Facile synthesis of diverse rotaxanes via successive supramolecular transformations†
Yang
Hu
a,
Wei
Wang
*a,
Rui
Yao
a,
Xu-Qing
Wang
a,
Yu-Xuan
Wang
a,
Bin
Sun
a,
Li-Jun
Chen
a,
Ying
Zhang
b,
Xiao-Li
Zhao
 a,
Lin
Xu
a,
Lin
Xu
 a,
Hong-Wei
Tan
a,
Hong-Wei
Tan
 b,
Yihua
Yu
c,
Xiaopeng
Li
b,
Yihua
Yu
c,
Xiaopeng
Li
 d and
Hai-Bo
Yang
d and
Hai-Bo
Yang
 *a
*a
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, Chang-Kung Chuang Institute, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062, P. R. China. E-mail: hbyang@chem.ecnu.edu.cn; weiwang@stu.ecnu.edu.cn
bCollege of Chemistry, Beijing Normal University, Beijing, 100875, P. R. China
cShanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai 200062, P. R. China
dDepartment of Chemistry, University of South Florida, East Fowler Ave., Florida 4202, USA
First published on 6th September 2019
Abstract
Facile synthesis of diverse rotaxanes was successfully realized through a simple and efficient supramolecular transformation strategy. Starting from an organometallic [3]rotaxane with a platinum-acetylide moiety as the bridge moiety and transformation site, employing a simple phosphine ligand exchange and sequential reductive elimination reactions resulted in the successful preparation of various rotaxanes with diverse structures and properties.
Rotaxanes, as a fundamental class of mechanically interlocked molecules (MIMs), have proven to be crucial candidates for the construction of artificial molecular machinery and electronic devices due to their shuttling and switching properties.1 For instance, the pioneering work on rotaxane-based molecular shuttles by 2016 Nobel laureate, J. Fraser Stoddart, initiated a new era of the design and synthesis of molecular machines.2 Relying on concepts such as molecular recognition and non-covalent synthesis, template-directed protocols have led to a great evolution in rotaxane synthesis.3 A number of templates, such as transition metal ions, anions, hydrogen bonding, and donor–acceptor interactions, have been employed in the highly efficient synthesis of rotaxanes and the related higher-order interlocked molecules. However, most of the current reports mainly focus on either one rotaxane with a specific structure or a family of rotaxanes with similar structural characteristics. The construction of diverse rotaxanes composed of a family of rotaxanes with varied structures and properties has rarely been explored, which might be due to the lack of efficient approaches towards the high throughput synthesis of diverse rotaxanes.4 Very few examples of the end-cap exchange of rotaxanes using suitable reactions such as the transesterification grafting reaction,4 condensation reaction,5 Wittig reaction,6 and Tsuji–Trost allylation reaction7 allowed for the successful structural derivations of rotaxanes. However, in these reports, the modifications were only limited to the stopper replacements, while the modification of the structure or properties of the main skeleton has not been realized. Since diverse rotaxane structures branched up from one principal structure would lead to attractive properties and potential applications in wide areas, the development of new synthetic protocols for the synthesis of rotaxanes with adequate diversity in both structures and properties remains an attractive proposition.
Inspired by biological transformation processes of fundamental biomacromolecules such as proteins, DNA, RNA etc., in which stimuli-induced structural changes lead to specific functions as the outputs,8 we envisaged that such a supramolecular transformation strategy could be employed for the synthesis of rotaxanes with adequate diversity in structures, properties, and applications. In order to realize such a strategy, a given rotaxane with a specific transformation site should be firstly prepared. Sequential supramolecular transformations derived from this reactive site under specific conditions could afford the formation of diverse rotaxanes with varied structures and properties. Based on our ongoing interest in organoplatinum chemistry,9 a trans-platinum-acetylide moiety with PPh3 ligands was chosen as the key transformation site. Under mild conditions, the trans-platinum-acetylide moiety could transform into its cis-analogue and further into a butadiyne moiety without the platinum atom, thus resulting in structural and property modification (Scheme 1).10 In addition, in this study, a pillar[5]arene/alkyl chain host–guest complex was selected as the key rotaxane moiety.11 The low polarity and good solubility of such a neutral rotaxane system would allow for convenient transformation and purification processes.
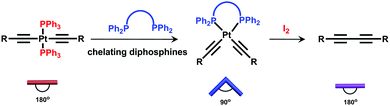 | ||
| Scheme 1 Schematic representation of the successive transformations of the platinum-acetylide moiety with PPh3 ligands. | ||
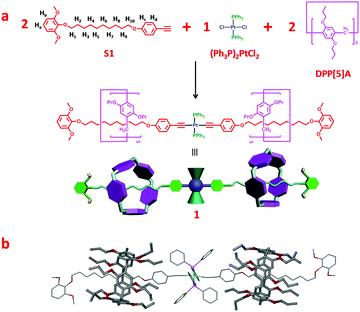
According to above-mentioned design strategy, pillar[5]arene-based organometallic [3]rotaxane 1 containing a trans-platinum-acetylide moiety with PPh3 ligands was prepared as the key rotaxane precursor through an efficient one-pot multicomponent reaction (“from five to one”), as shown in Fig. 1a. The 31P NMR spectrum of [3]rotaxane 1 revealed a single peak at 18.7 ppm, indicating the formation of only one dominant platinum containing species (Fig. S3, ESI†). In addition, in the 1H spectrum (Fig. 2a and e), the methylene peaks in the alkyl chain displayed an obvious upfield shift to even below 0 ppm due to the shielding effects. These changes were in accordance with previous reports on mechanically interlocked structures derived from pillar[5]arene with neutral alkyl chains,12 thus strongly supporting the existence of rotaxane structures.
 | ||
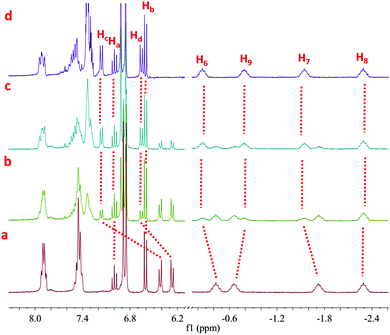
| Fig. 2 Partical 1H NMR spectra (400 MHz, CDCl3, 298 K) of the organometallic [3]rotaxane 1 (a and e), 2 (d), 3 (b) and organic [3]rotaxane 4 (c). | ||
Furthermore, the formation of rotaxane was further confirmed by a two-dimensional (2-D) NOESY NMR study (Fig. S4, ESI†), in which correlation signals between protons on the pillar[5]arene unit and methylene protons on the alkyl chain were observed. Finally, the structure of organometallic [3]rotaxane 1 was unambiguously confirmed by means of single-crystal X-ray diffraction (Fig. 1b). Slow diffusion of ethyl acetate (EtOAc) into a CH2Cl2 solution of 1 afforded pale yellow single crystals suitable for crystallographic analysis. The single crystal structure of [3]rotaxane 1 disclosed that two pillar[5]arene rings encircled two alkyl chain moieties on the dumbbell component. The platinum-acetylide moiety located in the center of the rotaxane and the acetylenic π-ligand displayed a coplanar geometry.
With the “reactive” rotaxane precursor 1 in hand, the investigation on the synthesis of diverse rotaxanes through supramolecular transformations of the platinum-acetylide moiety was then carried out. Four chelating diphosphines, including cis-bis(diphenylphosphino)ethylene (DPPEE), 1,3-bis(diphenylphosphino)propane (DPPP), (2S,3S)-bis(diphenylphosphino)butane (S,S-CHIRAPHOS), and (2R,3R)-bis(diphenylphosphino)butane (R,R-CHIRAPHOS), were chosen to replace the PPh3 ligands, thus leading to the transformation processes (Scheme 2). All the transformations proceeded under mild conditions (see general procedures in ESI†) and the details are shown below.
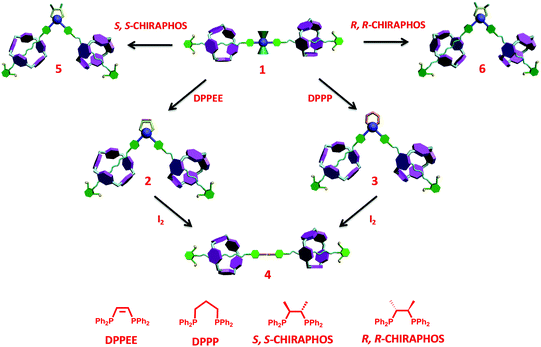 | ||
| Scheme 2 Construction of diverse rotaxanes 1–6via a successive supramolecular transformation approach. | ||
For the achiral chelating ligands DPPEE and DPPP, the transformation from the trans-platinum acetylide moiety in [3]rotaxane 1 to its cis-analogue proceeded efficiently under mild conditions, thus allowing for the formation of corresponding V-shaped [3]rotaxanes 2 and 3 in 95% and 90% yield, respectively. As shown in Fig. 2, after the transformation process, the 1H NMR spectra exhibited an obvious change of the peaks (Hc and Hd) attributed to phenyl rings near the platinum-acetylide moiety, while the peaks below 0 ppm that belonged to the rotaxane moiety remained intact. These results clearly indicated the maintenance of the threaded structures upon the ligand exchange processes. In addition, the shifts in the 31P NMR spectra from 18.7 ppm (1) to 52.2 ppm (2) and −5.5 ppm (3), respectively, with the decrease of the coupling constant (2671 Hz for 1, 2283 Hz for 2, and 2202 Hz for 3) further supported the formation of the corresponding V-shaped [3]rotaxanes. For both V-shaped rotaxanes, isotopically resolved peaks attributed to the targeted structures were observed in their MALDTI-TOF MS spectra (i.e. m/z = 3471.47 for [2 + H]+ and m/z = 3487.40 for [3 + H]+, respectively), thus supporting the successful synthesis of the desired rotaxanes through supramolecular transformation.
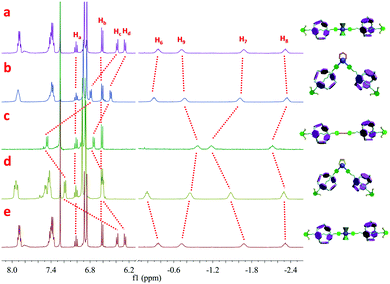
In order to gain an in-depth understanding of such efficient supramolecular transformations between rotaxanes of different shapes, NMR titration was carried out. As shown in Fig. 3, with the increase of the amount of DPPEE from 0.33 to 1.00 equiv., the peaks corresponding to the original organometallic [3]rotaxane 1 gradually disappeared accompanied by an increase of the new peaks attributed to V-shaped [3]rotaxane 2. In 31P{1H} NMR spectra (Fig. S22, ESI†), with the addition of DPPEE, the phosphine peak at 18.5 ppm, attributed to the PPh3 ligands on the platinum-acetylide moiety in the original [3]rotaxane 1, dramatically decreased along with the appearance of two new peaks at 51.6 and −5.5 ppm, which were ascribed to the DPPEE ligand in the newly-formed cis-platinum-acetylide moiety and the released free PPh3 ligand, respectively. Through the NMR titration study, supramolecular transformation from linear [3]rotaxane 1 to V-shaped [3]rotaxane 2 through the simple and highly efficient ligand exchange was undoubtedly confirmed.
Interestingly, the resultant V-shaped [3]rotaxanes 2 and 3 could undergo further transformations to afford the corresponding organic [3]rotaxane 4 with a linear butadiyne link. In order to realize the de-platinum process, [3]rotaxanes 2 and 3 were treated with I2 (4.0 eq.) at room temperature in CHCl3. TLC analysis revealed that such a reductive elimination reaction proceeded efficiently with complete conversion within 12 hours. Purification by flash column chromatography afforded the de-platinum [3]rotaxane 4 in 64% (from 2) and 59% (from 3) yield as well as the corresponding Pt complexes Pt(DPPEE)I2 (from 2) or Pt(DPPP)I2 (from 3), respectively, thus suggesting an oxidatively induced reductive elimination mechanism. As revealed by the 1H NMR spectrum, in the resultant organic [3]rotaxane, no peaks attributed to phosphine ligands were observed, while the peaks ascribed to the threaded structure were retained (Fig. 2c). As expected, no peaks were observed in the 31P NMR spectrum. Furthermore, the MALDI-TOF-MS spectrum of 4 clearly displayed a single peak at m/z = 2880.36, which was attributed to the species [4 + H]+ (Fig. S27, ESI†). All these data strongly indicated the generation of de-platinum [3]rotaxane 4. It is worth noting that by the successive supramolecular transformations shown above, we successfully realized a “three-station” shape transformation of [3]rotaxanes, i.e. from “linear” for 1 to “V-shaped” for 2 or 3 and further back to “linear” for 4. More importantly, along with such multiple structural interconversions, obvious changes in the length of rotaxane skeletons were achieved. For instance, according to the simulated structures (Fig. S28, ESI†), such successive structural transformation led to a contraction ratio of 0.17 from 1 to 2 and an extension ratio of 0.12 from 2 to 4 (Table S1, ESI†), respectively, thus making them excellent candidates for the construction of rotaxane-based dynamic materials.13
Inspired by an emerging asymmetric catalytic investigation with chiral rotaxanes,14 we envisioned that the employment of chiral chelating ligands would afford the corresponding chiral V-shaped [3]rotaxane upon similar supramolecular transformation processes. More importantly, if it works, from the same rotaxane precursor, both enantiomers of the rotaxane could be prepared by using enantiomeric chelating ligands, thus highlighting the feasibility of this supramolecular transformation strategy for the generation of diverse chiral rotaxanes.
To test our hypothesis, two types of classical chiral chelating diphosphine ligands, i.e.S,S-CHIRAPHOS and R,R-CHIRAPHOS, were employed in the ligand exchange reactions with 1. Treatment of 1 with S,S-CHIRAPHOS and R,R-CHIRAPHOS in CH2Cl2 at room temperature for 18 h resulted in the successful synthesis of the corresponding chiral V-shaped [3]rotaxanes 5 and 6 in 55% and 65% yield, respectively. As shown in Fig. S14 and S18 (ESI†), respectively, enantiomers 5 and 6 displayed the same 1H NMR spectra, in which the peaks attributed to the chiral ligands and interlocked structures were observed. Furthermore, a single peak at 44.7 ppm with a coupling constant of 1JP–Pt = 2234 Hz was also observed in the 31P NMR spectra of both enantiomers (Fig. S16 and S20, ESI†). The investigation of the chirality of chiral [3]rotaxanes 5 and 6 was performed by using circular dichroism (CD) spectra as shown in Fig. 4. The CD spectra of both 5 and 6 exhibited two major bands at approximately 240 nm and 260 nm, respectively. In addition, as expected, these two spectra mirrored each other in both form and intensity, which is consistent with an enantiomeric relationship.
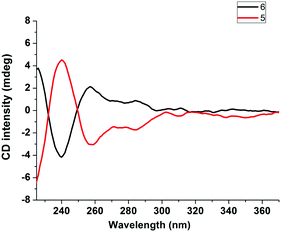 | ||
| Fig. 4 CD spectra of the chiral [3]rotaxane 5 derived from S,S-CHIRAPHOS (red line) and its enantiomer, i.e., the chiral [3]rotaxane 6 derived from R,R-CHIRAPHOS (black line). | ||
In summary, a simple yet efficient supramolecular transformation approach for the facile construction of diverse rotaxanes was successfully developed. By inserting a platinum-acetylide moiety as the transformation site in the rotaxane precursor, a simple ligand exchange and sequential reductive elimination reactions afforded the synthesis of rotaxanes with diversity in both structures and properties. Notably, successive “three-station” supramolecular transformations from linear organometallic [3]rotaxane 1 to V-shaped [3]rotaxanes 2 or 3 and back to linear organic [3]rotaxane 4 led to obvious changes in the length of rotaxane skeletons, thus making them excellent candidates for the construction of rotaxane-based dynamic materials. In addition, starting from the same [3]rotaxane 1, both enantiomeric rotaxanes 5 and 6 were prepared in a divergent way. Due to the diversity in both structures and properties, these resultant rotaxanes feature potential applications in many fields ranging from molecular devices to dynamic materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the NSFC/China (No. 21625202 and 21572066), the Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-05-E00012), and the Program for Changjiang Scholars and Innovative Research Team in University.Notes and references
- (a) J. F. Stoddart, Chem. Soc. Rev., 2009, 38, 1802 RSC; (b) X. Ma and H. Tian, Chem. Soc. Rev., 2010, 39, 70 RSC; (c) R. S. Forgan, J. P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434 CrossRef CAS PubMed; (d) E. A. Neal and S. M. Goldup, Chem. Commun., 2014, 50, 5128 RSC; (e) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081 CrossRef CAS PubMed; (f) C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines, John Wiley and Sons, Hoboken, 2016 CrossRef; (g) J. E. M. Lewis, M. Galli and S. M. Goldup, Chem. Commun., 2017, 53, 298 RSC; (h) M. Denis and S. M. Goldup, Nat. Rev. Chem., 2017, 0061 CrossRef CAS; (i) X.-Q. Wang, W.-J. Li, W. Wang and H.-B. Yang, Chem. Commun., 2018, 54, 13303 RSC; (j) J. P. Sauvage, Eur. J. Org. Chem., 2019, 3287 CrossRef CAS; (k) Z. Meng, J.-F. Xiang and C.-F. Chen, J. Am. Chem. Soc., 2016, 138, 5652 CrossRef CAS PubMed; (l) Z. Meng, J.-F. Xiang and C.-F. Chen, Chem. Sci., 2014, 5, 1520 RSC; (m) Q. Shi and C.-F. Chen, Chem. Sci., 2019, 10, 2529 RSC; (n) X. Fu, Q. Zhang, S.-J. Rao, D.-H. Qu and H. Tian, Chem. Sci., 2016, 7, 1696 RSC; (o) S.-J. Rao, Q. Zhang, J. Mei, X.-H. Ye, C. Gao, Q.-C. Wang, D.-H. Qu and H. Tian, Chem. Sci., 2017, 8, 6777 RSC; (p) D.-H. Qu, Q.-C. Wang, Q.-W. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543 CrossRef CAS PubMed; (q) Q. Zhang, S.-J. Rao, T. Xie, X. Li, T.-Y. Xu, D. W. Li, D.-H. Qu, Y.-T. Long and H. Tian, Chem, 2018, 4, 2670 CrossRef CAS; (r) Q. Jiang, H.-Y. Zhang, M. Han, Z.-J. Ding and Y. Liu, Org. Lett., 2010, 12, 1728 CrossRef CAS PubMed; (s) Z.-J. Zhang, H.-Y. Zhang, H. Wang and Y. Liu, Angew. Chem., Int. Ed., 2011, 50, 10834 CrossRef CAS PubMed.
- (a) P. L. Anelli, N. Spencer and J. F. Stoddart, J. Am. Chem. Soc., 1991, 113, 5131 CrossRef CAS PubMed; (b) P. R. Ashton, D. Philp, N. Spencer and J. F. Stoddart, J. Chem. Soc., Chem. Commun., 1992, 16, 1124 RSC; (c) P. R. Ashton, R. A. Bissell, N. Spencer, J. F. Stoddart and M. S. Tolley, Synlett, 1992, 914 CrossRef CAS; (d) P. R. Ashton, R. A. Bissell, R. Górski, D. Philp, N. Spencer, J. F. Stoddart and M. S. Tolley, Synlett, 1992, 919 CrossRef CAS; (e) P. R. Ashton, R. A. Bissell, N. Spencer, J. F. Stoddart and M. S. Tolley, Synlett, 1992, 923 CrossRef CAS; (f) R. A. Bissell, E. Córdova, A. E. Kaifer and J. F. Stoddart, Nature, 1994, 369, 133 CrossRef CAS; (g) K. Zhu, C. A. O'Keefe, V. N. Vukotic, R. W. Schurko and S. J. Loeb, Nat. Chem., 2015, 7, 514 CrossRef CAS PubMed; (h) K. Zhu, G. Baggi and S. J. Leob, Nat. Chem., 2018, 10, 625 CrossRef CAS PubMed.
- (a) J. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530 RSC; (b) J. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh and R. T. McBurney, Angew. Chem., Int. Ed., 2011, 50, 9260 CrossRef CAS PubMed; (c) W. R. Dichtel, O. S. Miljanić, W. Zhang, J. M. Spruell, K. Patel, I. Aprahamian, J. R. Heath and J. F. Stoddart, Acc. Chem. Res., 2008, 41, 1750 CrossRef CAS PubMed; (d) P. D. Beer, M. R. Sambrook and D. Curiel, Chem. Commun., 2006, 2105 RSC; (e) J. A. Faiz, V. Heitz and J.-P. Sauvage, Chem. Soc. Rev., 2009, 38, 422 RSC; (f) D.-H. Qu and H. Tian, Chem. Sci., 2011, 2, 1011 RSC; (g) G. T. Spence and P. D. Beer, Acc. Chem. Res., 2012, 46, 571 CrossRef PubMed; (h) M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398 CrossRef CAS PubMed; (i) J. E. M. Lewis, P. D. Beer, S. J. Loeb and S. M. Goldup, Chem. Soc. Rev., 2017, 46, 2577 RSC; (j) J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 11094 CrossRef CAS PubMed; (k) J.-P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080 CrossRef CAS PubMed.
- J. S. Hannam, S. M. Lacy, D. A. Leigh, C. G. Saiz, A. M. Z. Slawin and S. G. Stitchell, Angew. Chem., Int. Ed., 2004, 43, 3260 CrossRef CAS PubMed.
- D. W. Zehnder II and D. B. Smithrud, Org. Lett., 2001, 3, 2485 CrossRef PubMed.
- (a) S. J. Rowan and J. F. Stoddart, J. Am. Chem. Soc., 2000, 122, 164 CrossRef CAS; (b) S.-H. Chiu, S. J. Rowan, S. J. Cantrill, J. F. Stoddart, A. J. P. White and D. J. Williams, Chem. – Eur. J., 2002, 8, 5170 CrossRef CAS PubMed.
- N. Kihara, S. Motoda, T. Yokozawa and T. Takata, Org. Lett., 2005, 7, 1199 CrossRef CAS PubMed.
- (a) J. Monod, J. P. Changeux and F. Jacob, J. Mol. Biol., 1963, 6, 306 CrossRef CAS PubMed; (b) M. F. Perutz, Nature, 1970, 228, 726 CrossRef CAS PubMed; (c) H. R. Saibil, W. A. Fenton, D. K. Clare and A. L. Horwich, J. Mol. Biol., 2013, 425, 1476 CrossRef CAS PubMed; (d) W. Wang, Y.-X. Wang and H.-B. Yang, Chem. Soc. Rev., 2016, 45, 2656 RSC.
- (a) S. Chen, L.-J. Chen, H.-B. Yang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2012, 134, 13596 CrossRef CAS PubMed; (b) L. Xu, L.-J. Chen and H.-B. Yang, Chem. Commun., 2014, 50, 5156 RSC; (c) W. Wang and H.-B. Yang, Chem. Commun., 2014, 50, 5171 RSC; (d) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; (e) L.-J. Chen, G.-Z. Zhao, B. Jiang, B. Sun, M. Wang, L. Xu, J. He, Z. Abliz, H. Tan, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 13, 5993 CrossRef PubMed; (f) W. Wang, Y. Zhang, B. Sun, L.-J. Chen, X.-D. Xu, M. Wang, X. Li, Y. Yu, W. Jiang and H.-B. Yang, Chem. Sci., 2014, 5, 4554 RSC; (g) B. Sun, M. Wang, Z. Lou, M. Huang, C. Xu, X. Li, L.-J. Chen, Y. Yu, G. L. Davis, B. Xu, H.-B. Yang and X. Li, J. Am. Chem. Soc., 2015, 137, 1556 CrossRef CAS PubMed; (h) W. Wang, L.-J. Chen, X.-Q. Wang, B. Sun, X. Li, Y. Zhang, J. Shi, Y. Yu, L. Zhang, M. Liu and H.-B. Yang, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 5597 CrossRef CAS PubMed; (i) L.-J. Chen, Y.-Y. Ren, N.-W. Wu, B. Sun, J.-Q. Ma, L. Zhang, H. Tan, M. Liu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2015, 137, 11725 CrossRef CAS PubMed; (j) X.-Q. Wang, W. Wang, G.-Q. Yin, Y.-X. Wang, C.-W. Zhang, J.-M. Shi, Y. Yu and H.-B. Yang, Chem. Commun., 2015, 51, 16813 RSC; (k) L. Xu and H.-B. Yang, Chem. Rec., 2016, 16, 1274 CrossRef CAS PubMed; (l) W. Zheng, L.-J. Chen, G. Yang, B. Sun, X. Wang, B. Jiang, G.-Q. Yin, L. Zhang, X. Li, M. Liu, G. Chen and H.-B. Yang, J. Am. Chem. Soc., 2016, 138, 4927 CrossRef CAS PubMed; (m) B. Jiang, J. Zhang, J.-Q. Ma, W. Zheng, L.-J. Chen, B. Sun, C. Li, B.-W. Hu, H. Tan, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2016, 138, 738 CrossRef CAS PubMed; (n) W. Zheng, G. Yang, N. Shao, L.-J. Chen, B. Ou, S.-T. Jiang, G. Chen and H.-B. Yang, J. Am. Chem. Soc., 2017, 139, 13811 CrossRef CAS PubMed; (o) Y.-X. Wang, Q.-F. Zhou, L.-J. Chen, L. Xu, C.-H. Wang, X. Li and H.-B. Yang, Chem. Commun., 2018, 54, 2224 RSC; (p) W. Zheng, W. Wang, S.-T. Jiang, G. Yang, Z. Li, X.-Q. Wang, G.-Q. Yin, Y. Zhang, H. Tan, X. Li, H. Ding, G. Chen and H.-B. Yang, J. Am. Chem. Soc., 2019, 141, 583 CrossRef CAS PubMed; (q) X.-Q. Wang, W.-J. Li, W. Wang, J. Wen, Y. Zhang, H. Tan and H.-B. Yang, J. Am. Chem. Soc., 2019, 141, 13923 CrossRef CAS PubMed.
- (a) C.-K. Hui, B. W.-K. Chu, N. Zhu and V. W.-W. Yam, Inorg. Chem., 2002, 41, 6178 CrossRef CAS PubMed; (b) V. W.-W. Yam, C.-K. Hui, K. M.-C. Wong, N. Zhu and K.-K. Cheung, Organometallics, 2002, 21, 4326 CrossRef CAS; (c) K. Campbell, R. McDonald, M. J. Ferguson and R. R. Tykwinski, J. Organomet. Chem., 2003, 683, 379 CrossRef CAS; (d) T. Hasegawa, H. Goto, Y. Furusho, H. Katagiri and E. Yashima, Angew. Chem., Int. Ed., 2007, 46, 5885 CrossRef CAS PubMed; (e) S. Y.-L. Leung, W. H. Lam, N. Zhu and V. W.-W. Yam, Organometallics, 2010, 29, 5558 CrossRef CAS; (f) J. Stahl, W. Mohr, L. de Quadras, T. B. Peters, J. C. Bohling, J. M. Martín-Alvarez, G. R. Owen, F. Hampel and J. A. Gladysz, J. Am. Chem. Soc., 2007, 129, 8282 CrossRef CAS PubMed; (g) G. Fuhrmann, T. Debaerdemaeker and P. Bäuerle, Chem. Commun., 2003, 948 RSC; (h) P. Bäuerle, M. Ammann, M. Wilde, G. Götz, E. Mena-Osteritz, A. Rang and C. A. Schalley, Angew. Chem., Int. Ed., 2007, 46, 363 CrossRef PubMed; (i) V. W.-W. Yam, Acc. Chem. Res., 2002, 35, 55 CrossRef; (j) K. M.-C. Wong and V. W.-W. Yam, Acc. Chem. Res., 2011, 44, 424 CrossRef CAS PubMed; (k) V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589 CrossRef CAS PubMed; (l) A. K.-W. Chan and V. W.-W. Yam, Acc. Chem. Res., 2018, 51, 3041 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (b) W. Wang, B. Sun, X.-Q. Wang, Y.-Y. Ren, L.-J. Chen, J. Ma, Y. Zhang, X. Li, Y. Yu, H. Tan and H.-B. Yang, Chem. – Eur. J., 2015, 21, 6286 CrossRef CAS PubMed; (c) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed; (d) T. Ogoshi and T. Yamagishi, Chem. Commun., 2014, 50, 4776 RSC; (e) C. Li, Chem. Commun., 2014, 50, 12420 RSC; (f) N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2631 CrossRef CAS PubMed; (g) W. Si, P. Xin, Z.-T. Li and J.-L. Hou, Acc. Chem. Res., 2015, 48, 1612 CrossRef CAS PubMed; (h) T. Ogoshi, T.-a. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937 CrossRef CAS PubMed; (i) B. Jiang, W. Wang, Y. Zhang, Y. Lu, C.-W. Zhang, G.-Q. Yin, X.-L. Zhao, L. Xu, H. Tan, X. Li, G.-X. Jin and H.-B. Yang, Angew. Chem., Int. Ed., 2017, 56, 14438 CrossRef CAS PubMed; (j) X.-Q. Wang, W. Wang, W.-J. Li, L.-J. Chen, R. Yao, G.-Q. Yin, Y.-X. Wang, Y. Zhang, J. Huang, H. Tan, Y. Yu, X. Li, L. Xu and H.-B. Yang, Nat. Commun., 2018, 9, 3190 CrossRef PubMed; (k) S. Fa, T. Kakuta, T.-A. Yamagishi and T. Ogoshi, CCS Chem., 2019, 1, 50 Search PubMed; (l) X.-Q. Wang, W. Wang, W.-J. Li, Y. Qin, G.-Q. Yin, W.-L. Jiang, X. Li, S. Wu and H.-B. Yang, Org. Chem. Front., 2019, 6, 1686 RSC.
- (a) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668 CrossRef CAS PubMed; (b) T. Ogoshi, T. Aoki, R. Shiga, R. Iizuka, S. Ueda, K. Demachi, D. Yamafuji, H. Kayama and T.-A. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20322 CrossRef CAS PubMed; (c) T. Ogoshi, D. Yamafuji, T. Aoki, K. Kitajima, T.-A. Yamagishi, Y. Hayashi and S. Kawauchi, Chem. – Eur. J., 2012, 18, 7493 CrossRef CAS PubMed; (d) S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247 RSC; (e) S.-H. Li, H.-Y. Zhang, X. Xu and Y. Liu, Nat. Commun., 2015, 6, 7590 CrossRef PubMed; (f) G. Yu, D. Wu, Y. Li, Z. Zhang, L. Shao, J. Zhou, Q. Hu, G. Tang and F. Huang, Chem. Sci., 2016, 7, 3017 RSC.
- (a) C. J. Bruns and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2186 CrossRef CAS PubMed , and references therein; ; (b) A. Goujon, E. bMoulin, G. Fuks and N. Giuseppone, CCS Chem., 2019, 1, 83 Search PubMed.
- (a) D. A. Leigh, V. Marcos and M. R. Wilson, ACS Catal., 2014, 4, 4490 CrossRef CAS; (b) R. Bordolli and S. M. Goldup, J. Am. Chem. Soc., 2014, 136, 4817 CrossRef PubMed; (c) S. Hoekman, M. O. Kitching, D. A. Leigh, M. Papmeyer and D. Roke, J. Am. Chem. Soc., 2015, 137, 7656 CrossRef CAS PubMed; (d) M. Galli, J. E. M. Lewis and S. M. Goldup, Angew. Chem., Int. Ed., 2015, 54, 13545 CrossRef CAS PubMed; (e) Y. Cakmak, S. Erbas-Cakmak and D. A. Leigh, J. Am. Chem. Soc., 2016, 138, 1749 CrossRef CAS PubMed; (f) E. M. G. Jamieson, F. Modicom and S. M. Goldup, Chem. Soc. Rev., 2018, 47, 5266 RSC; (g) M. A. Jinks, A. de Juan, M. Denis, C. J. Fletcher, M. Galli, E. M. G. Jamieson, F. Modicom, Z. Zhang and S. M. Goldup, Angew. Chem., Int. Ed., 2018, 57, 14806 CrossRef CAS PubMed; (h) N. H. Evans, Chem. – Eur. J., 2018, 24, 3101 CrossRef CAS PubMed; (i) N. Pairault and J. Niemeyer, Synlett, 2018, 689 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 1938209. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qm00430k |
| This journal is © the Partner Organisations 2019 |