Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of zinc on glycosaminoglycan neutralisation during coagulation
Amélie I. S.
Sobczak
 ,
Samantha J.
Pitt
,
Samantha J.
Pitt
 and
Alan J.
Stewart
and
Alan J.
Stewart
 *
*
School of Medicine, University of St Andrews, Medical and Biological Sciences Building, St Andrews, Fife, UK. E-mail: ajs21@st-andrews.ac.uk; Fax: +44 (0)1334 463482; Tel: +44 (0)1334 463546
First published on 22nd August 2018
Abstract
Heparan sulfate (HS), dermatan sulfate (DS) and heparin are glycosaminoglycans (GAGs) that serve as key natural and pharmacological anticoagulants. During normal clotting such agents require to be inactivated or neutralised. Several proteins have been reported to facilitate their neutralisation, which reside in platelet α-granules and are released following platelet activation. These include histidine-rich-glycoprotein (HRG), fibrinogen and high-molecular-weight kininogen (HMWK). Zinc ions (Zn2+) are also present in α-granules at a high concentration and participate in the propagation of coagulation by influencing the binding of neutralising proteins to GAGs. Zn2+ in many cases increases the affinity of these proteins to GAGs, and is thus an important regulator of GAG neutralisation and haemostasis. Binding of Zn2+ to HRG, HMWK and fibrinogen is mediated predominantly through coordination to histidine residues but the mechanisms by which Zn2+ increases the affinity of the proteins for GAGs are not yet completely clear. Here we will review current knowledge of how Zn2+ binds to and influences the neutralisation of GAGs and describe the importance of this process in both normal and pathogenic clotting.
Introduction
Glycosaminoglycans (GAGs) including heparin sulfate (HS), dermatan sulfate (DS) and heparins are key molecules involved in several biological processes, including coagulation where they play an important anticoagulant role.1 HS is mostly synthesised by endothelial cells, where it lines the endothelium and participates in its intrinsic anticoagulant properties.2 DS is mostly synthesised in the sub-endothelium and is exposed to plasma proteins during injury.3 Heparins are synthesised by mast cells and may also be secreted following tissue injury,4,5 however, this has been disputed by some.6–8 The main relevance of heparins are in clinical settings, where they and heparin-based drugs are important agents used in the clinical treatment of coagulatory disorders.9 As GAGs are physiologically found in a variety of sizes, heparin drugs are administered clinically as unfractionated heparin (UFH), which have not been cleaved or separated by size or as low molecular weight heparin (LMWH), which are generally <8000 Da.10 All GAGs exercise their anticoagulant activity through their binding to serpins.1 The main partners for HS and heparin are antithrombin (AT) and heparin cofactor II (HCII) while DS can only bind to HCII.1 When bound together, the GAGs can change the conformation of the reactive centre loop of the serpin to increase the inhibitory activity of the molecule.11 During normal coagulation, when clotting is required, anticoagulant GAGs are neutralised by several proteins, including histidine-rich-glycoproteins (HRG), high-molecular-weight kininogen (HMWK) and fibrinogen.10,12After iron, zinc is the most abundant transition metal in the human body. Zinc is an important element in the body, playing key structural and catalytic roles as well as functioning as an extracellular and intracellular signalling molecule. Ionic zinc (Zn2+) is essential for physiological processes such as cell replication, tissue growth, immune functioning and coagulation.13–15 The importance of Zn2+ is best illustrated by the cases of zinc deficiency, which is defined as having a total plasma zinc concentration below 0.7 mg L−1 (normal range is 0.8–1.0 mg L−1).16–18 Zinc deficiency is associated with coagulatory abnormalities including a reduced ability for platelets to aggregate and longer bleeding times, which in most cases can be quickly corrected by zinc supplementation without secondary effects.16–18 In addition to the resting plasma Zn2+ level, during coagulation platelets release Zn2+ stored in their α-granules, thus initiating a signalling process.19–21 During this process Zn2+ acts to propagate several anticoagulation pathways as well as both pro- and anti-fibrinolytic pathways.13 In addition to Zn2+, platelet α-granules also release numerous proteins that impact on coagulation, among which are HRG, HMWK and fibrinogen.10,22 These proteins have both the ability to bind Zn2+ and to bind and neutralise anticoagulant GAGs.23–30 The mechanisms and impact by which Zn2+ influences GAG binding and neutralisation by these proteins is reviewed here.
Zn2+ repartition in plasma
Despite its requirement for various physiological processes, Zn2+ is toxic at mid-high micromolar levels,31,32 therefore its free/labile concentration is tightly regulated. The total concentration of Zn2+ in the plasma is approximately 20 μM.33 Those ions are mostly bound to serum albumin (75% of the total Zn2+ concentration in the body, i.e. around 15 μM).33 The remaining Zn2+ (around 5–6 μM) is bound to other proteins such as α2-macroglobulin.15,34 This fraction is regarded as non-exchangeable as the binding is very tight.15,34 The remaining Zn2+ is bound to small ligands and is considered “free” or “labile” because those ligands can easily be exchanged for proteins or other ligands (more easily than when Zn2+ is bound to serum albumin).15,34 The free Zn2+ concentration in plasma is generally thought to be in the micromolar range, between 0.5 to 1 μM.15,34The proportion of free/labile Zn2+ in plasma is dynamic (Fig. 1). For example, during coagulation, Zn2+ is released from platelets.35,36 Healthy platelets accumulate around 35 g L−1 of Zn2+ that they sequester into two pools, the cytoplasm (around 60% that is used to regulate platelet function)21,37 and the α-granules (around 40%).21 Variations of the total amount of Zn2+ present in the plasma affect the quantity present in the platelets, as well as the distribution of the two pools.37 There is still some uncertainty as to how Zn2+ is incorporated into the platelets. Some Zn2+ may be incorporated when the Zn2+-bound fibrinogen-coagulation factor XIII(a2) complex enters the platelet through binding to fibrinogen receptors.37 However the main mechanism for Zn2+ entry into platelets is likely to be through Zn2+ transporters (as reviewed by Taylor and Pugh);38 the exact mechanism however remains to be elucidated. When platelets are activated, up to half of the α-granule Zn2+ pool is released.35,36 This action has been reported to increase the labile/free plasma Zn2+ concentration to 7–10 μM in the proximities of activated platelets.35,36 The resultant increase in Zn2+ concentration can then facilitate its binding to coagulatory proteins and in-turn alter their affinity for other proteins or ligands to influence the coagulation process.19,20 Platelets are not the only cells in the blood that store Zn2+. Indeed, neutrophils, lymphocytes and erythrocytes all contain Zn2+ (reported levels of total zinc are 105 μg/1 × 1010 cells, 116 μg/1 × 1010 cells and 41 μg g−1 haemoglobin, respectively)39 and may therefore release Zn2+ under certain circumstances in a manner similar to platelets (such as at sites of injury, although this has yet to be confirmed). In addition, the epithelium contains around 60 μg Zn2+/g of dry weight,40 and epithelial cells release some of this when damaged (the exact amount is not known).41
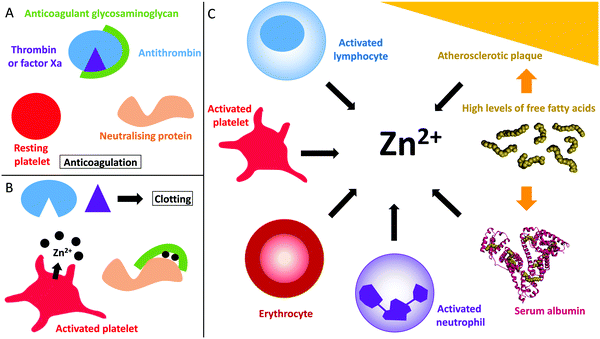 | ||
| Fig. 1 Coagulation control by glycosaminoglycans and Zn2+. (A) Anticoagulant glycosaminoglycans bind to antithrombin and enhance its neutralisation of thrombin (and/or factor Xa). (B) When platelets become activated, the Zn2+ released from the α-granules of platelets bind to the GAG neutralising proteins, increasing their affinity for GAGs and allowing them to neutralise them. Once neutralised, the GAG cannot promote the inhibition of thrombin and clotting occurs. (C) Sources of Zn2+ in plasma. During coagulation, Zn2+ is released by activated platelets. However, erythrocytes, lymphocytes and neutrophils contain Zn2+ which may be released under certain conditions. In some disease states, elevated levels of free fatty acids may also influence available Zn2+ levels through release from serum albumin. Atherosclerotic plaques contain up to six time more Zn2+ than healthy tissue and could potentially release Zn2+ when they rupture. The structure of human serum albumin (with stearate bound) was taken from PDB 1E7I.109 | ||
Relevance of Zn2+ in coagulation control
For a long time, the role of Zn2+ in coagulation had been ignored and was likely often masked by the use of citrate as an anticoagulant during blood collection (with citrate forming complexes with metallic cations). However, in more recent years, the importance of Zn2+ in coagulation and regulation of platelet function has started to emerge.13,38,42 A variety of blood proteins involved in coagulatory processes have been identified as Zn2+-binding proteins. In many cases the ability to bind Zn2+ has the potential to influence their activities and impact upon haemostasis. When looking at specific interactions, Zn2+ has been marked as an initiator of the contact activation pathway of coagulation through enhancing the interactions of contact proteins with polyanionic surfaces and their assembly on endothelial cells and platelets.13 Zn2+ enhances platelet aggregation and activation by increasing internal platelet signalling and external binding of platelets to their ligands.13 It also enhances fibrin formation while also attenuating thrombin activity.13 Simultaneously, Zn2+ is also a regulator of the anticoagulant and fibrinolytic pathways. It can inhibit platelet activation by increasing HMWK and factor XII competition with thrombin to bind GPIb on platelet surface. Zn2+ also attenuates FXa generation by FVIIa, both increases and reduces fibrinolysis and modulates the activities of protein C, protein S and heparin-mediated anticoagulant pathways and the pro- and anti-coagulation activities of HRG.13Many of the studies examining the impact of Zn2+ on coagulation have utilised purified protein systems where zinc-buffering or binding molecules normally found in plasma are absent. Therefore, it is not clear in some cases whether the labile Zn2+ concentrations used are physiologically (or pathophysiologically) attainable. With the involvement of Zn2+ in so many aspects of the clot process, it is difficult to tease out in which Zn2+ may be most involved. Dietary Zn2+ has been shown to exert a pronounced effect on platelet aggregation in humans and rats.16–18 Several studies have also investigated correlations between Zn2+ concentrations and clot formation and lysis. Generally, Zn2+ enhances clotting but reduces lysis – specific effects include an increase in fibrin diameter and clot porosity and reductions in clot stiffness.36,43 Yet, those studies were realised after dialysing the plasma and adding back Zn2+, a process which may have altered the concentration of certain (likely smaller) molecules that influence clotting.
Zn2+ binding by anticoagulant GAGs
HS, DS and heparin are highly negatively-charged; thus their binding to other proteins mainly occurs through electrostatic interactions.44 This type of interaction will increase with the degree of sulfation of the GAGs. HS is generally less sulfated than heparin but more so than DS. Metal ions are important binding partners of GAGs and, in plasma, both Ca2+ and Zn2+ have been shown to coordinate to them.21 Seo, Schenauer and Leary revealed that the binding of metal ions to a heparin octasaccharide, including Ca2+, Mn2+, Co2+, Fe2+ and Ni2+, triggers conformational changes that have the potential to affect their interactions with their ligands.45 The effect of Zn2+ was not examined in their study but is likely to mimic the effects of these other metal ions. As both Ca2+ and Zn2+ are released from platelets during coagulation and participate in the regulation of coagulation, this is of particular interest as it is likely that this mechanism alters the anti-coagulant activities of heparin and HS following platelet activation.21 UFH has been shown to bind Zn2+via two different mechanisms: the first represents a high-affinity form of binding (the equilibrium constant is 976 M−1) whilst the second is a low-affinity form of binding that only occurs at high Zn2+ concentrations (the equilibrium constant is 241 M−1).46 Both binding events are entropy driven and both involve sulfated side chains on the GAGs. The exact stoichiometry of these binding events has not yet been precisely defined but it is assumed that the first mode of binding involves one zinc ion binding per disaccharide and that the second mode intervenes only after saturation is reached for the first one.46 GAGs interact with basic amino acids on proteins, generally lysine and arginine side-chains that are not normally affected by the presence of cations.47 Those cations however often bind to exposed histidines, which are positively charged, and this binding may then facilitate the binding of the protein to GAGs by reducing the electrostatic repulsion between the two of them.47 As Zn2+ is released at the beginning of the coagulation process, its effect on GAG neutralisation reduces anticoagulation and promotes clotting.Impact of Zn2+ on protein–GAG interactions
Numerous proteins in plasma have the ability to neutralise anticoagulant GAGs, as reviewed previously.10 Among them, three are known to bind Zn2+: HRG,28 HMWK29 and fibrinogen.27 However, the Zn2+ binding properties of all GAG-neutralising proteins have not been examined and there are probably more that possess this ability. This ability to bind Zn2+ is important as Zn2+ has the potential to influence GAG binding and neutralisation by those proteins (Fig. 1). HRG, HMWK and fibrinogen are stored in platelet α-granules alongside Zn2+ and are therefore released together during coagulation. All three proteins are synthesised in the liver and, in addition to being stored in platelets, they are present at high nanomolar to low micromolar concentrations in plasma (1.3–2.0 μM for HRG,48,49 1–2 μM for HMWK50 and 12–24 μM for fibrinogen).51 Their specific roles in coagulation are diverse. Fibrinogen plays a prominent role as when it is cleaved, it polymerises to form fibrin clots.52 HRG plays a regulatory role by inhibiting fibrinolysis in addition to being incorporated in blood clots,53 while HMWK is a key activator of the contact activation pathway of the coagulation cascade.54 Thus all three proteins are in contact with endothelial GAGs and are likely to be important for GAGs neutralisation during coagulation. This section will examine the Zn2+ and GAG binding properties of these three proteins and how Zn2+ can influence GAG neutralisation.Histidine-rich glycoprotein (HRG)
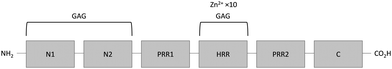
HRG is a single chain protein composed of several structural domains that include a histidine-rich region (HRR). This region is important in both proteins, as neutrally-charged histidine residues bind Zn2+via their imidazole side chains.28,48 HRG also contains two cystatin-like domains at its N-terminus (N1 and N2) and possesses a C-terminal domain, whilst its histidine-rich region is flanked by two proline-rich regions (Fig. 2).55 The structure of HRG has not yet been fully resolved. A crystal structure of the N2 domain has been reported (PDB: 4CCV),56 but structural information relating to the other domains (including the Zn2+-binding HRR) is lacking. Nevertheless, Human HRG has been demonstrated to bind up to 10 Zn2+ ions with an average Kd of 6.13 μM.48,57 Current evidences suggest that there are no clearly defined preferential binding sites for Zn2+ on HRG.58 When the net charge of HRG becomes positive, either through a change in protonation of the histidine residues (through a change in pH) or through binding of those residues to metal cations, the conformation of the molecule changes, influencing its affinity for binding its ligands.19,48,58–62HRG binds heparin with a Kd of 32.9 nM in the absence of Zn2+ and 5.1 nM in the presence of 1 μM Zn2+.57 Isothermal titration calorimetry studies have shown that there are two different modes of heparin binding, which are thought to occur at different binding sites.57 The first mode is Zn2+-dependent and thus most likely involves binding at the HRR.57,63 As Zn2+ only influences the binding of long chain heparins,57 this first binding site only involves long chain heparins (≥10 kDa).57,64 The second mode of binding is not dependent on chain length and is thought to occur at the N1 and N2 domains,20 although the exact location of this site is still unknown. The affinity of the second mode of binding is not directly affected by the presence of Zn2+, but Zn2+ binding to the HRR may induce conformation changes in HRG that would make this site more accessible to heparins. HRG forms mainly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with heparin, but it can form 2
1 complexes with heparin, but it can form 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with longer chain heparins in the presence of Zn2+.64 HRG has been shown to neutralise heparin in plasma with this ability (like binding) also dependent on the size of the heparin; with longer-chain heparins having a higher affinity for HRG.65 For example, even excess ratio of 500
1 complexes with longer chain heparins in the presence of Zn2+.64 HRG has been shown to neutralise heparin in plasma with this ability (like binding) also dependent on the size of the heparin; with longer-chain heparins having a higher affinity for HRG.65 For example, even excess ratio of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HRG
1 HRG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) heparin octasaccharide can neutralise less than half of the ability of heparin to accelerate the inactivation of factor Xa by AT.65 Zn2+-Dependent heparin binding by HRG only occurs when Zn2+ is released from activated platelets; otherwise, the metal concentration is too low and heparin preferentially binds the AT-thrombin complex.66 HRG can neutralise heparin-mediated thrombin inhibition by both AT and HCII, but it is much weaker in neutralising DS-mediated thrombin inhibition by HCII.67–69 HRG can also bind and neutralise HS in a Zn2+-dependent-manner.70 Thus, HRG is an important anticoagulant GAG neutraliser in plasma and this neutralisation is dependent upon the plasma Zn2+ concentration.
heparin octasaccharide can neutralise less than half of the ability of heparin to accelerate the inactivation of factor Xa by AT.65 Zn2+-Dependent heparin binding by HRG only occurs when Zn2+ is released from activated platelets; otherwise, the metal concentration is too low and heparin preferentially binds the AT-thrombin complex.66 HRG can neutralise heparin-mediated thrombin inhibition by both AT and HCII, but it is much weaker in neutralising DS-mediated thrombin inhibition by HCII.67–69 HRG can also bind and neutralise HS in a Zn2+-dependent-manner.70 Thus, HRG is an important anticoagulant GAG neutraliser in plasma and this neutralisation is dependent upon the plasma Zn2+ concentration.
High-molecular weight Kininogen (HMWK)
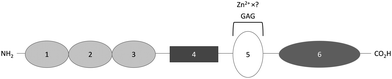
HMWK is a single chain protein that consists of 6 domains, one of which, domain 5, contains a HRR (Fig. 3).54 Kallikrein cleaves domain 4 of HMWK, releasing bradykinin and another peptide, while the rest of the protein forms a two chain HMWK, with the heavy chain being the N-terminal section composed of domain 1, 2 and 3 and the light chain being domain 5 and 6. Both chains are then linked together by a single disulfide bond.54 Like HRG, HMWK has not yet been fully crystallised and so the Zn2+ binding domain is also not fully characterised. It is known that Zn2+ binds mainly to histidine residues of the HRR located between the residues Gly440 and Lys458 of domain 5. Zn2+ binding is known to induce a conformational change in this domain.71 The affinity and stoichiometry of Zn2+ binding by HMWK have not yet been determined despite its potential to influence the binding of HMWK to its ligands.Like HRG, HMWK binds heparin in a Zn2+-dependent manner.25 The intact form of HMWK binds heparin with higher affinity than the cleaved form of the protein (the Kd values of intact and cleaved forms are 2.1 nM and 14.2 nM, respectively).29 The presence of 50 μM Zn2+ increases the binding affinity even further (Kd = 0.30 nM for intact HMWK).29 Heparin binds to the light chain portion of HMWK, at the HRR, in domain 5, which is known to mediate HMWK-binding to negatively-charged surfaces.25,29 Within this domain the binding of GAGs occurs at a combination of different sites, some of which are sensitive to Zn2+.29,47 In addition to histidines, this region is rich in lysine residues, which are involved in heparin binding.29,47 The heparin binding affinity of HMWK increases when the pH decreases and the histidine residues become protonated, regardless of the presence of cations.25 UFH and LMWH bind with similar affinity to HMWK in the absence of Zn2+ but the influence of the heparin chain length on the binding affinity in the presence of Zn2+ is not yet known.25 HMWK competes with AT, thrombin or the AT-thrombin complex for heparin-binding and can neutralise the anticoagulant effect of heparin in plasma.25 However, this binding may not be specific, as HMWK can bind all heparins regardless of whether or not they possess the saccharide sequence used to bind AT with high affinity, and thus several HMWK molecules may be required to fully neutralise one heparin molecule.25 Maximal neutralisation has been shown to occur in the presence of 10 μM Zn2+.25 In addition, HS proteoglycans located at the cell surface can bind HMWK in a Zn2+-dependent manner but the effect of this binding on the anticoagulant activity of HS has not been investigated directly.50 Thus HMWK appears to neutralise anticoagulant GAG in a similar manner to HRG. As HMWK is present in plasma at similar levels to HRG and binds heparin with similar affinity (Kd in low nanomolar range), these proteins may be of equal importance in anticoagulant GAG neutralisation.
Fibrinogen
Fibrinogen is a homodimer composed of two sets of three different polypeptides chains, Aα, Bβ and γ (Fig. 4).72 Most of the protein has been crystallised to some extent, with the exception of the highly variable αC domain (PDB 3GHG).73 Fibrinogen binds Zn2+ at two different regions. The first set of binding sites is located in the D-domains (Fig. 4, insert 1) and has a stoichiometry of six (three ions per D-domain).74 The sites predominantly consist of histidine residues located on the γ chain, with His-γ217 and His-γ234 thought to be involved,74 however the effect of Zn2+ binding at this region on the conformation of the protein is unknown. Another Zn2+-binding region has been identified in the αC-domain and also involves histidine residues (His-α544 and His-α545).75 Binding of Zn2+ to this region is thought to induce a change in the conformation of the protein.75 Based on several studies, Zn2+ binding to fibrinogen has an average Kd of ∼1–18 μM, but the individual contributions of the two groups of sites are unknown.27,36,75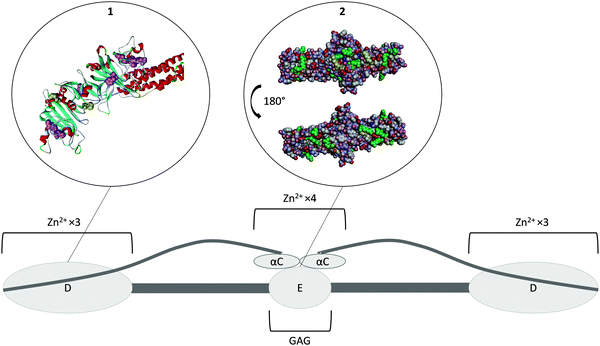 | ||
| Fig. 4 Structure of fibrinogen. The protein forms hexamer made of three different strands (Aα Bβ γ)2. All of the N-terminals are in the E domain which is the heparin binding domain. The three strands then coil together until they reach the D domains where the C-terminal of the β and γ strands are located. This domain is a Zn2+-binding domain. The α strand goes back toward the E domain where its C-terminal forms the αC domain, another Zn2+ binding domain. Insert 1. Crystal structure of fibrinogen D domain (one of the Zn2+ binding domain) and part of the coil–coil region (PDB structure 3GHG).73 The histidine residues which have the potential to be involved in Zn2+ binding are represented in pink with the residues His217 and His234 known to be involved represented in yellow. Most of those residues are hidden beneath the surface of the protein. Insert 2. Crystal structure of fibrinogen E domain (the heparin binding domain) and part of the coil–coil regions, (PDB structure 3GHG).73 The positive charges are represented in blue and the negative charges in red. The Lys and Arg residues which usually constitutes the main binding partners of GAGs are represented in green. The first few residues of the Bβ chain are mobile and so they are not visible in the crystal structure, with β58 the first residue that can be observed (represented in yellow). This residue is a protruding Lys that is believed to be important for GAG binding. As the GAG binding affinity of fibrinogen is enhanced when the protein is converted into fibrin by cleavage of the A and B peptides, the absence in the crystal structure of the first few residues of the Bβ chain may be the reason for exposure of the β58 residue. The αC domain is attached to the E domain; binding of Zn2+ ions to its His α544 and His α545 is thought to change the conformation of the protein and thus facilitate GAG binding to the E domain. | ||
Two different heparin binding modes have been identified on fibrinogen. The first occurs at a site located on the β chain in the E domain around the Bβ1-57 region (Fig. 4, insert 2). It has been shown that a synthetic peptide corresponding to this exact region binds heparin with a Kd of 16.5 μM,76 whilst a dimer of the peptide, (Bβ1-66)2 exhibits an almost two orders of magnitude higher affinity for heparin (Kd of 210 nM), compared to the monomer.76 As this Kd value is close to the Kd of intact fibrinogen (228 nM), this suggests that the binding of heparin to fibrinogen occurs predominantly via this binding mode and that the dimeric structure of fibrinogen plays an essential role in this binding.76 A second heparin-binding mode occurs when Zn2+ binds to the αC domains of fibrinogen.75 Binding of Zn2+ is thought to induce a change in the conformation of the protein promoting heparin-binding to the nearby E domain.75 In presence of 12.5 μM Zn2+, the average Kd for heparin binding to fibrinogen is 60 nM.75 This represents a 4-fold increase in affinity, contrasting to a lower affinity (Kd of 539 nM) for fibrinogen without the αC domain in the presence of Zn2+.75 The link between heparin chain length and binding affinity in the presence and absence of Zn2+ is not yet known. The binding of fibrinogen to heparin participates in the neutralisation of its anticoagulant activity. The direct study of fibrinogen-mediated GAG neutralisation has been complicated by the fact that thrombin cleaves fibrinogen. However, fibrinogen has been shown to be more effective at neutralising DS than HRG (and platelet factor 4).68 This neutralisation occurs at physiological fibrinogen concentrations and is not affected by the size or the degree of sulfation of DS.68 The mechanism of neutralisation appears not to occur through direct competition with the thrombin–HCII complex for DS binding but by controlling the rate of formation of this complex.68 In addition, fibrin can also form complexes with heparin, AT and thrombin to reduce thrombin inhibition by AT in a Zn2+-dependent manner.52,77 Thus, fibrinogen is an important anticoagulant GAG neutraliser, with the plasma concentration of fibrinogen being linked to heparin resistance in patients.78 The sensitivity of fibrinogen toward plasma Zn2+ levels relative to HRG and HMWK is not yet known.
Other anticoagulant GAG neutralising proteins
Zn2+ can also potentially affect the neutralisation of anticoagulant GAGs through, fibronectin, fibroblast growth factors (FGF), FGF-1 and FGF-7 and activated coagulation factor VII (FVIIa).30,79,80 Fibronectin can be found as the alternative spliced forms of cellular or plasma fibronectin. Plasma fibronectin is present in plasma at a concentration of 300–600 nM and is also stored in platelet α-granules and released during coagulation.81 Cellular fibronectin is synthesised by endothelial cells and can be released into plasma during wound healing.82 Zn2+ binding by fibronectin has not yet been fully characterised, however several regions have been shown to bind Zn2+in vitro. These include the collagen/gelatin binding domain (binding to which has been shown to elicit a conformational change in this region),30,83,84 the cell binding domain30 and the alternatively spliced type III connecting segment (IIICS) which is only fully present in cellular fibronectin and a small fraction of plasma fibronectin.85 It is still unclear whether plasma fibronectin binds Zn2+ physiologically or whether this only occurs with cellular fibronectin.85,86 Considering that Zn2+ induces a conformational change in the protein and may thus influence GAG binding, this is an important question to answer. Fibronectin possess 5–6 ionic GAG binding sites.26,81 The first binding site (often termed Hep1) is found in the N-terminal region. As Zn2+ binding to the neighbouring gleatin-binding region induces a conformation change in the protein, it is probable that this could impact on GAG binding in Hep1.83 The second GAG binding region (Hep2) has two distinct GAG binding sites and constitutes the high affinity GAG binding region.87 As the region is flanked by two Zn2+-binding regions, the cell-binding region and the IIICS, Zn2+ binding may also impact on GAG binding at this site. The global Kd of heparin binding to fibronectin is 0.9 μM for a 18–20 saccharide heparin (molecular weight 6000 Da).87 The affinity for UFH is not yet known. Fibronectin does interfere with AT binding to immobilised heparin as a function of heparin concentration. However AT is only completely displaced from heparin at fibronectin/AT ratios higher than are found physiologically.81 Fibronectin also binds HS and DS but its effect on their neutralisation is not known.88,89 Thus plasma Zn2+ levels have (at least in theory) the potential to influence GAG binding and neutralisation by fibronectin. This means that fibronectin has the potential to strongly react with the Zn2+ released during coagulation and to be major GAG-neutralisers during this time.Fibroblast growth factors are present at very low concentrations in plasma (ca. 28–48 pM for FGF-1 and 643 pM for FGF-7),90,91 nevertheless they are normally attached to GAGs of the endothelial surface layer and depend on this binding to exert their functions (including oligomerisation, binding to their cognate receptors and transport between cells).92 They are therefore important binding partners for GAGs. FGF-1 and FGF-7 bind UFH with Kd values of 29 nM and 140 nM, respectively.93 DS also interacts with FGF-7 and FGF-1 but with lower affinity than heparin and HS. FGF-7 and to a lesser extent FGF-1 have been shown to neutralise UFH.94 Unlike with HRG, HMWK and fibrinogen, the affinity of these interactions are reduced by the presence of metal ions (Na+, K+, Ca2+, Cu2+ and Zn2+).93 However, it is unknown whether metal ion binding influences the neutralisation of the GAG. It is possible that binding of metal ions by these FGFs is a mechanism to facilitate their release from the endothelium when the plasma concentrations of those ions are elevated. Nevertheless, FGF-1 and FGF-7 are unlikely to be important GAG neutralisers in vivo due to their low plasma levels.
FVIIa is a coagulatory protein involved in the contact activation pathway.79,80 It binds UFH with a Kd value of 3.38 μM in a Ca2+-dependent manner.95 FVIIa possesses two Zn2+ binding sites but it is not yet known if binding of Zn2+ plays any role in heparin binding.96 FVIIa can neutralise both UFH and LMWH, but its effects on HS and DS have not yet been studied. However, like FGF-1 and FGF-7, FVIIa is only present at a low concentrations in plasma (ca. 16 nM)97 and therefore it is not likely to be as relevant in anticoagulant GAG neutralisation as the proteins listed above. Nevertheless, the interaction of Zn2+ with HRG, HMWK and fibrinogen shows that Zn2+ is an important regulator of GAG binding and neutralisation by plasma protein and it is therefore important to investigate whether Zn2+ has the same effect on other GAG neutralising proteins.
Clinical significance of Zn2+-induced GAG neutralisation
In addition to being released during injury by epithelial cells and platelets, plasma Zn2+ levels are also increased in certain disease states (Fig. 1). Indeed, atherosclerotic plaques are also known to contain up to six-times more Zn2+ than healthy tissue.98 However, only total Zn2+ concentration has been measured and so the concentration of labile Zn2+ is not clear. The increase in Zn2+ concentration in atherosclerotic plaques correlates with an increase in Ca2+ concentration.98 In addition, both metal ions are present at high levels in areas of plaque mineralisation.99 This may signify that the accumulation in both metals occurs through a common mechanism that has not yet been identified. Accumulated Zn2+ could be released into the blood during plaque rupture, thus participating in the pro-thrombotic nature of these events.The concentration of available Zn2+ is also directly influenced by the plasma free fatty acid (FFA) levels. Serum albumin is the main plasma carrier for both Zn2+ and FFAs.57,100 When a FFA molecule binds at a high affinity binding site (called the FA2 site) adjacent to the main Zn2+ binding site, an allosteric interaction leading to perturbation of the Zn2+ binding site occurs. In healthy individuals around 75% of total plasma Zn2+ (around 15 μM) is bound to serum albumin and so this mechanism may result in the release of up to 15 μM Zn2+ in plasma in individuals with elevated FFA levels (when those levels are elevated enough to completely prevent Zn2+ binding by serum albumin). In certain conditions, the concentration of FFA can increase by up to six times: in diabetes FFA concentrations have been reported to be 0.62–0.82 mM in men and 0.82–0.98 mM in women (compared to controls of 0.59–0.68 mM for men and 0.74–0.83 mM for women).101 In non-alcoholic fatty liver disease, the corresponding concentrations are 0.12–3.4 mM (compared to controls of 0.11–0.9 mM).102 In obesity, FFA concentrations are 0.56–1.15 mM (compared to controls of 0.28–0.89 mM).103 Elevated FFA levels are also associated with some cancers; in malignant lymphoma FFA concentrations were found to be 0.55–1.8 mM (no controls);104 All of these conditions are associated with a higher incidence of developing thrombotic complications.105,106 In addition, under hyperglycemic conditions, serum albumin can undergo non-enzymatic glycation reactions which can disrupt the protein conformation and also directly affect its main Zn2+-binding site.107 This is another mechanism by which diabetic state can affect Zn2+ transport and speciation.
Another condition that may be associated with altered plasma Zn2+ homeostasis is analbuminemia (albumin deficiency), which is defined as having a plasma albumin level of <1 g L−1. In total, 78 cases have been reported in the analbuminemia register (http://www.albumin.org) and the prevalence of the disease is estimated to be less than 1 in 1 million.108 Individuals with analbuminemia have been reported to have elevated levels of other plasma proteins, including coagulation factors, as a compensatory mechanism.108 It is thought that those proteins take up most of the functions of albumin and therefore it is unclear if Zn2+ transport in plasma is affected or not. However, it has been shown that Ca2+ and Fe2+ have an altered protein-binding profile in these individuals.108 The transport of FFA is taken up by apolipoprotein B-100 and so FFA levels are close to normal (but dyslipidaemia is present).108 Follow-up studies of patients with analbuminemia have been limited, which limits the report of complications. Nevertheless, such follow-up studies have included reports of atherosclerosis and hypercoagulability in these individuals,108 but it is not clear if this may be due to alterations in plasma Zn2+ level/speciation or changes in plasma coagulatory protein levels.
The impact of Zn2+ on anticoagulant GAG-neutralisation is useful to consider, specifically as this process impacts on thrombin activation, an event that directly affects both platelet aggregation (through binding to cell surface receptors) and fibrin clot formation (through cleavage of fibrinogen). Indeed, by enhancing the neutralisation of the GAG present in the endothelium surface layer, Zn2+ induces a change in the natural anticoagulant properties of the endothelium.10 When coagulation is needed, this participates in the promotion of clotting. However, if Zn2+ speciation is chronically altered, such as is likely in certain diseases states (atherosclerosis, diabetes, obesity and cancer), then this could affect anticoagulant processes in the endothelium resulting in a pro- or hyper-thrombotic state through enhanced GAG neutralistaion.10 This phenomenon could also directly affect the efficiency of heparin-based treatments, which are widely used during surgeries and to manage thrombotic complications.10 In order to confirm this, it would be interesting to measure plasma Zn2+ levels in patients undergoing heparin treatment and to compare it to their response to this treatment. If such a relationship is confirmed then an option may be to control Zn2+ levels in those patients rather than to switch to another anticoagulant treatment whose efficiency could also be potentially affected by Zn2+.
Conclusion
Zn2+ plays a major role in the regulation of coagulation that is only starting to be understood. Because of this role, Zn2+ homeostasis in platelets and its speciation in plasma are especially relevant to the understanding and treatment of blood diseases. In particular, they can affect GAG binding and neutralisation by the platelet-stored proteins HRG, HMWK and fibrinogen. This mechanism is relevant to healthy coagulation processes through the natural anticoagulation properties of the endothelium, but also in anti-thrombotic treatments. Indeed, it may partly explain the observed variability in dose response to heparins and heparin-based drugs. This implies that free plasma Zn2+ levels need to be monitored in individuals with coagulatory disorders or at-risk of thrombotic events. The control of free plasma Zn2+ levels may also be a treatment lead for individuals suffering from high levels of plasma FFA who are at high risk of thrombotic disorders.Abbreviations
| AT | Antithrombin |
| DS | Dermatan sulfate |
| FGF | Fibroblast growth factor |
| FVIIa | Activated coagulation factor VII |
| GAG | Glycosaminoglycan |
| HCII | Heparin cofactor II |
| HMWK | High-molecular weight kininogen |
| HRG | Histidine-rich glycoprotein |
| HRR | Histidine-rich region |
| HS | Heparan sulfate |
| IIICS | Type III connecting segment of fibronectin |
| LMWH | Low molecular weight heparin |
| UFH | Unfractionated heparin |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the British Heart Foundation (grant codes: PG/15/9/31270 and FS/15/42/31556). SJP is supported by a Royal Society of Edinburgh Biomedical Fellowship.References
- E. M. Muñoz and R. J. Linhardt, Heparin-binding domains in vascular biology, Arterioscler., Thromb., Vasc. Biol., 2004, 24, 1549–1557 CrossRef PubMed.
- N. W. Shworak and T. Kobayashi, A. d. Agostini and N. C. Smits, Anticoagulant heparan sulfate to not clot--or not?, Prog. Mol. Biol. Transl. Sci., 2010, 93, 153–178 Search PubMed.
- D. M. Tollefsen, Vascular dermatan sulfate and heparin cofactor II, Prog. Mol. Biol. Transl. Sci., 2010, 93, 351–372 Search PubMed.
- J. A. Marcum, J. B. McKenney, S. J. Galli, R. W. Jackman and R. D. Rosenberg, Anticoagulantly active heparin-like molecules from mast cell-deficient mice, Am. J. Physiol.: Heart Circ. Physiol., 1986, 250, H879–H888 CrossRef PubMed.
- H. Engelberg and A. Dudley, Plasma heparin levels in normal man, Circulation, 1961, 23, 578–581 CrossRef PubMed.
- J. L. Zehnder and S. J. Galli, Mast-cell heparin demystified, Nature, 1999, 400, 714–715 CrossRef PubMed.
- E. Forsberg, G. Pejler, M. Ringvall, C. Lunderius, B. Tomasini-Johansson, M. Kusche-Gullberg, I. Eriksson, J. Ledin, L. Hellman and L. Kjellen, Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme, Nature, 1999, 400, 773–776 CrossRef PubMed.
- D. E. Humphries, G. W. Wong, D. S. Friend, M. F. Gurish, W.-T. Qiu, C. Huang, A. H. Sharpe and R. L. Stevens, Heparin is essential for the storage of specific granule proteases inmast cells, Nature, 1999, 400, 769–772 CrossRef PubMed.
- Venous thromboembolic diseases: diagnosis, management and thrombophilia testing, NICE Clinical guideline (CG144), https://www.nice.org.uk/guidance/cg144, (accessed November 23, 2017).
- A. I. S. Sobczak, S. J. Pitt and A. J. Stewart, Glycosaminoglycan Neutralization in Coagulation Control, Arterioscler., Thromb., Vasc. Biol., 2018, 38, 1258–1270 CrossRef PubMed.
- W. Li, D. J. Johnson, C. T. Esmon and J. A. Huntington, Structure of the antithrombin–thrombin–heparin ternary complex reveals the antithrombotic mechanism of heparin, Nat. Struct. Mol. Biol., 2004, 11, 857–862 CrossRef PubMed.
- M. Pai and M. A. Crowther, in Handbook of experimental pharmacology volume 207: Heparin – a century of progress, ed. R. Lever, B. Mulloy and C. P. Page, Springer, Berlin, 2012, pp. 265–277 Search PubMed.
- T. T. Vu, J. C. Fredenburgh and J. I. Weitz, Zinc, an important cofactor in haemostasis and thrombosis, Thomb. Haemostasis, 2013, 109, 421–430 CrossRef PubMed.
- K. Nishida and S. Yamasaki, in Zinc signals in cellular functions and disorders, ed. T. Fukada and T. Kambe, Springer, Japan, 2014, ch. 5, pp. 89–109 Search PubMed.
- B. L. Vallee and K. H. Falchuk, The biochemical basis of zinc physiology, Physiol. Rev., 1993, 73, 79–118 CrossRef PubMed.
- P. R. Gordon, C. W. Woodruff, H. L. Anderson and B. L. O'Dell, Effect of acute zinc deprivation on plasma zinc and platelet aggregation in adult males, Am. J. Clin. Nutr., 1982, 35, 113–119 CrossRef PubMed.
- M. P. Emery, J. D. Browning and B. L. O'Dell, Impaired hemostasis and platelet function in rats fed low zinc diets based on egg white protein, J. Nutr., 1990, 120, 1062–1067 CrossRef PubMed.
- M. P. Emery and B. L. O'Dell, Low zinc status in rats impairs calcium uptake and aggregation of platelets simulate by fluoride, Proc. Soc. Exp. Biol. Med., 1993, 203, 480–484 CrossRef PubMed.
- N. N. Gorgani, C. R. Parish, S. B. E. Smith and J. G. Altin, differential binding of histidine-rich glycoprotein (HRG) to human IgG subclasses and IgG molecules containing k and l light chains, J. Biol. Chem., 1999, 274, 29633–29640 CrossRef PubMed.
- A. L. Jones, M. D. Hulett and C. R. Parish, Histidine-rich glycoprotein binds to cell-surface heparan sulfate via its N-terminal domain following Zn2+ chelation, J. Biol. Chem., 2004, 279, 30114–30122 CrossRef PubMed.
- R. Gorodetsky, X. Mou, A. Blankenfeld and G. Marx, Platelet multielemental composition, lability, and subcellular localization, Am. J. Hematol., 1993, 42, 278–283 CrossRef PubMed.
- M. H. Fukami, H. Holmsen, M. A. Kowalska and S. Niewiarowski, in Hemostasis and Thrombosis: Basic Principles and Clinical Practice, ed. R. W. Colman, J. Hirsh, V. J. Marder, A. W. Clowes and J. N. George, Lippincott Williams & Wilkins, Philadelphia, 2001, pp. 561–573 Search PubMed.
- S. Raut and P. J. Gaffney, Interaction of heparin with fibrinogen using durface plasmon resonance technology: investigation of heparin binding site on fibrinogen, Thromb. Res., 1996, 15, 503–509 CrossRef.
- H. R. Lijnen, M. Hoylaerts and D. Collen, Heparin binding properties of human histidine-rich glycoprotein. Mechanism and role in the neutralization of heparin in plasma, J. Biol. Chem., 1983, 258, 3803–3808 Search PubMed.
- I. Bjoerk, S. T. Olson, R. G. Sheffer and J. D. Shore, Binding of heparin to human high molecular weight kininogen, Biochemistry, 1989, 28, 1213–1221 CrossRef.
- A. Ogamo, A. Nagai and K. Nagasawa, Binding of heparin fractions and other polysulfated polysaccharides to plasma fibronectin: effects of molecular size and degree of sulfation of polysaccharides, Biochim. Biophys. Acta, 1985, 841, 30–41 CrossRef.
- G. Marx, Zinc binding to fibrinogen and fibrin, Arch. Biochem. Biophys., 1988, 266, 285–288 CrossRef PubMed.
- W. T. Morgan, Human serum histidine-rich glycoprotein. I. Interactions with heme, metal ions and organic ligands, Biochim. Biophys. Acta, 1978, 535, 319–333 CrossRef.
- Y. Lin, R. A. Pixley and R. W. Colman, Kinetic analysis of the role of zinc in the interaction of domain 5 of high-molecular weight kininogen (HK) with heparin, Biochemistry, 2000, 39, 5104–5110 CrossRef PubMed.
- B. Gmeiner, H. Leibl, G. Zerlauth and C. Seelos, Affinity binding of distinct functional fibronectin domains to immobilized metal chelates, Arch. Biochem. Biophys., 1995, 321, 40–42 CrossRef PubMed.
- C. A. Blindauer, I. Harvey, K. E. Bunyan, A. J. Stewart, D. Sleep, D. J. Harrison, S. Berezenko and P. J. Sadler, Structure, properties, and engineering of the major zinc binding site on human albumin, J. Biol. Chem., 2009, 284, 23116–23124 CrossRef PubMed.
- H. Haase, S. Hebel, G. Engelhardt and L. Rink, The biochemical effects of extracellular Zn(2+) and other metal ions are severely affected by their speciation in cell culture media, Metallomics, 2015, 7, 102–111 RSC.
- B. Sarkar, Metal-protein interactions in transport, accumulation and excretion of metals, Biol. Trace Elem. Res., 1989, 21, 137–144 CrossRef PubMed.
- E. Kelly, J. Mathew, J. E. Kohler, A. L. Blass and D. I. Soybel, Redistribution of labile plasma zinc during mild surgical stress in the rat, Transl. Res., 2011, 157, 139–149 CrossRef PubMed.
- F. Mahdi, Z. S. Madar, C. D. Figueroa and A. H. Schmaier, Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1 on endothelial cell membranes, Blood, 2002, 99, 3585–3596 CrossRef PubMed.
- S. J. Henderson, A. R. Stafford, B. A. Leslie, P. Y. Kim, N. Vaezzadeh, R. Ni, J. C. Fredenburgh and J. I. Weitz, Zinc delays clot lysis by attenuating plasminogen activation and plasmin-mediated fibrin degradation, Thromb. Haemost., 2015, 113, 1278–1288 CrossRef PubMed.
- G. Marx, G. Korner, X. Mou and R. Gorodetsky, Packaging zinc, fibrinogen, and factor XIII in platelet alpha-granules, J. Cell. Physiol., 1993, 156, 437–442 CrossRef PubMed.
- K. A. Taylor and N. Pugh, The contribution of zinc to platelet behaviour during haemostasis and thrombosis, Metallomics, 2016, 8, 144–155 RSC.
- R. C. Whitehouse, A. S. Prasad, P. I. Rabbani and Z. T. Cossack, Zinc in plasma, neutrophils, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry, Clin. Chem., 1982, 28, 475–480 Search PubMed.
- G. Michaelsson, K. Ljunghall and B. G. Danielson, Zinc in epidermis and dermis in healthy subjects, Acta Derm.-Venereol., 1980, 60, 295–299 Search PubMed.
- H. Sharir, A. Zinger, A. Nevo, I. Sekler and M. Hershfinkel, Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair, J. Biol. Chem., 2010, 285, 26097–26106 CrossRef PubMed.
- S. Tubek, P. Grzanka and I. Tubek, Role of zinc in hemostasis: a review, Biol. Trace Elem. Res., 2008, 121, 1–8 CrossRef PubMed.
- S. J. Henderson, J. Xia, H. Wu, A. R. Stafford, B. A. Leslie, J. C. Fredenburgh, D. A. Weitz and J. I. Weitz, Zinc promotes clot stability by accelerating clot formation and modifying fibrin structure, Thromb. Haemostasis, 2016, 115, 533–542 CrossRef PubMed.
- I. Capila and R. J. Linhardt, Heparin-protein interactions, Angew. Chem., Int. Ed., 2002, 41, 390–412 CrossRef.
- Y. Seo, M. R. Schenauer and J. A. Leary, Biologically relevant metal-cation binding induces conformational changes in heparin oligosaccharides as measured by ion mobility mass spectrometry, Int. J. Mass Spectrom., 2011, 303, 191–198 CrossRef PubMed.
- N. E. Woodhead, W. F. Long and F. B. Williamson, Binding of zinc ions to heparin. Analysis by equilibrium dialysis suggests the occurrence of two, entropy-driven, processes, Biochem. J., 1986, 237, 281–284 CrossRef PubMed.
- R. A. Pixley, Y. Lin, I. Isordia-Salas and R. W. Colman, Fine mapping of the sequences in domain 5 of high molecular weight kininogen (HK) interacting with heparin and zinc, J. Thromb. Haemost., 2003, 1, 1791–1798 CrossRef PubMed.
- A. L. Jones, M. D. Hulett and C. R. Parish, Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems, Immunol. Cell Biol., 2005, 83, 106–118 CrossRef PubMed.
- J. J. Corrigan, M. A. Jeter, D. Bruck and W. M. Feinberg, Histidine-rich glycoprotein levels in children: the effect of age, Thromb. Res., 1990, 59, 681–686 CrossRef PubMed.
- T. Renné, J. Dedio, G. David and W. Müller-Esterl, High molecular weight kininogen utilizes heparan sulfate proteoglycans for accumulation on endothelial cells, J. Biol. Chem., 2000, 275, 33688–33696 CrossRef PubMed.
- G. A. Tennent, S. O. Brennan, A. J. Stangou, J. O'Grady, P. N. Hawkins and M. B. Pepys, Human plasma firbinogen is synthesized in the liver, Blood, 2007, 109, 1971–1974 CrossRef PubMed.
- P. J. Hogg and C. M. Jackson, Fibrin monomer protects thrombin from inactivation by heparin-antithrombin III: implications for heparin efficacy, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 3619–3623 CrossRef.
- I. K. H. Poon, K. K. Patel, D. S. Davis, C. R. Parish and M. D. Hulett, Histidine-rich glycoprotein: the SwissArmy knife of mammalian plasma, Blood, 2011, 117, 2093–2101 CrossRef PubMed.
- J. W. Weisel, C. Nagaswami, J. L. Woodhead, R. A. D. Cadena, J. D. Page and R. W. Colman, The shape of high molecular weight kininogen. Organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy, J. Biol. Chem., 1994, 269, 10100–10106 Search PubMed.
- T. K. D. Foster, S. Yoshitake and E. W. Davie, Amino acid sequence of human histidine-rich glycoproteiq derived from the nucleotide sequence of its cDNA, Biochemistry, 1986, 25, 2220–2225 CrossRef.
- O. Kassaar, S. A. McMahon, R. Thompson, C. H. Blotting, J. H. Naismith and A. J. Stewart, Crystal structure of histidine-rich glycoprotein N2 domain reveals redox activity at an interdomain disulfide bridge: implications for angiogenic regulation, Blood, 2014, 123, 1948–1955 CrossRef PubMed.
- O. Kassaar, U. Schwarz-Linek, C. A. Blindauer and A. J. Stewart, Plasma free fatty acid levels influence Zn2+-dependent histidine-rich glycoprotein–heparin interactions via an allosteric switch on serum albumin, J. Thromb. Haemostasis, 2015, 13, 101–110 CrossRef PubMed.
- E. M. Martin, F. D. L. Kondrat, A. J. Stewart, J. H. Scrivens, P. J. Sadler and C. A. Blindauer, Native electrospray mass spectrometry approaches to probe the interaction between zinc and an anti-angiogenic peptide from histidine-rich glycoprotein, Sci. Rep., 2018, 8, 8646 CrossRef PubMed.
- S. Mori, R. Shinohata, M. Renbutsu, H. K. Takahashi, Y. I. Fang, K. Yamaoka, M. Okamoto and M. Nishibori, Histidine-rich glycoprotein plus zinc reverses growth inhibition of vascular smooth muscle cells by heparin, Cell Tissue Res., 2003, 312, 353–359 CrossRef PubMed.
- J. L. MacQuarrie, A. R. Stafford, J. W. Yau, B. A. Leslie, T. T. Vu, J. C. Fredenburgh and J. I. Weitz, Histidine-rich glycoprotein binds factor XIIa with high affinity and inhibits contact-initiated coagulation, Blood, 2011, 117, 4134–4141 CrossRef PubMed.
- D. B. Borza and W. T. Morgan, Histidine-proline-rich glycoprotein as a plasma pH sensor, J. Biol. Chem., 1998, 273, 5493–5499 CrossRef PubMed.
- K. M. Priebatsch, M. Kvansakul, I. K. Poon and M. D. Hulett, Functional Regulation of the Plasma Protein Histidine-Rich Glycoprotein by Zn(2+) in Settings of Tissue Injury, Biomolecules, 2017, 7, 22 CrossRef PubMed.
- M. Vanwildemeersch, A. K. Olsson, E. Gottfridsson, L. Claesson-Welsh, U. Lindahl and D. Spillmann, The anti-angiogenic His/Pro-rich fragment of histidine-rich glycoprotein binds to endothelial cell heparan sulfate in a Zn2+-dependent manner, J. Biol. Chem., 2006, 281, 10298–10304 CrossRef PubMed.
- M. K. Burch, M. N. Blackburn and W. T. Morgan, Further characterization of the interaction of histidine-rich glycoprotein with heparin: evidence for the binding of two molecules of histidine-rich glycoprotein by high molecular weight heparin and for the involvement of histidine residues in heparin binding, Biochemistry, 1987, 26, 7477–7482 CrossRef PubMed.
- D. Lane, G. Pejler, A. Flynn, E. Thompson and U. Lindahl, Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor-4, J. Biol. Chem., 1986, 261, 3980–3986 Search PubMed.
- B. A. Kluszynski, C. Kim and W. P. Faulk, Zinc as a Cofactor for Heparin Neutralization by Histidine-rich Glycoprotein, J. Biol. Chem., 1997, 272, 13541–13547 CrossRef PubMed.
- D. M. Tollefsen and C. A. Pestka, Modulation of heparin cofactor II activity by histidine-rich glycoprotein and platelet factor 4, J. Clin. Invest., 1985, 75, 496–501 CrossRef PubMed.
- A. Zammit and J. Dawes, Fibrinogen inhibits the heparin cofactor II-mediated antithrombin activity of dermatan sulfate, Blood, 1995, 85, 720–726 Search PubMed.
- G. Cella, G. Boeri, G. Saggiorato, R. Paolini, G. Luzzatto and V. Terribile, Interaction between histidine-rich glycoprotein and platelet factor 4 with dermatan sulfate and low-molecular-weight dermatan sulfate, Angiology, 1992, 43, 59–62 CrossRef PubMed.
- A. L. Jones, M. D. Hulett and C. R. Parish, Histidine-rich glycoprotein binds to cell-surface heparan sulfate via its N-terminaldDomain following Zn2+ chelation, J. Biol. Chem., 2004, 279, 30114–30122 CrossRef PubMed.
- H. Herwald, M. Mörgelin, H. G. Svensson and U. Sjöbring, Zinc-dependent conformational changes in domain D5 of high molecular mass kininogen modulate contact activation, Eur. J. Biochem., 2001, 268, 396–404 CrossRef PubMed.
- J. M. Kollman, L. Pandi, M. R. Sawaya, M. Riley and R. F. Doolittle, Crystal structure of human fibrinogen, Biochemistry, 2009, 48, 3877–3886 CrossRef PubMed.
- R. F. Doolittle, Z. Yang and I. Mochalkin, Crystal structure studies on fibrinogen and fibrin, Ann. N. Y. Acad. Sci., 2001, 936, 31–43 CrossRef PubMed.
- M. F. Scully and V. V. Kakkar, Binding of fibrinogen fragment D to chelated zinc: localization of a possible binding site, Thromb. Res., 1983, 30, 297–300 CrossRef PubMed.
- J. C. Fredenburgh, B. A. Leslie, A. R. Stafford, T. Lim, H. H. Chan and J. I. Weitz, Zn2+ mediates high affinity binding of heparin to the aC domain of fibrinogen, J. Biol. Chem., 2013, 288, 29394–29402 CrossRef PubMed.
- S. Yakovlev, S. Gorlatov, K. Ingham and L. Medved, Interaction of fibrin(ogen) with heparin: further characterization and localization of the heparin-binding site, Biochemistry, 2003, 42, 7709–7716 CrossRef PubMed.
- H. H. Chan, B. A. Leslie, A. R. Stafford, R. S. Roberts, N. N. Al-Aswad, J. C. Fredenburgh and J. I. Weitz, By increasing the affinity of heparin for fibrin, Zn2+ promotes the formation of a ternary heparin-thrombin-fibrin complex that protects thrombin from inhibition by antithrombin, Biochemistry, 2012, 51, 7964–7973 CrossRef PubMed.
- T. Holger-Madsen and M. Schioler, Heparin resitsance (measured by the heparin thrombin time) and plasma fibrinogen in various dieases, Acta Haematol., 1962, 27, 294–305 CrossRef PubMed.
- L. Poller, Factor VII and heparin in thrombosis, J. Clin. Pathol., 1959, 12, 331–334 CrossRef PubMed.
- G. Young, K. E. Yonekawa, P. A. Nakagawa, R. C. Blain, A. E. Lovejoy and D. J. Nugent, Recombinant activated factor VII effectively reverses the anticoagulant effects of heparin, enoxaparin, fondaparinux, argatroban, and bivalirudin ex vivo as measured using thromboelastography, Blood Coagulation Fibrinolysis, 2007, 18, 547–553 CrossRef PubMed.
- Y. Byun, H. A. Jacobs, J. Feijen and S. W. Kim, Effect of fibronectin on the binding of antithrombin III to immobilized heparin, J. Biomed. Mater. Res., 1996, 30, 95–100 CrossRef PubMed.
- W. S. To and K. S. Midwood, Plasma and cellular fibronectin: distinct and independent functions during tissue repair, Fibrog. Tissue Repair, 2011, 4, 21–38 CrossRef PubMed.
- M. Graille, M. Pagano, T. Rose, M. R. Ravaux and H. V. Tilbeurgh, Zinc induces structural reorganization of gelatin binding domain from human fibronectin and affects collagen binding, Structure, 2010, 18, 710–718 CrossRef PubMed.
- S. L. Vidmar, F. Lottspeich, I. Emod, J. M. Imhoff and V. Keil-Dlouha, Collagen-binding domain of human plasma fibronectin contains a latent type-IV collagenase, Eur. J. Biochem., 1991, 201(1), 79–84 CrossRef.
- J. A. Askari, D. J. Thornton, J. D. Humphries, P. A. Buckley and M. J. Humphries, The alternatively spliced type III connecting segment of fibronectin is a zinc-binding module, Matrix Biol., 2007, 26, 485–493 CrossRef PubMed.
- M. J. Sinosich, M. W. Davey, B. Teisner and J. G. Grudzinskas, Comparative studies of pregnancy associated plasma protein-A and alpha 2-macroglobulin using metal chelate chromatography, Biochem. Int., 1983, 7, 33–42 Search PubMed.
- K. C. Ingham, S. A. Brew and D. H. Atha, Interaction of heparin with fibronectin and isolated fibronectin domains, Biochem. J., 1990, 272, 605–611 CrossRef PubMed.
- G. Schmidt, H. Robenek, B. Harrach, J. Glössl, V. Nolte, H. Hörmann, H. Richter and H. Kresse, Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin, J. Cell Biol., 1987, 104, 1683–1691 CrossRef PubMed.
- S. Tumova, A. Woods and J. R. Couchman, Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences, J. Biol. Chem., 2000, 275, 9410–9417 CrossRef PubMed.
- S. Kumara, H. Miyagaki, D. Giata, X. Yan, L. Njoh, C. Vesna, M. M. Alvarez-Downing and R. L. Whelan, Plasma levels of Keratinocyte Growth Factor, a proangiogenic protein, are significantly elevated for 3 weeks after minimally invasive colorectal resection (MICR) for cancer, Surg. Endosc., 2012, 26, 2751–2757 CrossRef PubMed.
- S. Wang, Q. Yang, S. Yu, R. Pan, D. Jiang, Y. Liu, H. Hu, W. Sun, X. Hong, H. Xue, W. Qian, D. Wang, L. Zhou, C. Mao and G. Yuan, Fibroblast growth factor 1 levels are elevated in newly diagnosed type 2 diabetes compared to normal glucose tolerance controls, Endocr. J., 2016, 63, 359–365 CrossRef PubMed.
- A. Beenken and M. Mohammadi, The FGF family: biology, pathophysiology and therapy, Nat. Rev. Drug Discovery, 2009, 8, 235–253 CrossRef PubMed.
- R. Xu, A. Ori, T. R. Rudd, K. A. Uniewicz, Y. A. Ahmed, S. E. Guimond, M. A. Skidmore, G. Siligardi, E. A. Yates and D. G. Fernig, Diversification of the structural determinants of fibroblast growth factor-heparin interactions: implications for binding specificity, J. Biol. Chem., 2012, 287, 40061–40073 CrossRef PubMed.
- Y. d. Luo, H. H. Cho and W. L. McKeehan, Biospecific extraction and neutralization of anticoagulant heparin with fibroblast growth factors (FGF), J. Pharm. Sci., 2003, 92, 2117–2127 CrossRef PubMed.
- I. Martínez-Martínez, A. Ordóñez, S. Pedersen, M. E. de la Morena-Barrio, J. Navarro-Fernández, S. R. Kristensen, A. Miñano, J. Padilla, V. Vicente and J. Corral, Heparin affinity of factor VIIa: implications on the physiological inhibition by antithrombin and clearance of recombinant factor VIIa, Thromb. Res., 2011, 127, 154–160 CrossRef PubMed.
- L. C. Petersen, O. H. Olsen, L. S. Nielsen, P. O. Freskgård and E. Persson, Binding of Zn2+ to a Ca2+ loop allosterically attenuates the activity of factor VIIa and reduces its affinity for tissue factor, Protein Sci., 2000, 9, 859–866 CrossRef PubMed.
- B. Blombäck and L. A. Hanson, Plasma proteins, Wiley, Chichester, 1979 Search PubMed.
- N. Stadler, N. Stanley, S. Heeneman, V. Vacata, M. J. Daemen, P. G. Bannon, J. Waltenberger and M. J. Davies, Accumulation of zinc in human atherosclerotic lesions correlates with calcium levels but does not protect against protein oxidation, Arterioscler., Thromb., Vasc. Biol., 2008, 28, 1024–1030 CrossRef PubMed.
- D. Kopriva, A. Kisheev, D. Meena, S. Pelle, M. Karnitsky, A. Lavoie and J. Buttigieg, The Nature of Iron Deposits Differs between Symptomatic and Asymptomatic Carotid Atherosclerotic Plaques, PLoS One, 2015, 10, e0143138 CrossRef PubMed.
- A. J. Stewart, C. A. Blindauer and P. J. Sadler, Plasma fatty acid levels may regulate the Zn2+-dependent activities of histidine-rich glycoprotein, Biochimie, 2009, 91, 1518–1522 CrossRef PubMed.
- M. Carlsson, Y. Wessman, P. Almgren and L. Groop, High levels of nonesterified fatty acids are associated with increased familial risk of cardiovascular disease, Arterioscler., Thromb., Vasc. Biol., 2000, 20, 1588–1594 CrossRef.
- J. Zhang, Y. Zhao, C. Xu, Y. Hong, H. Lu, J. Wu and Y. Chen, Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study, Sci. Rep., 2014, 4, 5832 CrossRef PubMed.
- P. Bjorntorp, H. Bergman and E. Varnauskas, Plasma free fatty acid turnover rate in obesity, Acta Med. Scand., 1969, 185, 351–356 CrossRef PubMed.
- J. Nuutinen, H. Minn, J. Bergman, M. Haaparanta, U. Ruotasalainen, H. Laine and J. Knuuti, Uncoupling of fatty acid and glucose metabolism in malignant lymphoma: a PET study, Br. J. Cancer, 1999, 80, 513–518 CrossRef PubMed.
- E. Privateli, P. Bucciarelli, S. M. Pasamonit and I. Martinelli, Risk factors vor venous and arterial thrombosis, J. Blood Transfus., 2011, 9, 120–138 Search PubMed.
- G. C. Connolly and A. A. Khorana, Risk stratification for cancer-associated venous thromboembolism, Best Pract. Res., Clin. Haematol., 2009, 22, 35–47 CrossRef PubMed.
- S. Iqbal, F. A. Qais, M. M. Alam and I. Naseem, Effect of glycation on human serum albumin–zinc interaction: a biophysical study, J. Biol. Inorg. Chem., 2018, 23, 447–458 CrossRef PubMed.
- B. G. Koot, R. Houwen, D. J. Pot and J. Nauta, Congenital analbuminaemia: biochemical and clinical implications. A case report and literature review, Eur. J. Pediatr., 2004, 163, 664–670 Search PubMed.
- A. A. Bhattacharya, T. Grune and S. Curry, Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin, J. Mol. Biol., 2000, 303, 721–732 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |