Open Access Article
Open Access ArticleDeuteration of terminal alkynes realizes simultaneous live cell Raman imaging of similar alkyne-tagged biomolecules†
Syusuke
Egoshi
 a,
Kosuke
Dodo
a,
Kosuke
Dodo
 *ab,
Kenji
Ohgane
*ab,
Kenji
Ohgane
 a and
Mikiko
Sodeoka
a and
Mikiko
Sodeoka
 *ab
*ab
aSynthetic Organic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: dodo@riken.jp; sodeoka@riken.jp
bRIKEN Center for Sustainable Resource Science, 2-1, Hirosawa, Wako, Saitama 351-0198, Japan
First published on 16th September 2021
Abstract
Alkynes were employed as tags to observe small molecules in cells by Raman microscopy. Herein, simple deuteration was found to shift the vibrational frequency of the alkyne by 135 cm−1. Two-color Raman imaging of D-alkynes and H-alkynes made it possible to distinguish between and observe similar small molecules in live cells.
Raman microscopy is a powerful imaging tool for observing endogenous biomolecules, as it can detect molecular vibrations in cells without exogenous labeling.1 However, cells contain many molecules and display complicated Raman spectra, making label-free Raman imaging of target molecules difficult. To overcome this limitation, several functional groups have been used as Raman tags, including alkynes (C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), nitriles (C
C), nitriles (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), and carbon–deuterium (C–D) bonds.2–6
N), and carbon–deuterium (C–D) bonds.2–6
The carbon–carbon triple bond of alkynes exhibits an intense Raman signal in a Raman-silent region (1800–2600 cm−1) that is free of interference from natural cellular molecules. Alkynes can be introduced without affecting the biological activities of small molecules and can be selectively detected using a Raman microscope with the technique referred to as alkyne-tag Raman imaging (ATRI).2,7 Since ATRI was first established by Sodeoka et al. in 2011, various alkyne tags (e.g., diynes,3,8–1113C-labeled alkynes,12 and gem-difluoroalkynes13) have been developed to observe biomolecules.
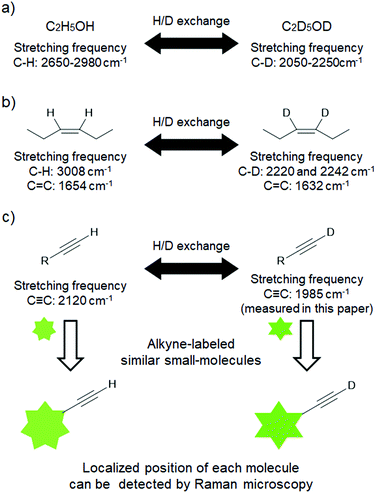
Since D is twice as heavy as H, the Raman scattering of the molecules was significantly affected by deuteration. In fact, C–H bond stretching frequencies of alkanes and alkenes appear in the region of 2650–3050 cm−1, while their corresponding C–D stretching frequencies appear in the region of 2050–2250 cm−1 (e.g., ethanol and ethanol-d6, Fig. 1a).14 Deuteration also affects the vibrational frequency of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, which shifts by approximately 20 to 30 cm−1 (e.g., 1654 cm−1 for 3-cis-hexene and 1632 cm−1 for 3-cis-hexene-3,4-d2, Fig. 1b).15 We recently reported Raman imaging of deuterated γ-linolenic acid based on the deuterated C
C bond, which shifts by approximately 20 to 30 cm−1 (e.g., 1654 cm−1 for 3-cis-hexene and 1632 cm−1 for 3-cis-hexene-3,4-d2, Fig. 1b).15 We recently reported Raman imaging of deuterated γ-linolenic acid based on the deuterated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching signal.16 Therefore, we anticipated that the C
C stretching signal.16 Therefore, we anticipated that the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C vibrational frequency also shifts upon deuteration; deuterated alkynes (D-alkynes) are expected to offer new tags for live cell Raman imaging (Fig. 1c). Our hypothesis was further supported by the density functional theory (DFT) calculations at the B3LYP/6-31G* level. The calculated C
C vibrational frequency also shifts upon deuteration; deuterated alkynes (D-alkynes) are expected to offer new tags for live cell Raman imaging (Fig. 1c). Our hypothesis was further supported by the density functional theory (DFT) calculations at the B3LYP/6-31G* level. The calculated C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C vibrational frequency of 1-hexyne is red-shifted by 148 cm−1 upon deuteration (from 2120 cm−1 to 1972 cm−1). This value is much larger than those observed in the replacement of the alkyne carbons with 13C (e.g., 48 cm−1 for mono-13C-alkyne and 77 cm−1 for di-13C-alkyne).12 Herein, we demonstrate the potential of D-alkynes as Raman tags for live cell Raman imaging.
C vibrational frequency of 1-hexyne is red-shifted by 148 cm−1 upon deuteration (from 2120 cm−1 to 1972 cm−1). This value is much larger than those observed in the replacement of the alkyne carbons with 13C (e.g., 48 cm−1 for mono-13C-alkyne and 77 cm−1 for di-13C-alkyne).12 Herein, we demonstrate the potential of D-alkynes as Raman tags for live cell Raman imaging.
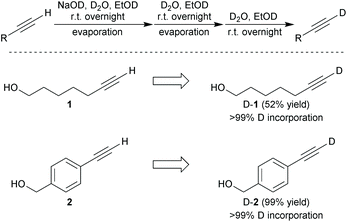
First, to determine the potential of D-alkynes as Raman tags, we examined their physical and chemical properties. D-alkynes were synthesized by the treatment of H-alkynes in heavy water (D2O) containing strong bases.17,18 We synthesized deuterated 6-heptyn-1-ol (D-1) from 1 as a non-conjugated terminal alkyne and deuterated 4-ethynylbenzyl alcohol (D-2) from 2 as a conjugated terminal alkyne using the procedure shown in Scheme 1. High incorporation of D (>99%, determined by 1H-NMR spectroscopy) was achieved by repeating the same reaction three times (Scheme 1). 1 exhibited an alkynyl C–H stretch at 3304 cm−1 and D-1 displayed an alkynyl C–D stretch at 2595 cm−1 (Fig. S1a†). In addition, 2 exhibited alkynyl C–H stretches at 3268 cm−1 and 3289 cm−1, and D-2 displayed alkynyl C–D stretches at 2575 cm−1 and 2585 cm−1 (Fig. S1b†). However, the Raman intensity of the C–H/C–D stretching frequencies was too weak to be observed in cells. In addition, the C–H stretching frequency was difficult to detect in aqueous solutions because the Raman signal of water appears as a broad peak from 2800 cm−1 to 3800 cm−1.19
On the other hand, the effect of deuteration on the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C vibrational frequency was very interesting. Upon deuteration, the C
C vibrational frequency was very interesting. Upon deuteration, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C vibrational frequency of 1 shifted from 2119 cm−1 to 1985 cm−1 (D-1), while that of 2 also shifted similarly from 2110 cm−1 to 1974 cm−1 for D-2 (Fig. S1†). These changes are more pronounced than those from the deuteration of alkenes (∼20 cm−1). The Raman peak area ratio corresponding to deuterated and protonated C
C vibrational frequency of 1 shifted from 2119 cm−1 to 1985 cm−1 (D-1), while that of 2 also shifted similarly from 2110 cm−1 to 1974 cm−1 for D-2 (Fig. S1†). These changes are more pronounced than those from the deuteration of alkenes (∼20 cm−1). The Raman peak area ratio corresponding to deuterated and protonated C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C (<0.01) was in good agreement with the ratio estimated by 1H-NMR analysis (Fig. S1† and 1H-NMR). Furthermore, strong correlations between the molar ratios of the H-/D-alkynes and the Raman peak area ratios of the H-/D-alkynes were confirmed (Fig. S2†). These results indicate that the real-time H/D exchange of terminal alkynes can be quantitatively evaluated by Raman microscopy. Moreover, the difference in vibrational frequencies was approximately 135 cm−1 between the H-/D-alkynes, even though they are nearly identical in structure. We therefore speculated that H-/D-alkynes could be employed as Raman tags to distinguish between and observe similar alkyne-tagged small molecules in cells.
C (<0.01) was in good agreement with the ratio estimated by 1H-NMR analysis (Fig. S1† and 1H-NMR). Furthermore, strong correlations between the molar ratios of the H-/D-alkynes and the Raman peak area ratios of the H-/D-alkynes were confirmed (Fig. S2†). These results indicate that the real-time H/D exchange of terminal alkynes can be quantitatively evaluated by Raman microscopy. Moreover, the difference in vibrational frequencies was approximately 135 cm−1 between the H-/D-alkynes, even though they are nearly identical in structure. We therefore speculated that H-/D-alkynes could be employed as Raman tags to distinguish between and observe similar alkyne-tagged small molecules in cells.
In order to pursue the use of H-/D-alkynes in cells, we first verified the stability of the D-alkynes by measuring the time-course Raman spectra in phosphate buffer (pH 6.0, 7.0, or 8.0) containing 20 mM D-1 or D-2 at 37 °C. Surprisingly, D/H exchange of the D-alkynes was observed at every pH. Moreover, conjugated D-2 reacted faster than non-conjugated D-1 (Fig. 2). The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C Raman peak of D-1 disappeared under slightly alkaline conditions after 8 h at pH 8.0. After 12 h at neutral pH, more than 40% of the C
C Raman peak of D-1 disappeared under slightly alkaline conditions after 8 h at pH 8.0. After 12 h at neutral pH, more than 40% of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C signal of D-1 remained. At slightly acidic pH (pH 6.0), more than 90% of the original C
C signal of D-1 remained. At slightly acidic pH (pH 6.0), more than 90% of the original C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C signal was observed after 12 h (Fig. 2a). Conversely, the C
C signal was observed after 12 h (Fig. 2a). Conversely, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C Raman peak of D-2 was not detected after only 1 h at pH 8.0 and after 4 h at pH 7.0. At pH 6.0, only 20% of the original C
C Raman peak of D-2 was not detected after only 1 h at pH 8.0 and after 4 h at pH 7.0. At pH 6.0, only 20% of the original C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C signal remained after 12 h (Fig. 2b). Furthermore, we found that D/H exchange of the D-alkynes at pH 7.0 depends on the temperature of the solution (Fig. S3†). These results suggest that non-conjugated D-alkynes under neutral or acidic conditions can be employed as Raman tags in cells.
C signal remained after 12 h (Fig. 2b). Furthermore, we found that D/H exchange of the D-alkynes at pH 7.0 depends on the temperature of the solution (Fig. S3†). These results suggest that non-conjugated D-alkynes under neutral or acidic conditions can be employed as Raman tags in cells.
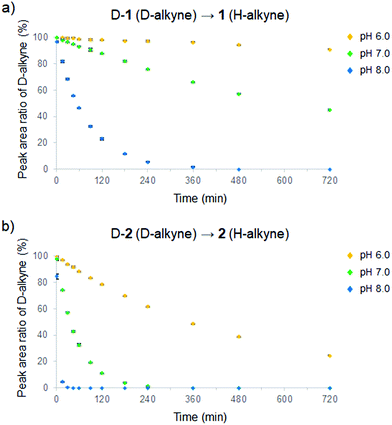 | ||
| Fig. 2 Real-time analysis of D/H exchange from D-alkynes to H-alkynes at various pH (6.0–8.0) in phosphate buffer at 37 °C of (a) D-1 and (b) D-2. Data are presented as mean ± SD (n = 3). | ||
We also investigated the H/D exchange from the H-alkynes to the D-alkynes in deuterated buffer. The buffer pD was adjusted according to pD = pH + 0.41.20 As a result, H/D exchange of the H-alkynes was also observed at all pD, with 2 reacting faster than 1 (Fig. S4†) in the same manner as D-1 and D-2. We then calculated the apparent rate constants for D/H and H/D exchange of the terminal alkynes (Tables 1 and 2). As the pH/pD value increases by 1.0 or the temperature increases by ∼15 °C, the rate constants increase by an order of magnitude. Furthermore, D/H exchange in the pH buffer was faster than H/D exchange in pD buffer of the same value. These results indicate that the D/H or H/D exchange rate strongly depends on the amount of [OH−] or [OD−] in the buffer. A proportional relationship exists between the D/H or H/D exchange rate constants and the amount of [OH−] or [OD−] in each buffer, which were calculated from the pKw values at several temperatures.21 Theoretically, the calculated amount of [OH−] should be 10 times higher than the amount of [OD−] for the same pH and pD values, and thus expected D/H exchange rate constants could be 10 times higher than that of H/D exchange. However, the reaction rate constants of D/H exchange differed from the rate constants of H/D exchange by a factor of approximately three in our experiments (Tables 1 and 2). This difference can be attributed to the slower D/H exchange of the D-alkynes than H/D exchange of the H-alkynes, presumably due to the kinetic isotope effect (KIE) of deuterium.
| pH | °C | OH−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
D-1 to 1 | D-2 to 2 |
|---|---|---|---|---|
| a Obtained from data in Fig. 2 and S3.† b The value (mol L−1) of [OH−] was calculated according to ref. 21. | ||||
| 6.0 | 37 | 2.32 × 10−8 | (1.26 ± 0.005) × 10−4 | (1.97 ± 0.005) × 10−3 |
| 7.0 | 4 | 1.86 × 10−8 | (1.49 ± 0.03) × 10−5 | (1.26 ± 0.007) × 10−4 |
| 20 | 6.50 × 10−8 | (1.05 ± 0.005) × 10−4 | (1.69 ± 0.003) × 10−3 | |
| 37 | 2.32 × 10−7 | (1.16 ± 0.01) × 10−3 | (1.84 ± 0.01) × 10−2 | |
| 8.0 | 37 | 2.32 × 10−6 | (1.17 ± 0.02) × 10−2 | (1.94 ± 0.02) × 10−1 |
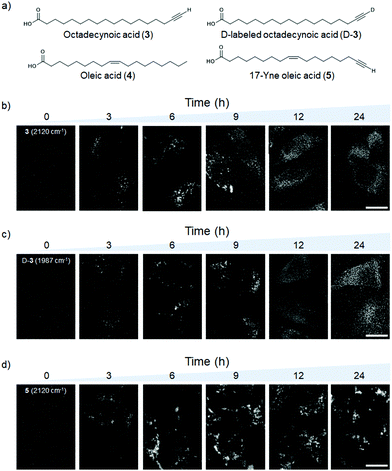
To demonstrate Raman imaging of D-alkynes in live cells, we next turned our attention to long-chain fatty acids (FAs). Saturated FAs (SFAs) exhibit different intracellular metabolism/distribution from unsaturated FAs (UFAs), even with chains of the same carbon number. For example, the C18 SFA octadecynoic acid (3) exhibits a cytoplasmic distribution after accumulating in lipid droplets (LDs),12 whereas the C18 UFA oleic acid (4) remains accumulating in LDs.22 In order to perform simultaneous live cell Raman imaging of C18 SFAs and UFAs labeled with H-/D-alkyne, the appropriate FAs were prepared. Deuterated 17-octadecynoic acid (D-3, 99% D incorporation by 1H NMR spectroscopy) was synthesized from 3 following a procedure similar to that shown in Scheme 1 (see Scheme S1†). Moreover, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C vibrational frequency shifted from 2115 cm−1 to 1980 cm−1 upon deuteration of 3 to obtain D-3, and the Raman peak area ratio between the C
C vibrational frequency shifted from 2115 cm−1 to 1980 cm−1 upon deuteration of 3 to obtain D-3, and the Raman peak area ratio between the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C of D-3 and the C
C of D-3 and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C of 3 resulted in 1
C of 3 resulted in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.013, which is in good agreement with the NMR analysis (Fig. S5a and b† and 1H-NMR).
0.013, which is in good agreement with the NMR analysis (Fig. S5a and b† and 1H-NMR).
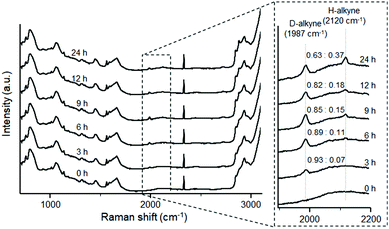
Next, we performed ATRI of living HeLa cells treated with 3, D-3, and alkyne-tagged oleic acid (5; Raman spectra are shown in Fig. S5c†). SFA 3 and D-3 initially accumulated in LDs during the first 6 h of treatment, and then spread throughout the cells during the remaining treatment time (Fig. 3b and c). Conversely, UFA 5 remained within the LDs during the 24 h treatment time (Fig. 3d). Since time-course Raman imaging of D-3 and 3 showed almost the same images (Fig. 3b and c), we concluded that deuteration has little effect on the intracellular metabolism of these labeled compounds. D/H exchange of D-3 in the lipid region was calculated; only 10% of D-3 was converted to 3 after treatment with HeLa cells for 6 h (Fig. 4). These results clearly indicate that D-alkynes are viable as Raman tags, exhibiting Raman vibrational frequencies that are distinct from the H-alkynes. Simultaneous Raman imaging of D-3 and EdU (an alkyne-tagged dT analog that is known to incorporate in nuclear DNA)23 was performed by treating HeLa cells with D-3 for 6 h after 22 h cultivation with EdU to obtain two-color Raman images (Fig. S6†).
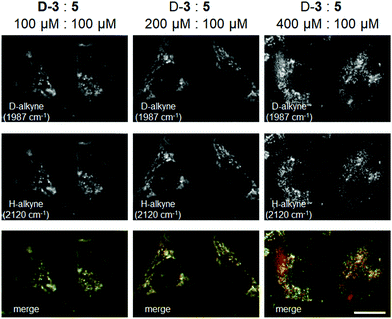
To further demonstrate the utility of D-alkynes as Raman tags, we performed simultaneous live cell Raman imaging of D-3 and 5 at several concentration ratios (Fig. 5). At higher concentrations, both 3 and D-3 spread faster throughout the cells (Fig. S7†). Raman imaging of D-3 and 5 at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio displayed clear differences in HeLa cell distribution: 5 localized in the LDs, whereas D-3 was observed in both LDs and the cytoplasm. However, at molar ratios of 1
1 molar ratio displayed clear differences in HeLa cell distribution: 5 localized in the LDs, whereas D-3 was observed in both LDs and the cytoplasm. However, at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the Raman images of D-3 and 5 appeared the same, with both FAs only detected in the LDs. Interestingly, despite the same conditions for D-3 alone and D-3 co-treatment with 5 (200 μM treatment for 6 h), D-3 alone began to spread in cells (Fig. S7b†), whereas D-3 with 5 resulted in both remaining in the LDs (Fig. 5). Previous metabolic studies demonstrated that 4 aided palmitic acid (a C16 SFA) distribution to LDs,24,25 and we surmised that 5 could also function as a guide to facilitate D-3 delivery to LDs in this experiment.
1, the Raman images of D-3 and 5 appeared the same, with both FAs only detected in the LDs. Interestingly, despite the same conditions for D-3 alone and D-3 co-treatment with 5 (200 μM treatment for 6 h), D-3 alone began to spread in cells (Fig. S7b†), whereas D-3 with 5 resulted in both remaining in the LDs (Fig. 5). Previous metabolic studies demonstrated that 4 aided palmitic acid (a C16 SFA) distribution to LDs,24,25 and we surmised that 5 could also function as a guide to facilitate D-3 delivery to LDs in this experiment.
To confirm the consistency of these results (Fig. 5), Raman imaging of HeLa cells treated with SFA 3 and UFA 4 was performed under the same conditions (Fig. S8†). Since previously SFA palmitate and 4 were reported to be metabolized into triglycerides (TGs) in LDs after 6 h,24 most of the C–H Raman signal for the olefin detected at 3015 cm−1 was considered to be TGs derived from 4 in this experiment.26 Raman images derived from the alkyne of 3 and vinylic protons of 4 appeared the same in HeLa cells treated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios, with both 3 and 4 localized in the LDs. However, at a 4
1 molar ratios, with both 3 and 4 localized in the LDs. However, at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, the Raman images differed slightly: 3 diffused into the cytosol, whereas 4 was detected only in the LDs. These results confirm that the two-color Raman images of D-3 and 5 are not artifacts. Taken together, we successfully demonstrated the utility of D-alkynes and H-alkynes as Raman tags for simultaneous Raman imaging of similar small molecules in live cells.
1 molar ratio, the Raman images differed slightly: 3 diffused into the cytosol, whereas 4 was detected only in the LDs. These results confirm that the two-color Raman images of D-3 and 5 are not artifacts. Taken together, we successfully demonstrated the utility of D-alkynes and H-alkynes as Raman tags for simultaneous Raman imaging of similar small molecules in live cells.
Conclusions
In this study, we investigated the effect of deuteration on the alkyne signal in Raman microscopy. We quantitatively measured the H/D or D/H exchange rate of terminal alkynes in aqueous solutions using spontaneous Raman microscopy. The H/D or D/H exchange of terminal alkynes was dependent on its structure (whether conjugated or non-conjugated), pH (pD), and temperature. Furthermore, deuterated compounds are widely used in various research fields (e.g., synthetic chemistry, pharmaceutical science, and materials science) and the D-alkyne functional group is valuable for the synthesis of other deuterated functional groups, such as D-alkenes or D-alkanes,13,27–29 D-ketones,30 and D-aromatics.31 Our findings related to D/H exchange of terminal alkynes provide further insights for the synthesis of D-labeled compounds using D-alkynes.Furthermore, we demonstrated the utility of a deuterated non-conjugated alkyne as a Raman tag for live cell imaging, and we obtained simultaneous Raman imaging of a C18 SFA and C18 UFA in HeLa cells by monitoring the D-alkyne and H-alkyne signals. D-alkynes and H-alkynes can serve as Raman tags to distinguish between and observe similar small molecules in cells. Live cell Raman imaging of the non-conjugated D-alkyne tag makes it possible to detect short-term accumulation and localization at slightly acidic or neutral pH, or in the hydrophobic environment. D-alkynes are therefore promising for various applications – such as for markers of acidic organelles, as pH sensors, and as indicators to distinguish between hydrophobicity and hydrophilicity – and we expect that they will be more widely employed in diverse scientific fields in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JST CREST Grant Number JPMJCR1925 (M.S.), JSPS KAKENHI Grant Number JP18K14360 (S.E.), and JSPS KAKENHI Grant Number JP20K15418 (S.E.). S.E. was supported by the Special Postdoctoral Researchers Program of RIKEN. We thank RIKEN CSRS Molecular Structure Characterization Unit for HRMS measurement.Notes and references
- G. J. Puppels, M. Grond and J. Greve, Appl. Spectrosc., 1993, 47, 1256–1257 CrossRef CAS.
- H. Yamakoshi, K. Dodo, M. Okada, J. Ando, A. Palonpon, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2011, 133, 6102–6105 CrossRef CAS PubMed.
- H. Yamakoshi, K. Dodo, A. Palonpon, J. Ando, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2012, 134, 20681–20689 CrossRef CAS PubMed.
- H. Yamakoshi, A. F. Palonpon, K. Dodo, J. Ando, S. Kawata, K. Fujita and M. Sodeoka, Chem. Commun., 2014, 50, 1341–1343 RSC.
- L. Li, H. Wang and J.-X. Cheng, Biophys. J., 2005, 89, 3480–3490 CrossRef CAS PubMed.
- E. O. Potma and X. S. Xei, ChemPhysChem, 2005, 6, 77–79 CrossRef CAS PubMed.
- S. Bakthavatsalam, K. Dodo and M. Sodeoka, RSC Chem. Biol., 2021 10.1039/d1cb00116g.
- H. J. Lee, W. Zhang, D. Zhang, Y. Yang, B. Liu, E. L. Barker, K. K. Buhman, L. V. Slipchenko, M. Dai and J.-X. Cheng, Sci. Rep., 2015, 5, 7930 CrossRef CAS PubMed.
- M. Ueda, S. Egoshi, K. Dodo, Y. Ishimaru, H. Yamakoshi, T. Nakano, Y. Takaoka, S. Tsukiji and M. Sodeoka, ACS Cent. Sci., 2017, 3, 462–472 CrossRef CAS PubMed.
- M. Ueda, K. Hayashi, S. Egoshi, Y. Ishimaru, Y. Takaoka, H. Yamakoshi, K. Dodo and M. Sodeoka, Org. Biomol. Chem., 2018, 16, 3348–3352 RSC.
- M. M. Gaschler, F. Hu, H. Feng, A. Linkermann, W. Min and B. R. Stockwell, ACS Chem. Biol., 2018, 13, 1013–1020 CrossRef CAS PubMed.
- Z. Chen, D. W. Paley, L. Wei, A. L. Weisman, R. A. Friesner, C. Nuckolls and W. Min, J. Am. Chem. Soc., 2014, 136, 8027–8033 CrossRef CAS PubMed.
- T. Okamura, S. Egoshi, K. Dodo, M. Sodeoka, Y. Iwabuchi and N. Kanoh, Chem. – Eur. J., 2019, 25, 16002–16006 CrossRef CAS PubMed.
- S. Mizushima, Y. Morino, Y. Kitasato and S. Nakamura, Proc. Imp. Acad., 1940, 16, 549–554 CrossRef.
- H. W. Schrötter and E. G. Hoffmann, Justus Liebigs Ann. Chem., 1964, 672, 44–54 CrossRef.
- K. Dodo, A. Sato, Y. Tamura, S. Egoshi, K. Fujiwara, K. Oonuma, S. Nakao, N. Terayama and M. Sodeoka, Chem. Commun., 2021, 57, 2180–2183 RSC.
- S. P. Bew, G. D. Hiatt-Gipson, J. A. Lovell and C. Poullain, Org. Lett., 2012, 14, 456–459 CrossRef CAS PubMed.
- Y. Yabe, Y. Sawama, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2013, 19, 484–488 CrossRef CAS PubMed.
- D. M. Carey and G. M. Korenowski, J. Chem. Phys., 1998, 108, 2669–2675 CrossRef CAS.
- A. K. Covington, M. Paabo, R. A. Robinson and R. G. Bates, Anal. Chem., 1968, 40, 700–706 CrossRef CAS.
- A. K. Covington, R. A. Robinson and R. G. Bates, J. Phys. Chem., 1966, 70, 3820–3824 CrossRef CAS.
- C. Matthäus, C. Krafft, B. Dietzek, B. R. Brehm, S. Lorkowski and J. Popp, Anal. Chem., 2012, 84, 8549–8556 CrossRef PubMed.
- A. Salic and T. J. Mitchison, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2415–2420 CrossRef CAS PubMed.
- L. L. Listenberger, X. Han, S. E. Lewis, S. Cases, R. V. Farese Jr., D. S. Ory and J. E. Schaffer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3077–3082 CrossRef CAS PubMed.
- Y. Shen, Z. Zhao, L. Zhang, L. Shi, S. Shahriar, R. B. Chan, G. Di Paolo and W. Min, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 13394–13399 CrossRef CAS PubMed.
- D. Fu, Y. Yu, A. Folick, E. Currie, R. V. Farese, T.-H. Tsai, X. S. Xie and M. C. Wang, J. Am. Chem. Soc., 2014, 136, 8820–8828 CrossRef CAS PubMed.
- D. Orain and J.-C. Guillemin, J. Org. Chem., 1999, 64, 3563–3566 CrossRef CAS PubMed.
- J. E. Baldwin and J. A. Kapecki, J. Am. Chem. Soc., 1970, 92, 4874–4879 CrossRef CAS.
- J. M. Brown and G. C. Lloyd-Jones, J. Am. Chem. Soc., 1994, 116, 866–878 CrossRef CAS.
- J. Dheur, M. Sauthier, Y. Castanet and A. Mortreux, Adv. Synth. Catal., 2007, 349, 2499–2506 CrossRef CAS.
- R. N. Nair, P. J. Lee and D. B. Grotjahn, Catalysis, 2010, 53, 1045–1047 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, supplemental data, and spectral data. See DOI: 10.1039/d1ob01479j |
| This journal is © The Royal Society of Chemistry 2021 |