Nucleophilic reactivity of a mononuclear cobalt(III)–bis(tert-butylperoxo) complex†
Bongki
Shin‡
 ,
Younwoo
Park‡
,
Donghyun
Jeong
and
Jaeheung
Cho
,
Younwoo
Park‡
,
Donghyun
Jeong
and
Jaeheung
Cho
 *
*
Department of Emerging Materials Science, DGIST, Daegu 42988, Korea. E-mail: jaeheung@dgist.ar.kr
First published on 11th July 2020
Abstract
A mononuclear cobalt(III)–bis(tert-butylperoxo) adduct (CoIII–(OOtBu)2) bearing a tetraazamacrocyclic ligand was synthesized and characterized using various physicochemical methods, such as X-ray, UV-vis, ESI-MS, EPR, and NMR analyses. The crystal structure of the CoIII–(OOtBu)2 complex clearly showed that two OOtBu ligands bound to the equatorial position of the cobalt(III) center. Kinetic studies and product analyses indicate that the CoIII–(OOtBu)2 intermediate exhibits nucleophilic oxidative reactivity toward external organic substrates.
Transition metal–alkylperoxo (M–OOR) species play an important role in oxidation reactions such as industrial and biological catalytic oxidation.1–6 In industrial processes, M–OOR intermediates, such as CoIII–OOR complexes, were proposed as key intermediates in hydrocarbon catalytic oxidation under harsh conditions.1,7 Mononuclear nonheme M–OOR complexes have been suggested to play a significant role in the oxidation reaction of metalloenzyme systems (e.g., lipoxygenase and homoprotocatechuate 2,3-dioxygenase).8,9 Biomimetic studies of M–OOR complexes enabled catalysts to be developed that produce high value-added organic products under mild conditions.
Mononuclear heme and nonheme first-row M–OOR intermediates (M = Mn, Fe, Co, Ni, and Cu) have been investigated as model complexes for the active sites of metalloenzymes.2,5,6,10–13 A number of M–OOR complexes were mainly studied in the investigation of electrophilic reactions (e.g., oxygen and hydrogen atom transfer reaction).14–25 It has been reported that the reaction proceeds via the ˙OOR and ˙OR radicals from decomposition of the M–OOR species.1 However, only a few examples of nucleophilic reactivity with M–OOR intermediates (M = Fe, Ni, and Cu) have been reported.19,20,23
In the Sharpless–Katsuki epoxidation, Ti–(OOR)n (n = 1–4) species were proposed as reactive intermediates.26,27 Furthermore, in the formation of Fe–OOtBu complexes, Fe–(OOtBu)2 species, [FeIII(TPP)(OOtBu)2]− (TPP = 5,10,15,20-tetraphenylporphyrinate) and [FeIII(BPMCN)(OOtBu)(HOOtBu)]2+ (BPMCN = N,N′-bis(2-pyridylmethyl-N,N′-dimethyl-trans-1,2-diaminocyclohexane)) adducts, have been proposed as short-lived intermediates.28–31 However, definitive evidence of bis(alkylperoxo) binding first-row transition metal compounds has not been reported yet. In this work, we report a fully characterized CoIII–(OOtBu)2 complex bearing a tetraazamacrocyclic ligand, [CoIII(Me3-TPADP)(OOtBu)2]+ (2, Me3-TPADP = 3,6,9-trimethyl-3,6,9-triaza-1(2,6)-pyridinacyclodecaphane). Intermediate 2 was investigated in nucleophilic reactions such as aldehyde oxidation. Only one of the two OOtBu ligands in 2 is able to oxidize external substrates. In order to compare the structure and the reactivity of an alkylperoxo and bis(alkylperoxo) binding cobalt species, CoIII–(OOtBu)(X) complexes, [CoIII(Me3-TPADP)(OOtBu)(X)]+ (X = N3 for 4, NCS for 5), were prepared as well.
The cobalt(II) starting complex, [CoII(Me3-TPADP)(CH3CN)2]2+ (1), was synthesized by using a published method.32 When 10 equiv. of tert-butyl hydroperoxide (tBuOOH) was added to 1 in the presence of 2 equiv. of triethylamine (TEA) in CH3CN at 25 °C, the CoIII–(OOtBu)2 adduct, [CoIII(Me3-TPADP)(OOtBu)2]+ (2), was generated and the solution color changed from purple to dark green (Scheme S1, ESI†). Intermediate 2 is thermally metastable in CH3CN at 25 °C, which allowed us to use it for characterization and reactivity studies.
The UV-vis spectrum of 2 in CH3CN at 25 °C shows electronic absorption bands at λmax = ∼360 (ε = 1100 M−1 cm−1) and 583 nm (ε = 190 M−1 cm−1) (Fig. 1a). Electrospray ionization mass spectrometry (ESI-MS) analysis of 2 exhibits a prominent ion peak at m/z 485.3, whose mass and isotope distribution pattern correspond to [CoIII(Me3-TPADP)(OOtBu)2]+ (calcd m/z 485.3) (Fig. 1b). The X-band electron paramagnetic resonance (EPR) silence (Fig. 1a, inset) and 1H NMR spectral features (Fig. S1, ESI†) in the diamagnetic region confirm that complex 2 is a low-spin S = 0 cobalt(III) species.
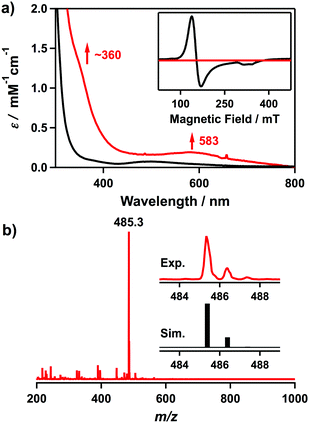 | ||
| Fig. 1 (a) UV-vis spectra of [CoII(Me3-TPADP)(CH3CN)2]2+ (1) (the black line) and [CoIII(Me3-TPADP)(OOtBu)2]+ (2) (the red line) in CH3CN at 25 °C.32 Inset shows the X-band EPR spectra of 1 (the black line) in frozen CH3CN at 5 K and 2 (the red line) in frozen CH3CN at 113 K.32 The parameters for the measurement of 2: microwave power = 1.0 mW, frequency = 9.176 GHz, sweep width = 0.40 T, and modulation amplitude = 0.60 mT. (b) ESI-MS of 2 in CH3CN at −40 °C. Insets show experimental (upper) and simulated (lower) isotope distribution patterns. | ||
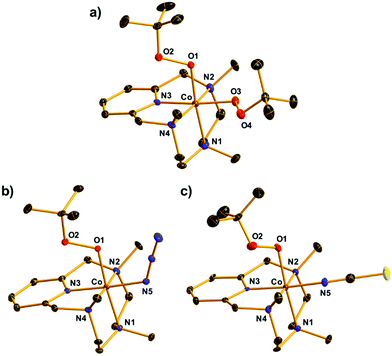
The X-ray crystal structure of [CoIII(Me3-TPADP)(OOtBu)2](BPh4)(Et2O) (2-BPh4·Et2O) revealed a distorted octahedral geometry where two tert-butyl peroxide ligands coordinate to the cobalt(III) center in the cis positions (Fig. 2a). To the best of our knowledge, this is the first crystal structure of a mononuclear CoIII–(OOtBu)2 complex. The average Co–O (1.8590 Å) and O–O (1.4757 Å) bond distances of 2 are comparable to those of the CoIII–OOtBu complexes (Table S2, ESI†).1,33
Thermal decomposition of 2 produced a CoIII–(OOtBu)(OH) complex, [CoIII(Me3-TPADP)(OOtBu)(OH)]+ (3), in CH3CN at 25 °C (Fig. S2, ESI†).34 Formation of 3 was confirmed by cold spray ionization spectrometry (CSI-MS). The CSI-MS spectrum of 3 shows a prominent signal at m/z 413.17 (calcd m/z 413.20) (Fig. S3, ESI†). Upon adding isotopically labeled H218O and D2O into the solution of 3, mass peaks at 415.21 and 414.22 corresponding to [CoIII(Me3-TPADP)(OOtBu)(18OH)]+ (calcd m/z 415.20) and [CoIII(Me3-TPADP)(OOtBu)(OD)]+ (calcd m/z 414.20), respectively, were observed (Fig. S3, ESI,† the inset). These mass shifts demonstrate that 3 contains a hydroxide ligand. In a previous study, the Fe–(OOtBu)2 species was also proposed as a precursor of the Fe–(OOtBu) species.29 Tajima et al. insisted that [FeIII(TPP)(OOtBu)2]−, generated by adding an excess amount of sodium methoxide (NaOCH3) and tBuOOH to the [FeIII(TPP)]+ solution, reacted with additional NaOCH3, affording the formation of the [FeIII(TPP)(OOtBu)(OCH3)]− species.29
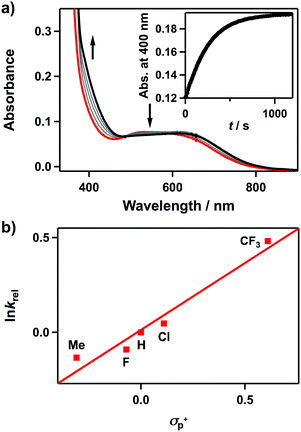
We then investigated the electrophilic and nucleophilic reactivities of 2. The electrophilic reaction of 2 was performed by using styrene and 2,3-dimethyl-2-butene. Upon addition of substrates to the solution of 2 in CH3CN at 10 °C, the intermediate remained intact without showing specific UV-vis spectral changes, and product analysis of these reaction solutions did not show oxidized products (Scheme 1). In contrast, the nucleophilic reactivity of 2 was observed in the oxidation of aldehydes (Scheme 1). Upon the addition of benzaldehyde to 2 in CH3CN at 15 °C, the characteristic absorption band of 2 disappeared with a pseudo-first-order decay (Fig. 3a, the inset and Table S3, ESI†). The product analysis of the reaction solution revealed that benzoic acid (95(1)%) was produced in the oxidation of benzaldehyde (Scheme S2, ESI†). In addition, the cobalt(II)-benzoato complex, [CoII(Me3-TPADP)(C6H5COO)]+, was generated after the reaction was completed (Fig. S7, ESI† for CSI-MS analysis). The reactivity of 2 was further investigated with para-substituted benzaldehydes, para-X-Ph-CHO (X = Me, F, Cl, and CF3) (Table S3, ESI†). The Hammett plot of the pseudo-first-order rate constants versus σp+ gave a ρ value of 0.7(1) (Fig. 3b). The positive ρ value indicates that 2 has nucleophilic character. The reactivity of 2 was further examined by using primary (1-pentanal for 1°-CHO), secondary (2-methylbutanal for 2°-CHO), and tertiary (pivalaldehyde for 3°-CHO) aldehydes, and the observed reactivity order of 1°-CHO > 2°-CHO > 3°-CHO supports the nucleophilic character of 2 as well (Fig. S8, ESI†). Product analyses of the resulting solutions revealed that pentanoic acid (94(3)%), 2-methylbutanoic acid (94(4)%), and 2,2-dimethylpropanoic acid (94(1)%) were produced in the oxidation of 1-pentanal, 2-methylbutanal, and pivalaldehyde, respectively (Table S4, ESI†).
Upon the addition of 2-phenylpropionaldehyde (2-PPA) to 2 in CH3CN at 25 °C under aerobic conditions, the UV-vis absorption band of 2 slightly changed with isosbestic points at 390 and 452 nm, which follows a pseudo-first-order decay profile (Fig. S9, ESI†). The pseudo-first-order rate constants increased proportionally with the 2-PPA concentration, giving a second-order rate constant (k2) of 4.1(3) × 10−1 M−1 s−1 at 25 °C (Fig. S10a, ESI†). After the reaction of 2 with 2-PPA, product analysis revealed that acetophenone (95(1)%) was produced as a final product. The CSI-MS spectrum of the reaction solution revealed the formation of 3 and a small amount of cobalt(II)-formato species was also detected under an inert atmosphere (Fig. S11, ESI†). The temperature dependence of the k2 values was examined in the range of 273–298 K, where a linear Eyring plot was obtained with the activation parameters of ΔH‡ = 11(1) kcal mol−1 and ΔS‡ = −22(3) cal mol−1 K−1 (Fig. S10b, ESI†). The observed negative entropy value and the second-order kinetics suggest that the oxidation of 2-PPA by 2 is performed through a bimolecular mechanism.
Interestingly, the same reaction performed under a N2 atmosphere gives different products. The reaction of 2 with 2-PPA under N2 in CH3CN at 25 °C gave a new absorption band at 480 nm (Fig. S12, ESI†). By analysing the resulting solution with CSI-MS, we found that a cobalt(II)–enolate complex, [CoII(Me3-TPADP)(OCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)Ph)]+, was formed as a decomposed product (Fig. S13, ESI†). The product analysis of the reaction solution indicated that trace amounts of acetophenone were produced (<1%) after the reaction. When the cobalt(II)–enolate complex was exposed to O2, a cobalt(II)–formato complex was obtained as a major product, as observed by CSI-MS (Fig. S14, ESI†). These results are very similar to Tolman's recent mechanism of the aldehyde deformylation pathway via a copper(II)–enolate species.35 Based on the kinetic studies and product analyses under N2 and O2, the possible reaction mechanisms for 2-PPA oxidation by 2 are summarized in Scheme 2. The reaction of 2 and 2-PPA in the presence of water afforded enolate and a putative cobalt(III)–(OOtBu) species through α-deprotonation of 2-PPA by one of the alkylperoxides of 2. The putative cobalt(III)–(OOtBu) species decomposed to the cobalt(II)–enolate complex under N2. In the presence of O2, the enolate is oxidized to acetophenone, and complex 3 is produced as a final product.
C(Me)Ph)]+, was formed as a decomposed product (Fig. S13, ESI†). The product analysis of the reaction solution indicated that trace amounts of acetophenone were produced (<1%) after the reaction. When the cobalt(II)–enolate complex was exposed to O2, a cobalt(II)–formato complex was obtained as a major product, as observed by CSI-MS (Fig. S14, ESI†). These results are very similar to Tolman's recent mechanism of the aldehyde deformylation pathway via a copper(II)–enolate species.35 Based on the kinetic studies and product analyses under N2 and O2, the possible reaction mechanisms for 2-PPA oxidation by 2 are summarized in Scheme 2. The reaction of 2 and 2-PPA in the presence of water afforded enolate and a putative cobalt(III)–(OOtBu) species through α-deprotonation of 2-PPA by one of the alkylperoxides of 2. The putative cobalt(III)–(OOtBu) species decomposed to the cobalt(II)–enolate complex under N2. In the presence of O2, the enolate is oxidized to acetophenone, and complex 3 is produced as a final product.
In the aldehyde oxidation, only one OOtBu ligand in 2 was able to participate in the oxidation of 2-PPA (Scheme 1). To compare the reactivity properties of CoIII–(OOtBu) and CoIII–(OOtBu)2 complexes, CoIII–(OOtBu)(X) complexes, [CoIII(Me3-TPADP)(OOtBu)(X)]+ (X = N3 for 4, NCS for 5), were synthesized. 4 and 5 were prepared by adding 1.1 equiv. of NaX (X = N3, NCS) to the reaction solution of 1 in CH3CN at 25 °C and then 5 equiv. of tBuOOH and 2 equiv. of TEA were added (Scheme S1, ESI†). Characterization of 4 and 5 was performed by UV-vis, ESI-MS, EPR, and 1H NMR analyses (Experimental section and Fig. S15–S19, ESI†).
The single crystals of 4 and 5 revealed a similar distorted octahedral geometry to that of 2 in which one OOtBu ligand bound to the cobalt(III) center was located in the trans position of the amine group and the other anionic monodentate ligand, X, was located in the trans position of the pyridine ring (Fig. 2b and c). These data clearly indicate that the OOtBu ligand in the trans position of the pyridine ring in 2 was substituted with an anionic ligand in 3 and 4. The Co–O1 bond distances (1.862(3) Å for 4, 1.880(4) Å for 5) and O1–O2 bond distances (1.479(4) Å for 4, 1.430(6) Å for 5) were within the range of those of the reported CoIII–OOtBu complexes and similar to those of 2 (Table S2, ESI†).1,33
In the reactions of 4 and 5 with 2-PPA, we could not observe any change in the UV-vis spectra. Based on the reactivity and structural comparison of 2, 4, and 5, the reaction site of 2 is presumed to be the OOtBu ligand in the trans position of the pyridine ring. Further theoretical calculations on the detailed reaction mechanism of 2 with substrates are underway and will clarify the reaction site of 2.
In conclusion, we have synthesized and characterized a mononuclear CoIII–(OOtBu)2 intermediate, [CoIII(Me3-TPADP)(OOtBu)2]+ (2), with various physicochemical methods including UV-vis, ESI-MS, EPR, X-ray, and NMR analyses. In the kinetic studies, under mild conditions, one of the two OOtBu ligands in 2 is capable of performing a nucleophilic reaction (i.e., aldehyde oxidation). A CoIII–(OOtBu)(OH) complex, 3, was generated by thermal decomposition of 2 and/or deformylation reaction of 2-PPA by 2 in the presence of O2. Furthermore, CoIII–(OOtBu) complexes, [CoIII(Me3-TPADP)(OOtBu)(X)]+ (X = N3 for 4, NCS for 5), which have a OOtBu ligand in the trans position of the amine group were prepared, and 4 and 5 did not undergo aldehyde oxidation.
This research was supported by the NRF (2019R1A2C2086249 and 2018R1A5A1025511) and the Ministry of Science, ICT and Future Planning (CGRC 2016M3D3A01913243) of Korea.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- F. A. Chavez and P. K. Mascharak, Acc. Chem. Res., 2000, 33, 539–545 CrossRef CAS PubMed.
- S. Hikichi, M. Akita and Y. Moro-Oka, Coord. Chem. Rev., 2000, 198, 61–87 CrossRef CAS.
- M. Costas, M. P. Mehn, M. P. Jensen and L. Que, Jr., Chem. Rev., 2004, 104, 939–986 CrossRef CAS PubMed.
- B. de Bruin, P. H. M. Budzelaar and A. W. Gal, Angew. Chem., Int. Ed., 2004, 43, 4142–4157 CrossRef CAS PubMed.
- S. Itoh, Acc. Chem. Res., 2015, 48, 2066–2074 CrossRef CAS PubMed.
- A. T. Fiedler and A. A. Fischer, J. Biol. Inorg. Chem., 2017, 22, 407–424 CrossRef CAS PubMed.
- J. F. Black, J. Am. Chem. Soc., 1978, 100, 527–535 CrossRef CAS.
- E. G. Kovaleva and J. D. Lipscomb, Science, 2007, 316, 453–457 CrossRef CAS PubMed.
- E. Skrzypczak-Jankun, R. A. Bross, R. T. Carroll, W. R. Dunham and M. O. Funk, Jr., J. Am. Chem. Soc., 2001, 123, 10814–10820 CrossRef CAS PubMed.
- H. Komatsusuzaki, N. Sakamoto, M. Satoh, S. Hikichi, M. Akita and Y. Moro-oka, Inorg. Chem., 1998, 37, 6554–6555 CrossRef PubMed.
- S. Hong, Y.-M. Lee, K.-B. Cho, M. S. Seo, D. Song, J. Yoon, R. Garcia-Serres, M. Clémancey, T. Ogura, W. Shin, J.-M. Latour and W. Nam, Chem. Sci., 2014, 5, 156–162 RSC.
- J. A. Kovac, Acc. Chem. Res., 2015, 48, 2744–2753 CrossRef PubMed.
- M. Sankaralingam, Y.-M. Lee, W. Nam and S. Fukuzumi, Coord. Chem. Rev., 2018, 365, 41–59 CrossRef CAS.
- N. Kitajima, T. Katayama, K. Fujisawa, Y. Iwata and Y. Morooka, J. Am. Chem. Soc., 1993, 115, 7872–7873 CrossRef CAS.
- J. Kim, E. Larka, E. C. Wilkinson and L. Que, Jr., Angew. Chem., Int. Ed. Engl., 1995, 34, 2048–2051 CrossRef CAS.
- M. S. Seo, T. Kamachi, T. Kouno, K. Murata, M. J. Park, K. Yoshiza-wa and W. Nam, Angew. Chem., Int. Ed., 2007, 46, 2291–2294 CrossRef CAS PubMed.
- S. Gosiewska, H. P. Permentier, A. P. Bruins, G. van Koten and R. J. M. Gebbink, Dalton Trans., 2007, 3365–3368 RSC.
- A. Kunishita, H. Ishimaru, S. Nakashima, T. Ogura and S. Itoh, J. Am. Chem. Soc., 2008, 130, 4244–4245 CrossRef CAS PubMed.
- S. Hikichi, H. Okuda, Y. Ohzu and M. Akita, Angew. Chem., Int. Ed., 2009, 48, 188–191 CrossRef CAS PubMed.
- J. Stasser, F. Namuswe, G. D. Kasper, Y. Jiang, C. M. Krest, M. T. Green, J. Penner-Hahn and D. P. Goldberg, Inorg. Chem., 2010, 49, 9178–9190 CrossRef CAS PubMed.
- T. Tano, M. Z. Ertem, S. Yamaguchi, A. Kunishita, H. Sugimoto, N. Fujieda, T. Ogura, C. J. Cramer and S. Itoh, Dalton Trans., 2011, 40, 10326–10336 RSC.
- S. Paria, T. Ohta, Y. Morimoto, T. Ogura, H. Sugimoto, N. Fujieda, K. Goto, K. Asano, T. Suzuki and S. Itoh, J. Am. Chem. Soc., 2015, 137, 10870–10873 CrossRef CAS PubMed.
- B. Kim, D. Jeong and J. Cho, Chem. Commun., 2017, 53, 9328–9331 RSC.
- T. Abe, Y. Morimoto, K. Mieda, H. Sugimoto, N. Fujieda, T. Ogura and S. Itoh, J. Inorg. Biochem., 2017, 177, 375–383 CrossRef CAS PubMed.
- J. Lewiński, Z. Ochal, E. Bojarski, E. Tratkiewicz, I. Justyniak and J. Lipkowski, Angew. Chem., Int. Ed., 2003, 42, 4643–4646 CrossRef PubMed.
- T. Katsuki and K. B. Sharpless, J. Am. Chem. Soc., 1980, 102, 5974–5976 CrossRef CAS.
- D. E. Babushkin and E. P. Talsi, J. Mol. Catal. A: Chem., 2003, 200, 165–175 CrossRef CAS.
- M. Rivera, G. A. Caignan, A. V. Astashkin, A. M. Raitsimring, T. K. Shokhireva and F. A. Walker, J. Am. Chem. Soc., 2002, 124, 6077–6089 CrossRef CAS PubMed.
- K. Tajima, K. Tada, J. Jinno, T. Edo, H. Mano, N. Azuma and K. Makino, Inorg. Chim. Acta, 1997, 254, 29–35 CrossRef CAS.
- M. P. Jensen, M. Costas, R. Y. N. Ho, J. Kaizer, A. Mairata i Payeras, E. Münck, L. Que, Jr., J.-U. Rohde and A. Stubna, J. Am. Chem. Soc., 2005, 127, 10512–10525 CrossRef CAS PubMed.
- M. P. Jensen, A. M. I. Payeras, A. T. Fiedler, M. Costas, J. Kaizer, A. Stubna, E. Münck and L. Que, Jr., Inorg. Chem., 2007, 46, 2398–2408 CrossRef CAS PubMed.
- B. Shin, K. D. Sutherlin, T. Ohta, T. Ogura, E. I. Solomon and J. Cho, Inorg. Chem., 2016, 55, 12391–12399 CrossRef CAS PubMed.
- F. A. Chavez, C. V. Nguyen, M. M. Olmstead and P. K. Mascharak, Inorg. Chem., 1996, 35, 6282–6291 CrossRef CAS.
- Interestingly, the decay of 2 was facilitated by adding excess water in 2, and a cobalt(II)–hydroxo complex was observed as a major peak in CSI-MS (Fig. S4–S6, ESI†).
- W. D. Bailey, N. L. Gagnon, C. E. Elwell, A. C. Cramblitt, C. J. Bouchey and W. B. Tolman, Inorg. Chem., 2019, 58, 4706–4711 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization data and kinetic details. CCDC 1906994–1906996. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc03385e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: