMed.
- A. M. Tothill, M. Partridge, S. W. James and R. P. Tatam, J. Micromech. Microeng., 2017, 27, 035018 CrossRef.
- A. E. Ongaro, I. Keraite, A. Liga, G. Conoscenti, S. Coles, H. Schulze, T. T. Bachmann, K. Parvez, C. Casiraghi, N. Howarth, V. La Carubba and M. Kersaudy-Kerhoas, ACS Sustainable Chem. Eng., 2018, 6(4), 4899–4908 CrossRef CAS.
- V. Romanov, R. Samuel, M. Chaharlang, A. R. Jafek, A. Frost and B. K. Gale, Anal. Chem., 2018, 90, 10450 CrossRef CAS PubMed.
- A. E. Ongaro, D. Di Giuseppe, A. Kermanizadeh, A. Miguelez Crespo, A. Mencattini, L. Ghibelli, V. Mancini, K. L. Wlodarczyk, D. P. Hand, E. Martinelli, V. Stone, N. Howarth, V. La Carrubba, V. Pensabene and M. Kersaudy-Kerhoas, Anal. Chem., 2020, 92, 6693–6701 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2010, 82, 3–10 CrossRef CAS PubMed.
- C. Carrell, A. Kava, M. Nguyen, R. Menger, Z. Munshi, Z. Call, M. Nussbaum and C. Henry, Microelectron. Eng., 2019, 206, 45–54 CrossRef CAS.
- J. Reboud, G. Xu, A. Garrett, M. Adriko, Z. Yang, E. M. Tukahebwa, C. Rowell and J. M. Cooper, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 4834–4842 CrossRef CAS PubMed.
- A. Andar, M. S. Hasan, V. Srinivasan, M. Al-Adhami, E. Gutierrez, D. Burgenson, X. Ge, L. Tolosa, Y. Kostov and G. Rao, Anal. Chem., 2019, 91, 11004–11012 CrossRef CAS PubMed.
- C. Brigham, Green Chem. An Incl. Approach, 2018, pp. 753–770 Search PubMed.
- P. Wu and C. Zhang, Lab Chip, 2015, 15(6), 1598–1608 RSC.
- M. F. Ulum, L. Maylina, D. Noviana and D. H. B. Wicaksono, Lab Chip, 2016, 16, 1492–1504 RSC.
- G. Xiao, J. He, X. Chen, Y. Qiao, F. Wang, Q. Xia, L. Yu and Z. Lu, Cellulose, 2019, 26, 4553–4562 CrossRef CAS.
- G. M. Stojanović, M. M. Radetić, Z. V. Šaponjić, M. B. Radoičić, M. R. Radovanović, Ž. V. Popović and S. N. Vukmirović, Appl. Sci., 2020, 10, 4392 CrossRef.
- World Economic Forum, The New Plastics Economy: Rethinking the future of plastics, 2016 Search PubMed.
- S. Thakur, J. Chaudhary, B. Sharma, A. Verma, S. Tamulevicius and V. K. Thakur, Curr. Opin. Green Sustainable Chem., 2018, 13, 68–75 CrossRef.
- H. Karan, C. Funk, M. Grabert, M. Oey and B. Hankamer, Trends Plant Sci., 2019, 24, 237–249 CrossRef CAS PubMed.
- E. Corradini, P. S. Curti, A. B. Meniqueti, A. F. Martins, A. F. Rubira and E. C. Muniz, Int. J. Mol. Sci., 2014, 15, 22438–22470 CrossRef CAS PubMed.
- E. Castro-Aguirre, F. Iñiguez-Franco, H. Samsudin, X. Fang and R. Auras, Adv. Drug Delivery Rev., 2016, 107, 333–366 CrossRef CAS PubMed.
- E. T. H. Vink, D. A. Glassner, J. J. Kolstad, R. J. Wooley and R. P. O'Connor, Ind. Biotechnol., 2007, 3, 58–81 CrossRef CAS.
- M. I. Mohammed, M. N. H. Zainal Alam, A. Kouzani and I. Gibson, J. Micromech. Microeng., 2016, 27, 015021 CrossRef.
- B. K. Gale, A. R. Jafek, C. J. Lambert, B. L. Goenner, H. Moghimifam, U. C. Nze and S. K. Kamarapu, Inventions, 2018, 3, 60 CrossRef.
- L. P. Bressan, C. B. Adamo, R. F. Quero, D. P. De Jesus and J. A. F. Da Silva, Anal. Methods, 2019, 11, 1014–1020 RSC.
- G. Gaál, T. A. da Silva, V. Gaál, R. C. Hensel, L. R. Amaral, V. Rodrigues and A. Riul, Front. Chem., 2018, 6, 151 CrossRef PubMed.
- S. Ghoshal, M. A. Khan, R. A. Khan, F. Gul-E-Noor and A. M. S. Chowdhury, J. Polym. Environ., 2010, 18, 216–223 CrossRef CAS.
- T. Lin, C. Lu, L. Zhu and T. Lu, AAPS PharmSciTech, 2011, 12, 172 CrossRef CAS PubMed.
- M. A. Elsawy, K. H. Kim, J. W. Park and A. Deep, Renewable Sustainable Energy Rev., 2017, 79, 1346–1352 CrossRef CAS.
- J. G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed.
- C. Gaberščik, S. Mitchell and A. Fayne, Smart Innov. Syst. Technol., 2021, vol. 200, pp. 205–214 Search PubMed.
- Y. He, Y. Wu, J. Z. Fu and W. Bin Wu, RSC Adv., 2015, 5, 78109–78127 RSC.
- J. A. Adkins and C. S. Henry, Anal. Chim. Acta, 2015, 891, 247–254 CrossRef CAS PubMed.
- Lia Diagnostics has launched its eco-friendly, flushable pregnancy test to consumers - Technical.ly, https://technical.ly/startups/lia-diagnostics-launched-flushable-pregnancy-test/, (accessed 14 April 2022).
- (12) Post | LinkedIn, https://www.linkedin.com/posts/okosdiagnostics_covid-sustainable-testing-activity-6905073401137098752-TVsL/?utm_source=linkedin_share&utm_medium=member_desktop_web, (accessed 14 April 2022).
- X. Li, J. Tian and W. Shen, ACS Appl. Mater. Interfaces, 2010, 2, 1–6 CrossRef CAS PubMed.
- D. Agustini, F. Abio, R. Caetano, R. Fernandes Quero, J. J. José, A. Fracassi Da Silva, M. Arcio, F. Bergamini, A. Luiz, H. Marcolino-Junior and D. Pereira De Jesus, Anal. Methods, 2021, 13, 4830–4857 RSC.
- D. Agustini, M. F. Bergamini and L. H. Marcolino-Junior, Lab Chip, 2016, 16, 345–352 RSC.
- S. Thakur, M. Misra and A. K. Mohanty, ACS Sustainable Chem. Eng., 2019, 7, 8766–8774 CrossRef CAS.
- R. Thavarajah, V. K. Mudimbaimannar, J. Elizabeth, U. K. Rao and K. Ranganathan, J. Oral. Maxillofac. Pathol., 2012, 16, 400 CrossRef PubMed.
- H. A. Alturkistani, F. M. Tashkandi and Z. M. Mohammedsaleh, Glob. J. Health. Sci., 2015, 8, 72–79 CrossRef PubMed.
- R. Ananthalakshmi, S. Ravi, N. Jeddy, R. Thangavelu and S. Janardhanan, J. Clin. Diagn. Res., 2016, 10, EE01 Search PubMed.
- S. Patil, R. S. Rao, B. S. Ganavi and B. Majumdar, J. Nat. Sci., Biol. Med., 2015, 6, 67–70 CrossRef PubMed.
- S. Patil, P. B. R, R. S. Rao and G. B. S, J. Int. Oral. Health., 2013, 5, 31 Search PubMed.
- B. Sabarinath, B. Sivapathasundharam and M. Sathyakumar, J. Histotechnol., 2014, 37, 21–25 CrossRef.
- V. Lalwani, R. Surekha, M. Vanishree, A. Koneru, S. Hunasgi and S. Ravikumar, J. Oral. Maxillofac. Pathol., 2015, 19, 342 CrossRef PubMed.
- C. Zanini, E. Gerbaudo, E. Ercole, A. Vendramin and M. Forni, Environ. Health: Glob. Access Sci. Source, 2012, 11, 1–14 CrossRef PubMed.
- S. C. Tan and B. C. Yiap, J. Biomed. Biotechnol., 2009 DOI:10.1155/2009/574398.
- Z. Ndlovu, E. Fajardo, E. Mbofana, T. Maparo, D. Garone, C. Metcalf, H. Bygrave, K. Kao and S. Zinyowera, PLoS One, 2018, 13, e0193577 CrossRef PubMed.
- P. Rodrigues, A. Venâncio and N. Lima, Lett. Appl. Microbiol., 2018, 66, 32–37 CrossRef CAS PubMed.
- T. Nakagawa, T. Tanaka, D. Niwa, T. Osaka, H. Takeyama and T. Matsunaga, J. Biotechnol., 2005, 116, 105–111 CrossRef CAS PubMed.
- B. Yoza, M. Matsumoto and T. Matsunaga, J. Biotechnol., 2002, 94, 217–224 CAS.
- W. Cao, C. J. Easley, J. P. Ferrance and J. P. Landers, Anal. Chem., 2006, 78, 7222–7228 CrossRef CAS PubMed.
- Y. Shin, A. P. Perera, C. C. Wong and M. K. Park, Lab Chip, 2014, 14, 359–368 RSC.
- EFSA Panel on Contaminants in the Food Chain (CONTAM), EFSA Journal, 2016, DOI:10.2903/J.EFSA.2016.4501/ABSTRACT.
- WHO global model regulatory framework for medical devices including in vitro diagnostic medical devices, https://apps.who.int/iris/handle/10665/255177, (accessed 8 April 2022).
- E. Manzini and C. Vezzoli, J. Cleaner Prod., 2003, 11, 851–857 CrossRef.
- F. Ceschin and I. Gaziulusoy, Des. Stud., 2016, 47, 118–163 CrossRef.
- G. Clark, J. Kosoris, L. N. Hong and M. Crul, Sustainability, 2009, 1, 409–424 CrossRef.
- Sustainability Guide – Tools to secure a sustainable future for generations to come, https://sustainabilityguide.eu/, (accessed 8 April 2022).
- X. Guo, M. A. Khalid, I. Domingos, A. L. Michala, M. Adriko, C. Rowel, D. Ajambo, A. Garrett, S. Kar, X. Yan, J. Reboud, E. M. Tukahebwa and J. M. Cooper, Nat. Electron., 2021, 4, 615–624 CrossRef CAS.
- C. S. Wood, M. R. Thomas, J. Budd, T. P. Mashamba-Thompson, K. Herbst, D. Pillay, R. W. Peeling, A. M. Johnson, R. A. McKendry and M. M. Stevens, Nature, 2019, 566, 467–474 CrossRef CAS PubMed.
- E. Osei, D. Kuupiel, P. N. Vezi and T. P. Mashamba-Thompson, BMC Med. Inf. Decis. Making, 2021, 21, 1–18 CrossRef PubMed.
- D. Erickson, D. O'Dell, L. Jiang, V. Oncescu, A. Gumus, S. Lee, M. Mancuso and S. Mehta, Lab Chip, 2014, 14, 3159–3164 RSC.
- D. Xu, X. Huang, J. Guo and X. Ma, Biosens. Bioelectron., 2018, 110, 78–88 CrossRef CAS PubMed.
- I. Hernández-Neuta, F. Neumann, J. Brightmeyer, T. Ba Tis, N. Madaboosi, Q. Wei, A. Ozcan and M. Nilsson, J. Intern. Med., 2019, 285, 19 CrossRef PubMed.
- Life Cycle Analysis (LCA) - A Complete Guide to LCAs, https://www.bpf.co.uk/sustainable_manufacturing/life-cycle-analysis-lca.aspx, (accessed 11 March 2022).
- M. Ranjbari, Z. Shams Esfandabadi, T. Shevchenko, N. Chassagnon-Haned, W. Peng, M. Tabatabaei and M. Aghbashlo, J. Hazard. Mater., 2022, 422, 126724 CrossRef CAS PubMed.
- Pioneering circularity in the healthcare industry: Royal Philips, https://ellenmacarthurfoundation.org/circular-examples/pioneering-circularity-in-the-healthcare-industry-royal-philips, (accessed 7 June 2022).
- A. J. Macneill, H. Hopf, A. Khanuja, S. Alizamir, M. Bilec, M. J. Eckelman, L. Hernandez, F. McGain, K. Simonsen, C. Thiel, S. Young, R. Lagasse and J. D. Sherman, Health. Aff., 2020, 39, 2088–2097 CrossRef PubMed.
- Pharmafilter - Pharmafilter, https://pharmafilter.nl/en/, (accessed 8 June 2022).
- ECOSTERYL: Medical waste treatment and recycling, https://www.ecosteryl.com/en/, (accessed 8 June 2022).
- P. K. Mallick, K. B. Salling, D. C. A. Pigosso and T. C. McAloone, J. Diabetes Sci. Technol., 2022 DOI:10.1177/19322968221088329.
| This journal is © The Royal Society of Chemistry 2022 |

