Gold-catalyzed annulations of N-aryl ynamides with benzisoxazoles to construct 6H-indolo[2,3-b]quinoline cores†
Meng-Han
Tsai
a,
Cheng-Yu
Wang
ab,
Antony Sekar
Kulandai Raj
a and
Rai-Shung
Liu
 *a
*a
aFrontier Research Center for Fundamental and Applied Science of Matters and Department of Chemistry, National Tsing-Hua University, 101, Sec. 2, Kuang-Fu Rd., Hsinchu, 30013, Taiwan, Republic of China. E-mail: rsliu@mx.nthu.edu.tw
bSchool of Chemistry and Chemical Engineering, Linyi University, Linyi 276000, Shandong, P. R. China
First published on 4th September 2018
Abstract
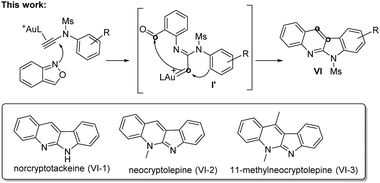
This work reports new annulations of N-aryl ynamides with benzisoxazoles to form 6H-indolo[2,3-b]quinoline derivatives. The synthetic utility of this new method is manifested by its applicability to access naturally occurring alkaloids including norcryptotackeine, neocryptolepine and 11-methylneocryptol-epine. Our experimental data indicate that high-temperature conditions allow N-aryl nucleophiles to become conformationally flexible, rendering the attack at gold carbenes effective to generate reactive indoles that attack again the benzaldehyde to furnish the observed products.
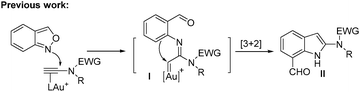
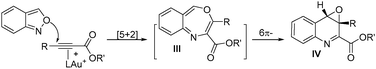
Interest in the gold-catalyzed annulations of isoxazoles or benzisoxazoles with alkynes is rapidly growing because of their easy access to five- and six-membered azacycles.1–4 The reactions of isoxazoles2 and benzisoxazoles3 on the same alkynes typically afforded two distinct products. In the case of benzisoxazoles, Hashmi reported their [3 + 2]-annulations with ynamides via N-attack regioselectivity to form α-imino gold carbenes I, further preceding to 2-amino-7-formylindole products II.3a In contrast, the gold-catalyzed annulations of benzisoxazoles with propiolate derivatives proceeded through a distinct O-attack regioselectivity to enable [5 + 2]-annulation/6π-electrocyclization cascades, yielding valuable quinoline oxides IV.4 In these reactions, gold π-alkynes bear no nucleophiles; we envisage that new reactions likely occur when these alkynes comprise a potent nucleophile to functionalize further gold carbene intermediates. To test this hypothesis, as depicted in eqn (3), ynamides bearing an electron-rich N-aryl sulfonamide trigger a cascade reaction on gold carbene intermediates I′, involving a N-aryl attack at gold carbenes, then at the benzaldehyde sequentially to construct 6H-indolo[2.3-b]quinoline frameworks.5a,b Such a framework matches well with several naturally occurring alkaloids such as norcryptotackeine, (VI-1)5a,6a, neocryptolepine (VI-2)6b,c and 11-methylneocryptolepine (VI-3),6d which exhibit potent activity in the treatment of infectious diseases, fever and malaria. Application of our new method for the synthesis of these three bioactive molecules will be described herein.
 | (1) |
 | (2) |
 | (3) |
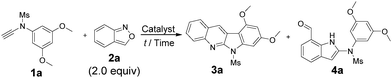
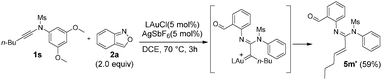
In the gold-catalyzed annulations of benzisoxazole 2a with ynamide 1a, two possible products 3a and 4a are likely to form; the former arises from our new process (eqn (3)) whereas the latter is produced from the known system3a (eqn (1)). We tested the reactions with LAuCl/AgSbF6 (L = P(t-Bu)2(o-biphenyl)), IPr, PPh3 and P(OPh)3 (Table 1, entries 1–4) in hot DCE (70 °C, 0.25 h); only P(t-Bu)2(o-biphenyl)AuSbF6 showed chemoselectivity to yield 6H-indolo[2,3-b]quinoline 3a, in up to 88% yield, together with the pyrrole derivative 4a in only 7% yield. Herein, electron-rich IPrAuNTf2 is envisaged to generate gold carbenes I′ favorably (eqn (3)), but its tethered benzaldehyde becomes an active nucleophile to facilitate the formation of the pyrrole product 4a. Other silver salts such as AgNTf2 and AgOTf were not chemoselective to afford compounds 3a and 4a in significant proportions (entries 5 and 6). As noted in entries 7 and 8, the reactions at mild temperatures 50 °C and 25 °C preferably delivered side product 4a with 43% and 58% yields, respectively (entries 7 and 8); this temperature-dependent chemoselectivity is attributed to the rigid conformation of a sulfonamide group at low temperatures, rendering its nucleophilic attack difficult. Other solvents gave 3a in the following yields: toluene 70%, 1,4-dioxane 19% and MeCN 66% (entries 9–11). Under the conditions in entry 1, we examined the reaction with P(t-Bu)2(o-biphenyl)AuCl alone, which gave the desired product 3a in 57% yield together with 4a in 29% yield. (entry 12). Accordingly, the preferable chemoselectivity toward 3a was affected largely by the nature of the gold catalyst. AgSbF6 (20 mol%) alone gave the undesired pyrrole derivative 4a efficiently (entry 13). Compound 3a was characterized by X-ray diffraction to confirm its 6H-indolo[2.3-b]quinoline framework.7
| Entry | Catalyst (mol%) | Solvent | t (°C)/Time (h) | Isolated yieldsa (%) | |
|---|---|---|---|---|---|
| 3a | 4a | ||||
| 1a (0.1 M, 1.0 equiv.).a Product yields are obtained after purification from a silica column.b L = P(t-Bu)2(o-biphenyl).c IPr = 1,3-Bis(diisopropylphenyl)imidazol-2-ylidene. | |||||
| 1 | LAuCI (5)/AgSbF6 (5)b | DCE | 70/0.25 | 88 | 7 |
| 2 | IPrAuCI (5)/AgSbF6 (5)c | DCE | 70/0.25 | 44 | 50 |
| 3 | PPh3AuCI (5)/AgSbF6 (5) | DCE | 70/0.25 | 68 | 21 |
| 4 | (PhO)3AuCI (5)/AgSbF6 (5) | DCE | 70/0.25 | 61 | 31 |
| 5 | LAuCI (5)/AgNTf2 (5) | DCE | 70/0.25 | 52 | 47 |
| 6 | LAuCI (5)/AgOTf (5) | DCE | 70/0.25 | 57 | 39 |
| 7 | LAuCI (5)/AgSbF6 (5) | DCE | 50/0.47 | 50 | 43 |
| 8 | LAuCI (5)/AgSbF6 (5) | DCE | 25/0.75 | 35 | 58 |
| 9 | LAuCI (5)/AgSbF6 (5) | Toluene | 70/0.25 | 70 | 24 |
| 10 | LAuCI (5)/AgSbF6 (5) | 1,4-Dioxane | 70/0.25 | 19 | 72 |
| 11 | LAuCI (5)/AgSbF6 (5) | MeCN | 70/0.25 | 66 | 29 |
| 12 | LAuCI (5) | DCE | 70/0.25 | 57 | 29 |
| 13 | AgSbF6 (20) | DCE | 70/0.25 | 8 | 88 |
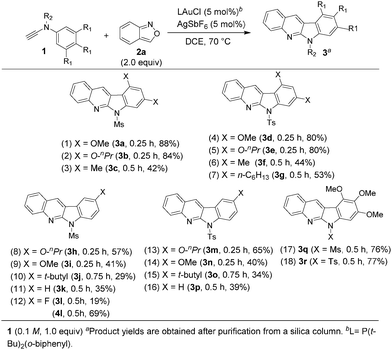
These new annulations were tested further with various ynamides 1 and benzisoxazole 2a to assess the reaction generality; the results are summarized in Scheme 1. Pyrrole products 4 would also be produced when the desired reactions (eqn (3)) became inefficient. For mesyl-derived 3,5-substituted phenyl sulfonamides 1a–1c bearing X = O-nPr, OMe and Me, their resulting products 3a–3c were obtained in 42–88% yields (entries 1–3). These cascade annulations were successfully extended to their tosylate-derived products 3d–3f in 44–80% yields (entries 4–6). In comparison with the 3,5-dimethylphenyl analogue 1f, 3,5-di(n-hexyl)phenyl 3g provided a slightly increased yield, i.e. 53% (entry 7). We tested the reactions with para-substituted phenyl derivatives 1h–1l (X = O-nPr, OMe, t-butyl and F), their corresponding products 3h–3l were obtained in 19–57% yield (entries 8–12). The fluoroderivative 1l gave the desired product in 19% yield together with species 4l in 69% yield (entry 12). Notably, their tosyl-derived analogues gave improved yields (34–65%) of the desired compounds 3m–3p (entries 13–16). Unsubstituted ynamides 1k and 1p were also applicable substrates to afford the desired products 3k and 3p in 35% and 39% yields, respectively (entries 11 and 16). Finally, we tested the reactions on 3,4,5-trimethoxyphenyl sulfonamides 1q and 1r, producing the desired products 3q and 3r in 76–77% yields (entries 17 and 18).
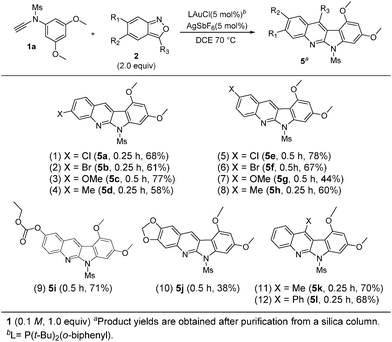
As shown in Scheme 2, the scope of these annulations is significantly expanded with various applicable benzisoxazoles 2a–2l. For species 2a–2d bearing various 6-substituted benzisoxazoles (X = Cl, Br, OMe, and Me), their annulations with the model ynamide 1a afforded the desired products 5a–5d in reasonable yields (58–77%, entries 1–4). In the case of 5-substituted benzisoxazoles (X = Cl, Br, OMe, Me and OCO2Et), their corresponding products 5e–5i were obtained in 44–78% yields (entries 5–9). The reaction of dioxolo benzoisoxazole 2j with 1a delivered the desired product 5j, albeit in 38% yield (entry 10). We examined the reactions on 3-substituted benzisoxazoles 2k and 2l (X = Me and Ph), yielding the desired products 5k and 5l in 68–70% yields (entries 11 and 12).
 | (4) |
 | (5) |
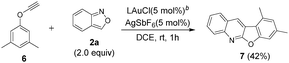
We tested the reaction of one internal ynamide 1s that reacted with benzisoxazole 2a, in the presence of a gold catalyst, to form compound 5m′ in 59% yield; this information confirms the intermediacy of gold carbene intermediates (eqn (4)). To expand the reaction scope, we examined such cascade annulations of phenoxyalkyne 6,8a–c which reacted with benzisoxazole 2a under standard conditions to yield distinct heterocyclic compound 7 in 42% yield (eqn (5)).
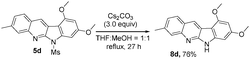
Indoloquinoline 5d undergoes a facile deprotection to remove the mesyl group, producing 8d in 76% yield (eqn (6)). Compound 5d was treated with Cs2CO3 in THF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
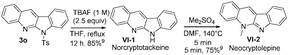
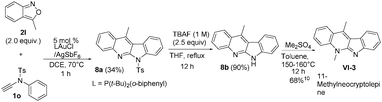
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under reflux in 27 h, from which we obtained compound 8d in 76% yield. In the literature,5a,b,9 compound VI-1 and its methyl derivative, neocryptolepine VI-2 were prepared smoothly from our resulting indolo[2,3-b]quinoline 3o (eqn (7)). We launched a formal synthesis of the third target, methylneocryptolepine VI-3 based on our new method. To our pleasure, the gold-catalyzed reactions of 3-methylbenzisoxazole 2l with ynamide 1o in hot DCE (70 °C, 1 h) afforded the desired product 8a in 34% yield, which was convertible to the key intermediate 8b before preceding to the desired alkaloid VI-3 (eqn (8)).10
1) under reflux in 27 h, from which we obtained compound 8d in 76% yield. In the literature,5a,b,9 compound VI-1 and its methyl derivative, neocryptolepine VI-2 were prepared smoothly from our resulting indolo[2,3-b]quinoline 3o (eqn (7)). We launched a formal synthesis of the third target, methylneocryptolepine VI-3 based on our new method. To our pleasure, the gold-catalyzed reactions of 3-methylbenzisoxazole 2l with ynamide 1o in hot DCE (70 °C, 1 h) afforded the desired product 8a in 34% yield, which was convertible to the key intermediate 8b before preceding to the desired alkaloid VI-3 (eqn (8)).10
 | (6) |
 | (7) |
 | (8) |
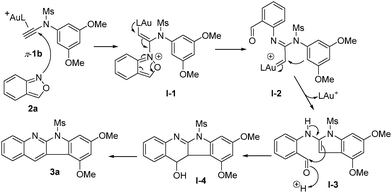
Scheme 3 shows a proposed mechanism of this gold catalysis. An initial N-attack3 of benzisoxazole 2a at π-alkyne complex 1a yielded alkenyl gold species I-1, further producing gold carbenes I-2via a cleavage of the N–O bond. At high temperatures, the N-aryl nucleophile is conformationally flexible to attack gold carbenes I-2 preferably, generating reactive indole species I-3. An addition of this indole at the benzaldehyde is expected to yield the azacyclic product 3avia the dehydration of species I-4.
In summary, we have designed a new path for the gold-catalyzed annulations11 of N-aryl ynamides12,13 with benzisoxazoles to construct 6H-indolo[2,3-b]quinoline frameworks. Besides the electron-rich property of the N-aryl group, the success of these reactions relies on the operations at high temperatures to enable a N-aryl group to attack gold carbenes, forming indoles that attack again at the benzaldehyde. This high-temperature effect avoids the formation of 2-amino-7-formylindoles by the Hashmi's reaction.3a The utility of this method is manifested by a satisfactory range of N-aryl ynamides and benzisoxazoles. Our preliminary results indicate that a phenoxyethyne is also an applicable substrate. To manifest the utility of this new method, we have completed the formal synthesis of three naturally occurring alkaloids based on this new catalysis.
We thank the Ministry of Education (MOE 106N506CE1) and the Ministry of Science and Technology (MOST 107-3017-F-007-002), Taiwan, for financial support of this work.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) D. B. Huple, S. Ghorpade and R.-S. Liu, Adv. Synth. Catal., 2016, 358, 1348 CrossRef; (b) L. Li, T.-D. Tan, Y.-Q. Zhang, X. Liu and L.-W. Ye, Org. Biomol. Chem., 2017, 15, 8483 RSC.
- (a) A.-H. Zhou, Q. He, C. Shu, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L.-W. Ye, Chem. Sci., 2015, 6, 1265 RSC; (b) X.-Y. Xiao, A.-H. Zhou, C. Shu, F. Pan, T. Li and L.-W. Ye, Chem. – Asian J., 2015, 10, 1854 CrossRef PubMed; (c) W.-B. Shen, X.-Y. Xiao, Q. Sun, B. Zhou, X.-Q. Zhu, J.-Z. Yan, X. Lu and L.-W. Ye, Angew. Chem., Int. Ed., 2017, 56, 605 CrossRef PubMed; (d) S. S. Giri and R.-S. Liu, Chem. Sci., 2018, 9, 2991 RSC; (e) B. D. Mokar, P. D. Jadhav, Y. B. Pandit and R.-S. Liu, Chem. Sci., 2018, 9, 4488 RSC.
- (a) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794 CrossRef PubMed; (b) H. Jin, B. Tian, X. Song, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 12688 CrossRef PubMed.
- (a) R. L. Sahani and R.-S. Liu, Angew. Chem., Int. Ed., 2017, 56, 1026 CrossRef PubMed; (b) R. L. Sahani and R.-S. Liu, Angew. Chem., Int. Ed., 2017, 56, 12736 CrossRef PubMed.
- (a) H. K. Kadam and S. G. J. Tilve, Heterocycl. Chem., 2016, 53, 2066 CrossRef; (b) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Curr. Org. Chem., 2011, 15, 1036 CrossRef.
- (a) G. V. Subbaraju, J. Kavitha, D. Rajasekhar and I. J. Jimenez, J. Nat. Prod., 2004, 67, 461 CrossRef PubMed; (b) K. Cimanga, T. D. Bruyne, L. Pieters and A. J. Vlietinck, J. Nat. Prod., 1997, 60, 688 CrossRef PubMed; (c) S. V. Miert, S. Hostyn, B. U. W. Maes, K. Cimanga, R. Brun, M. Kaiser, P. Mátyus, R. Dommisse, G. Lemiére, A. Vlietinck and L. J. Pieters, Nat. Prod., 2005, 68, 674 CrossRef PubMed; (d) P.-C. Wanda, F. Pognan, E. Kaczmarek and J. Boratyliski, J. Med. Chem., 1994, 37, 3503 CrossRef.
- X-ray crystallographic data of compound 3a were deposited at Cambridge Crystallographic Data Center (CCDC 1840927)†.
- For the gold-catalyzed reactions of alkoxyalkynes, see selected examples: (a) A. Zeiler, M. J. Ziegler, M. Rudolph, F. Rominger and A. S. K. Hashmi, Adv. Synth. Catal., 2015, 357, 1507 CrossRef; (b) J. S. Alford and H. M. L. Davies, J. Am. Chem. Soc., 2014, 136, 10266 CrossRef PubMed; (c) Y. Nishimoto, K. Kang and M. Yasuda, Org. Lett., 2017, 19, 3927 CrossRef PubMed.
- P. Molina, P. M. Molina and S. Delgado, Synthesis, 1999, 326 CrossRef.
- G. S. M. Sundaram, C. Venkatesh, U. K. Syamkumar, H. Ila and H. Junjappa, J. Org. Chem., 2004, 69, 5760 CrossRef PubMed.
- (a) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef PubMed; (b) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef PubMed; (c) S. Abu Sohel and R. S. Liu, Chem. Soc. Rev., 2009, 38, 2269 RSC; (d) M. E. Muratore, A. Homs, C. Obradors and A. M. Echavarren, Chem. – Asian J., 2014, 9, 3066 CrossRef PubMed.
- Recent reviews for ynamides, see: (a) X. N. Wang, H. S. Yeom, L. C. Fang, S. He, Z. X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560 CrossRef PubMed; (b) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef PubMed; (c) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840 CrossRef PubMed; (d) G. Evano, C. Theunissen and M. Lecomte, Aldrichimica Acta, 2015, 48, 59 Search PubMed; (e) T. Lu, Z. Lu, Z.-X. Ma, Y. Zhang and R. P. Hsung, Chem. Rev., 2013, 113, 4862 CrossRef PubMed.
- For ynamides bearing a reactive N-aryl group, see: (a) C. Shu, Y.-H. Wang, B. Zhou, X.-L. Li, Y.-F. Ping, X. Lu and L.-W. Ye, J. Am. Chem. Soc., 2015, 137, 9567 CrossRef PubMed; (b) L. Li, X.-M. Chen, Z.-S. Wang, B. Zhou, X. Liu, X. Lu and L.-W. Ye, ACS Catal., 2017, 7, 4004 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1840927. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc04264k |
| This journal is © The Royal Society of Chemistry 2018 |