Open Access Article
Open Access ArticleDual-ion charge–discharge behaviors of Na–NiNc and NiNc–NiNc batteries†
Jinkwang
Hwang
 a,
Rika
Hagiwara
a,
Rika
Hagiwara
 a,
Hiroshi
Shinokubo
a,
Hiroshi
Shinokubo
 b and
Ji-Young
Shin
b and
Ji-Young
Shin
 *b
*b
aGraduate School of Energy Science, Kyoto University, Kyoto 606-8501, Japan
bGraduate School of Engineering, Nagoya University, Nagoya 464-8601, Japan. E-mail: jyshin@chembio.nagoya-u.ac.jp; Fax: 81-52-747-6771
First published on 9th March 2021
Abstract
Dual-ion sodium-organic secondary batteries were produced with anti-aromatic porphyrinoid, NiNc, as an active electrode material, which exhibited inherent charge–discharge behavior with high discharge capacity, high stability, and high Coulombic efficiency at high current density (132.6 mA h g−1 discharge capacity and 99.4% efficiency at the 100th cycle with 1 A g−1 of current density and 95.3 mA h g−1 discharge capacity and 99.3% efficiency at the 100th cycle with 2 A g−1 of current density).
Advanced electrochemical energy storage is an important topic of investigation in our world. The urgency of our global situation provides challenges for the invention of eco-friendly systems.1 Innovative approaches in current research in secondary or rechargeable lithium and lithium-ion-associated organic and inorganic batteries in portable electronic devices have been tried by many different organizations for commercialization.2 And inexpensive and highly abundant sodium and sodium-ion (sodium being the fourth most abundant element: 2.36% of the Earth's crust) complementary batteries have been investigated to understand the redox durability of organic frameworks for electrode materials.3 However, achieving transparent dual-ion mobility, fast charge–discharge rate, enhanced discharge capacity, and Coulombic efficiency with diminished electrolyte parasitic reactions has been challenging in order to succeed in achieving superior battery systems, and an enormous number of in-depth studies have investigated these features.4 Furthermore, investigations of rechargeable batteries without an alkali metal anode have been pursued to achieve freely redox-switchable and accumulating organic batteries that are capable of satisfying safety considerations. So far, no organic battery has been produced that satisfies this goal.
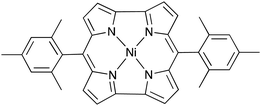
Anti-aromatic compounds are known to possess unsuitable conformations for practical applications due to anti-aromatic destabilization, which easily distorts the compound's planarity. However, an exceedingly active electrode material with anti-aromaticity, nickel norcorrole (NiNc, Fig. 1), has been reported.5 NiNc showed significantly stable charge–discharge performances in both lithium-cooperating Li–NiNc and lithium-free NiNc–NiNc secondary batteries.6 The batteries exhibited appropriately high capacities that do not change over long periods, of over 100 cycles. A maximum discharge capacity of about 207 mA h g−1 was obtained with Li–NiNc batteries. NiNc has been noted to be a realistically stable anti-aromatic molecule that is suitable for practical application as an active battery material.
Considering this, the possibility of dual-ion conducting materials was investigated with anti-aromatic NiNc as the cathode and inexpensive alkali metal, sodium, as the anode. The charge–discharge durability of Na–NiNc cells was examined with coin cells fabricated with 70 wt% NiNc cathode (NiNc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB
CB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVDF = 70
PVDF = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; CB, carbon black; PVDF, polyvinylidene fluoride), sodium metal anode, and 50 mol% Na[FSA]-[C2C1im][FSA] (C2C1im, 1-ethyl-3-methylimidazolium; FSA, bis(fluorosulfonyl)amide) ionic liquid electrolyte. Surprisingly, the battery cell exhibited high redox durability and showed precise ion insertion processes, whose behaviors are consistent with the charge–discharge curves of NiNc investigated through a three-electrode cell configuration. Symmetric NiNc batteries were built using different electrolytes. The ionic insertion behavior was understood by means of comparison of the different electrolytes. Herein, we report the first sodium-organic batteries fabricated with an anti-aromatic active electrode. Furthermore, to the best of our knowledge, this is the first non-alkali metal cooperating dual-ion battery with active organic electrodes.
5; CB, carbon black; PVDF, polyvinylidene fluoride), sodium metal anode, and 50 mol% Na[FSA]-[C2C1im][FSA] (C2C1im, 1-ethyl-3-methylimidazolium; FSA, bis(fluorosulfonyl)amide) ionic liquid electrolyte. Surprisingly, the battery cell exhibited high redox durability and showed precise ion insertion processes, whose behaviors are consistent with the charge–discharge curves of NiNc investigated through a three-electrode cell configuration. Symmetric NiNc batteries were built using different electrolytes. The ionic insertion behavior was understood by means of comparison of the different electrolytes. Herein, we report the first sodium-organic batteries fabricated with an anti-aromatic active electrode. Furthermore, to the best of our knowledge, this is the first non-alkali metal cooperating dual-ion battery with active organic electrodes.
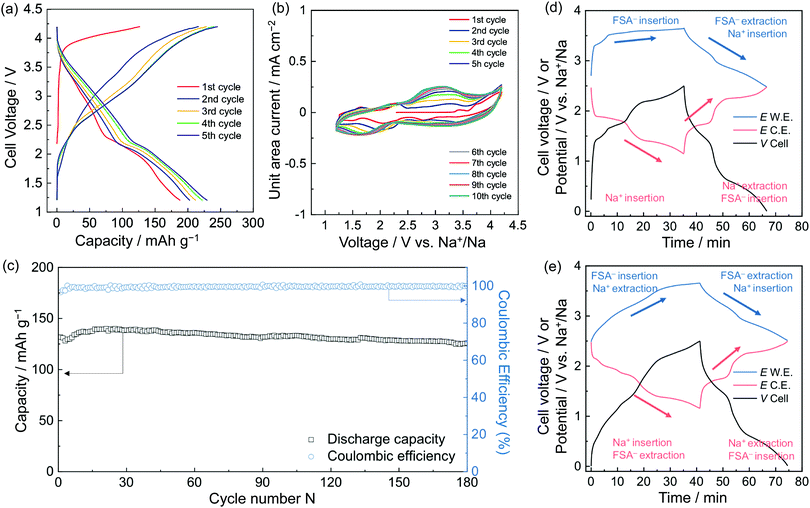
Na–NiNc batteries were prepared using different electrolytes (Fig. S1, ESI†). When 50 mol% Na[FSA]-[C2C1im][FSA] electrolyte was applied to the batteries, the charge–discharge performance improved by twice as much as the battery system prepared with 1 M Na[PF6]-EC/DMC electrolyte (Fig. S2, ESI†). It was considered that the performance improvement was achieved by minimizing the dissolution of high composite NiNc battery electrodes in common organic electrolytes by participating in additional ion pairs (Fig. S3, ESI†). The upper potential of the charging and discharging potential window was optimized to 4.2 V. The 100 cycles of battery performance were monitored in the voltage range of 2.0–4.2 V, with a slow cycling speed (0.2 A g−1 of current density), with a plot that successively gave rise to around three electrons involved in the Coulombic efficiency (Fig. S4, ESI†). An optimal cut-off voltage was then fixed in the range of 1.2–4.2 V, where the charge and discharge curves of the Na–NiNc batteries gave sequentially characteristic chemical properties (Fig. 2a). The first redox cycle in the cyclic voltammogram of the Na–NiNc battery was silent until 3.4 V (Fig. 2b and Fig. S5a, ESI†), where Na+ extraction was appreciably excluded, which can be explained by the three-electrode cell curves in the different charging steps (Fig. 2d and 3a). The durability of the dual-ion charge and discharge performance was evident after the first cycle (Fig. 2e and 3b). Long-term battery performance was then examined to ensure the durability of the discharge capacity and Coulombic efficiency, as shown in Fig. 2c and Fig. S6 (ESI†).
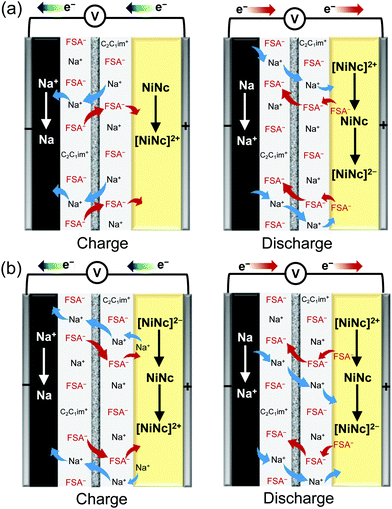 | ||
| Fig. 3 Schematic perspectives of dual-ion insertion/deletion processes for the first and later cycles: (a) the 1st cycle and (b) the 2nd cycle. | ||
As current density increased, the discharge capacity decreased due to the lack of time to adjust the entirely supplementary polarization (Fig. S7, ESI†). When the current density was set at 1 A g−1 for a long-term experiment, as shown in Fig. 2c, 132.6 mA h g−1 of discharge capacity and 99.4% efficiency for the 100th cycle were achieved, and these values were retained up to 180 cycles. This performance is in good agreement with involvement of three electrons per molecule. An increased current density, 2 A g−1, resulted in discharge capacities of 95.3, 92.5, 93.1, 91.5, and 90.4 mA h g−1 and Coulombic efficiencies of 99.3, 99.6, 99.8, 99.9, and 99.9% for the 100th, 200th, 300th, 400th, and 500th cycles, respectively (Fig. S6, ESI†). The charge–discharge processes offered stabilized performances to give nearly 100% efficiency, which was preserved over 500 cycles. It is a delight that two electrons are involved in the discharge capacities even with the speeded-up charging process.
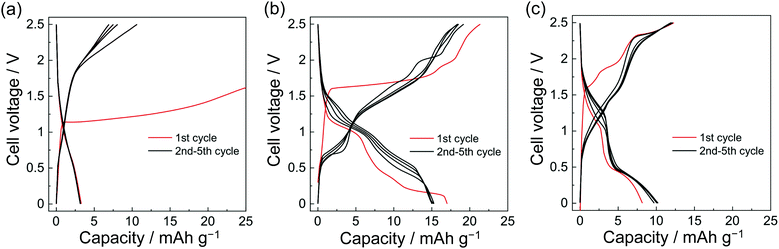
The NiNc electrode behavior was established in the potential ranges 1.2–2.5 V and 2.5–3.7 V vs. Na+/Na for negative and positive electrodes, respectively (Fig. 2d, e and 3). Significantly, the NiNc–NiNc cell processes provided sufficient charge–discharge curves in a range of 0.0–2.5 V, which verified the dual organic electrode conducting networks of the NiNc electrodes (Fig. 4).
The dual organic electrode batteries, NiNc–NiNc batteries, also showed ion-conducting performance (Fig. 4 and Fig. S8, ESI†). The NiNc–NiNc batteries exhibited significantly lower discharge capacities than the Na–NiNc batteries. However, it is notable that those batteries signified dual organic battery behaviors associated with electrolyte ions. The symmetric NiNc–NiNc batteries were assembled with organic solvents of ethylene carbonate (EC)/dimethyl carbonate (DMC), ionic liquids [FSA]-[C2C1im][FSA], and 50 mol% Na[FSA]-[C2C1im][FSA] electrolytes to refine the influence of dual-ion behavior.
In contrast, the best electrolyte (50 mol% Na[FSA]-[C2C1im] [FSA]), which afforded excellent charge–discharge performance for Na–NiNc batteries, did not result in the highest capacity for NiNc–NiNc batteries. When Na+ was removed from the electrolyte, the performance was nearly doubly improved. It was presumed that this was due to the effectiveness of frequent redox switches in the channels of totally symmetric organic electrodes.
The strain of dual-ion insertion/extraction processes confused the mobilities of distinct charged electrolyte ions, resulting in a narrow operating voltage range for actual charge–discharge performance. The systematic insertion/extraction of anions was only established with [C2C2im][FSA] for ion flow, and the absence of ions in the ED/DMC electrolyte failed to improve the charge and discharge performance.
Conclusions
Transparent dual-ion mobility, fast charge–discharge rate, enhanced discharge capacity, and high Coulombic efficiency with diminished electrolyte parasitic reactions for Na-organic batteries were successfully achieved using NiNc active electrodes. A high composition of 70% for the NiNc electrodes satisfied inherent charge–discharge behaviors with high discharge capacity, high stability, high Coulombic efficiency and high current densities for charging. Furthermore, symmetric NiNc–NiNc batteries exhibited significantly conserved charge–discharge processes with the dual organic electrodes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by JSPS KAKENHI Grant Number JP18K04925. J.-Y. Shin acknowledges the Graduate School of Engineering, Nagoya University, for analytical instrumental support. J.-Y. Shin and J. Hwang acknowledge Prof. K. Matsumoto at the Graduate School of Energy Science, Kyoto University, for his appreciated support and advice.Notes and references
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- (a) A. R. Armstrong and P. G. Bruce, Nature, 1996, 381, 499 CrossRef CAS; (b) J. M. Tarascon and M. Armand, Nature, 2001, 414, 356 CrossRef PubMed; (c) B. Scrosati, Nat. Nanotechnol., 2007, 2, 598 CrossRef CAS; (d) H. Nishida and K. Oyaizu, Science, 2008, 319, 737 CrossRef PubMed; (e) A. S. Aricó, P. Bruce, B. Scrosati, J.-M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 368 CrossRef PubMed; (f) T. Nokami, T. Matsuo, Y. Inatomi, N. Hojo, T. Tsukagoshi, H. Yoshizawa, A. Shimizu, H. Kuramoto, K. Komae, H. Tsuyama and J.-I. Yoshida, J. Am. Chem. Soc., 2012, 134, 19694 CrossRef CAS; (g) Y. Hanyu and I. Honma, Sci. Rep., 2012, 49, 453 CrossRef PubMed; (h) Z. Luo, L. Liu, Q. Zhao, F. Li and J. Chen, Angew. Chem., 2017, 129, 12735 CrossRef (Angew. Chem. Int. Ed., 2017, 56, 12561); (i) Z. Song, T. Xu, M. L. Gordin, Y.-B. Jiang, L.-T. Bae, Q. Ziao, H. Zhan, J. Liu and D. Wang, Nano Lett., 2012, 12, 2205 CrossRef CAS PubMed; (j) X. Li, Z. Hou, W. Huang, H.-S. Xu, X. Wang, W. Yu, R. Li, K. Zhang, L. Wang, Z. Chen, K. Xie and K. P. Loh, ACS Energy Lett., 2020, 5, 3498 CrossRef CAS.
- (a) J. M. Lee, G. Siingh, W. Cha, S. Kim, J. Yi, S.-J. Hwang and A. Vinu, ACS Energy Lett., 2020, 5, 1939 CrossRef CAS; (b) S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859 CrossRef CAS; (c) W. Wang, W. Li, S. Wang, Z. Miao, H. K. Liu and S. Chou, J. Mater. Chem. A, 2018, 6, 6183 RSC; (d) Y. Lee, S. M. Oh, B. Park, B. U. Ye, N.-S. Lee, J. M. Baik, S.-J. Hwang and M. H. Kim, CrystEngComm, 2017, 19, 5028 RSC; (e) S. M. Oh, I. Y. Kim, S. B. Patil, B. Park, J. M. Lee, K. Adpakpang, S. A. Chae, O. H. Han and S.-J. Hwang, ACS Appl. Mater. Interfaces, 2017, 9, 2249 CrossRef CAS PubMed; (f) W. Cha, I.-Y. Kim, J. M. Lee, S. Kim, K. Ramadass, K. Gopalakrishnan, S. Premkumar, S. Umapathy and A. Vinu, ACS Appl. Mater. Interfaces, 2019, 11, 27192 CrossRef CAS PubMed; (g) S. Y. Lim, J. H. Lee, S. Kim, J. Shin, W. Choi, K. Y. Chung, D. S. Jung and J. W. Choi, ACS Energy Lett., 2017, 2, 998 CrossRef CAS; (h) L. Wang, C. Wang, N. Zhang, F. Li, F. Cheng and J. Chen, ACS Energy Lett., 2017, 2, 256 CrossRef CAS; (i) J. Ni, L. Li and J. Lu, J. Nanosci. Nanotechnol., 2010, 10, 21 CrossRef; (j) S. Nandal, M. V. Lee, J. P. Hill, A. Vinu and K. Ariga, J. Nanosci. Nanotechnol., 2010, 10, 21 CrossRef PubMed; (k) I. Y. Kim, S. Kim, X. Jin, S. Premkumar, G. Chandra, N.-S. Lee, G. P. Mane, S.-J. Hwang, S. Umapathy and A. Vinu, Angew. Chem., 2018, 130, 17381 ( Angew. Chem., Int. Ed. , 2018 , 57 , 17137 ) CrossRef; (l) B. Park, S. M. Oh, Y. K. Jo and S.-J. Hwang, Mater. Lett., 2016, 178, 79 CrossRef CAS; (m) B. Park, S. M. Oh and X. Jin, Chem. – Eur. J., 2017, 23, 6544 CrossRef CAS PubMed; (n) P. Xiong, R. Ma, N. Sakai, L. Nurdiwijayanto and T. Sasaki, ACS Energy Lett., 2018, 3, 997 CrossRef CAS; (o) Y. Shynkarenko, M. I. Bodnarchuk, C. Benrnasconi, Y. Berezovska, V. Verteletskyi, S. T. Ochsenbein and M. V. Kovalenko, ACS Energy Lett., 2020, 5, 2835 CrossRef; (p) H. Wan, W. Weng, F. Han, L. Cai, C. Wang and X. Yao, Nano Today, 2020, 33, 100860 CrossRef CAS; (q) L. Yang, P. Wang, S. Zhang, Y. Wang, L. Zang, H. Zhu, J. Yin and H. Y. Yang, J. Mater. Chem. A, 2020, 8, 22791 RSC; (r) H. Wan, L. Cai, Y. Yao, W. Weng, Y. Feng, J. P. Mwizerwa, G. Liu, Y. Yu and X. Yao, Small, 2020, 2001574 CrossRef CAS PubMed; (s) M. Sheng, F. Zhang, B. Ji, X. Tong and Y. Tang, Adv. Energy Mater., 2017, 7, 1601963 CrossRef; (t) D. Xie, M. Zhang, L. Xiang and Y. Tang, Adv. Funct. Mater., 2020, 30, 1906770 CrossRef CAS; (u) A. Yu, Q. Pan, M. Zhang, D. Xie and Y. Tang, Adv. Funct. Mater., 2020, 30, 2001440 CrossRef CAS; (v) S. Mu, Q. Liu, P. Kidkhunthod, X. Zhou, W. Wang and Y. Tang, Natl. Sci. Rev., 2020 DOI:10.1093/nsr/nwaa178.
- (a) H. Sun, Z. Cao, T. Wang, R. Lin, Y. Liu, L. Zhang, F. Lin, Y. Huang and W. Luo, Mater. Today Energy, 2019, 13, 145 CrossRef; (b) A. Manthiram, A. V. Murugan, A. Sarkar and T. Muraliganth, Energy Environ. Sci., 2008, 1, 621 RSC; (c) Z. Wu, S. Cui, Z. Zhuo, W. Yang, X. Wang and F. Pan, ACS Appl. Mater. Interfaces, 2015, 7, 25105 CrossRef PubMed; (d) X. Liu, S. Wang, L. Wang, K. Wang, X. Wu, P. Zhou, Z. Miao, Y. Zhao and S. Zhuo, J. Power Sources, 2019, 438, 227017 CrossRef CAS; (e) H. H. Sun, H.-H. Ryu, U.-H. Kim, J. A. Weeks, A. Heller, Y.-K. Sun and C. B. Mullins, ACS Energy Lett., 2020, 5, 1136 CrossRef CAS.
- T. Ito, Y. Hayashi, S. Shimizu, J.-Y. Shin, N. Kobayashi and H. Shinokubo, Angew. Chem., 2012, 124, 8670 CrossRef (Angew. Chem. Int. Ed., 2012, 51, 8542).
- J.-Y. Shin, T. Yamada, H. Yoshikawa, K. Awaga and H. Shinokubo, Angew. Chem., 2014, 126, 3160 CrossRef (Angew. Chem. Int. Ed., 2014, 53, 3096).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00007a |
| This journal is © The Royal Society of Chemistry 2021 |