Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of electric fields for controlling crystallization†
Lee Fiona
Alexander
and
Norbert
Radacsi
 *
*
Institute for Materials and Processes, School of Engineering, The University of Edinburgh, Robert Stevenson Road, Edinburgh, EH9 3FB, UK. E-mail: n.radacsi@ed.ac.uk
First published on 31st July 2019
Abstract
This highlight investigates the different aspects of electric fields controlling crystallization, focusing on strong electric fields. Application of both internal and external electric fields, as well as utilizing both alternating and direct currents (AC and DC) are discussed, with an emphasis on protein crystallization. Attention is drawn to the similarities and opposing findings within the papers published in the field to date. It has been demonstrated that the crystallization process can be significantly enhanced by the application of electric fields. Namely, electric fields can reduce the nucleation time, control the location of nucleation, increase the product yield, control the product crystal size, enhance overall crystal quality, control the crystal orientation and control the polymorphism. In addition, recent advances including the use of an electric field for separation of multicomponent mixtures, electric field-assisted crystallization in a continuous flow, and transformation of amorphous material into crystalline are discussed.
Introduction
Crystallization is one of the oldest separation and purification techniques and has not changed in the past centuries significantly, until recently.1 The technique is used abundantly in industry, with practical applications in pharmaceutical, chemical, petrochemical, food industries and biotechnology,2–4 making it a multitrillion-dollar business.5 It is crucial for most industrial crystallization processes to control the crystallization, as the properties of the final product are dependent on the extent of crystallinity, polymorphism, and the magnitude and quality of the final crystals obtained. Furthermore, it is of vital significance in today's industry to have the ability to control the promotion or suppression of crystallisation.6 Electric fields comprise one method of achieving this. They are of great attention due to their tunable strength and direction leading to the possibility of enhanced crystal properties. Understanding the effects of electric fields on the crystallization process can potentially lead to control and enhancement of the crystalline product.In literature, key topics regarding protein crystallization enhancement via the application of electric fields have been reviewed by Al-Haq et al. (2007),7 Frontana-Uribe and Moreno (2008),8 Hammadi and Veesler (2009)9 and most recently, by Nanev (2017).10
Considering the methods already established for crystallization purposes, the application of electric fields stands out as a technique that incites a considerable amount of attention among researchers today. Comprehending the growth of crystals and their nucleation mechanisms under the presence of electric fields is fundamental, with regards to its utilization in a practical environment, e.g. for inducing crystallization and controlling the crystal polymorphic outcome,2,11,12 improving the quality of protein crystals,13–17 separation of components from their suspensions in multicomponent mixtures,18 and for crystallization of food systems.4
The aim of this highlight is to provide a helicopter view on all the different aspects of electric fields affecting crystallization, focusing on recent studies made with strong electric fields. The influence of both internal and external electric fields, as well as utilizing both alternating and direct currents (AC and DC), and the similarities and opposing findings are discussed.
Background
The effects of an electric field on crystallization from solution is a relatively new and active field of research, with a vast amount of research conducted in the early 21st century. Only a few published papers on the topic were available prior to the research of Taleb et al. (1999).19 The electric field effects on crystallization have only in recent times been studied extensively.Internal and external fields
Electric-field-induced crystallization experiments are executed in two different configurations: (1) if the electrodes are immersed in the solution then it is classed as an internal electric field. In this case, the strength of the electric fields used are restricted to small voltages or currents to limit faradic reactions that would be expected to occur at higher applied strengths and (2) if the solution has no contact with the electrodes when the electric field is applied, then it is an external electric field.Electric field effects on the thermodynamics of nucleation and crystal growth
The chemical potential and therefore the chemical potential difference between the liquid and solid are altered by the application of an electric field to a solution or crystalline bulk material. It may, therefore, modify the nucleation work by altering the chemical potential difference.20The subsequent equation denotes the addition of the electric field, E, to the chemical potential difference.20
| Δμε = Δμ + cεE2 | (1) |
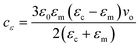 | (2) |
The subsequent equation expresses the nucleation work, W*, under the influence of an electric field:20
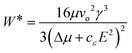 | (3) |
Nucleation is enhanced when εc > εm, here, cε is positive and the chemical potential difference is enlarged.20,21 In a situation where εc < εm, the nucleus creation is impeded by the applied field, and therefore nucleation is inhibited.20,21 If εc = εm, the electric field does not influence nucleation.20 A decrease in nucleation work is related to a rise in the nucleation frequency.20,22
By substituting eqn (5) into eqn (1), and noting that the supersaturation is given as Δμ = kTlnS, where S is the supersaturation ratio, the resulting equation for the rate of stationary nucleation accounting for the applied electric field is:20
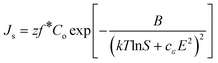 | (4) |
The theory developed by Kashchiev assumed the electric field to be uniform (before the nucleus is formed) and the same at different distances from the nucleus after it has been formed).20,22 Furthermore, the system containing the nuclei is often immersed inside a medium during crystallization, which decreases the electrostatic energy of the system of fixed charges.21 Thus, for crystallization processes, the effect of the electrostatic energy to the chemical potential should be considered from the Gibbs and Helmholtz free energy.23,24 From these the following equations are obtained when the electric field is accounted for:23
| dG = −SdT + Vdp + ∑μidni − VcPdE | (5) |
| dA = −SdT − pdV + ∑μidni + VcEdP | (6) |
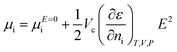 | (7) |
This equation should be used in the case of crystal growth since the relative permittivities are different between the liquid and solid phases.
Koizumi et al.25 discussed the electric field effects on thermodynamics by investigating the effects of crystal growth rate on entropy. They discussed in their study that both the electrostatic energy added to the energy required for step formation and the entropy related to the shape of the step must be considered when calculating the free energy on the crystal surface Fs, which be expressed from the Helmholtz free energy:25
| Fs = Us − TSs | (8) |
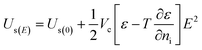 | (9) |
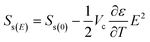 | (10) |
Thus, both the entropy related to the shape of step and the energy required to form the step depends on the volume to which the external electric field is applied, the absolute temperature and the magnitude of the applied electric field.
Electric field effects on protein crystallization
The study of electric field effects on protein crystallization was initiated approximately twenty years ago by Aubry's group.13,19 Since then, this area of research has been widely studied by the teams of Moreno,8,14,26–30 Koizumi16,24,31–34 and Veesler.9,35 Conventionally, the majority of experimental investigations have been undertaken using hen egg white lysozyme (HEWL) as a model protein. Four different approaches were taken to study the effects on proteins. Depending on their nature, electric fields can be generated by (static) direct current (DC) or (dynamic) alternating current (AC). Furthermore, defined by the design, electric fields can internal or external in regard to the studied material.External DC electric field
In 1999, Taleb et al.19 designed two basic devices to study the crystallization of proteins under a direct current external electric field. The study found that the external electric field affects both the size and quality of the produced crystals, resulting in fewer crystals being formed of greater size, in the presence of an electric field than in its absence.19 The crystals developed at the surface of the droplet close to the cathode.19Altering conditions such as pH, temperature and precipitation agent composition can be utilized along with external electric fields to impact crystalline properties of proteins.19 Taleb et al.19 demonstrated that an external DC electric field had a sizeable impact on the nucleation rate of lysozyme crystallization. Furthermore, the electric field impacted the size and quality of crystals; fewer crystals of greater size were produced, and their quality was enhanced as measured by the detected mosaic spread.
In a subsequent study, an external electric potential of 7.5 × 105 Vm−1 was used. Nucleation did not occur as the protein solution remained liquid phase throughout the experiment.13 For a pH controlled HEWL solution with no NaCl addition, there was a detected increase in protein concentration near to the cathode, which confirms why crystals were favorably found within close proximity of the cathode in the earlier experiment.19 The experiment clearly showed a concentration gradient between the electrodes, resulting from the effects of an external DC electric field.13 It was also demonstrated that the variance in concentration between the electrodes is dependent on the overall charge of the protein, with the effect of the electric field increasing relative to the magnitude of the charge.13 Furthermore, the authors noted that the protein crystalline solubility is not altered by the presence of the field.13
Findings concluded that the presence of the electric field significantly reduced the time to reach equilibrium.13 It was also noted that when crystallizing in a low ionic strength solution and at a pH far from the isoelectric point of the protein, the effects were greatest.13 After four weeks, the protein concentration was much lower when under the influence of an external electric field, than in the absence of the electric field.13
Another group36,37 repeated the original experiment of Taleb et al.19 using the SDVD method. The Nanev group,36 using custom made two-dimensional glass cells,36 (Fig. 1) investigated the effect of HEWL crystallization as a function of temperature. In order to aid comparison, the experiments were undertaken using a pair of cells, the first had an applied DC external electric field of 1.5 × 105 Vm−1 and the second cell had no exposure. Other conditions, for example, the temperature and pH, were identical in both cells. The control of temperature decreased convection, and microscopic observation are all benefits of this setup.36
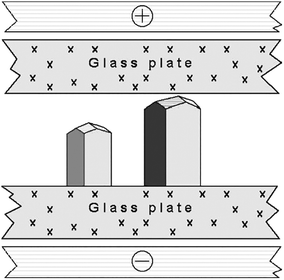 | ||
| Fig. 1 Representation of the growth cell displaying two lysozyme crystals with the c-axis orientation (normal to the glass plate, with respect to all other orientations).36 Reprinted from ref. 36 with permission of J. of Crystal Growth, Elsevier. | ||
Following Taleb's work regarding a concentration gradient within the cell, the authors confirmed that it is primarily at the cathode where nucleation happens and that crystals develop more rapidly at this electrode due to the electric field. Furthermore, their studies also found that at a relatively low temperature the presence of an electric field has a more significant effect on the induction time and nucleation rate.36 Nanev's studies demonstrated that the orientation of the crystals is greatly influenced by the presence of an external electric field.36,37 They found that operating at temperatures less than 5–7 °C, combined with the applied electric field, results in a considerably larger number of growing crystals having a preferred c-axis orientation.36 Interestingly, this action of the crystals orientating due to the electric field was not observed at 18 °C, irrespective of the applied electric field strength, which was up to 4.0 × 105 Vm−1.36 Nanev assumed that at higher temperatures the presence of an electric field was not orienting the molecules as their thermal motion disturbed and repositioned them.10 From this, one can deduce that reducing the temperature (kinetic energy of the molecules) facilitates the electric field to control the orientation of the crystals in solution, or crystallization is influenced differently at larger ΔT.4 Moreover, Taleb et al. have deduced that crystal quality was enhanced for those grown under the influence of an electric field.13,19 In opposition to this, Nanev's group reported that in some cases, a rough crystal morphology was evident.36 The differences are, however, recognizable. Taleb et al. used higher temperatures in their studies combined with a higher protein concentrated solution.36 The authors stated that this rough crystal morphology could be a consequence of strain that occurs due to the nucleation temperature being low.36 Potential reasoning for the aforementioned deductions can be accredited to the fact that, in the solution (pH 4.5), HEWL molecules hold a positive charge under conditions of nucleation.
In one of the latest advances regarding the growth of protein crystals by the influence of an external electric field, glucose isomerase crystals at ambient temperature were developed via the microbatch method, established by Chayen et al.,38 and used recently by Rubin et al.17 Firstly, tests using the microbatch method were conducted with HEWL at four field potentials (1, 2, 4 and 6 kV). Comparisons were made with and without the application of the electric field. These revealed that the lysozyme crystals experienced growth, while the quantity of nucleation was reduced when applied to the field, as well as the number of crystals per well being reduced in relation to the burgeoning electric field intensities17 (Fig. 2).
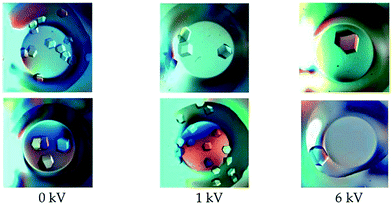 | ||
| Fig. 2 Glucose isomerase crystals grown under the application of a DC electric field of varying intensities. Each row corresponds to experimental results obtained on different dates.17 Reprinted with permission from ref. 17 Copyright (2017) American Chemical Society. | ||
Analogous findings were recorded by various authors9,13,14,16,19,26,31,33,36,38–40 containing records that the crystals develop favorably near the interface of the oil-growth solution.31 Examination from X-ray diffraction crystallography has confirmed that under the influence of the electric field, the quality of crystals improves as the intensity of the electric field increases.14 Furthermore, raising the potential difference led to an improvement in the resolution of diffraction.14
During the process of increasing the potential difference, several variations were noticed in comparison to the crystals that were grown without an electric field present. These included the mosaicity and the number of nucleation centers, which were seen to decrease in the presence of an applied voltage.17 These observations indicated that electric fields have a noticeable effect on the processes of crystallization and nucleation. Furthermore, light microscopy showed that crystals exhibited nucleation rate reduction and crystal size enlargement when experiencing an electric field.17
The significantly different magnitude of the electric field strength necessary for lysozyme crystallization (of magnitude ∼105 Vm−1) compared with glucose isomerase (∼100–1000 Vm−1) could be explained by the differences detected in the dipole moments, resultant molecular charges, and ratios between the quantity of positive and negative residues.17 This explanation may also provide reasoning for the different behavior observed when HEWL and glucose isomerase crystals were formed using the same microbatch equipment, in the presence of the electric field: glucose isomerase crystals were prone to develop near the bottom and walls of the well, while HEWL crystals were detected favorably at the interface of the oil-growth solution. Sui et al. used a significantly lower DC electric field, up to 103 Vm−1, in a microfluidic crystallization setup.41 They found that the crystals grown in the presence of an electric field diffracted to a higher resolution than crystals grown in the absence of the field.41 Thus, when performing X-ray diffraction analysis, the signal-to-noise ratio is improved,41 which can help in determining the crystal structures of proteins and other biological molecules. The improved X-ray diffraction signal-to-noise ratio was also reported by other scientists.42
Thus, it can be concluded that applying an electric field to macromolecule crystallization results in a reduction of nucleation sites, and larger crystals of enhanced crystallographic quality being produced.
Internal DC electric field
Electric-field-induced protein crystallization was first introduced in a cutting-edge paper in 1976 by Chin et al.39 Studies on internal DC electric fields on protein crystallization have since been carried out by Mirkin et al.,43 Sazaki et al.,29 Moreno and Sazaki,30 Nieto-Mendoza et al.,40 Flores-Hernández et al.26 and Sui et al.41The preliminary work by Mirkin et al.43 for the purpose of examining the impact of an internal electric field on HEWL and thaumatin crystallization, saw the application of a revised design of the gel-acupuncture arrangement,44 so as to apply an electric field by use of electrodes within the crystallization medium.
The conditions classically used for HEWL and thaumatin crystallization by the gel acupuncture method44 were the same as those applied in this experiment.
Mirkin et al. proposed that one of the key benefits of using their technique for the growth of crystals is that less time is required to achieve crystals in comparison to the standard gel-acupuncture setup.43 Thaumatin crystals were obtained in just five days using their method under the influence of the internal electric field, which was seven days quicker than using the conventional method.43 Therefore, for both lysozyme and thaumatin, a reduction in both induction time and quantity of crystals forming was detected by Mirkin et al. This supports Taleb's earlier findings.13,19
With regard to the crystal orientation, Mirkin et al.43 detected that for every crystal joined to the anode, there appeared to be favored orientation under the application of the electric field towards the c-axis of the lysozyme crystal.43 It was confirmed by X-ray crystallography that the structure of the thaumatin crystals was not altered by the application of the internal electric field.43 The study also confirmed that the closer the electrodes were to each other (i.e. the higher the electric field strength was), the faster the crystal growth was.43
In the work published by Moreno and Sazaki.30
Tests were carried out at three varying distances between the electrodes to study the impact of the internal electric field. Bubbles of gas were apparent as a result of water electrolysis at currents greater than 3 μA in the initial experiments. To override this issue, the current was reduced to a maximum of 2 μA in the subsequent experiments. To assist with comparisons, every experiment had a reference in which no current was applied. Under the influence of a DC current the authors made a number of observations: (1) nucleation time of HEWL was observed to have reduced for both gel and supersaturated solutions; (2) a smaller quantity of nuclei was favored and as a result, crystals were larger in size; (3) it was only within close proximity of the cathode that HEWL crystals appeared, whereas amorphous precipitation was seen near the anode. These observations are consistent with the prior observations stated for effects on crystallization processes under the influence of an external electric field.13,19
The groups of Nanev and Aubry13,19,37 solely used external electric fields of magnitude 1.5–7.5 × 105 Vm−1 in their investigations, Moreno and Sazaki,30 however, stated substantial effects could be achieved even when applying electric fields of reduced size. In their methodology, a significantly lower electric field of 0.19 Vm−1 was used and the electrodes were placed directly in contact with the solution (internal DC field). From this observation, they concluded that they must account for three different effects: (1) electromigration; (2) redox reactions on the surface of the electrode, but this was discounted as throughout the crystallization process, there was no noticeable production of gas; (3) impact of electric potential produced by the electric field on the chemical potential difference.30
Moreno and Sazaki claimed that both the formation of crystals occurring near the cathode, and the reduction in induction time could be interpreted by (1) electromigration; (2) the polarity difference between the electrodes and the protein molecules; (3) interactions among protein molecules and the Cl− ions within the vicinity of the electrodes.30 The decline in the number of deposited crystals could also be accredited to the electric potential created by the field. It was also observed that the crystals exhibited a preferred orientation along the c axis (normal to the glass support, in relation to all other orientations) of the tetragonal HEWL. This effect was also detected by Nanev et al. in the presence of a 1.5 × 105 Vm−1 DC external electric field, and by Mirkin et al. when they made use of an internal electric field.43
According to the findings of Moreno and Sazaki,30 one of the primary benefits of their technique for crystal growth was mainly the control of the crystal quantity. As a result, this method enabled an increase in the size of the crystals and enhanced the diffraction intensity for X-ray crystallography purposes.
A transparent crystallization cell, comprised of two glass plate electrodes coated with conductive indium tin oxide (ITO), was utilized by Gil-Alvaradejo et al.42
The improved intensity was observed from X-ray diffraction analysis of lysozyme crystals grown at 6 μA, and of ferritin crystals grown between 2–6 μA (both cases DC power was used).42 Even though the crystal quality was improved, no conformational changes in the 3D protein molecule structures were observed.42
A subsequent paper was published in 2013 by Flores-Hernández et al.26 exhibiting similar effects and in unanimous agreement to the aforementioned papers. The authors yet again made use of the model protein, lysozyme, but also investigated a more challenging protein, 2TEL–Lys. They created a novel crystallization device (using again ITO-covered-glass-electrodes), combining an electric current with a sitting drop vapor diffusion setup, which, would enable it to be used with an extensive range of proteins. They implemented this device by reengineering their previous device with the reasoning that the batch method is limited to merely simple proteins. Their results indicated that 50 times fewer crystals were obtained around the cathode when grown in the presence of an internal DC electric field than in its absence.26 In addition, these crystals were drastically larger in size and exhibited enhanced quality.26 This design is said to be straightforward to use and would allow for a smooth scale up for production of larger crystals.26
Internal AC electric field
Compared to DC electric fields, there has been a lack of investigation in the area of AC electric field application to crystallization. This could be attributed to the fact that it presents a supplementary variable – the current frequency.9 A major advance in 2008, and the initial study carried out to investigate the effects of an AC electric field on protein crystallization, was carried out by Hou and Chang.15 They used an internal AC electric field to examine both the frequency and voltage impacts of the electric field. Diminishing Faradaic reactions on the electrodes, thereby enabling greater applied voltages to be used, is the first known benefit of an AC field.15 The authors tested HEWL solutions in the presence of various different AC fields for signs of nucleation in 24 hours, and observed three different zones in relation to the applied voltages and frequencies (Fig. 3): (1) the first zone, beneath the solid line, contained no crystals in 24 hours.15 With the prolonged application of the field, however, crystals developed at rates and dimensions equipollent to crystals grown in the absence of electric field.15 This indicates that in this region, the AC electric field had minimal impact on the crystallization process; (2) crystals were detected in the zone between the solid and dashed lines; (3) the final zone (above the dashed lines) was the production of a gel, from which the largest single, and least defected crystals were produced (in comparison to case 2).15 The minimum of zone 2 represents the most favorable frequency with the lowest required voltage for crystallization in a 24 hour period (Fig. 3).15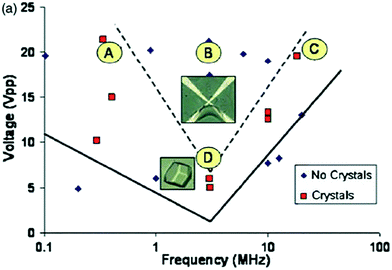 | ||
| Fig. 3 Results of Hou and Chang15 of HEWL solutions in the presence of different internal AC fields, having been observed for 24 hours for signs of nucleation. A and C are within the crystallization window during this time, while gelation takes place for B. The point D indicates the frequency, where the lowest voltage is required for crystallization. Reprinted from ref. 15 with the permission of AIP Publishing. | ||
To summarize, Hou and Chang have successfully shown that the voltage and frequency of the applied AC electric field could create reversible gelation.15 Eradication of the field then resulted in a small number of high-quality crystals being produced as the induced gel phase was converted. Their investigations thus confirmed enhanced HEWL crystal quality (large single crystals with few defects) when an AC electric field is applied, in agreement with aforementioned studies on external electric fields affecting crystallization.16,19 A phase diagram similar to Fig. 3 would be required to choose the most suitable voltage, frequency, and length of exposure for alternative proteins.
Using an alternative protein, thaumatin, Wakamatsu45 assessed the effect of applying an internal AC electric field (1.06 × 103 Vm−1) during crystallization.45 The author utilized a transparent cell containing two ITO thin film electrodes and demonstrated that the application of the field encouraged crystallization.45 Wakamatsu and Ohnishi have also made use of ITO-coated conductive glass cells to investigate HEWL crystallization in an earlier study.46 Utilizing a similar transparent cell, an internal electric field and a low-angle (<8deg;) forward light scattering technique, the authors observed electric field-induced aggregate formation from lysozyme solutions.47 The analysis of the lysozyme aggregation is detailed in a later paper by the same group.48
External AC electric field
There are many examples of research focusing on the use of DC external fields to control nucleation.2,11,13,17,19,36,37,49,50 These articles have primarily demonstrated a reduction in the nucleation rate with an applied electric field. Nonetheless, making use of an external AC electric field with suitable frequency to successfully control an increase and a reduction in the rate of nucleation for crystallization processes had not been accomplished until the work of Koizumi et al.24 in 2009. The impact on the rates of nucleation under the influence of an external electric field has been examined theoretically by Kashchiev20,22 and Isard.21 Their analysis proposed that whether the rate of nucleation is increased or reduced, is dependent on the variance in the electrical permittivity between solid and liquid phases.20 Koizumi et al.24 have examined their theory experimentally, by using an AC electric field on protein crystallization. These authors suggested that there was a potential of a reverse in the electrical permittivity between the two phases.24 They anticipated that the dielectric constant of the HEWL crystals would be greater than that of the medium at frequencies <500 kHz and vice versa for frequencies >500 kHz.24 The group has carried out a thorough study in the field over the last decade to understand how AC electric fields could impact protein crystallization. They proved that the rate of nucleation of HEWL increases in the presence of a 1 MHz electric field, however it decreases under the presence of the same field at 10 kHz.24 Demonstrating an improvement and impedance on the rate of nucleation of lysozyme crystals, the group effectively succeeded in controlling the nucleation process.24 In a later study, using a different compound, they also showed that the rate of nucleation of porcine insulin was found to increase under the presence of an external AC electric field at 3 MHz.32 The authors credited this phenomenon to the electrostatic energy which was added to the chemical potentials of each phase, and to the formed electric double layer (EDL) that forms at the interface between the dissimilar phases.32 The chemical potential of the solid material was considerably altered in comparison to the liquid, which resulted in a burgeoning nucleation driving force in agreement with the classical nucleation theory.32 Koizumi et al. have successfully communicated that the observed increase or decrease in lysozyme rate of nucleation was a consequence due to the variance between the electrical permittivity of the liquid and solid phases.24,31,32 The authors concluded that the use of an AC field can be a valuable practice for crystallization of proteins, and could subsequently be applied to a vast majority of proteins on the market.32Following on from this study, another paper submitted by the same group exhibited crystallization experiments at room temperature using the batch method.16 They applied a 4 × 104 Vm−1 external AC electric field at a frequency of 1 MHz to the protein solution, and further confirmed by X-ray diffraction rocking-curve measurements, that HEWL crystal quality could be enhanced in the presence of an external AC electric field. Fig. 4 shows the batch setup used in their study. Further investigations by the same group led to the conclusion that there was an optimum frequency to advance the protein crystal quality.33
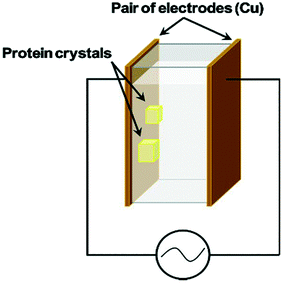 | ||
| Fig. 4 Schematic diagram of the batch setup of Koizumi et al.51 | ||
More recently, Pareja-Rivera et al.14 demonstrated that it is not only the number of appearing crystals that are influenced by an applied AC field but that their size is also affected subject to the electric fields' frequency. Using an AC electric field (2 and 8 Hz) they revealed for both glucose isomerase and lysozyme crystals, that the greater the applied AC frequency, the greater the number of crystals produced (Fig. 5).14
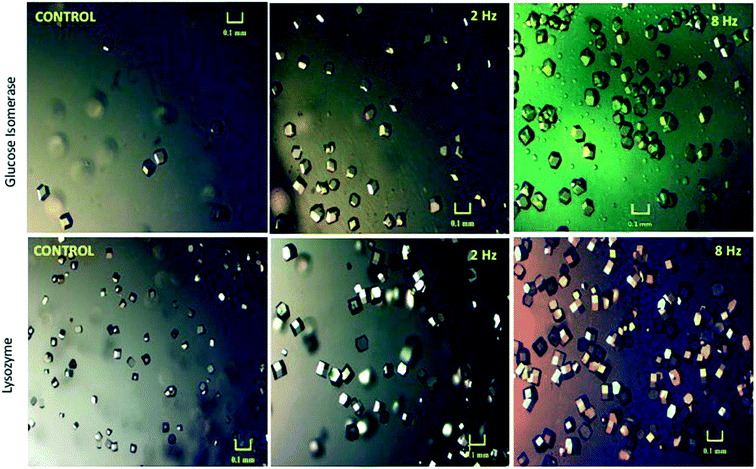 | ||
| Fig. 5 Microscopic images of the crystals developed under application of an AC electric field for 24 hours for both glucose isomerase and lysozyme crystals in comparison to those crystals grown in the absence of the electric field (control experiment).14 Reprinted with permission from ref. 14 (Copyright (2017) American Chemical Society). | ||
The majority of investigations conducted to date make use of uniform electrodes. Hou and Chang constructed quadrupole and interdigitated Ti/Au electrodes for their research and concluded that the quantity of nucleation sites reduced.15 In addition, the number of crystals was enhanced under the application of the nonuniform electric field arising from the patterned electrodes.15
Numerous devices utilizing ITO electrodes have been constructed for enhanced control over nucleation rate and the final crystal quality.25,26,52,53 Li and Lakerveld53 examined the influence of alternating electric fields on protein crystallization (using insulin and HEWL as model compounds) in microfluidic devices. The authors used ITO-coated glass slides as the electrodes; the bottom of which was ITO-patterned and the top uniformly coated. Eight different patterns in combination with different surface areas in parallel-plate configurations were used in their research. It was demonstrated that depending on the parameters of the design of an electrode (shape, pattern and surface area), both an enhanced and inhibited effect on crystallization could be observed.53 Their results further proposed that the location of nucleation was manipulated by the non-uniform electric field brought about by the characteristic electrode designs and by the ITO layer, which acted as a template for nucleation.53
Further applications of electric fields on crystallization
Another study from 2019 by Fallah-Joshaqani et al.55 used an external electric DC electric field up to 9.6 × 105 Vm−1 to study the electric field effects on freezing ice, NaCl solution and mushrooms. The researchers found that the supercooling degree was significantly reduced during the water freezing process in the presence of the electric field, and nucleation started earlier with the electric field.55 Surprisingly, this trend was not linear, and when the electric field was increased further, from 6.4 × 105 Vm−1 to 9.6 × 105 Vm−1, both the supersaturation degree and the nucleation time increased.55 This phenomenon was explained by the authors as the electric field aligned the dipole of the water molecules in the direction of the electric field, decreasing the free energy barrier, which increased the probability of nucleation.55 However, increasing the electric field further might have caused the water molecules to deviate from the optimal structure, increasing the nucleation time.55 When the NaCl solution was frozen in the presence of the electric field, first the nucleation time decreased, but at larger electric fields (6.4 × 105 Vm−1 and 9.6 × 105 Vm−1) the nucleation time increased and was even larger than the nucleation time in the absence of the electric field.55 The mushroom samples showed similar trends to the NaCl solution.55 Thus, it seems that the electric fields benefit freezing mainly when used with high water-containing products.
The authors introduce a new application to crystallize polymers, utilizing both DC and AC fields (application of DC bias across the PVDF films). The maximum electric field used in their research was 8 × 106 Vm−1. Here, the application of external bias brings about a resultant difference in domain configuration and size and is interlinked with an ‘amorphous to crystalline’ transfiguration of PVDF films.57 Their experiments show that the head to head and tail-to-tail arrangements of PVDF molecules are more favorable than the head-to-tail arrangements of the domains.57 The key finding of this paper is that the electrostatic field within the PVDF during piezoresponse force microscopy affects the polymer chain, resulting in the monomer turning in the direction of the electric field. Consequently, change arises in the configuration of the irregular amorphous region, which is transformed into the crystalline phase.57
Another recent study by Qi et al.58 describes the use of an external electric field for the synthesis of the piezoelectric ZnO nanorods. The authors used an electric field of 5 × 104 Vm−1 during the crystallization process of ZnO, and they obtained long nanorods in the presence of the electric field, while short ‘rice-like’ ZnO nanoparticles in the absence of the electric field.58
Thus, electric fields can enhance the fabrication of piezoelectric crystals.
| SS | Nucleation conditions | Sample size | Percent samples pure α | Percent samples pure γ | Percent samples mixed α + γ |
|---|---|---|---|---|---|
| 1.85 | Spontaneous | 22 | 86 | 14 | 0 |
| 1.85 | DC field | 4 | 50 | 50 | 0 |
| 1.9–2.0 | Spontaneous | 15 | 13 | 60 | 27 |
| 1.9–2.0 | DC field | 5 | 0 | 100 | 0 |
A later study in 2013 by Di Profio et al.12 investigated glycerin crystallization using membrane crystallization technology (which allowed for enhanced control of supersaturation in solution, by adjusting the removal rate of solvent, in the presence of an AC electric field). Experiments were carried out using the apparatus depicted in Fig. 7, by varying the operating conditions: removal rate of solvent, pH, and the use of a pulsed electric field. The setup is comprised of one internal and one external electrode. The former being the cathode, a stainless-steel conductor directly in contact with the glycerin solution, and the latter being the copper wire anode. This setup, combined with the pulsed AC field, delivered polarization that was adequate to prevent electrolysis (which could be present if a highly conductive solution and two internal electrodes were to be used).12
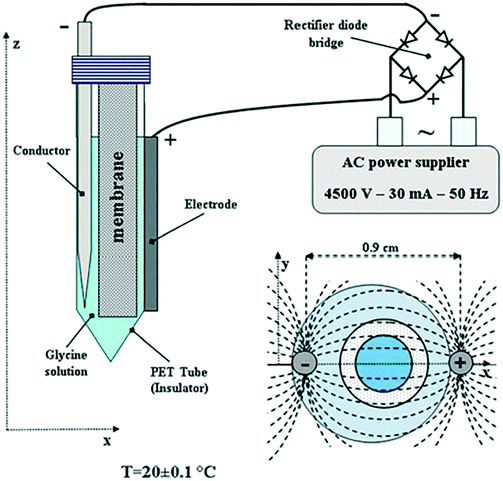 | ||
| Fig. 7 Schematic illustrating the experimental arrangement of Di Profio et al.12 reprinted from ref. 12 with permission of Physical Chemistry Chemical Physics, Royal Society of Chemistry. | ||
Di Profio et al. claimed that, from their experimental work with and without an applied electric field, the factors influencing glycerin polymorphism are: electric field > pH > rate of supersaturation > concentration of solute.12 Their findings disprove the classical justification grounded on the ‘self-poisoning’ mechanism and standard ‘cyclic dimer hypothesis’ for the crystallization of α and γ polymorphic forms from solution.12 They proposed that the rates of nucleation, in relation to the respective progress in the kinetics of both polymorphs affected by differing conditions, have a leading part in shaping the polymorphic outcome.12 In opposition to the aforementioned disproval, the authors advocate a molecular nucleation process where open chain dimers can act as the foundations for each polymorphic form in the rate-limiting stage of the multi-step mechanism for nucleation.12
In contrast to Aber et al.,2 who provided evidence that the γ-polymorph could be preferentially crystallized under application of a DC electric field, Di Profio et al.12 demonstrated in their study that the application of the electric field consistently gave rise to the α form of the polymorph, even under the circumstances in which only the γ-polymorph was predicted to crystallize.12
Using a different model compound, Radacsi59 explored the impact of a DC internal electric field (5.6 × 105 Vm−1) on the polymorphic outcome of isonicotinamide (a highly polar molecule) from solution throughout a cooling crystallization method from 1,4-dioxane (non-polar solvent). The use of the non-polar solvent facilitates crystallization without interacting with the electric field, and also prevents redox reactions and short-circuits (sparks) from occurring. Two experimental arrangements were used in this study as a novel way to produce polymorphs, namely ‘parallel-plate setup’ and ‘parallel-rod setup’ (Fig. 8). The author found, by structural analysis, that the polymorphic form changed under the influence of an electric field.59 When no electric field was applied to the solution, the isonicotinamide crystals grown were the metastable form I. However, when a homogeneous electric field of 5.6 × 105 Vm−1 was applied, the crystals formed on the anode were of the stable form II. Moreover, the author observed that when an inhomogeneous electric field was applied of a potential lower than 5.6 × 105 Vm−1, a combination of both polymorphs forms was present.59 The study found that raising the strength of the field led to the transformation of the polymorphic structure from the metastable form I to the stable form II, and thus had the ability to alter the crystallization kinetics.59 Additionally, Radacsi confirmed that the crystals grew in less time under application of the electric field in comparison to those grown without.59 The crystal growth rate increased from 8.3 μm min−1 to 126 μm min−1.59 This observation is in agreement with the previous authors13–16 who have studied crystallization under an electric field. They concluded, from the recrystallization of isonicotinamide, that the control of crystallization (localized growth, induction time and polymorphism) was attributed to the increased local supersaturation due to electromigration by application of the electric field (likewise established by prior authors).30,35,40
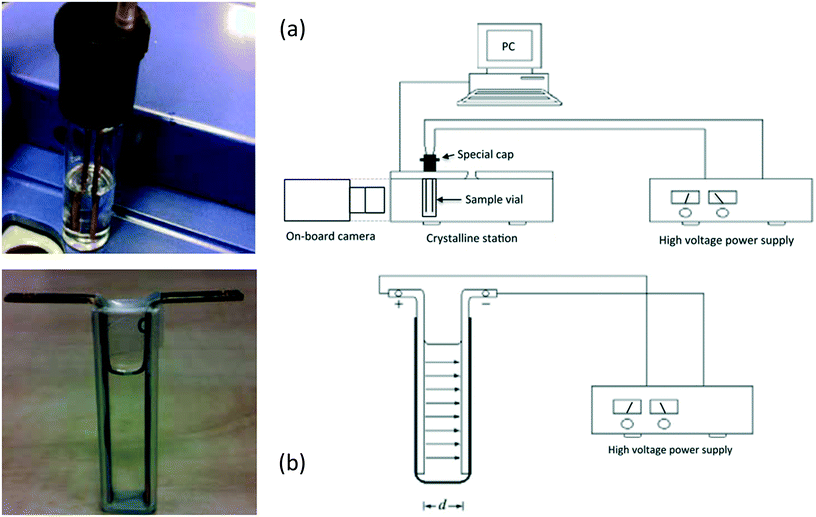 | ||
| Fig. 8 (a) The parallel-rod arrangement of Radacsi,59 along with the schematic representation of the scheme and (b) the parallel-plate arrangement with the equivalent schematic. Reprinted with permission from ref. 59. | ||
Adrjanowicz et al. published a paper on DC electric field effects on crystal quality using cooling crystallization.60 They reported that application of an external DC electric field (4.0 × 106–2.0 × 107 Vm−1) resulted in the crystallization behaviors of a subcooled, glass-forming liquid (4-vinyl-propylene carbonate) being altered, and thereby stimulating the development of a new polymorphic crystal that could not be formed in the absence of the field. Furthermore, in agreement with Radacsi, their results show that under the influence of an electric field, changes are made in the overall crystallization tendency and a large proportion of a different form of the polymorph is created. The time for crystallization is reduced when the potential is applied, in comparison to its absence. Thus, their results agree with the findings of Radacsi,59 even though Adrjanowicz et al. used an external DC electric field (while Radacsi used and internal DC electric field).
Furthermore, molecular dynamics simulations by Parks et al.11 demonstrated that a strong DC field of 1.5 × 109 Vm−1, had the ability to create an innovative paracetamol polymorph. Through simulation, they assessed the influence of an external DC electric field on dissolution and growth rates, the morphology of the crystals and the polymorphic form obtained. From the dynamics they found that the crystal growth rate of the supersaturated nanocrystals was suppressed by application of the field, claiming a 40% reduction in growth rate under the applied intensities. This finding, although contrasting with previous findings, is in accordance with magnetic field crystallization studies of paracetamol.11 In addition, for paracetamol nanocrystals which are not saturated, the simulations indicated that the electric field could both enhance and impede the rate of dissolution.11 Finally, the newly created polymorph was found to be metastable under the influence of the electric field, exhibiting improved aqueous solubility and thus it is suspected that this form will have improved bioavailability and show distinctly new characteristics when compared to the already known forms of paracetamol.11 This is of particular importance in pharmaceutical applications.
The aforementioned studies have shown that an applied electric field has the potential to be used for controlling polymorphic formation in crystallization processes.
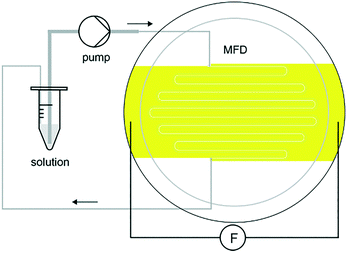
In their seminal paper, Li et al.18 studied the impact of a continuous DC electric field on the result of nucleation and polymorphic form of crystals throughout cooling crystallization experiments. Fluid dynamics and the rate of growth of the crystals were observed to change under application of the field. This work, to the best of our knowledge, was the first separation technique based on coupling manipulation of particles by application of a strong internal DC electric field, with cooling crystallization for in situ product recovery and separation. Initial experiments carried out showed that in the presence of an internal electric field (above 2 kV), particle movement was induced (see Video in ESI† and Fig. 9). Crystals of isonicotinamide began to move from their suspension in 1,4-dioxane, and attach on the anode. As the potential difference was raised, the number of crystals around the anode also increased. A crystal bridge was created at potential differences higher than 5 kV as the number of crystals participating in the movement was amplified. Even though the crystals connected the anode with the cathode, no short-circuit or reactions were observed (see Video in ESI†). Removal of the field resulted in all the crystals detaching from the anode and falling to the base of the vial.18 The authors claimed that the motion of the particles was primarily related to the interaction of the crystals and the applied field.18 They observed no liquid motion when the experiment was carried out in the presence of the field but in the absence of crystals in the solution.18 They also stated that electrochemical reactions could not be responsible for this phenomenon as there was no gas formation.18
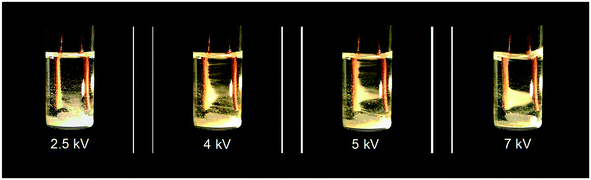 | ||
| Fig. 9 Experimental observations of Li et al.18 of particle movement when an inhomogeneous static electric field was applied to a suspension of isonicotinamide in 1,4-dioxane. Reprinted from ref. 18 with permission of Angewandte, Chemie, John Wiley and Sons. | ||
Using this newly discovered phenomenon, phenazine and caffeine (two organic crystalline compounds) were shown to be individually removed from their suspension in 1,4-dioxane (Fig. 10).18 Application of an inhomogeneous electric field to this mixture at 30 °C led to crystals adhering to the surface of both electrodes (phenazine on the cathode, and caffeine on the anode). The suspension was gently cooled to 15 °C under the application of the electric field, in order to immobilize the crystals on the electrode thus allowing recovery of the deposited crystals.18 Solubility had been reduced during the cooling process, enabling the growth of accumulated crystals. Crystals were then removed from the bulk solution by withdrawing the electrodes, allowing them to be separately collected. These crystals were of different color, shape and thickness.18 The purities were found to be greater than 91%,18 which is slightly lower than the 99% purity that can be obtained in theory by a single crystallization process step.6
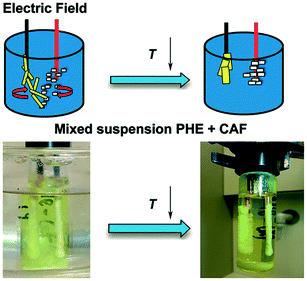 | ||
| Fig. 10 The experimental arrangement of Li et al.18 in the presence of an electric field and subsequent cooling crystallisation. Phenazine and caffeine were separately recovered from their suspension in 1,4-dioxane. Reprinted from ref. 18 with permission of Angewandte, Chemie, John Wiley and Sons. | ||
These results proved that the hybrid of particle manipulation, brought about by the presence of the electric field, and cooling crystallization can be used for separation and recovery of two crystalline substances from their multicomponent solution.18
Although promising, this new and unique technique exhibits limitations in its design. It requires additional work and improvements to be made in the design in order to allow a higher yield to be obtained to prove satisfactory in industry scale-up.
Summary
This highlight was undertaken in order to investigate all the different aspects of strong electric fields controlling crystallization and has underlined the importance of electric fields in significantly enhancing the crystallization process. Substantial progress has been made in investigating electric field-assisted crystallization within the last two decades. The results to date have been very promising, depicting clear advantages of crystallization in the presence of electric fields. Electric fields have been proven to be a valuable manipulation instrument for controlling the crystallization process and the crystal product. The studies have been undertaken by the application of four primary approaches; the influence of both internal and external electric fields, as well as utilizing both alternating and direct currents (AC and DC). Table 2 summarizes the different types and magnitudes of electric fields applied on various model compounds, using different electrode materials and crystallization method, that are presented in this highlight.| Type of electric field | Method | Material studied | Electric field strength | Electrode material | Solution concentration | Ref no. |
|---|---|---|---|---|---|---|
| BPTI = bovine pancreatic trypsin inhibitor, CAF = caffeine, HDVD = hanging drop vapor diffusion, HEWL = hen egg white lysozyme, INA = isonicotinamide, ITO = indium tin oxide, PHE = phenazine, PVDF = poly(vinylidenefluoride), SDVD = sitting drop vapor diffusion. | ||||||
| External DC | HDVD SDVD | HEWL | 2.0 × 105 Vm−1 | Metal | 20, 30, and 40 mg mL−1 | Taleb et al., 1999 (ref. 19) |
| External DC | SDVD | HEWL | 7.5 × 105 Vm−1 | Metal | 10 mg mL−1 | Taleb et al., 2001 (ref. 61) |
| 4.8 × 105 Vm−1 | ||||||
| External DC | HDVD SDVD | HEWL | 1.5 × 105 Vm−1 | Metal | 20 and 24 mg mL−1 | Nanev and Penkova, 2001 (ref. 62) |
| External DC | HDVD | HEWL | 1.5 × 105 Vm−1 | Metal | 20 and 24 mg mL−1 | Nanev and Penkova, 2002 (ref. 37) |
| External DC | Laser-induced nucleation | Glycine | 6 × 105 Vm−1 | Brass | 4.1–4.5 mg mL−1 | Aber et al., 2005 (ref. 63) |
| External DC | Microbatch | Glucose isomerase | 100–1000 Vm−1 | Copper | 30 mg mL−1 | Rubin, Owen and Stojanoff, 2017 (ref. 17) |
| External DC | Molecular dynamic simulations | Paracetamol | 1.5 × 109 Vm−1 | N/A | N/A | Parks et al., 2017 (ref. 11) |
| External DC | Protein film organized by external electric field | HEWL | 6.0 × 103 Vm−1 | Siliconized cover-slips | 0.00143 mg mL−1 | Walter et al., 2018 (ref. 50) |
| External DC bias | Cooling crystallization | 4-vinyl-propylene carbonate | 4.0 × 106–2.0 × 107 Vm−1 | Invarsteel plates | N/A | Adrjano-wicz et al., 2018 (ref. 60) |
| External DC bias | Chemical synthesis | PVDF | Maximum 8.0 × 106 Vm−1 | Pt and Co/Cr coated tips | N/A | Ganguly et al., 2018 (ref. 57) |
| External DC | Chemical synthesis | ZnO | 5 × 104 Vm−1 | Stainless steel | N/A | Qi et al., 2018 (ref. 58) |
| External DC | Cooling crystallization | Water, NaCl solution, mushroom (extract) | 3.2–9.6 × 105 Vm−1 | Copper | 0.713% w/w NaCl | Fallah-Joshaqani et al., 2019 (ref. 55) |
| External DC | Microbatch | HEWL, thaumatin | ∼2.7 × 105 Vm−1 | Pt ITO graphite | N/A | Al-Haq et al., 2007 (ref. 7 and 49) |
| External DC | Microfluidic device | HEWL | Maximum 103 Vm−1 | Graphene | 3.85 mg mL−1 | Sui et al., 2018 (ref. 41) |
| Internal DC | Electrophoretic diffusion | Estradiol 17β-dehydrogenase | 1500–4000 Vm−1 | N/A | 0.02–6.9 mg mL−1 | Chin et al. 1976 (ref. 39) |
| Internal DC | Gel | HEWL, Thaumatin | N/A (applied current 0.9–1 μA) | Pt wire Graphite | 100; 100 mg mL−1 | Mirkin et al., 2003 (ref. 43) |
| Internal DC | Gel | HEWL | N/A (applied current 2.0 μA) | Pt | 10 mg mL−1 | Sazaki et al., 2004 (ref. 29) |
| Internal DC | Gel | HEWL | 0.19 Vm−1 | Pt | N/A | Moreno and Sazaki, 2004 (ref. 30) |
| Internal DC | Gel | HEWL | N/A (applied current 2.0 μA) | Pt | 30 mg mL−1 | Nieto-Mendoza et al., 2005 (ref. 40) |
| Internal DC | Crystallization cell where at least one of the electrodes is a sharp tip | HEWL, BPTI | Maximum 108 Vm−1 | Tungsten wire | 25 (HEWL), 20 (BPTI) mg mL−1 | Hammadi et al., 2007 (ref. 35) |
| Internal DC | Cooling crystallization | INA | Maximum 5.6 × 105 Vm−1 | Cu | 17, 20, 25, 30 and 35 mg mL−1 | Radacsi, 2012 (ref. 59) |
| Internal DC | SDVD | HEWL, 2TEL–Lys | 0.2 Vm−1 | ITO | 60 (HEWL), 4.5 (2TEL–Lys) mg mL−1 | Flores-Hernández et al., 2013 (ref. 26) |
| Internal DC | Cooling crystallization | INA, CAF, PHE | Maximum 1.25 × 106 Vm−1 | Cu | 18 (INA), 13 (CAF), 29 (PHE) mg mL−1 | Li et al., 2016 (ref. 18) |
| Internal DC | Microfluidic device | HEWL | 400–600 Vm−1 | Graphene | 80 mg mL−1 | Sui et al., 2018 (ref. 41) |
| External AC | Batch | HEWL | 6.6 × 104 and 8.6 × 104 Vm−1 | Cu | 57 mg mL−1 | Koizumi, et al., 2009 (ref. 24) |
| External AC | Batch | HEWL | 8.0 × 104 Vm−1 | N/A | 57 mg mL−1 | Koizumi et al., 2010 (ref. 31) |
| External AC | Containerless batch | Porcine insulin | 9.0 × 104 Vm−1 | N/A | 10 mg mL−1 | Koizumi et al., 2012 (ref. 32) |
| External AC | Batch | HEWL | 4.0 × 104 Vm−1 | Cu | 57 mg mL−1 | Koizumi et al., 2013, 2015, 2016 (ref. 16, 33 and 51) |
| External AC | Batch | HEWL | 1.1 × 105 Vm−1 | ITO | 40 mg mL−1 | Koizumi et al., 2017 (ref. 25) |
| External AC | Microfluidic device with patterned electrodes | HEWL, insulin | Maximum 1.2 × 104 Vm−1 | ITO | 30 (HEWL); 30 (insulin) mg mL−1 | Li and Lakerveld, 2017 (ref. 53) |
| External AC | Microfluidic device flow. | HEWL | 5.0 × 104 Vm−1 | Ti/Au | 30 mg mL−1 | Li and Lakerveld, 2018 (ref. 54) |
| AC (one internal and one external electrode) | Membrane crystallization | Glycine | 5.6 × 103 Vm−1 in solution | Cu wire | 199.9–468.2 mg mL−1 | Di Profio et al., 2013 (ref. 12) |
| External (AC/DC) | Vapor diffusion | Glucose isomerase lysozyme | N/A (applied current 2.0–6.0 μA) | ITO | 30 mg mL−1 | Pareja-Rivera et al., 2017 (ref. 14) |
| Internal AC | Batch | Thaumatin | 1.06 × 103 Vm−1 | ITO | 90 mg mL−1 | Wakamatsu, 2016 (ref. 45) |
Discussion and challenges
Several factors have been deduced to affect crystallization processes induced by application of an electric field. One such factor is the respective strength of the field, and therefore the efficiency in the ability to control the crystal quantity. It appears that, unsurprisingly, external electric fields involve the application of greater field strengths up to 7.5 × 105 Vm−1 to obtain significant impacts on the kinetics of crystallization, whereas internal fields can generally obtain similar effects at much lower intensities. However, it seems that there is an optimal field strength for these positive effects, and increasing the electric field above a certain limit might decrease or diminish the benefits seen during nucleation.55 The groups of Nanev and Aubry13,19,37 solely used external electric fields of magnitude between 1.5–7.5 × 105 Vm−1 in their investigations, whereas Moreno and Sazaki30 stated that substantial effects could be reached when applying a weak electric field (in their methodology, an electric field of 0.19 Vm−1 was used). In addition, Rubin, Owen and Stojanoff17 suggested that the large variance in the magnitude of the external electric field strength stated necessary for lysozyme crystallization (of magnitude × 105 Vm−1) in comparison to glucose isomerase (100–1000 Vm−1) could be explained by the differences detected in the dipole moments, resultant molecular charges, and ratios between the quantity of positive and negative residues.17Moreover, Taleb et al.13,19 deduced that crystal quality was enhanced for those grown under the influence of an electric field. In opposition to this, Nanev's group36 reported that in some cases, a rough crystal morphology was evident. The differences are, however, recognizable. Taleb et al. used higher temperatures in their studies combined with a higher protein concentrated solution.19 The authors stated that this rough crystal morphology could be a consequence of strain that occurs due to the nucleation temperature being low.36 Potential reasoning for the aforementioned deductions can be accredited to the fact that, in the solution (pH 4.5), HEWL molecules hold a positive charge under conditions of nucleation.36 From this, one can deduce that reducing the temperature disrupts the orientation of the crystals in solution. Nanev suggested in a recent article that the relation between the electric field effects and the supersaturation ratio should be studied to understand this phenomena.10
Frontana-Uribe and Moreno noted the significant difference between electrochemically-assisted crystallization and true electrochemical reaction.64 When the crystallization is assisted by the electric field, there are no redox reactions occurring between the forming crystals and the inert electrodes in the solution, which is similar to the electrophoresis process, and it causes molecules concentrating around the cathode or the anode.64 While in case of direct electro-crystallization, current flows through the electrolyte between the immersed electrodes in a solution containing small-molecule ions, and electromigration occurs (positively charged ions migrate towards the cathode and negatively charged ions travel towards the anode).64 When an ion reaches the electrode material, it loses its charge, and gas bubbles are released. However, these bubbles are not noticeable, and they dissolve in the solution when the current is low.64
It is also crucial to investigate if the effects of the electric field are the same for different types of molecules. However, it is hard to compare the effects, as different molecules can have substantially different solvent systems, with different properties. Al-Haq et al., 2007 (ref. 7) did not notice any major differences in the nucleation behaviour between HEWL and thaumatin crystallized in the presence of electric fields, except for the location of the crystal growth. While HEWL grew around the cathode, thaumatin crystallized on the surface of the anode.7 They explained the difference with the presence of the electric double layer that is formed around an electrode dipped in a solution.7 Li et al.18 and Radacsi59 also found different crystallization locations when crystallizing different small molecules under the influence of an electric field. While some chemicals crystallized on the anode, other ones crystallized on the cathode, making it possible to use electric fields for separation of molecules.18
Fallah-Joshaqani et al.55 found opposing nucleation behavior when crystallizing water and NaCl solution in the presence of the electric field. While the nucleation time was significantly decreased for water at 9.6 × 105 Vm−1, the nucleation time increased for the NaCl solution.55
From the classical nucleation theory by Kashiev,22 it was claimed that if εc = εm, the electric field does not influence the nucleation. However, to the best of our knowledge, this theory had not been experimentally proven.20 For the instance of the INA-1,4-dioxane system, as utilized in Radacsi's research,59 the relative permittivities of INA and that of the medium are εc = 2.209 and εm = 2.25 respectively.60,65 Being nearly equal, the contribution of the electric field to the chemical potential difference and therefore on the nucleation work is estimated to be less than 10−7kT. Consequently, the electric field should have no effect on nucleation thermodynamics in the used solution system. In disagreement to this theory, Radacsi found that a strong electric field does affect the nucleation and crystallization of isonicotinamide in 1,4-dioxane.59 Therefore, the main challenge ahead is to understand how electric fields change the nucleation and crystallization kinetics. In order to control the crystalline product quality, it is essential to understand all the process parameters, influencing the crystallization process. The strength of the electric field, as well as its nature (external, internal, DC or AC), is affecting the crystalline product directly. Once the effects of electric fields on crystallization are controlled, the next issue is the scale-up. As the crystallization vessel is often metal, the applied high voltage might raise safety concerns. This could be justified by the low current in the system, meaning that the system is non-lethal, and proper design and material use (e.g. glass instead of metal) of the setup, along with safety signs and interlock system, could solve the safety concerns. For industrial applications, the increased costs could be also a concern. However, the power of a high voltage power supply is usually low due to the low current, meaning low operational costs. The increased yield and crystal quality should overcome the financial investments in an electric field-assisted crystallization system. Finally, the scale-up of such systems is still a challenge. If used in a batch system, the applied potential difference might be too high, leading to sparks and safety concerns. Therefore, the application of electric fields would be more realistic and safe in continuous-flow crystallization systems, as was demonstrated by Li and Lakerveld.54
Conclusions
This highlight has underlined the importance of electric fields in significantly enhancing the crystallization process. These fields have proven to be a valuable manipulation instrument for both the dynamics of crystallization and the crystal size distribution. This highlight has shown and confirmed by using an extensive range of conditions and methods, that utilizing electric fields is one successful method of enhancing crystallization through controlling the nucleation rate and location of nucleation.Crystallization under a DC field7–9 has primarily been employed for protein crystallization in both internal26,29,30,40,43 or external13,19,36,37,49 electrode arrangements. Effects resulting from the application of AC fields on crystallization have also been studied by Hou and Chang,15 Li and Lakerveld,53 and a series of publications by Koizumi et al.16,24,25,31,33,34,51 Each author contributed findings on an effect of the applied electric field on the crystallization process. It has been demonstrated that application of electric fields have the ability to control both an increase and a reduction in the rate of nucleation19,24,29,30,36,37,43 and crystal size,7,14,17,19,26,29,57 an increase in the final crystal quality was obtained,14–17,19,25,50 improved X-ray diffraction signal-to-noise ratio was observed,41,42 and control of crystal orientation14,30,36,37,43,57 and polymorphic form.2,11,12,59,60
The findings drawn from past studies show in general, positive effects on the behavior of crystal growth under application of an electric field. Table 3 summarizes the observed effects on crystallization induced by an electric field as outlined in this literature review.
| Observed effect by application of electric field | Research groups | Ref. number |
|---|---|---|
| Reduced nucleation time | Taleb et al., 1999; Nanev and Penkova, 2001, 2002; Mirkin et al., 2003; Moreno and Sazaki, 2004; Sazaki, Moreno and Nakajima, 2004; Koizumi et al., 2009; Radacsi, 2012; Flores-Hermandez et al., 2013; Adrjanowicz, Pauch and Richard, 2018; Walter et al., 2018; Sui et al., 2018 | 19, 24, 26, 30, 37, 41, 43, 50, 59, 60, 62, 66 |
| Increased nucleation rate | Koizumi et al., 2009, 2012; Jha et al., 2017 | 4, 24, 32 |
| Enhanced overall crystal quality | Taleb et al., 1999; Hou and Chang, 2008; Koizumi et al., 2013; Pareja-Rivera et al., 2017; Rubin, Owen and Stojanoff, 2017; Koizumi et al., 2017; Walter et al., 2018 | 14–17, 19, 25, 50 |
| Less crystals produced, and with an enlarged size | Taleb et al., 1999; Sazaki, Moreno and Nakajima, 2004; Al-Haq, Lebrasseur, Tsuchiya, et al., 2007; Flores-Hermandez et al., 2013; Pareja-Rivera et al., 2017; Owen and Stojanoff, 2017 | 7, 14, 17, 19, 26, 29 |
| Substantial effect of crystalline orientation was induced – Crystals exhibited a preferred orientation along c axis | Nanev and Penkova, 2001, 2002; Mirkin et al., 2003; Moreno and Sazaki, 2004; Pareja-Rivera et al., 2017 | 14, 30, 37, 43, 62 |
| Crystals grew better on cathode when the protein was positively charged | Taleb et al., 1999; Nanev and Penkova, 2001, 2002; Sazaki, Moreno and Nakajima, 2004; Pareja-Rivera et al., 2017 | 14, 19, 29, 37, 62 |
| Polymorphic outcome was successfully controlled | Aber et al., 2005; Radacsi, 2012; Di Profio et al., 2013; Parks et al., 2017; Adrjanowicz, Paluch and Richert, 2018 | 11, 12, 59, 60, 63 |
| Electric field had a greater impact on nucleation rate and induction time at lower temperatures | Nanev and Penkova, 2001, 2002; Nieto-Mendoza et al., 2005 | 37, 40, 62 |
| Using optimized AC frequency to improve the crystal quality for protein crystals | Koizumi et al., 2009, 2015 | 24, 33 |
| Increasing charge of the protein led to increasing electric field effect | Taleb et al., 2001 | 61 |
| Increased X-ray diffraction signal-to-noise ratio | Sui et al., 2018; Gil-Alvaradejo et al., 2011 | 41, 42 |
| The position of crystals depended on the polarity of the electric field | Nanev and Penkova, 2001, 2002 | 37, 62 |
| Decreased growth rate | Parks et al., 2017; Koizumi et al., 2017 | 11, 25 |
| Promotion or inhibiting of protein crystallization | Li and Lakerveld, 2017; Wang, Li and Lakerveld, 2018 | 53, 67 |
| Separation and recovery of two crystalline substances from their multicomponent solution | Li et al., 2016 | 18 |
| Increased crystallization yield | Hou and Chang, 2008; Pareja-Rivera et al., 2017; Walter et al., 2018; Li and Lakerveld, 2018 | 14, 15, 50, 54 |
| Transformation from amorphous to crystalline form | Ganguly et al., 2018 | 57 |
As a conclusion, there are several benefits brought by application of the applied electric field in controlling crystallization, which are: reduction of the nucleation time, control of the location of nucleation, control of the product crystal size, enhancement of overall crystal quality, increase of the crystalline product yield, control of the crystal orientation and control the polymorphism. In addition, electric fields can be also used for separation of multicomponent mixtures, polymer transformation into crystalline material and crystallization in continuous flows.
Outlook
Regardless of the progress, information regarding a thorough and uniform understanding of electric field promoted nucleation is yet to be ascertained. There remain questions to be unanswered regarding the range of crystallizing compounds and appropriate solvents that can be used under these conditions, as well as process engineering design and scale-up from laboratory work and miniature devices. The upcoming decade is likely to see this gap of knowledge converging, and thus promoting several opportunities in areas such as pharmaceutical science, food industry, chemical industry or life science. The knowledge obtained thus far can be tested on more challenging compounds that poorly diffract e.g. membrane proteins, or complex cellular proteins, like ribosomes, which are difficult to crystallize for X-ray analysis. Furthermore, the future could see an inclination in research concentrated on polymorphism in proteins. Recently, results have proved that the hybrid of particle manipulation, brought about by the presence of the electric field, and cooling crystallization can be used for separation and recovery of two crystalline substances from their multicomponent solution. In addition to this, supplementary research could be conducted on merging various crystal growth approaches as advanced methods of controlling crystallization, which would deepen our understanding regarding the effect of the electric field on the process of crystallization. With regards to crystallization in a continuous flow, as this is a novel approach, there is a lot of opportunity for optimization in this area. As an example: flow rate (shear), flow direction, distribution of the electric-field potential, and frequency may each be altered to fully study and comprehend the effects of crystallization under continuous flow. As the emphasis has been placed on model proteins and compounds, it is worthwhile investigating the varied reactions of different materials to electric fields. This would enable the researcher to see if there is a common effect with the model compounds, and therefore allow them to completely comprehend the nucleation and crystal development mechanisms in these circumstances. It is anticipated that numerous advances will arise to further control the properties of crystals, and thus obtain a set of great quality products in the near future.Author contributions
The manuscript was written through contributions of both authors. Both authors have given approval to the final version of the manuscript.Funding sources
No funding sources to be claimed.Abbreviations
| AC | Alternating current |
| CAF | Caffeine |
| DC | Direct current |
| HDVD | Hanging drop vapor diffusion |
| SDVD | Sitting drop vapor diffusion |
| INA | Isonicotinamide |
| ITO | Indium tin oxide |
| PHE | Phenazine |
| HEWL | Hen egg white lysozyme |
| BPTI | Bovine pancreatic trypsin inhibitor |
| PVDF | Poly(vinylidenefluoride) |
| SDVD | Sitting drop vapor diffusion |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Professor Ulrich Loening for his useful comments.References
- Z. Gao, S. Rohani, J. Gong and J. Wang, Engineering, 2017, 3, 343–353 CrossRef.
- J. E. Aber, S. Arnold, B. A. Garetz and A. S. Myerson, Phys. Rev. Lett., 2005, 94, 145503 CrossRef PubMed.
- G. Di Profio, M. T. Reijonen, R. Caliandro, A. Guagliardi, E. Curcio and E. Drioli, Phys. Chem. Chem. Phys., 2013, 15, 9271 RSC.
- P. K. Jha, M. Sadot, S. A. Vino, V. Jury, S. Curet-Ploquin, O. Rouaud, M. Havet and A. Le-Bail, Innovative Food Sci. Emerging Technol., 2017, 42, 204–2019 CrossRef CAS.
- S. Panda, The Internet of Things: Breakthroughs in Research and Practice, IGI Global, 2017 Search PubMed.
- A. S. Myerson and R. Ginde, in Industrial Crystallization of Melts, 2004, pp. 183–240 Search PubMed.
- M. I. Al-Haq, E. Lebrasseur, H. Tsuchiya and T. Torii, Crystallogr. Rev., 2007, 13, 29–64 CrossRef CAS.
- B. A. Frontana-Uribe and A. Moreno, Cryst. Growth Des., 2008, 8, 4194–4199 CrossRef CAS.
- Z. Hammadi and S. Veesler, Prog. Biophys. Mol. Biol., 2009, 101, 38–44 CrossRef CAS PubMed.
- C. Nanev, Crystals, 2017, 7, 310 CrossRef.
- C. Parks, A. Koswara, H. H. Tung, N. Nere, S. Bordawekar, Z. K. Nagy and D. Ramkrishna, Cryst. Growth Des., 2017, 17, 3751–3765 CrossRef CAS.
- G. Di Profio, M. T. Reijonen, R. Caliandro, A. Guagliardi, E. Curcio and E. Drioli, Phys. Chem. Chem. Phys., 2013, 15, 9271 RSC.
- M. Taleb, C. Didierjean, C. Jelsch, J. P. Mangeot and A. Aubry, J. Cryst. Growth, 2001, 232, 250–255 CrossRef CAS.
- C. Pareja-Rivera, M. Cuéllar-Cruz, N. Esturau-Escofet, N. Demitri, M. Polentarutti, V. Stojanoff and A. Moreno, Cryst. Growth Des., 2017, 17, 135–145 CrossRef CAS.
- D. Hou and H.-C. Chang, Appl. Phys. Lett., 2008, 92, 223902 CrossRef.
- H. Koizumi, S. Uda, K. Fujiwara, M. Tachibana, K. Kojima and J. Nozawa, J. Appl. Crystallogr., 2013, 46, 25–29 CrossRef CAS.
- E. Rubin, C. Owen and V. Stojanoff, Crystals, 2017, 7, 206 CrossRef.
- W. W. Li, N. Radacsi, H. J. M. Kramer, A. E. D. M. van der Heijden and J. H. ter Horst, Angew. Chem., Int. Ed., 2016, 55, 16088–16091 CrossRef CAS PubMed.
- M. Taleb, C. Didierjean, C. Jelsch, J. P. Mangeot, B. Capelle and A. Aubry, J. Cryst. Growth, 1999, 200, 575–582 CrossRef CAS.
- D. Kashchiev, Nucleation: Basic Theory with Applications, 2000 Search PubMed.
- J. O. Isard, Philos. Mag., 1977, 35, 817–819 CrossRef CAS.
- D. Kashchiev, J. Cryst. Growth, 1972, 13–14, 128–130 CrossRef CAS.
- E. A. Guggenheim, Thermodynamics: An Advanced Treatise for Chemists and Physicists, North-Holland Publishing Company, Vancouver, 1967 Search PubMed.
- H. Koizumi, K. Fujiwara and S. Uda, Cryst. Growth Des., 2009, 9, 2420–2424 CrossRef CAS.
- H. Koizumi, S. Uda, K. Fujiwara, J. Okada and J. Nozawa, Crystals, 2017, 7, 170 CrossRef.
- E. Flores-Hernández, V. Stojanoff, R. Arreguín-Espinosa, A. Moreno and N. Sánchez-Puig, J. Appl. Crystallogr., 2013, 46, 832–834 CrossRef PubMed.
- S. Martínez-Caballero, M. Cuéllar-Cruz, N. Demitri, M. Polentarutti, A. Rodríguez-Romero and A. Moreno, Cryst. Growth Des., 2016, 16, 1679–1686 CrossRef.
- A. Rodríguez-Romero, N. Esturau-Escofet, C. Pareja-Rivera and A. Moreno, Crystals, 2017, 7, 179 CrossRef.
- G. Sazaki, A. Moreno and K. Nakajima, J. Cryst. Growth, 2004, 262, 499–502 CrossRef CAS.
- A. Moreno and G. Sazaki, J. Cryst. Growth, 2004, 264, 438–444 CrossRef CAS.
- H. Koizumi, K. Fujiwara and S. Uda, Cryst. Growth Des., 2010, 10, 2591–2595 CrossRef CAS.
- H. Koizumi, Y. Tomita, S. Uda, K. Fujiwara and J. Nozawa, J. Cryst. Growth, 2012, 352, 155–157 CrossRef CAS.
- H. Koizumi, S. Uda, K. Fujiwara, M. Tachibana, K. Kojima and J. Nozawa, J. Appl. Crystallogr., 2015, 48, 1507–1513 CrossRef CAS.
- H. Koizumi, S. Uda, K. Fujiwara, M. Tachibana, K. Kojima and J. Nozawa, AIP Conf. Proc., 2014, 1618, 265–268 CrossRef CAS.
- Z. Hammadi, J. P. Astier, R. Morin and S. Veesler, Cryst. Growth Des., 2007, 7, 1472–1475 CrossRef CAS.
- C. N. Nanev and A. Penkova, J. Cryst. Growth, 2001, 232, 285–293 CrossRef CAS.
- C. N. Nanev and A. Penkova, Colloids Surf., A, 2002, 209, 139–145 CrossRef CAS.
- N. E. Chayen, P. D. Shaw Stewart and D. M. Blow, J. Cryst. Growth, 1992, 122, 176–180 CrossRef CAS.
- C. C. Chin, J. B. Dence and J. C. Warren, J. Biol. Chem., 1976, 251, 3700–3705 CAS.
- E. Nieto-Mendoza, B. A. Frontana-Uribe, G. Sazaki and A. Moreno, J. Cryst. Growth, 2005, 275, 1437–1446 CrossRef.
- S. Sui, Y. Wang, C. Dimitrakopoulos and S. Perry, Crystals, 2018, 8, 76 CrossRef.
- G. Gil-Alvaradejo, R. R. Ruiz-Arellano, C. Owen, A. Rodríguez-Romero, E. Rudiño-Piñera, M. K. Antwi, V. Stojanoff and A. Moreno, Cryst. Growth Des., 2011, 11, 3917–3922 CrossRef CAS.
- N. Mirkin, B. A. Frontana-Uribe, A. Rodríguez-Romero, A. Hernández-Santoyo and A. Moreno, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2003, 59, 1533–1538 CrossRef CAS PubMed.
- J. M. García-Ruiz and A. Moreno, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1994, 50, 484–490 CrossRef.
- T. Wakamatsu, Trans. Mater. Res. Soc. Jpn., 2016, 41, 13–15 CrossRef CAS.
- T. Wakamatsu and Y. Ohnishi, Jpn. J. Appl. Phys., 2011, 50, 048003 CrossRef.
- T. Wakamatsu, S. Toyoshima and H. Shimizu, Appl. Phys. Lett., 2011, 99, 153701 CrossRef.
- T. Wakamatsu, Rev. Sci. Instrum., 2015, 86, 015112 CrossRef PubMed.
- M. I. Al-Haq, E. Lebrasseur, W. K. Choi, H. Tsuchiya, T. Torii, H. Yamazaki and E. Shinohara, J. Appl. Crystallogr., 2007, 40, 199–201 CrossRef CAS.
- T. K. Walter, C. F. da G. Ferreira, J. Iulek and E. M. Benelli, ACS Omega, 2018, 3, 8683–8690 CrossRef CAS.
- H. Koizumi, S. Uda, K. Fujiwara, M. Tachibana, K. Kojima, J. Nozawa, H. Koizumi, S. Uda, K. Fujiwara, M. Tachibana, K. Kojima and J. Nozawa, Crystals, 2016, 6, 95 CrossRef.
- A. Rodríguez-Romero, N. Esturau-Escofet, C. Pareja-Rivera and A. Moreno, Crystals, 2017, 7, 179 CrossRef.
- F. Li and R. Lakerveld, Cryst. Growth Des., 2017, 17, 3062–3070 CrossRef CAS.
- F. Li and R. Lakerveld, Cryst. Growth Des., 2018, 18, 2964–2971 CrossRef CAS.
- S. Fallah-Joshaqani, N. Hamdami, E. Keshavarzi, J. Keramat and M. Dalvi-Isfahan, Int. J. Refrig., 2019, 99, 30–36 CrossRef CAS.
- Z. Zhang, C. Yao, Y. Yu, Z. Hong, M. Zhi and X. Wang, Adv. Funct. Mater., 2016, 26, 6760–6765 CrossRef CAS PubMed.
- R. Ganguly, S. Bandyopadhyay, K. Miriyala, V. Gunasekaran, S. Bhattacharya, A. Acharyya and R. Ramadurai, Polym. Cryst., 2018, e10027 Search PubMed.
- J. Qi, J. Chang, X. Han, R. Zhong, M. Jiang, Z. Chen and B. Liu, Mater. Chem. Phys., 2018, 211, 168–171 CrossRef CAS.
- N. Radacsi, Process intensification in crystallization: Submicron particle generation using alternative energy forms, IPSKAMP DRUKKERS, 2012 Search PubMed.
- K. Adrjanowicz, M. Paluch and R. Richert, Phys. Chem. Chem. Phys., 2018, 20, 925–931 RSC.
- M. Taleb, C. Didierjean, C. Jelsch, J. Mangeot and A. Aubry, J. Cryst. Growth, 2001, 232, 250–255 CrossRef CAS.
- C. N. Nanev and A. Penkova, J. Cryst. Growth, 2001, 232, 285–293 CrossRef CAS.
- J. Aber, S. Arnold, B. Garetz and A. Myerson, Phys. Rev. Lett., 2005, 94, 1–4 CrossRef PubMed.
- B. A. Frontana-Uribe and A. Moreno, Cryst. Growth Des., 2008, 8, 4194–4199 CrossRef CAS.
- J. A. Riddick, W. B. Bunger and T. K. Sakano, Organic solvents: physical properties and methods of purification, John Wiley and Sons, New York, NY, 1986, vol. 2 Search PubMed.
- G. Sazaki, A. Moreno and K. Nakajima, J. Cryst. Growth, 2004, 262, 499–502 CrossRef CAS.
- J. Wang, F. Li and R. Lakerveld, Chem. Eng. Process., 2018, 127, 111–126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ce00755e |
| This journal is © The Royal Society of Chemistry 2019 |