Magnetic nanoarchitectures for cancer sensing, imaging and therapy
Nikola Ž.
Knežević
 a,
Ivana
Gadjanski
ab and
Jean-Olivier
Durand
a,
Ivana
Gadjanski
ab and
Jean-Olivier
Durand
 *c
*c
aBioSense Institute, University of Novi Sad, Dr Zorana Djindjica 1, Novi Sad 21000, Serbia
bBelgrade Metropolitan University, Tadeusa Koscuska 63, 11000 Belgrade, Serbia
cInstitut Charles Gerhardt Montpellier, UMR 5253, CNRS-Univ Montpellier-ENSCM CC1701, Place Eugene Bataillon, 34095 Montpellier Cedex 05, France. E-mail: durand@univ-montp2.fr
First published on 26th November 2018
Abstract
The use of magnetic nanoparticles for sensing and theranostics of cancer has grown substantially in the last decade. Since the pioneering studies, which reported magnetic nanoparticles for bio-applications more than fifteen years ago, nanomaterials have increased in complexity with different shapes (nanoflowers, nanospheres, nanocubes, nanostars etc.) and compositions (e.g. core–shell) of nanoparticles for an increase in the sensitivity (imaging or sensing) and efficiency through synergistic treatments such as hyperthermia and drug delivery. In this review, we describe recent examples concerning the use of magnetic nanoparticles for bio-applications, from the surface functionalization methods to the development of cancer sensors and nanosystems for magnetic resonance and other imaging methodologies. Multifunctional nanosystems (nanocomposites, core shell nanomaterials) for theranostic applications involving treatments such as hyperthermia, photodynamic therapy, targeted drug delivery, and gene silencing are also described. These nanomaterials could be the future of medicine, although their complexity raises concerns about their safety.
1. Introduction
Despite a variety of molecular therapeutics for cancer currently being applied in clinics and the promising achievements in this research area,1,2 serious adverse effects of the molecular cytotoxic agents led to the design of nanoparticulate therapeutics for selective cancer targeting.3 Several specific properties of nanomaterials enable their use for selective cancer treatment. These characteristics include: (a) their size, which leads to preferential accumulation of nanoparticles (NP) in tumors through the leaky vasculature of cancer, i.e. the enhanced permeability and retention (EPR) effect;4 (b) high surface area to attach different molecular therapeutics and (c) possibility to functionalize the surface with cancer-homing ligands to further enhance their cancer targeting properties. In the case of using magnetic nanoparticles (MNPs), all these advantages are further supplemented with possibilities for sensing and imaging through external magnetic field-related measurements and for enhanced targeting through exposure to disease-localized magnetic force. Superparamagnetic iron oxide nanoparticles (SPIONs) are the most widely used type of MNPs, and are typically magnetite (Fe3O4), with monodomain particles in the size range of about 5–20 nm and characterized by high magnetic saturation and high biocompatibility. High potential of SPIONs for biomedical applications has been demonstrated in the scientific literature for quite some time,5 and certain formulations of surface coated magnetite-based nanotherapetics have already gained U S Food and Drug Administration (FDA) approval for use in humans as iron deficiency therapeutics and as magnetic resonance imaging (MRI) contrast agents (e.g. Feraheme®, Feridex I.V.® and Gastromark®).6The recent research developments have progressed towards more elaborate and complex multifunctional magnetic designs. This multifunctionality reflects in achieving several functions at the same time, such as simultaneous cancer targeting, imaging and therapy.7
The concept of applying MNPs for cancer sensing is based on the selective interaction between functionalized MNPs and a cancer-specific biomolecule or cancer cell, typically through specific antibody–antigen interactions, which leads to a change in the measured magnetic property of the nanoparticle. MNPs also enable MRI of the tissues by acting as contrast agents. While SPIONs typically act as contrast agents by shortening the T2 relaxation times of the nearby nuclei, other magnetic nanomaterials are being increasingly developed for acting as T1-contrast agents.8 Contrast agents functioning by shortening T1 are considered as “positive” contrast agents, giving a bright signal for the surrounding tissue, which is beneficial for increasing the resolution of the imaging. However, “negative”, darkening T2 contrast agents typically do not require short proximity between the nuclei and the contrast agents, as in the case of T1 agents, which can be highly useful for achieving selective imaging, e.g. for detection of brain metastases by MRI with antibody-functionalized microparticles of iron oxide as the T2 contrast agent.9 Different therapeutic approaches are available through application of MNPs, which may include magnetic-field responsive drug delivery, hyperthermia or both processes at the same time for the synergistic effect against cancer.
Hence, the magnetic nanomaterials exhibit high potential for clinical applications in targeted treatment of diseased tissues and simultaneous MRI for monitoring the treatment. Synthesis, functionalization strategies and different application potentials of SPIONs have been reviewed in detail in the literature.10–12 Reviews focusing on theranostics with magnetic nanomaterials,13–20 and studies detailing the research contributions in magnetic mesoporous silica nanoparticle (MMSN)-based multifunctional systems21,22 have also been reported. In the current review article, we focus on recent, most relevant functionalization strategies of multifunctional, multicore, MNPs and their demonstrated application potentials for cancer sensing, imaging and therapy (Fig. 1).
2. Functionalization of magnetic nanoparticles
Direct attachment of desired molecules to the surface of MNPs is often possible due to the presence of electrophilic metal ions or nucleophilic anions on their surface. However, in the case of insufficient affinity for the nanoparticle surface, functionalization with the desired molecules is achieved through different sequential linking strategies. The attachment of the linker to the molecule may be initially achieved in solution through a homogeneous reaction, followed by a heterogeneous reaction where such a newly formed conjugate is grafted to the surface of the nanoparticle. On the other hand, the desired functionalized nanoparticle may be built in sequence, through sequential heterogeneous reactions of MNPs with the linker, followed by conjugation with the desired molecule.Employment of homogeneous reactions is certainly preferred as much as possible due to higher reaction yields. However, the methodology with sequential heterogeneous reactions is often favored because of the facile separation of the products from byproducts through filtration, centrifugation or magnetic separation. Hence, this methodology may be useful in the case of multicomponent functionalization reactions, in the presence of catalysts, or in the case of difficulties in separation of surface-reactive side products after the homogeneous couplings. On the other hand, in cases where catalytic molecules and byproducts of the homogeneous coupling processes are not reactive with the nanomaterial's surface it may be desirable to perform in situ functionalization of nanomaterials with linker-modified molecules immediately after the homogeneous coupling reaction, and hence to remove all of the soluble side-products and catalysts by a single separation of the final functionalized material.
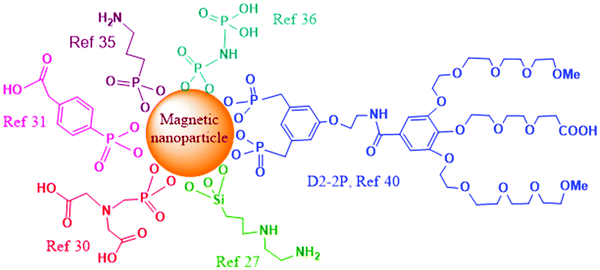
Nanomaterials which contain hydroxylated surfaces of metal ions (Fe, Al, Ti) or silicon (e.g. Fe3O4, Al2O3, TiO2, mesoporous silica nanoparticles (MSNs)) can be functionalized with silicon-based bifunctional reagents.23–26 Various commercially available alkoxysilanes can be grafted on the surface of these nanoparticles, to introduce different functional groups (thiol, amine, halide), which are useful in subsequent conjugations. The grafting process is usually performed in dry solvents at an increased temperature, in order to promote the formation of covalent linkage between nucleophilic surface hydroxyls and the reagent's electrophilic Si atoms. This reaction is possible even in the presence of water, though various side reactions are promoted under these conditions through hydrolysis of organosilane reagents and hence their homologous couplings. As an example, covalent functionalization of genomic DNA to magnetic nanoparticles was recently reported.27 Initial functionalization of the magnetite surface was investigated through two pathways, and both utilize silanization procedures with organosilane reagents. In the case of using N-(2-aminoethyl)-3-aminopropyltrimethoxysilane for surface functionalization (Fig. 2), the chelating diamine functionality was further reacted with potassium tetrachloroplatinate to create an analogue of cisplatin on the surface and thus enable attachment of DNA to platinum through guanine bases. The second approach involved silanization of the surface with 4-aminobutyltriethoxysilane, where subsequent alkylation with tris(2-chloroethyl)amine hydrochloride yielded an analogue of nitrogen mustard chemotherapy agents on the surface, which was capable of binding covalently to the genomic DNA. The prepared DNA-functionalized MNPs were demonstrated for application in removing excess chemotherapy agents (doxorubicin, cisplatin, and epirubicin) from biological solutions.
Besides hydroxyl groups, magnetic iron-oxide and other metal-based nanoparticles may also contain surface-metal ions. This type of surface is typically functionalized with bifunctionalized ligands, where one of the functional groups is a metal-coordinating group while the other functional group has weaker affinity for metal-coordination and remains free for further modification. Thus, magnetic iron-oxide nanoparticles could be functionalized through carboxylate28 or phosphonate coordination.29 Phosphonate coordination is increasingly used and has been recently shown to be very efficient for surface modification of iron oxide nanoparticles. Indeed, attachment of cytochrome P450 BM3 enzyme that contains both monooxygenase and reductase domains to magnetic nanoparticles was achieved through the usage of phosphonate bifunctionalized ligand N-phosphonomethyl iminodiacetic acid ((PMIDA), Fig. 2).30 Attachment of PMIDA to the surface of magnetite nanoparticles occurs through the phosphonate group, leaving the carboxylate moieties for further reaction. In this study, coordination of Ni2+ ions to the carboxylates was first employed, followed by the coordination of the histidine tag of the enzyme to the remaining coordination sites of Ni2+. Increased stability and prolonged activity of the magnetite-functionalized enzyme was also achieved through cross-linking the enzyme with glutaraldehyde.
A recent article investigated functionalization of piezoelectric/ferroelectric bismuth ferrite (BiFeO3, BFO) films and particles with bifunctional ligands benzene-1,4-dicarboxylic acid (BDC) or with phosphonobenzoic acid (PBA, Fig. 2), after a pre-activation step with hydrogen peroxide (H2O2) to produce –OH groups on the surface.31 The authors concluded that in the case of PBA the surface functionalization occurred preferentially through the phosphonate group. In the case of BDC as the ligand, the functionalization was also successful though a significant portion of BDC molecules was lying down parallel to the surface with both carboxylic terminations deprotonated and attached to the surface. In another study, Sandre and co-workers synthesized multifunctionalized iron oxide nanoparticles and nanoflowers.32 The surface functionalization of the nano-objects with PEG groups and the near-infrared fluorescent probe DY700 was carried out in one step through a very efficient convergent strategy using phosphonate anchors. The nanoflowers were demonstrated to be more efficient than single core nanoparticles for the destruction of cancer glioblastoma cells through magnetic hyperthermia, leading to 80–90% cell death.
Functionalization of cell penetrating peptides (CPP) on MNPs was recently investigated to enhance the lysosomal escape and cell uptake of the nanoparticles. One of the strategies for functionalization of SPIONs,33 obtained using the Massart protocol,34 with cyanine fluorescent dye emitting in far red, polyethylene glycol (PEG5000) coating, and the membranotropic peptide gH625, involved initial functionalization of the magnetite surface with 3-aminopropyltrimethoxy silane (APTES), followed by subsequent dicyclohexylcarbodiimide–n-hydroxysuccinimide (DCC–NHS) and thiol–maleimide coupling. In a different functionalization approach, the initial surface functionalization was achieved by reaction with the phosphonate moiety from 3-aminopropylphosphonic acid (Fig. 2), and the free amine moiety was further involved for DCC–NHS coupling with folic acid, PEG and gH625 peptide.35 In both cases the increased cell uptake of CPP-functionalized magnetic nanoparticles was demonstrated by in vitro experiments.
Radiolabeling of magnetite nanoparticles with 90Y, a high energy β-emitter, was recently demonstrated for potential applications in magnetic field-guided delivery of radionuclides to the tumor and imaging.36 The attachment of the radionuclide was enabled through coordination to phosphonate groups, by surface functionalization of magnetite with multiphosphonate ligands imidodiphosphate (IDP, Fig. 2) and inositol hexaphosphate (IHP).
Strong coordination of the phosphonate group to Gd3+ was utilized to construct renal clearable peptide–NaGdF4 nanodots with active tumor targeting.37 Functionalization with cell penetrating peptide (CPP), phosphorylated cytosol-localizing internalization peptide 6 (pCLIP6), and phosphorylated retro-inverso tumor-affinity peptide (pD-SP5) (pD-SP5), was achieved through ligand exchange with oleate on the surface of NaGdF4 nanodots. In vitro and in vivo experiments revealed that the functionalized nanodots have low toxicity and allow excellent MR imaging of orthotopic colorectal tumors at low dose of the contrast agent. High affinity of Fe3+ ions to phosphonate ligands was previously utilized to functionalize the MNPs with different sugars, through ligand exchange of surface oleates.38
A similar principle of exchange with phosphonate ligands was recently used to covert hydrophobic iron nanoparticles into water-soluble iron–iron oxide core–shell nanoparticles.39 The nanoparticles contain a monocrystalline body-centered cubic iron core of ca. 10 nm in size surrounded by a spinel oxide shell of ca. 2 nm thickness, having promising magnetization (156 A m2 kgFe−1) and relaxivity (r2 = 335 mM−1 s−1) values for biomedical applications.
Ligands of an oligoethylene glycol dendron structure, containing a phosphonic anchor (D2) or a biphosphonic tweezer (D2-2P, Fig. 2) moiety were functionalized by the ligand exchange methodology on superparamagnetic spherical iron oxide nanoparticles of 10 nm in diameter, synthesized by thermal decomposition.40In vivo MRI studies on mice, upon intravenous injections, showed high contrast enhancement and evidence for continuous renal and hepatobiliary excretions of both dendronized NPs, with no signs of toxicity during the period of the experiment (48 h). Particularly interesting is the observation that the D2-2P-functionalized magnetic nanoparticle showed no uptake in three different cancer cell lines, which would be beneficial for devising safe early cancer diagnosis and imaging agents, or for achieving specific active targeting through additional functionalization with targeting ligands.
Polyelectrolytes having different iron oxide complexing units (carboxylic vs. phosphonic acid), along with different structures and properties have been investigated for direct functionalization of the surface of magnetofluorescent nanostructures, which are multimodal imaging agents facilitating high resolution diagnosis.41 As determined, using comblike polyelectrolytes containing carboxylate units (n ≈ 25 repetitive units) based on poly(methyl methacrylate) led to the highest colloidal stability of magnetofluorescent nanostructures in an aqueous environment. However, application of polyelectrolytes with phosphonic acid moieties lead to disruption of magnetofluorescent nanostructures presumably due to strong interaction of phosphonates with Fe3+ ions on the surface, leading to separation of magnetic nanoparticles from the original organic fluorescent framework.
Other research studies also determined that the surface-functionalized molecules can exert an effect on the magnetic properties of the nanoparticles.42–44
Further modification of the surface of iron oxide nanosystems can be performed through many different chemical conjugation strategies, which have been reviewed in detail.45,46 The thus obtained multifunctionalized nanoparticles have been showcased for stimuli-responsive therapy and diagnostics of cancer,47 as well as for image-guided targeted therapy.48
3. Applications of magnetic nanoarchitectures for cancer sensing and imaging
Magnetic biosensors are being increasingly investigated in the context of cancer nanotheranostics. The key approach is the use of magnetic micro/nanoparticles or magnetic nanostructures coupled to the molecular recognition elements (bioreceptors) such as antibodies, aptamers, DNA probes, small peptides, and bacteriophages. The biofunctionalized magnetic nanoparticles (BMNPs) produce the stray field when magnetized by an externally applied field, which provides a signal detectable by the magnetic sensors. The concentration of the BMNPs bound to the analyte is proportional to the analyte concentration. Many conventional assays that were using fluorescent labeling have been adapted for magnetic sensing using BMNPs, e.g. magnetic immunoassay (MIA).49,50 Some of the main advantages of magnetic sensing are high sensitivity, high signal-to-noise ratio (SNR), detectability of MNPs in colored medium and opaque structures, as well as the possibility of using a convenient option for manipulation of the magnetically labeled entities within the microfluidic channels of the sensing device, using externally applied high gradient magnetic fields. Kokkinis et al. harnessed this option to produce a microfluidic platform containing integrated magnetic sensors for counting the cancer cells pre-isolated from the whole blood samples.51 The innovative aspect of the proposed cell chip is that it utilizes BMNPs to label and separate the cancer cells using integrated conducting microstructures. Magnetically labeled and separated cancer cells can be subjected to further complex analyses, which renders the described chip a very useful low-cost tool that has potential to simplify workflow in the clinical setting.Another characteristic potentially useful in a clinical setting is the reusability of the MNPs, as after collecting the BMNPs from the mixed system by an external magnetic field, the captured biomolecules can be washed out, allowing reuse of the magnetic particles.52 Nevertheless, there are still a number of technical challenges to solve, particularly regarding separation of bound biomolecules, in order to achieve full BNMP recyclability.
In contrast to sensing with fluorescent labels, magnetic biosensing does not face the problem of background noise, since there is no detectable magnetic content in the biological samples, which enables development of sensing systems with low limit of detection (LOD). However, there are a few disadvantages to the use of BMNPs as well, such as their stability, with respect to the surrounding environment, especially when considering nanoparticles below 100 nm.53 These disadvantages might be overcome with new developments in the surface functionalization methods. Another issue when using such small particles that exhibit small magnetic moment is the need for more sensitive measurement systems and sensors, which is another technical challenge for the magnetic biosensing field.
In terms of detection of the magnetic signal, most current applications in life sciences use the magnetoresistance effect. In biosensing applications several different magnetoresistance sensor technologies (giant magnetoresistive, spin valves, and tunnel magnetoresistive) are used, presented in detail in the recently published perspective article by Giouroudi and Hristoforou.54
However, a particularly interesting approach for detecting the magnetic signal was developed by Nikitin et al., who use magnetic particle quantification (MPQ) based on the non-linear magnetization of MNPs subjected to an alternating magnetic field at AC frequencies f1 and f2, and subsequently recording the MNP response at a combinatorial frequency f = f1 + f2. The MPQ approach is robust and permits registration of minute magnetic signals in the presence of strong noise, providing high sensitivity and precision.55,56 The Nikitin group developed several MPQ based platforms for quantitative multiplex magnetic immunoassays for different cancer markers, such as prostate-specific antigen (PSA), using the 50 nm or 100 nm superparamagnetic microspheres conjugated to antibodies. The platforms include (a) one-run tests on 3D porous filters where the signal was read out from the entire volume of the nontransparent 3D fiber filters employed as a solid phase for sandwich immunoassays, with LOD of 12 pg mL−1 and the dynamic range exceeding 3 orders of concentration; (b) disposable flat and micropillar biomagnetic sensor chips where the capture antibodies are placed in several recognition spots on the chip. The target molecule binds to the capture antibodies and subsequently the immunosandwich structure is formed between the captured target molecule and the magnetic label functionalized with the antibody specific for the target molecule, with LOD of 90–100 pg mL−1 for PSA; (c) a lateral flow assay (LFA) test strip with several test lines with magnetic signals simultaneously recorded from each line independently using a multichannel MPQ reader. This design brings the problem of cross-reactivity of different immunoreagents and BMNPs since they are all simultaneously present in solution and can potentially interact with each other. This problem is reduced with the fourth platform of magnetic biosensors based on a 3D modular architecture where several spatially separated LFA test strips of different specificity are used and simultaneous volumetric detection of MNPs from all recognition zones was performed. The described platforms are useful for rapid, simple, sensitive quantitative measurements of different cancer markers and other small molecules.57
Immunomagnetic nanosensors are a useful tool not only for quantitative detection but for imaging of cancer stem cells (CSCs) as well. Wang et al. developed a nanosensor for real-time molecular imaging of targeted glioblastoma CSCs, that harnesses specific interaction between cell-membrane marker antigen CD133 of glioblastoma CSCs and anti-CD133 monoclonal antibody (mAb) coupled to MNPs.50 Superparamagnetic γ-Fe2O3 iron oxide nanoparticles were fabricated as nanosensor cores of approximately 10–15 nm in size, and coated with carboxymethyl chitosan via sodium tripolyphosphate crosslinking, and then chemically modified with polyethylenimine (PEI). Anti-CD133 mAb was conjugated to the PEI-MNPs using coupling reagents sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate and Traut's Reagent. The formed anti-CD133 mAb-conjugated-MNPs were tested in vitro for cell toxicity, specificity and effect on the cell cycle and subsequently delivered to cultured human brain glioblastoma CSCs for fluorescence imaging and MRI. The mAb–MNP conjugate used for fluorescence imaging was additionally coupled to the rhodamine B isothiocyanate (RBITC) fluorescent probe. Nanosensor particles showed high biocompatibility, specificity and no toxicity. Glioblastoma CSCs targeted with the mAb–MNP conjugate displayed strong fluorescence signal and negative MRI contrast enhancement (signal darkening) in T2-weighted MRI in comparison to the cells treated with non-mAb-functionalized MNPs, leading to the conclusion that the described immunomagnetic sensor has high potential for use as a fluorescent nanoprobe and MR contrast agent for glioblastoma CSC real-time monitoring. However, additional evaluations are needed to confirm the sensor's in vivo usability.
Nebu et al. used a different approach to ensure targeting of the biofunctionalized MNPs-BMNPs to the cells while achieving the theranostic effect in parallel.58 They conjugated MNPs to erlotinib, clinically approved as an efficient inhibitor of epidermal growth factor receptor (EGFR) that is commonly over-expressed by pancreatic cancer cells (PCCs).59 Firstly, superparamagnetic Fe3O4 cores were produced having gold nanoclusters (AuNCs) adsorbed on the surface through electrostatic attraction. The rationale for adding the luminescent AuNCs to MNPs is based on the good photo-stability of AuNCs, and their ultrafine size, large Stokes shift, size-dependent optical properties and good biocompatibility.60 The thus formed Fe3O4@AuNCs with negatively charged shells were then conjugated to the positively charged erlotinib by electrostatic attraction. The in vitro drug release study of erlotinib was carried out in PBS buffers mimicking two physiological environments with pH = 7.4 and 5.0 at 37 °C, evidencing clear enhancement of drug delivery at lower pH values, which are characteristic for cancer tissues. The erlotinib-functionalized MNP–AuNC nanoprobe provided fluorescence enhancement in the confocal microscopy of the cultured PCCs (PANC-1 cell line). Taken together, these results indicate good potential of the described anticancer drug-MNP-gold cluster nanoprobe for both imaging (MRI or optical) and drug delivery, two criteria crucial for efficient nanotheranostics.
The general tendency in the field of biosensors is to reduce costs of production and to simplify their use for achieving low-cost point-of-care (POC) usability. One of the attempts with regards to POC cancer diagnostics is described by Tian et al. who devised a microRNA detection biosensor based on combining MNP assemblies with DNAzyme-assisted target recycling, which can lower costs of sensor production in comparison to the protein–enzyme based microRNA detection methods.61 MicroRNAs are regarded as one of the crucial developments in cancer biology in recent years, with high potential of circulating microRNAs to be used as cancer biomarkers, even providing a specific microRNA signature for specific cancer types, prognosis and response.62 Bioresponsive DNA-based scaffolds possess several characteristics such as controllable length, specific hybridization and reactivity with enzymes, which make them a good choice for use in nanotheranostics, in combination with nanoparticle assemblies, particularly since production of functional DNA sequences such as deoxyribozymes (DNAzymes), aptamers and aptazymes has been improved by the use of SELEX (systematic evolution of ligands by exponential enrichment) technology.63 DNAzymes – DNA enzymes or catalytic DNA, are single stranded DNA oligonucleotides with nuclease- or peroxidase-mimicking activity, used frequently in combination with nanoparticles as biosensors, particularly for amplified sensing and as proofreading units, especially as multi-component DNAzymes (MNAzymes).64,65
Tian et al. devised an interesting concept by using MNP assemblies in combination with DNA scaffolds that also comprise substrate sequences for DNAzyme.61 When target DNA or microRNA sequences are present, DNAzyme will react with them, catalyzing the cleavage that will further cause the rupture of the scaffolds and disintegration of the MNP assemblies. The number of released MNPs, proportional to the concentration of the target, is quantified by a 405 nm laser-based optomagnetic sensor. Importantly, nucleic acid target sequences are not destroyed during the cleavage reaction, meaning that they can be released further to the suspension to induce digestion of multiple DNA scaffolds. The diffusion time for target recycling can be optimized i.e. reduced by increasing the concentration of the substrate sequences in the MNP assemblies. An interesting aspect of this study is that the MNPs were employed for multiple functions – as labels, carriers and building blocks. The proposed biosensor was tested with short (app. 20 nucleotides) single-stranded DNA and RNA targets and showed a limit of detection (LOD) of 1.5 pM target DNA in buffer and LOD of 6 pM target microRNA in 10% fetal bovine serum. However, further studies are needed to confirm the efficacy of the described biosensor with cancer-specific microRNA sequences. In addition, further technical optimization is needed, since the current sensor performance is heavily dependent on experimental conditions, such as reaction temperature and reaction time – DNAzymes start denaturization at temperatures higher than 50 °C, while the signal intensity and subsequent sensor sensitivity depends on the reaction time – it is necessary to provide at least a 30 min long interval as the reaction time for the described biosensor.
Circulating tumor cells (CTCs), considered as a valuable biomarker for early cancer detection, are released from primary and metastatic tumors into the bloodstream. A recent report demonstrated the application of a magnetic mesoporous silica nanoparticle (MMSN) type of material, functionalized with anti-MUC1 aptamer for selective interaction with mucin 1 protein (MUC1) which is over-expressed in more than 90% of human breast carcinomas, to capture CTCs from the bloodstream (Fig. 3).66 The amount of breast cancer cells was quantified by additional specific reaction between overexpressed folate receptors on CTCs and the fluorescent probe (FA–BSA–FITC), prepared by linking folic acid and fluorescein isothiocyanate (FITC) to bovine serum albumin (BSA). Hence, CTCs are sandwiched between the fluorescent probe and the AntiMUC1-MMSN, which are isolated from the sample by a magnet and further quantified by fluorescence. The constructed detection system showed excellent selectivity in the presence of different cell lines, good reproducibility and accuracy, with a LOD of 100 cells per mL.
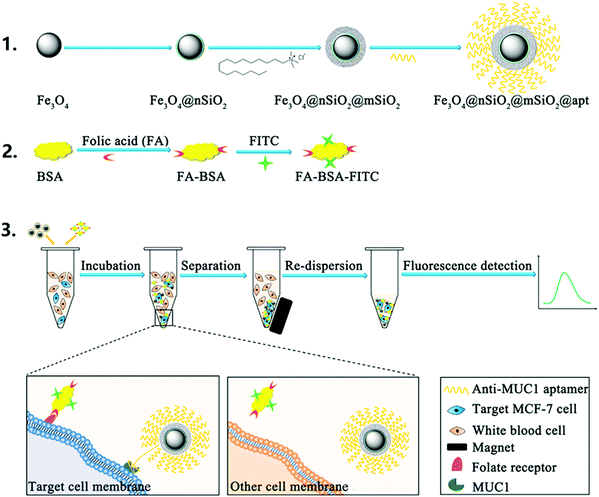 | ||
| Fig. 3 Principle of the dual-target recognition sandwich assay for MCF-7 cell detection. Reprinted from ref. 66 with permission from Elsevier. | ||
A superparamagnetic-upconversion nanocomposite was also demonstrated for deep tissue imaging and treatment of cancers.67 Namely, folic acid (FA)-targeted, photosensitizer (PS)-loaded Fe3O4@NaYF4:Yb/Er nanocomposites were synthesized for dual imaging capability, fluorescence and T2-weighted MRI, along with the ability for treatment of cancer by photodynamic therapy (PDT) with near-infrared (NIR) irradiation. In vivo experiments on mice revealed the efficient capability of PDT with the synthesized nanocomposite to reduce the volume of tumors with preferential accumulation of the nanomedicine in tumor tissues.
Previously, triple-functional core–shell structured nanomaterial NaGdF4:Yb,Er@CaF2@SiO2-PS was synthesized and its in vitro applicability for PDT, MRI and fluorescence/luminescence imaging of tumors was demonstrated.68 Therein, the upconversion nanoparticles NaGdF4:Yb,Er@CaF2 were first prepared as cores and the mesoporous silica shell was then formed, containing covalently attached PSs (silicon phthalocyanine dihydroxide and haematoporphyrin) for PDT activity. Under excitation at 980 nm, the nanomaterial gives luminescence emissions at 550 and 660 nm. The former is used for fluorescence imaging and the latter is used for energy absorption of PS to generate singlet oxygen and hence PDT. Magnetic measurements revealed that the synthesized material is paramagnetic, attributed to the seven unpaired inner 4f electrons of Gd3+ ions. This feature was showcased as applicable for T1-weighted imaging, evidently through penetration of water within the mesopores of the nanotheranostic agent. The constructed NaGdF4:Yb,Er@CaF2@SiO2-PS nanomaterial showed similar capability for MRI to Gd–DTPA, which is readily applicable in clinics.
Another type of magnetic-upconverting nanorattle core–shell particle has been demonstrated for efficient application in magnetic field-targeted cancer therapy and imaging in vivo.69 The nanoparticles were composed of upconverting hydrophilic, rare-earth doped NaYF4 shells for imaging, each containing a loose magnetic nanoparticle inside the hollow compartments. Doxorubicin filled nanomaterial was intravenously injected into the tumour-bearing mice while the neodymium disk magnets were located at the tumour site, which resulted in the enhanced efficiency of the drug delivery process and shrinkage of tumor volumes. This study demonstrated a facile methodology for increasing the activity of designed nanotherapeutics through active magnetic targeting.
Recently an innovative principle for analyte dependent on/off switching of MRI was described.70 The authors attached a T1 contrast agent (Gd(III) complex) to the surface of silica-embedded superparamagnetic nanoparticles (12 nm Zn0.4Fe2.6O4). The distance between Gd(III) and the superparamagnetic nanoparticles was modified by controlling the thickness of the SiO2 separating layer from 18 to 2 nm. In this system, the superparamagnetic nanoparticles exhibited quenching of T1 weighted MRI in a distance-dependent manner, which was termed as a novel magnetic resonance tuning (MRET) principle between a paramagnetic ‘enhancer’ and a superparamagnetic ‘quencher’. Hence, the intensity of T1 MRI signals increases significantly with increasing the separation from the quencher, which was utilized for sensing specific molecules, by designing a H2O2 cleavable sulfonate linker or a matrix metalloproteinase-2 (MMP-2) cleavable peptide linker between the enhancer and the quencher. Thus, in the presence of H2O2 or MMP-2, a cancer biomarker, the T1 MRI signals intensify and allow in vitro and in vivo cancer tracking.
Novel cobalt oxide nanoprisms containing doxorubicin were recently demonstrated as responsive to intratumoral oxidizing and acidifying environments, with switching the MRI imaging from T2 to T1 and luminescence recovery of doxorubicin.71 This principle allowed efficient multimodal in vivo imaging and simultaneous treatment of cancer. For further studies regarding the applications of magnetic nanomaterials in MRI the focused review articles on this topic should be consulted.72,73
4. Magnetic-field responsive therapy and drug delivery
Hyperthermia of cancer through exposure of MNPs to an alternating magnetic field (AMF) has been demonstrated in recent years as a particularly promising treatment option. The capability of magnetic nanomaterials to generate heat upon exposure to AMF can be expressed by specific loss power (SLP), which is the value of heat dissipation in water per unit mass of magnetic material. Different iron oxide-based nanoparticles, such as nanocubes,74,75 nanoparticles with a magnetically hard core and magnetically soft shell,76 and multi-core nanoparticles,77 have been reported recently with SLP values of up to 1000 W g−1. However, a recent study demonstrated that unprecedented SLP values of up to 5000 W g−1 can be obtained when cubic MNPs are exposed to near infrared irradiation simultaneously to AMF.78 This synergistic effect of magnetic hyperthermia and photothermal effects of magnetic nanocubes is demonstrated as very applicable for the treatment of tumors in vitro and in vivo. Similar effects have been observed in the case of multifunctional silver/magnetite nanoflowers (Ag/Fe3O4), with the increase in SLP value by an order of magnitude under the application of both an external magnetic field and simultaneous laser irradiation.79MNPs of 11 nm mean diameter with 2000 MW polyethylene (PEG) coating have been showcased to achieve preferential accumulation in tumor tissues,80 through the EPR effect, which helped in efficient ablation of tumor tissues upon exposure of MNP-treated mice to AMF. The authors demonstrated that much higher temperature was achievable in tumor tissues compared to healthy muscle upon exposure to AMF, which is due to accumulation of the nanoparticles in tumors. Also, the survivability of AMF treated tumor-bearing mice was much higher to the untreated ones. To further increase the efficiency of hyperthermia, more sophisticated and very diverse nanosystems have been reported. Recently, wüstite Fe0.6Mn0.4O nanoflowers have been demonstrated as effective theranostic agents with T1–T2 dual-mode magnetic resonance imaging (MRI) capabilities and efficient magnetic hyperthermia treatment ability.81In vitro and in vivo experiments revealed enhanced cancer cell apoptosis and complete tumor regression in mice upon the treatment with the nanoflowers and exposure to alternating magnetic field. Exposure to multiple repeatable magnetic hyperthermia treatments has also shown enhanced therapeutic effect.82 The authors assembled magnetic nanoparticles within biodegradable poly(organophosphazene) hydrogel nanocapsules, which showed excellent MRI capabilities and induced necrosis of cancer cells in vivo after four cycles of exposure to magnetic hyperthermia at mild temperatures, without severe damage of the surrounding healthy tissues.
Hyperthermia can be used in combination with drug delivery, to facilitate the delivery of the payload from the nanoparticle at the cancer site. J. Liu et al. prepared thermo-, glucose- and pH-responsive poly(vinyl alcohol)-b-poly(N-vinylcaprolactam)-based nanogels conjugated to 7.5 nm maghemite nanoparticles through boronate linkages.83 The nanogel was tested with loaded Nil Red as a model hydrophobic drug, and no premature release was noticed in the absence of glucose and at physiologic conditions. The release was triggered upon exposure to glucose or acidic pH, while exposure to AMF was showcased to lead to hyperthermia and enhancement of the dye release. Furthermore, the capability of the hybrid material for T2-weighted MRI was demonstrated, as well as the glucose- and acidification-induced toxicity of the drug Tamoxifen loaded nanogel against mouse fibroblast-like L929 and human melanoma MEL-5 cell lines. In another study, Park et al. performed the synthesis of Fe–Co core nanoparticles and wrapped them with a graphitic carbon shell (Fe–Co/C).84 The nanoparticles of 11 nm were coated with dextran for biocompatibility and were modified with antibodies or cyclic RGD peptide to target glioblastoma. The nanoparticles were also applicable for MRI and Raman bimodal imaging, endowing them with theranostic capabilities, as showcased by in vitro and in vivo experiments. Layer by layer functionalization of the Fe–Co/C nanoparticles was employed for surface modification with PEI and subsequently with siRNA. In vitro experiments were carried out in order to deliver siRNA to glioblastoma U87 cells and effective silencing of the oncogenic EGFRvIII gene was demonstrated, as well as the synergistic therapeutic effect of magnetic field-induced hyperthermia. Zn-Doped iron oxide nanoparticles of 15.4 nm hydrodynamic diameter were also coated with PEI and then grafted with a pro-apoptotic mitochondria-targeting peptide (ATAP) along with a polyethylene glycol (PEG)-conjugated tumor targeting peptide (iRGD).85 Both peptides were attached to the nanoparticle's surface through disulfide linkages and their anticancer effect was showcased on glioblastoma multiforme (U87vIII) and metastatic breast cancer cells (MDA-MB-231). Magnetic field induced hyperthermia further enhanced cancer cell death through a synergistic effect. Preliminary in vivo testing was carried out with an esophageal cancer xenograft model and showed suppression of tumor growth. Omar et al. prepared large pore (20–60 nm) iron oxide–MSN composites of 100 nm diameter for the delivery of proteins to cancer cells.86 The nanoparticles were biodegradable and were functionalized with APTES in order to immobilize negatively charged proteins in the pores of the nanoparticles by electrostatic interactions. The model protein was ferritin, which was released in an aqueous environment by lowering the pH or upon exposure to AMF. The ferritin-loaded nanoparticles were also tested on HeLa cancer cells, and the protein was successfully delivered due to the acidic compartment of lysosomes, as shown by confocal microscopy.
MNPs can cause damage to the cells even without production of heat. For example, exposure to a low frequency pulsed magnetic field was observed to cause cell killing through mechanical damage.87 A similar mechanical effect on cancer cells was observed in the case of the movement of cancer cell-treated MMSNs in response to the magnetic pull.88
5. Magnetic nanoparticle–polymer core–shell nanotheranostics
Various hybrid materials containing polymers and magnetic nanoparticles have been developed in recent years for battling cancer through different therapeutic and diagnostic methodologies.89 Complex heat-responsive anticancer nanomedicines can be designed by exploiting the heat-producing feature of magnetic nanoparticles upon exposure to AMF. Core–shell nanoparticles containing a magnetite core of 20 nm in diameter, a 20 nm-thick mesoporous silica shell and a covalently attached thermo-sensitive polymer, poly[(ethylene glycol)-co-(L-lactide)] (P(EO-co-LLA)) coating, as a “gatekeeper” onto the mesopores, have been showcased for efficient simultaneous treatment of cancer by AMF-induced hyperthermia and thermo/pH-responsive drug delivery.90In vitro studies on HeLa cancer cells revealed that hyperthermia alone causes a decrease in viable cells but it is augmented by the presence of mesopore-loaded drugs which caused killing of cancer cells with increase in temperature or by acidification inside the endosomal compartments. Recently, a sacrificial mesoporous carbon template was employed for the construction of a mesoporous iron oxide nanosystem, which was capped with a thermoresponsive polymer shell and entrapping doxorubicin within the material (Fig. 4).91 Gradual release of the drug was observed in an acidic environment, which was significantly enhanced upon exposure to an alternating magnetic field. Therapeutic effects of the nanosystem were observed on different cancer cells and even treatment resistant cancer cells, while no effect was observed on healthy cells, ascribed to a low internalization of nanoparticles. Combination of the anticancer effect of magnetic hyperthermia and photodynamic therapy was recently demonstrated by magnetite nanoparticles functionalized with photosensitizer conjugated hyaluronic acid, which showed cooperative inhibition of tumor growth.92 Capability for dual imaging (optical and MRI) with the same nanomaterial was also demonstrated.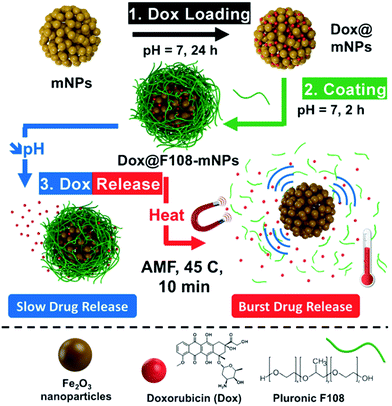 | ||
| Fig. 4 Schematic representation of the application of polymer-capped mesoporous iron oxide nanoparticles for loading doxorubicin and its pH- and hyperthermia-responsive release. Reproduced from ref. 91 with permission from Wiley. | ||
Hyaluronic acid-modified Fe3O4@Au core/shell nanostars (Fe3O4@Au-HA NSs) were also demonstrated for a triple-mode imaging (magnetic resonance (MR), computed tomography (CT), and thermal imaging) with capabilities for photothermal therapy of tumors.93 Ferritin was showcased as an efficient shell for construction of biocompatible magnetic nanoarchitectures.94 A series of uniform magnetic/plasmonic Fe3O4/Au core/shell nanoparticles with precisely controlled core diameters and shell thicknesses were recently developed by employing amphiphilic star-like P4VP-b-PtBA-b-PEO triblock copolymers as nanoreactors.95 A core–shell nanostructure consisting of Fe3O4 nanoparticles as the core, organic alginate as the shell, and cell-targeting ligands (i.e.D-galactosamine) decorated on the outer surface were reported for targeted treatment of cancer through alternating magnetic field-induced hyperthermia.96
A new imaging modality with magnetic nanoparticles, magnetic particle imaging (MPI) was also recently utilized for cancer-cell labeling and in vivo tracking, by Janus iron oxide nanoparticles, functionalized with a semiconducting polymer.97 The obtained nanoparticles showed higher efficacy for MPI-in vivo particle tracking than commercially available agents and showcased an effective alternative to MRI with high sensitivity, unlimited tissue penetration, linear quantitativity and nearly no background from tissues.
6. Core–shell silicon-based magnetic cancer nanotheranostics
Nanoparticles based on mesoporous silica are particularly attractive scaffolds for construction of complex nanotherapeutics, due to high surface area, isostructural mesoporosity and a particle diameter (100–200 nm) in a range suitable for exploiting the EPR effect of cancerous tissue, to enhance the selectivity of the treatment. Magnetic core/shell analogues of MSN materials (MMSN) are readily obtained by including magnetic nanoparticles during the sol–gel surfactant templated synthesis procedure.98–101 Transmission electron micrographs of some of the literature-reported MMSN materials are represented in Fig. 5, characterized by various pore and particle morphologies.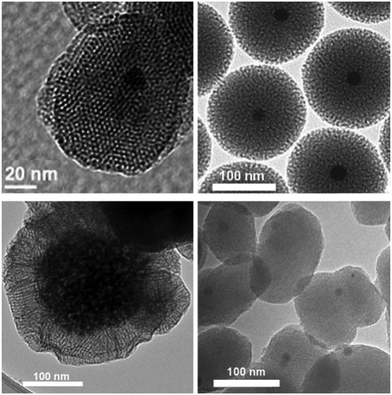 | ||
| Fig. 5 Transmission electron micrograph of MMSN materials. Reproduced from literature reports of the authors.98–101 | ||
Moreover, the functionalization can be selectively applied to different surfaces of MSNs, i.e. the external surface can be functionalized with one moiety while the surface inside the mesopores can contain another type of functional group. This property enables construction of complex nanodevices, with separate functionalized moieties performing different functions simultaneously (evading immune response, selective cancer targeting, on desire-responsiveness of the treatment activity, imaging). Cancer targeting can be enhanced beyond the EPR-related accumulation by attaching cancer specific ligands on the external surface of nanodevices,102 while at the same time the MSN-based nanodevice can contain a magnetically active core to produce MMSNs and to open possibilities for magnetic field targeting.100,103 Ultimately, the activity of the drug-carrying nanodevice can be designed in such a manner to render them responsive to various intratumoral or to externally applicable stimuli, thus enabling “on desire” drug release from the nanotherapeutic agent.104
Capabilities for MRI imaging and drug delivery with MMSN type materials were demonstrated in 2006–2008 by three independent groups. Mou's group prepared Fe3O4@silica core–shell nanoparticles and fused them with FITC-functionalized MSNs to prepare Mag-Dye@MSNs which were able to act as bimodal imaging probes and drug reservoirs.105 These nanocomposites, 120 nm in diameter, were used to labeled cells in vitro and for in vivo tracking experiments.106 After administration of Mag-Dye@MSNs though eye vein injection of mice, the nanoparticles accumulated in the liver and spleen as shown by T2 weighted-MRI and histological sections of tissue organs. An MRI long term tracking (three months) revealed that Mag-Dye@MSNs were not easily excreted and were resistant to decomposition. No toxicity or abnormalities were noticed. Zink's group prepared multifunctional MSNs of 100–200 nm diameter encapsulating iron oxide nanocrystals of 20 nm diameter, by aqueous transfer of iron oxide nanocrystals coated with CTAB and by subsequent basic hydrolysis-polycondensation of TEOS in the presence of CTAB. The MMSNs were labeled with FITC for fluorescence imaging, and coated with phosphonate and folic acid to prevent aggregation and to target cancer cells respectively. The MMSNs were used for MRI in solution and inside cancer cells, for paclitaxel and camptothecin delivery in cancer cells with significant efficiency.107
Hyeon's group independently used a similar procedure to synthesize MMSNs. The authors showcased EPR-induced accumulation of MMSNs in tumor tissues, which resulted in dark areas in T2-weighted images due to the presence of superparamagnetic nanoparticles.108 Studying relaxivities of core–shell MMSNs of different diameters, i.e. different silica shell thicknesses, revealed that r2-relaxivity does not decrease significantly in comparison to the superparamagnetic nanoparticles without mesoporous silica coating up to the particles of 75 nm in diameter.109 Therefore, mesoporosity of the silica shell allows unhindered water penetration into the proximity of superparamagnetic nanoparticles, which allows influence on r2-relaxation. In contrast, when a non-porous silica shell is grafted on the magnetite core, r2-relaxation decreases rapidly already at the thickness of the shell at 20 nm.110 In the same study, the authors demonstrated the enhancement of MRI accuracy in the case of the presence of T1-contrast agent in addition to the superparamagnetic nanoparticles as T2-contast agents within the same nanoparticle.
Another study reported combination of paramagnetic and superparamagnetic nanomaterials, as T1 and T2 contrast agents, respectively, which is capable of eliminating false errors (artifacts) from the raw images to enhance accuracy of the MRI.111 This effect is achieved through the capability to perform an “AND logic gate” algorithm, with the contribution of the filtering effect. The constructed artifact filtering imaging agents (AFIAs) have a core–shell structure, containing different superparamagnetic nanoparticles as a T2 contrast agent in the core, SiO2 as the separating layer and various paramagnetic materials as the shell and T1 contrast agent, as represented in Fig. 6. The authors showcase that the thickness of the separation layer of 16 nm is suitable to have both T1 and T2 signals of high magnitude at the same time and demonstrate the efficacy of the constructed AFIAs for artifact-free imaging by in vitro and in vivo experiments.
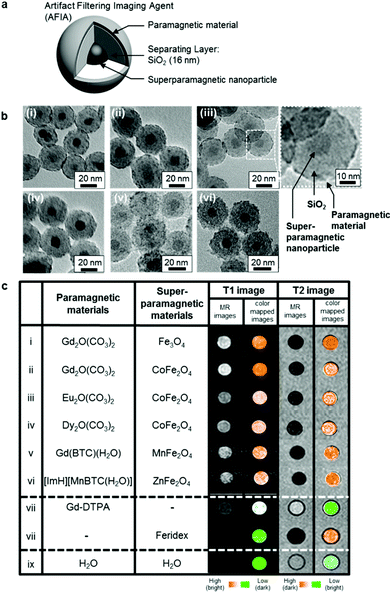 | ||
| Fig. 6 A variety of AFIAs and their MRI contrast effects. (a) Schematic illustration of core–shell structured AFIA. (b) TEM images of various core–shell structured AFIAs and (c) MRI images and their color-coded images of AFIAs and conventional contrast agents. Reprinted from ref. 111 with permission from Copyright 2014 American Chemical Society. | ||
Synthesis and biomedical applications of different MMSN materials were reviewed in depth in 2013,112 however many attractive important achievements on this topic have been published since. The synergic effect between the intracellular hyperthermia and chemotherapy triggered by exposure to AMF was recently demonstrated by in vivo experiments.113 The authors utilized MMSN material containing core magnetite and drug loaded mesopores, entrapped by the presence of temperature-responsive polymer as the gatekeeper. The analogues of MMSN materials with radial porosity and surface-attached folic acid were also demonstrated, both in vitro and in vivo, for AMF-responsive hyperthermia and simultaneous MRI-guided and AMF-responsive chemotherapy for breast cancer.114
Vascular endothelial growth factor (VEGF)-small interfering RNA (siRNA) was encapsulated into an MMSN-based, polyethylenimine (PEI)-capped, polyethylene glycol (PEG)-grafted, fusogenic peptide (KALA)-functionalized siRNA delivery system, which showed significant effectiveness with regard to VEGF gene silencing in vitro and in vivo, yielding an efficient antiangiogenesis effect on ovarian cancer tissues.115
A more recent study showed application of complex MMSN nanoarchitectures for in vivo MRI imaging and precise therapy.116 In this study doxorubicin was loaded inside the mesopores of MMSN and β-cyclodextrin (β-CD) was used as the gatekeeper, which was linked to the pore entrances through a platinum(IV) prodrug. In addition, adamantane-PEG8-glycine-arginine-glycineaspartic-serine (AD-PEG8-GRGDS) was functionalized on the MMSNs through the strong AD-β-CD host–guest interaction, for additional targeting of cancer by binding to cancer-overxpressed αvβ3 integrin. The functioning principle of this nanomedicine is that the drug delivery is intracellularly activated by reduction of Pt(IV) to the toxic platinum(II) drug. The authors showcased that the material efficiently reduced the volume of cancer tissue in vivo and that magnetic targeting is an effective method for increasing the accumulation of MMSNs in cancer tissue and to enhance the therapeutic effect.
In another study, J. Li and co-workers coated MMSNs with red blood cell membrane (RBC@MMSNs).117 Photosensitizer hypocrellin A was adsorbed inside the mesoporous structure. In vivo experiments after tail vein injection of RBC@MMSNs in tumor-xenografted mice demonstrated long circulation time and stealth behavior of RBC@MMSNs. Magnetic targeting allowed accumulation of RBC@MMSNs in the tumor and light irradiation led to efficient photodynamic therapy with necrosis of cancer tissues showing the efficiency of RBC@MMSNs.
A novel multifunctional MMSN type of material was reported containing magnetite and gold nanoparticles as the core and photosensitizer chlorin e6 (Ce6) and doxorubicin inside the mesopores of the mesoporous silica shell (Fig. 7).118 These molecules were entrapped by layer-by-layer assembly of alginate and chitosan as pH-sensitive gatekeepers with the final layer containing small hairpin RNA (shRNA) which downregulates P-glycoprotein (P-gp), a protein involved in drug efflux that contributes to the occurrence of treatment resistance. Real-time imaging-guided simultaneous PDT therapy, chemotherapy and gene therapy was enabled in vivo by the presence of iron oxide and Au nanoparticles for MRI and CT imaging, with efficient combination of PDT and chemotherapy through overcoming multidrug resistance by simultaneous gene silencing.
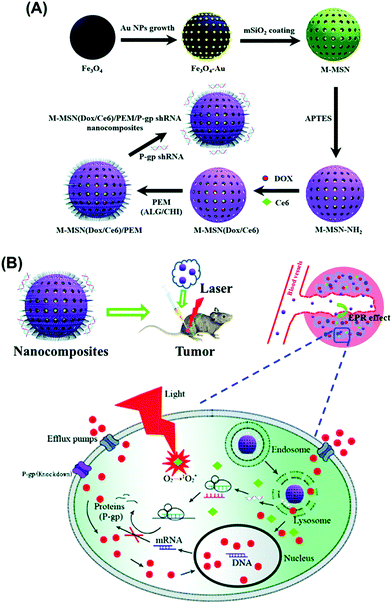 | ||
| Fig. 7 Schematic illustration of the preparation process of MMSN(Dox/Ce6)/PEM/P-gp shRNA nanocomposites (A) and intracellular pH-triggered release in the tumor cells (B). Reproduced from ref. 118 with permission from The Royal Society of Chemistry. | ||
7. Perspectives
Magnetic nanomaterials and complex magnetic nanoarchitectures have indeed bright prospects regarding their possibilities for biomedical applications. Superparamagnetic iron oxide nanoparticles (SPIONs) are already showing high potential for application in clinics for magnetic resonance imaging and magnetic hyperthermia of cancer.119 Novel research studies have discovered a plethora of possible complex designs of magnetic nanomaterials, with magnetic nanoparticles forming the core and the shell consisting of polymers, surfactants or mesoporous silica. These nanomaterials showcase significantly enhanced therapeutic and imaging capabilities compared to SPIONs. The shell, therefore, brings additional features to the magnetic nanomaterial with tunable morphology and porosity, which is typically reflected in embedding drugs, imaging agents or genes120 within their framework and possibilities for surface functionalization with additional therapeutic, and targeting ligands. Hence, highly selective multi-functionalized nanotherapeutics can be constructed, capable of utilizing magnetic field and/or ligands for homing the drug loaded nanocarriers to the therapeutic area, along with possibilities for magnetic hyperthermia treatment of the ill tissues and live monitoring of the treatment through magnetic resonance or/and other types of imaging. In addition, sensing of cancer-related biomolecules (cancer biomarkers) and imaging of the tumor microenvironment is a promising methodology for enabling early and accurate detection of cancer,121 as well as for obtaining information about the responses, mechanisms, and efficacy of the nanotherapeutics, which is of paramount importance for treatment optimization and obtaining personalized nanomedicines. These types of versatile multifunctional nanomaterials, combining sensing, imaging and therapy within one nanotheraputic agent, could be the future of clinical procedures, though their complexity brings concerns regarding the adverse effects on healthy tissues. Particularly the degradability,122 pharmacology-biodistribution, elimination and excretion of these nanomaterials need to be thoroughly addressed for designing novel clinically applicable nanotherapeutics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
For financial support, NK is thankful to the Ministry of Science and Technological Development of the Republic of Serbia (Grant no. III44006). The PHC grant Pavle Savic No. 40800RD (451-03-01963/2017-09/05) is gratefully acknowledged.References
- S. Hoelder, P. A. Clarke and P. Workman, Mol. Oncol., 2012, 6, 155–176 CrossRef CAS PubMed.
- M. Marković, N. Knezević, M. Momcilović, S. Grgurić-Sipka, L. Harhaji, V. Trajković, M. Mostarica Stojković, T. Sabo and D. Miljković, Eur. J. Pharmacol., 2005, 517, 28–34 CrossRef PubMed.
- N. Ž. Knežević and J.-O. Durand, ChemPlusChem, 2015, 80, 26–36 CrossRef.
- K. Greish, J. Drug Targeting, 2007, 15, 457–464 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- R. A. Revia and M. Zhang, Mater. Today, 2016, 19, 157–168 CrossRef CAS PubMed.
- M. Wankhede, A. Bouras, M. Kaluzova and C. G. Hadjipanayis, Expert Rev. Clin. Pharmacol., 2012, 5, 173–186 CrossRef CAS PubMed.
- M. Magro, D. Baratella, E. Bonaiuto, J. de A Roger and F. Vianello, Curr. Med. Chem., 2018, 25, 540–555 CrossRef CAS.
- S. Serres, M. S. Soto, A. Hamilton, M. A. McAteer, W. S. Carbonell, M. D. Robson, O. Ansorge, A. Khrapitchev, C. Bristow, L. Balathasan, T. Weissensteiner, D. C. Anthony, R. P. Choudhury, R. J. Muschel and N. R. Sibson, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6674 CrossRef CAS PubMed.
- K. Turcheniuk, A. V. Tarasevych, V. P. Kukhar, R. Boukherroub and S. Szunerits, Nanoscale, 2013, 5, 10729–10752 RSC.
- D. Ling, N. Lee and T. Hyeon, Acc. Chem. Res., 2015, 48, 1276–1285 CrossRef CAS PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- A. Malik, T. Tahir Butt, S. Zahid, F. Zahid, S. Waquar, M. Rasool, M. H. Qazi and A. M. Qazi, J. Nanotechnol., 2017, 2017, 8 Search PubMed.
- D. Yoo, J.-H. Lee, T.-H. Shin and J. Cheon, Acc. Chem. Res., 2011, 44, 863–874 CrossRef CAS.
- M. Angelakeris, Biochim. Biophys. Acta, 2017, 1861, 1642–1651 CrossRef CAS.
- G. Kandasamy and D. Maity, Int. J. Pharm., 2015, 496, 191–218 CrossRef CAS PubMed.
- M. K. Lima-Tenório, E. A. Gómez Pineda, N. M. Ahmad, H. Fessi and A. Elaissari, Int. J. Pharm., 2015, 493, 313–327 CrossRef PubMed.
- R. A. Revia and M. Zhang, Mater. Today, 2016, 19, 157–168 CrossRef CAS PubMed.
- O. L. Gobbo, K. Sjaastad, M. W. Radomski, Y. Volkov and A. Prina-Mello, Theranostics, 2015, 5, 1249–1263 CrossRef CAS.
- A. J. Cole, V. C. Yang and A. E. David, Trends Biotechnol., 2011, 29, 323–332 CrossRef CAS.
- J. E. Lee, N. Lee, T. Kim, J. Kim and T. Hyeon, Acc. Chem. Res., 2011, 44, 893–902 CrossRef CAS.
- Y. Wang and H. Gu, Adv. Mater., 2015, 27, 576–585 CrossRef CAS.
- R. A. Bini, R. Fernando, C. Marques, F. J. Santos, J. A. Chaker and M. Jafelicci, J. Magn. Magn. Mater., 2012, 324, 534–539 CrossRef CAS.
- Z. Guo, T. Pereira, O. Choi, Y. Wang and H. T. Hahn, J. Mater. Chem., 2006, 16, 2800–2808 RSC.
- J. Zhao, M. Milanova, M. M. C. G. Warmoeskerken and V. Dutschk, Colloids Surf., A, 2012, 413, 273–279 CrossRef CAS.
- N. Ž. Knežević and J.-O. Durand, ChemPlusChem, 2015, 80, 26–36 CrossRef.
- C. M. Blumenfeld, M. D. Schulz, M. S. Aboian, M. W. Wilson, T. Moore, S. W. Hetts and R. H. Grubbs, Nat. Commun., 2018, 9, 7 CrossRef PubMed.
- M. Radović, M. P. Calatayud, G. F. Goya, M. R. Ibarra, B. Antić, V. Spasojević, N. Nikolić, D. Janković, M. Mirković and S. Vranješ-Đurić, J. Biomed. Mater. Res., Part A, 2015, 103, 126–134 CrossRef PubMed.
- D. Benedetto, F. Benyettou and Y. Lalatonne, Phys. Chem. Chem. Phys., 2011, 13, 10020–10027 RSC.
- A. Bahrami, T. Vincent, A. Garnier, F. Larachi, J. Boukouvalas and M. C. Iliuta, Ind. Eng. Chem. Res., 2017, 56, 10981–10989 CrossRef CAS.
- C. Tudisco, A. L. Pellegrino, G. Malandrino and G. G. Condorelli, Surf. Coat. Technol., 2018, 343, 75–82 CrossRef CAS.
- G. Hemery, C. Genevois, F. Couillaud, S. Lacomme, E. Gontier, E. Ibarboure, S. Lecommandoux, E. Garanger and O. Sandre, Mol. Syst. Des. Eng., 2017, 2, 629–639 RSC.
- E. Perillo, K. Hervé-Aubert, E. Allard-Vannier, A. Falanga, S. Galdiero and I. Chourpa, J. Colloid Interface Sci., 2017, 499, 209–217 CrossRef CAS.
- R. Massart, IEEE Trans. Magn., 1981, 17, 1247–1248 CrossRef.
- C. Tudisco, M. T. Cambria, A. E. Giuffrida, F. Sinatra, C. D. Anfuso, G. Lupo, N. Caporarello, A. Fa1langa, S. Galdiero, V. Oliveri, C. Satriano and G. G. Condorelli, Nanoscale Res. Lett., 2018, 13, 45 CrossRef CAS PubMed.
- M. Radović, M. Mirković, M. Perić, D. Janković, A. Vukadinović, D. Stanković, Đ. Petrović, M. Bošković, B. Antić, M. Marković and S. Vranješ-Đurić, J. Mater. Chem. B, 2017, 5, 8738–8747 RSC.
- H. Chen, X. Li, F. Liu, H. Zhang and Z. Wang, Mol. Pharmaceutics, 2017, 14, 3134–3141 CrossRef CAS.
- L. Lartigue, C. Innocenti, T. Kalaivani, A. Awwad, M. del, M. Sanchez Duque, Y. Guari, J. Larionova, C. Guérin, J.-L. G. Montero, V. Barragan-Montero, P. Arosio, A. Lascialfari, D. Gatteschi and C. Sangregorio, J. Am. Chem. Soc., 2011, 133, 10459–10472 CrossRef CAS PubMed.
- K. Gharbi, F. Salles, P. Mathieu, C. Amiens, V. Collière, Y. Coppel, K. Philippot, L. Fontaine, V. Montembault, L. S. Smiri and D. Ciuculescu-Pradines, New J. Chem., 2017, 41, 11898–11905 RSC.
- C. Bordeianu, A. Parat, C. Affolter-Zbaraszczuk, R. N. Muller, S. Boutry, S. Begin-Colin, F. Meyer, S. Laurent and D. Felder-Flesch, J. Mater. Chem. B, 2017, 5, 5152–5164 RSC.
- C. Linot, J. Poly, J. Boucard, D. Pouliquen, S. Nedellec, P. Hulin, N. Marec, P. Arosio, A. Lascialfari, A. Guerrini, C. Sangregorio, M. Lecouvey, L. Lartigue, C. Blanquart and E. Ishow, ACS Appl. Mater. Interfaces, 2017, 9, 14242–14257 CrossRef CAS PubMed.
- D. Peddis, F. Orrù, A. Ardu, C. Cannas, A. Musinu and G. Piccaluga, Chem. Mater., 2012, 24, 1062–1071 CrossRef CAS.
- Y. Tanaka, S. Saita and S. Maenosono, Appl. Phys. Lett., 2008, 92, 7 CrossRef.
- J. Salafranca, J. Gazquez, N. Pérez, A. Labarta, S. T. Pantelides, S. J. Pennycook, X. Batlle and M. Varela, Nano Lett., 2012, 12, 2499–2503 CrossRef CAS.
- N. Ž. Knežević, L. Raehm and J.-O. Durand, in Bionanocomposites: Integrating Biological Processes for Bioinspired Nanotechnologies, ed. C. Aime and T. Coradin, John Wiley & Sons, Inc., 2017, ch. 4.2, pp. 153–168 Search PubMed.
- L. Adumeau, M.-H. Delville and S. Mornet, in Clinical Applications of Magnetic Nanoparticles From Fabrication to Clinical Applications, ed. N. T. Thanh, CRC Press, 2018, p. 20 Search PubMed.
- T. Kang, F. Li, S. Baik, W. Shao, D. Ling and T. Hyeon, Biomaterials, 2017, 136, 98–114 CrossRef CAS.
- J. Huang, Y. Li, A. Orza, Q. Lu, P. Guo, L. Wang, L. Yang and H. Mao, Adv. Funct. Mater., 2016, 26, 3818–3836 CrossRef CAS.
- S.-H. Liao, H.-S. Huang, J.-H. Chen, Y.-K. Su and Y.-F. Tong, RSC Adv., 2018, 8, 4057–4062 RSC.
- X. Wang, B. Li, R. Li, Y. Yang, H. Zhang, B. Tian, L. Cui, H. Weng and F. Wei, Sens. Actuators, B, 2018, 255, 3447–3457 CrossRef CAS.
- G. Kokkinis, S. Cardoso, F. Keplinger and I. Giouroudi, Sens. Actuators, B, 2017, 241, 438–445 CrossRef CAS.
- Z. Liu, Y. Liu, S. Shen and D. Wu, J. Mater. Chem. B, 2018, 6, 366–380 RSC.
- S. Fathi Karkan, M. Mohammadhosseini, Y. Panahi, M. Milani, N. Zarghami, A. Akbarzadeh, E. Abasi, A. Hosseini and S. Davaran, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 1–5 CrossRef CAS.
- I. Giouroudi and E. Hristoforou, J. Appl. Phys., 2018, 124, 30902 CrossRef.
- S. L. Znoyko, A. V. Orlov, A. V. Pushkarev, E. N. Mochalova, N. V. Guteneva, A. V. Lunin, M. P. Nikitin and P. I. Nikitin, Anal. Chim. Acta, 2018, 1034, 161–167 CrossRef CAS.
- V. O. Shipunova, M. P. Nikitin, P. I. Nikitin and S. M. Deyev, Nanoscale, 2016, 8, 12764–12772 RSC.
- M. P. Nikitin, A. V. Orlov, S. L. Znoyko, V. A. Bragina, B. G. Gorshkov, T. I. Ksenevich, V. R. Cherkasov and P. I. Nikitin, J. Magn. Magn. Mater., 2018, 459, 260–264 CrossRef CAS.
- J. Nebu, J. S. Anjali Devi, R. S. Aparna, K. Abha and G. Sony, Sens. Actuators, B, 2018, 257, 1035–1043 CrossRef CAS.
- A. V. Sheahan, A. V. Biankin, C. R. Parish and L. M. Khachigian, Oncotarget, 2018, 9, 21613–21627 CrossRef PubMed.
- D. Chen, B. Li, S. Cai, P. Wang, S. Peng, Y. Sheng, Y. He, Y. Gu and H. Chen, Biomaterials, 2016, 100, 1–16 CrossRef CAS PubMed.
- B. Tian, Y. Han, E. Wetterskog, M. Donolato, M. F. Hansen, P. Svedlindh and M. Stromberg, ACS Sens., 2018, 3, 1884–1891 CrossRef CAS.
- M. A. Macha, P. Seshacharyulu, S. R. Krishn, P. Pai, S. Rachagani, M. Jain and S. K. Batra, Curr. Pharm. Des., 2014, 20, 5287–5297 CrossRef CAS.
- L. H. Tan, H. Xing and Y. Lu, Acc. Chem. Res., 2014, 47, 1881–1890 CrossRef CAS PubMed.
- I. Willner, B. Shlyahovsky, M. Zayats and B. Willner, Chem. Soc. Rev., 2008, 37, 1153–1165 RSC.
- E. Mokany, S. M. Bone, P. E. Young, T. B. Doan and A. V. Todd, J. Am. Chem. Soc., 2010, 132, 1051–1059 CrossRef CAS PubMed.
- W. Wang, S. Liu, C. Li, Y. Wang and C. Yan, Talanta, 2018, 182, 306–313 CrossRef CAS.
- L. Zeng, L. Luo, Y. Pan, S. Luo, G. Lu and A. Wu, Nanoscale, 2015, 7, 8946–8954 RSC.
- C.-H. Yan, X.-F. Qiao, J.-C. Zhou, J.-W. Xiao, Y.-F. Wang and L.-D. Sun, Nanoscale, 2012, 4, 4611–4623 RSC.
- F. Zhang, G. B. Braun, A. Pallaoro, Y. Zhang, Y. Shi, D. Cui, M. Moskovits, D. Zhao and G. D. Stucky, Nano Lett., 2012, 12, 61–67 CrossRef CAS.
- J. Choi, S. Kim, D. Yoo, T.-H. Shin, H. Kim, M. D. Gomes, S. H. Kim, A. Pines and J. Cheon, Nat. Mater., 2017, 16, 537 CrossRef CAS.
- Y. Liu, Q. Jia, Q. Guo, W. Wei and J. Zhou, Biomaterials, 2018, 180, 104–116 CrossRef CAS.
- J. Huang, X. Zhong, L. Wang, L. Yang and H. Mao, Theranostics, 2012, 2, 86–102 CrossRef CAS PubMed.
- Z. Shen, A. Wu and X. Chen, Mol. Pharmaceutics, 2017, 14, 1352–1364 CrossRef CAS.
- P. Guardia, R. Di Corato, L. Lartigue, C. Wilhelm, A. Espinosa, M. Garcia-hernandez, F. Gazeau, L. Manna and T. Pellegrino, ACS Nano, 2012, 3080–3091 CrossRef CAS.
- C. Martinez-boubeta, K. Simeonidis, A. Makridis, M. Angelakeris, D. Serantes, D. Baldomir, Z. Saghi and P. A. Midgley, Sci. Rep., 2013, 3, DOI:10.1038/srep01652.
- J.-H. Lee, J.-T. Jang, J.-S. Choi, S. H. Moon, S.-H. Noh, J.-W. Kim, J.-G. Kim, I.-S. Kim, K. I. Park and J. Cheon, Nat. Nanotechnol., 2011, 6, 418–422 CrossRef CAS.
- P. Hugounenq, D. Alloyeau, S. P. Clarke, M. Le, R. Bazzi, D. F. Brougham, C. Wilhelm and F. Gazeau, ACS Nano, 2012, 10935–10949 Search PubMed.
- A. Espinosa, R. Di Corato, J. Kolosnjaj-Tabi, P. Flaud, T. Pellegrino and C. Wilhelm, ACS Nano, 2016, 10, 2436–2446 CrossRef CAS PubMed.
- R. Das, N. Rinaldi-Montes, J. Alonso, Z. Amghouz, E. Garaio, J. A. García, P. Gorria, J. A. Blanco, M. H. Phan and H. Srikanth, ACS Appl. Mater. Interfaces, 2016, 8, 25162–25169 CrossRef CAS PubMed.
- H. S. Huang and J. F. Hainfeld, Int. J. Nanomed., 2013, 8, 2521–2532 Search PubMed.
- X. L. Liu, C. T. Ng, P. Chandrasekharan, H. T. Yang, L. Y. Zhao, E. Peng, Y. B. Lv, W. Xiao, J. Fang, J. B. Yi, H. Zhang, K.-H. Chuang, B. H. Bay, J. Ding and H. M. Fan, Adv. Healthcare Mater., 2016, 5, 2092–2104 CrossRef CAS PubMed.
- Z.-Q. Zhang and S.-C. Song, Biomaterials, 2016, 106, 13–23 CrossRef CAS PubMed.
- J. Liu, C. Detrembleur, A. Debuigne, M.-C. De Pauw-Gillet, S. Mornet, L. Vander Elst, S. Laurent, E. Duguet and C. Jérôme, J. Mater. Chem. B, 2014, 2, 1009–1023 RSC.
- J. K. Park, J. Jung, P. Subramaniam, B. P. Shah, C. Kim, J. K. Lee, J.-H. Cho, C. Lee and K.-B. Lee, Small, 2011, 7, 1647–1652 CrossRef CAS.
- B. P. Shah, N. Pasquale, G. De, T. Tan, J. Ma and K.-B. Lee, ACS Nano, 2014, 8, 9379–9387 CrossRef CAS PubMed.
- H. Omar, J. G. Croissant, K. Alamoudi, S. Alsaiari, I. Alradwan, M. A. Majrashi, D. H. Anjum, P. Martins, R. Laamarti, J. Eppinger, B. Moosa, A. Almalik and N. M. Khashab, J. Controlled Release, 2017, 259, 187–194 CrossRef CAS PubMed.
- S. Hapuarachchige, Y. Kato, E. J. Ngen and B. Smith, PLoS One, 2016, 11, e0156294 CrossRef PubMed.
- N. Ž. Knežević, J. Nanosci. Nanotechnol., 2016, 16, 4195–4199 CrossRef.
- A. M. Bonilla and P. H. Gonzalez, Curr. Pharm. Des., 2017, 23, 5392–5402 CAS.
- W. Guo, C. Yang, H. Lin and F. Qu, Dalton Trans., 2014, 43, 18056–18065 RSC.
- F. Benyettou, A. Ocadiz, F. Ravaux, R. Rezgui, M. Jouiad, S. I. Nehme, K. Parsapur and J. Olsen, Chem. – Eur. J., 2016, 22, 17020–17028 CrossRef CAS PubMed.
- K. S. Kim, J. Kim, J. Y. Lee, S. Matsuda, S. Hideshima, Y. Mori, T. Osaka and K. Na, Nanoscale, 2016, 8, 11625–11634 RSC.
- J. Li, Y. Hu, J. Yang, P. Wei, W. Sun, M. Shen, G. Zhang and X. Shi, Biomaterials, 2015, 38, 10–21 CrossRef CAS PubMed.
- E. Fantechi, C. Innocenti, M. Zanardelli, M. Fittipaldi, E. Falvo, M. Carbo, V. Shullani, L. Di, C. Mannelli, C. Ghelardini, A. M. Ferretti, A. Ponti, C. Sangregorio and P. Ceci, ACS Nano, 2014, 8, 4705–4719 CrossRef CAS PubMed.
- D. Yang, X. Pang, Y. He, Y. Wang, G. Chen, W. Wang and Z. Lin, Angew. Chem., 2015, 127, 12259–12264 CrossRef.
- S.-H. Liao, C.-H. Liu, B. P. Bastakoti, N. Suzuki, Y. Chang, Y. Yamauchi, F.-H. Lin and K. C.-W. Wu, Int. J. Nanomed., 2015, 10, 3315–3328 CrossRef CAS PubMed.
- G. Song, M. Chen, Y. Zhang, L. Cui, H. Qu, X. Zheng, M. Wintermark, Z. Liu and J. Rao, Nano Lett., 2018, 18, 182–189 CrossRef CAS PubMed.
- N. Ž. Knežević, I. I. Slowing and V. S.-Y. Lin, ChemPlusChem, 2012, 77, 48–55 CrossRef.
- S. Dib, M. Boufatit, S. Chelouaou, F. Sadi-Hassaine, J. Croissant, J. Long, L. Raehm, C. Charnay and J.-O. Durand, RSC Adv., 2014, 4, 24838–24841 RSC.
- N. Knezevic, Process. Appl. Ceram., 2014, 8, 109–112 CrossRef CAS.
- N. Z. Knezevic, C. Mauriello Jimenez, M. Albino, A. Vukadinovic, A. Mrakovic, E. Illes, D. Janackovic, J.-O. Durand, C. Sangregorio and D. Peddis, MRS Adv., 2017, 1–9 Search PubMed.
- N. Ž. Knežević, J. Mrđanović, I. Borišev, S. Milenković, Đ. Janaćković, F. Cunin and A. Djordjevic, RSC Adv., 2016, 6, 7061–7065 RSC.
- N. Z. Knezevic, I. I. Slowing and V. S.-Y. Lin, ChemPlusChem, 2012, 77, 48–55 CrossRef CAS.
- N. Z. Knezevic, RSC Adv., 2013, 3, 19388–19392 RSC.
- Y.-S. Lin, S.-H. Wu, Y. Hung, Y.-H. Chou, C. Chang, M.-L. Lin, C.-P. Tsai and C.-Y. Mou, Chem. Mater., 2006, 18, 5170–5172 CrossRef CAS.
- S.-H. Wu, Y.-S. Lin, Y. Hung, Y.-H. Chou, Y.-H. Hsu, C. Chang and C.-Y. Mou, ChemBioChem, 2008, 9, 53–57 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef CAS PubMed.
- J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47, 8438–8441 CrossRef CAS PubMed.
- F. Ye, S. Laurent, A. Fornara, L. Astol, J. Qin, A. Roch, A. Martini, M. S. Toprak, R. N. Muller and M. Muhammed, Contrast Media Mol. Imaging, 2012, 7, 460–468 CrossRef CAS PubMed.
- J. S. Choi, J. H. Lee, T. H. Shin, H. T. Song, E. Y. Kim and J. Cheon, J. Am. Chem. Soc., 2010, 132, 11015–11017 CrossRef CAS PubMed.
- T. Shin, J. Choi, S. Yun, I. Kim, H. Song and Y. Kim, ACS Nano, 2014, 8, 3393–3401 CrossRef CAS PubMed.
- N. Ž. Knežević, E. Ruiz-Hernández, W. E. Hennink and M. Vallet-Regí, RSC Adv., 2013, 3, 9584 RSC.
- E. Guisasola, L. Asín, L. Beola, J. M. de la Fuente, A. Baeza and M. Vallet-Regí, ACS Appl. Mater. Interfaces, 2018, 10, 12518–12525 CrossRef CAS PubMed.
- Q. Gao, W. Xie, Y. Wang, D. Wang, Z. Guo, F. Gao, L. Zhao and Q. Cai, RSC Adv., 2018, 8, 4321–4328 RSC.
- Y. Chen, X. Wang, D. S. Zhang and H. Gu, Int. J. Nanomed., 2015, 10, 2579–2594 CAS.
- W. Chen, G. Luo, Q. Lei, F. Cao, J. Fan and W. Qiu, Biomaterials, 2016, 76, 87–101 CrossRef CAS PubMed.
- M. Xuan, J. Shao, J. Zhao, Q. Li, L. Dai and J. Li, Angew. Chem., Int. Ed., 2018, 57, 6049–6053 CrossRef CAS PubMed.
- H. Yang, Y. Chen, Z. Chen, Y. Geng, X. Xie, X. Shen, T. Li, S. Li, C. Wu and Y. Liu, Biomater. Sci., 2017, 5, 1001–1013 RSC.
- F. K. H. van Landeghem, K. Maier-Hauff, A. Jordan, K.-T. Hoffmann, U. Gneveckow, R. Scholz, B. Thiesen, W. Brück and A. von Deimling, Biomaterials, 2009, 30, 52–57 CrossRef CAS PubMed.
- J. Liu, B. Wang, S. Budi Hartono, T. Liu, P. Kantharidis, A. P. J. Middelberg, G. Q. (Max) Lu, L. He and S. Z. Qiao, Biomaterials, 2012, 33, 970–978 CrossRef CAS PubMed.
- Z. Zhou and Z.-R. Lu, Adv. Drug Delivery Rev., 2017, 113, 24–48 CrossRef CAS PubMed.
- J. G. Croissant, Y. Fatieiev and N. M. Khashab, Adv. Mater., 2017, 29, 1604634 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |