Deciphering the biochemical similarities and differences among mouse embryonic stem cells, somatic and cancer cells using ATR-FTIR spectroscopy
Günnur
Güler
 *a,
Eda
Acikgoz
*bc,
N. Ülkü
Karabay Yavasoglu
d,
Buket
Bakan
d,
Erik
Goormaghtigh
*a,
Eda
Acikgoz
*bc,
N. Ülkü
Karabay Yavasoglu
d,
Buket
Bakan
d,
Erik
Goormaghtigh
 e and
Huseyin
Aktug
b
e and
Huseyin
Aktug
b
aCenter for Drug Research & Development and Pharmacokinetic Applications (ARGEFAR), Ege University, 35100, Izmir, Turkey. E-mail: gunnurgorucu@gmail.com; Tel: +90 232 390 4171
bDepartment of Histology and Embryology, Faculty of Medicine, Ege University, 35100, Izmir, Turkey. E-mail: acikgozedaa@gmail.com; Tel: +90 232 390 5900
cDepartment of Histology and Embryology, Faculty of Medicine, Yuzuncu Yil University, 65080, Van, Turkey
dDepartment of Biology, Faculty of Science, Ege University, 35100, Izmir, Turkey
eLaboratory of Structure and Function of Biological Membranes, Center of Structural Biology and Bioinformatics, Université Libre de Bruxelles, Brussels, Belgium
First published on 9th February 2018
Abstract
Cellular macromolecules play important roles in cellular behaviors and biological processes. In the current work, cancer (KLN205), normal (MSFs) and mouse embryonic stem cells (mESCs) are compared using ATR-FTIR spectroscopy. Modifications in the composition, concentration, structure and function-related changes in the cellular components were deciphered using the infrared spectra. Our results revealed that cancer and embryonic stem cells are very similar but highly different from the normal cells based on the spectral variations in the protein, lipid, carbohydrate and nucleic acid components. The longest lipid acyl chains exist in mESCs, while cancer cells harbor the lowest lipid amount, short lipid acyl chains, a high content of branched fatty acids and thin cell membranes. The highest cellular growth rate and accelerated cell divisions were observed in the cancer cells. However, the normal cells harbor low nucleic acid and glycogen amounts but have a higher lipid composition. Any defect in the signaling pathways and/or biosynthesis of these cellular parameters during the embryonic-to-somatic cell transition may lead to physiological and molecular events that promote cancer initiation, progression and drug resistance. We conclude that an improved understanding of both similarities and differences in the cellular mechanisms among the cancer, normal and mESCs is crucial to develop a potential clinical relevance, and ATR-FITR can be successfully used as a novel approach to gain new insights into the stem cell and cancer research. We suggest that targeting the cellular metabolisms (glycogen and lipid) can provide new strategies for cancer treatment.
Introduction
Embryonic stem cells (ESCs), derived from the inner cell mass of the blastocyst, have two unique properties: they are able to proliferate without differentiation by a process of self-renewal and to differentiate into specialized cell types in the body.1 Thus, ESCs are very promising candidates for replacement cell/tissue therapy.2 Unlike normal (somatic) cells, cancer cells have different characteristics such as the proliferation rate, cell cycle, energy metabolism, changes in the biological structure and control of biological processes,3,4 and are known to have different properties in cellular biochemicals.2 Cancer cells share abnormal behavior patterns, involving uncontrolled growth, the ability to invade other tissues and their social relationship with the neighboring cells.3–5Cells show great differences in terms of behaviour, determination, and creating their own internal dynamic balances during the embryonic, fetal and adult life. From the earliest stage of life, cells are drifting in the triangle of proliferation, migration and apoptosis with their metabolic processes, energy distribution, entropy and their own divisional dynamics. The reason for choosing embryonic pluripotent cells (mouse embryonic stem cells), somatic cells (fibroblasts) and cancer cells (lung Ca) in our study is to determine what factors are effective in this chaos. In fact, ESCs, cancer and normal cells share several common features. Particularly, there are many connections between the ESCs and cancer cells, which are important to figure out the cancerous mechanism and embryogenesis at the molecular level.6 Metabolic changes associated with the cellular biochemicals play crucial roles in cellular behaviors and biological processes. The biochemical characterization of ESCs is important for determining how they will act during proliferation and differentiation processes, and thus, can provide important novel knowledge on how these cells behave and relate with the cancer cells. In the field of cancer research, it was formerly proven that lipid signaling is essential in cell signaling.7 In this regard, several types of lipids were determined as signaling molecules and cellular messengers.8 Particularly, the association of free fatty acids, fatty acid synthesis and β-oxidation catabolic processes (degradation of fatty acid molecules) with cancer development and progression were also reported.9 Glycogen metabolism, upregulated in many cancer types, plays a pivotal role in cancer.10 Although there is detailed knowledge on these cells, the molecular mechanisms and regulatory pathways involved in carcinogenesis (transformation of normal cells into the cancer cells), tumor recurrence and metastasis are still under debate.
Fourier transform infrared (FTIR) spectroscopy has been successively used for the sensitive and precise probing of biological specimens such as tissue sections, cells and body fluids. The analysis of cells with the FTIR technique provides significant information on the structure, dynamics and biochemical content of cellular macromolecules such as proteins, lipids, nucleic acids and carbohydrates. This approach also enables us to obtain comprehensive information regarding the cellular events (cell growth, cell cycle, proliferation, differentiation, cell death and apoptosis).11–14 Recently, FTIR spectroscopy as a sensitive and cost-effective technique has been applied to the study of a variety of cell lines (i.e., cancer cells and stem cells) to figure out the biochemical alterations induced under environmental conditions, during cell cycle or upon the addition of drugs.11,15–17 This technique is capable of showing the structural changes of cells at the molecular level in various types of cancers. Formerly, it was demonstrated that the vibrational spectra of nucleic acids in the cancer and normal cells are different from each other and the spectral differences are attributable to identify unusual RNA and DNA levels.18 Thus, the spectral differences between the normal and cancer cells could provide important clues for understanding tumorigenesis. Previous FTIR studies on ESCs have shown that the changes of the protein composition and lipid content of ESCs were associated with the differentiation process.19–21 Although there are numerous studies on the differentiation of embryonic stem cells or on the comparison of the normal cells and cancer cells for diagnostic purposes, we have encountered no study so far in the literature, which is associated with embryogenesis and carcinogenesis based on the investigation and comparison of the normal, cancer and stem cells. The number of studies that compare the embryonic stem cells and cancer cells is limited. In our previous study, we compared the embryonic stem cells, cancer and normal cells (not with ATR-FTIR spectroscopy) and we found significant similarities and differences in the molecular mechanisms between embryogenesis and carcinogenesis which may be crucial in developing novel therapeutics that specifically target the cancer cells.6 At present, we wanted to go a step further using ATR-FTIR spectroscopy, which is rapid, non-destructive and does not require special sample preparation in comparison with other techniques.
To the best of our knowledge, this is the first study reporting the use of a label-free FTIR technique to identify similarities and differences in the proteomic, lipidemic, genomic as well as the metabolic states of the mouse embryonic stem cells (mESCs), mouse skin fibroblast cells (MSFs) as somatic cells and mouse lung squamous carcinoma cells (KLN205) simultaneously. The comparison of these cells might help us understand the mechanisms of embryogenesis and carcinogenesis, and thus, the pathways involved in the transformation of the normal cells into the cancer cells. Here, we show that the FTIR technique paves a way for understanding the mechanisms of cellular processes at the molecular scale as well as for the holistic assessment of cell functionalities. In this work, we were able to determine the metabolic changes and specific IR markers of cellular components that play pivotal roles in the progress of a cancerous mechanism. Thus, this study sheds light on how cellular biochemical structures can contribute to the molecular mechanisms of tumorigenesis, and thus, may serve us to develop new therapeutics against cancer.
Materials and methods
Cell culture
Mouse embryonic stem cells (mESCs) were purchased from Celprogen (Torrance, CA, USA) and cultured in a mouse embryonic stem cell expansion medium with serum and antibiotics (Celprogen) using extracellular matrix-coated cell culture plates as recommended by the supplier. Mouse skin fibroblast cells (MSFs; Clone III8C, ATCC-CRL-2017™) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The MSF cells were cultured in McCoy's 5a medium supplemented with 10% FBS. Mouse lung squamous carcinoma cells (SqCLCs, KLN205) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Bio. Ind., Kibbutz Beit-Haemek, Israel). All cell lines were maintained under an atmosphere of 5% CO2 at 37 °C. The growth and morphology were daily checked microscopically to ensure cell health.Hematoxylin and eosin staining protocol
To examine morphological changes, the mESC, KLN205 and MSF cells at a density of 5 × 105 cells per mL were seeded in chamber slides under a 5% CO2 atmosphere at 37 °C in a humidified chamber, and subjected to hematoxylin and eosin staining. Briefly, the cells were fixed in 4% paraformaldehyde for 15 minutes and washed with PBS (phosphate buffered saline) three times. The cells were stained with hematoxylin, and incubated at room temperature for 10 min and washed with tap water for the development of blue color. Then, the cells were subsequently dyed with eosin for 45 s and dehydrated with 70% ethanol. The cells were washed with PBS and mounted on a mounting medium. All slides were photographed with an Olympus C-5050 digital camera mounted on an Olympus BX51 microscope. The hematoxylin and eosin staining is based on differentiating the nuclei from the cytoplasm. The hematoxylin stains the nuclei blue/purple whereas the eosin stains the cytoplasm pink/red.Sample preparation
For FTIR spectroscopy, three kinds of cell lines (mESCs, MSFs and KLN205) were cultured in T25 flasks. The medium was changed every 2 days and cells were transferred from a T-25 flask to a T-75 flask using 0.05% trypsin (Sigma-Aldrich) as they reached confluence. Before harvesting the cells, the medium was thrown away and the cells were quickly washed with 1 ml 0.05% trypsin (Sigma-Aldrich) to remove the dead cell debris. After this, the cells were detached from their culture support by means of a five-minute treatment with 1 ml of trypsin buffer at 37 °C in a 5% CO2 environment. The reaction was stopped by rinsing the cells with 5 ml of culture medium and with 10% FBS (fetal bovine serum), and was centrifuged (Nuve NF200; Laboratory and Sterilization Technology, Ankara, Turkey) at 300g for 2 min. After centrifugation, the cells were washed three times with sterile isotonic solution (NaCl, 0.9%) to ensure the removal of trypsin and the culture medium completely. The cells were then resuspended in the NaCl solution for ATR-FTIR analysis. Three independent cultures were grown for each condition.ATR-FTIR spectroscopic measurements
All measurements were performed at room temperature with an IRTracer-100 FTIR spectrometer (Shimadzu, Japan) combined with an attenuated total reflection (ATR) unit and equipped with a DLATGS detector. A 2 μl amount of the cell suspension (about 1 × 106 cells per ml) was deposited on a Diamond/ZnSe lens single reflection ATR plate and dried at room temperature for about 10 minutes under dry air purge conditions to eliminate water excess before measurements. Three independent cultures were grown for each condition and three samples taken from each culture were measured so that the measurements were triplicated. At least five spectra per each sample were recorded in the range of 4000–800 cm−1 after drying the sample on the ATR plate (3 cultures, 3 samples per culture, 5 spectra per sample gives at least 15 spectra per cell type). A total of 128 scans were averaged for each interferogram at a 4 cm−1 spectral resolution. The air spectrum was recorded as the background when the ATR plate was empty.Data analyses
The correction of the IR spectra, Kolmogorov–Smirnov test, difference spectra and Student's t-tests as well as hierarchical cluster analysis were carried out using the software Kinetics (provided kindly by Prof. Dr Erik Goormaghtigh from Université Librede Bruxelles, Belgium) running under MATLAB.For each cell type, the normality of the distribution of the absorbances was controlled at every wavenumber by the Kolmogorov–Smirnov test in comparison with a standard normal distribution, with a confidence level α = 0.5% (data not shown) as described previously.17 The results showed that all absorbance points were normally distributed.
Results and discussion
Cellular morphology of the mESC, KLN205 and MSF cells in vitro
Cellular morphology is the reflection of the biological processes of cells. The morphological characterization of cells is important to study the cellular organization and the physiological state of the cells. Microscopy is routinely used to examine the cellular morphology (Fig. 1). mESCs appear as round cells with clear margins and exhibit prominent nucleoli and a high nucleus-to-cytoplasm volume ratio. The KLN205 cells are characterized by a highly irregular shape and a large nucleus; the nucleus and the nucleolus are plainly visible, and the cytoplasm is scarce and intensely colored or, on the contrary, less stained than the nucleus. The MSF cells have an elongated-spindle shape with a centrally placed oval or round nucleus. When compared with the MSF cells, the mESC and KLN205 cells exhibit a high nucleus/cytoplasm volume ratio (Fig. 2). It is well known that the nucleus/cytoplasm volume ratio is high in the embryonic stem cells and cancer cells.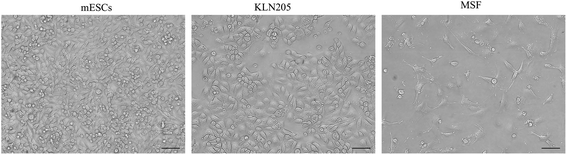 | ||
| Fig. 1 Morphology of the mESC, KLN205 and MSF cells using an inverted light microscope (magnification 20×). | ||
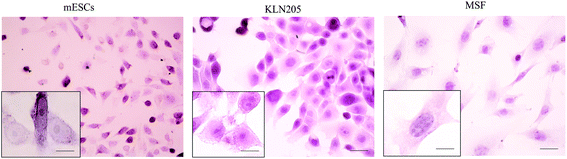 | ||
| Fig. 2 Hematoxylin–eosin staining results of the mESC, KLN205 and MSF cells in vitro (magnification 40× and 100×). | ||
FTIR-difference spectra and Student's t-test
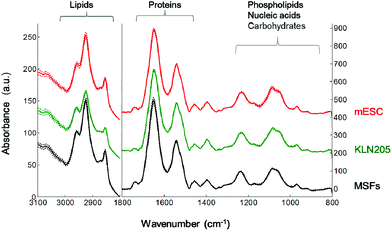
Fig. 3 represents the mean FTIR spectra of the mouse embryonic stem cells, somatic and cancer cells recorded in the mid-IR spectral region of 4000–800 cm−1. The region is dominated by the spectral features of cellular macromolecules that exhibit characteristic absorption bands. The mean absorbance spectra of the mESC, MSF and KLN205 cells seem to be very similar at first glance. However, significant spectral variations are noticed in the lipid CH region (3000–2800 cm−1), protein amide I band (1700–1600 cm−1) and the fingerprint region (1300–800 cm−1). The latter is dominated by the absorbance of phospholipids, nucleic acids, carbohydrates and metabolites. Thus, in the current study, the FTIR spectroscopic approach enables a rapid and label-free monitoring of the proteomic, lipidemic, genomic and metabolic variations among the mESC, MSF and KLN205 cells.The FTIR spectral features were examined more in detail by applying the difference spectrum. The IR-difference spectrum is frequently used to capture the subtle spectral alterations that are not easily detected in the absorbance spectrum. Fig. 4 displays the difference spectrum calculated from the mean absorbance spectra shown in Fig. 3. Absorbance differences (ΔA) are represented by the positive (+) and negative (−) signals, indicating the minute spectral changes in the biochemical variations of these cells. Overall, these minor spectral modifications can be due to the conformational changes, dynamics and/or relative concentrations of the cellular macromolecules.
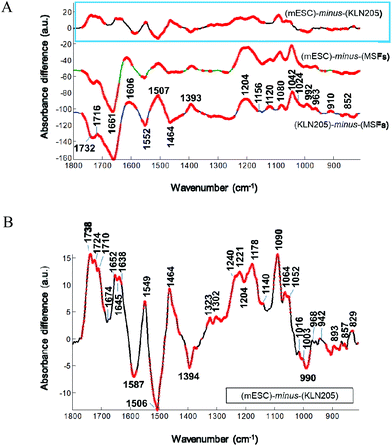
When the absorbance spectrum of the MSFs was subtracted separately from the KLN205 and mESC cell lines, represented by ΔA = (KLN205) − (MSFs) and ΔA = (mESCs) − (MSFs), respectively, the band shapes of their resultant difference spectra are quite similar (Fig. 4A). Herein, thicker lines represent the significant differences in absorbance calculated by the Student's t-test (α = 0.1%). The negative and positive peaks are detected at similar wavenumbers with comparable intensities, indicating variations in the concentrations of cellular macromolecules. In comparison with the MSFs, the absorbance value at around 1732 cm−1, attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band mode of the fatty acid esters,23 decreases both in the case of KLN205 and mESCs. The observation of the negative peaks at 1661 cm−1 and 1552 cm−1 (due to α-helices)22,24,25 clearly demonstrates a reduction of the amount of regular protein structures. Additionally, the positive peaks at 1600–1580 and at 1393 cm−1, which correspond to the anionic carboxylate groups (anti-symmetric and symmetric stretching vibrations of COO− groups, respectively),26 were detected suggesting an increment in the content of anionic metabolites (strongest in the KLN205 cells). Furthermore, the detection of multiple positive peaks below 1250 cm−1 indicates a change in the content of cellular macromolecules containing phosphate groups and carbohydrates. Particularly, broad and strong positive peaks at around 1230–1204 cm−1 and 1080 cm−1 were detected for both KLN205 and mESCs, respectively. These IR peaks could arise most likely from the anti-symmetric and symmetric P–O stretching vibrations, respectively, tentatively assigned to nucleic acids (DNA/RNA)16,23 due to the fact that the absorbance values of the fatty acid moieties (ca. 3000–2800 cm−1 in Fig. 6) are low in both KLN205 and mESCs with respect to the MSFs and this will be discussed later. Significant increments in the IR peaks at 963, 910 and 852 cm−1, which are due to sugar–phosphate skeletal motions in nucleic acids23,27 as well as a positive signal at around 1716 cm−1 due to the DNA base pair vibration23,27,28 were detected in the difference spectra as well. These altogether suggest the existence of a less compact, a higher amount and/or a conformational change of DNA in both the cancer and mouse embryonic stem cells in comparison with the normal cells. These observations are consistent with the former studies.16,29–31 They reported that a very compact DNA has a low spectral absorbance due to the loss of Beer law linearity while a less densely packed DNA gives rise to a high absorbance value because of the increased nucleic acid synthesis activity. In our work, the detection of the positive bands at around 1120 and 992 cm−1, tentatively assigned to the RNA ribose ring and uracil,27,28 can be explained by an increased amount of RNA molecules in the KLN205 (highest) and mESC cells. A higher absorption at these wavenumbers associated with the nucleic acids for the cancer cells and mouse embryonic stem cells may be indicative of cellular events such as cell cycle phases, and accelerated cell growth and division. Thus, the observation of spectral alterations in the phosphate region of nucleic acids clearly demonstrates the potential difference in the nucleic acid abundance, differentiation and conformation among the cancer, normal and embryonic stem cells. Additionally, the detection of pronounced positive bands located around 1024 (shoulder) and 1042 cm−1 is a strong indicative of a higher amount of polymeric sugars like glycogen in the KLN205 and mESC cells with respect to the MSFs (for band assignments see ref. 23 and 29).
O stretching band mode of the fatty acid esters,23 decreases both in the case of KLN205 and mESCs. The observation of the negative peaks at 1661 cm−1 and 1552 cm−1 (due to α-helices)22,24,25 clearly demonstrates a reduction of the amount of regular protein structures. Additionally, the positive peaks at 1600–1580 and at 1393 cm−1, which correspond to the anionic carboxylate groups (anti-symmetric and symmetric stretching vibrations of COO− groups, respectively),26 were detected suggesting an increment in the content of anionic metabolites (strongest in the KLN205 cells). Furthermore, the detection of multiple positive peaks below 1250 cm−1 indicates a change in the content of cellular macromolecules containing phosphate groups and carbohydrates. Particularly, broad and strong positive peaks at around 1230–1204 cm−1 and 1080 cm−1 were detected for both KLN205 and mESCs, respectively. These IR peaks could arise most likely from the anti-symmetric and symmetric P–O stretching vibrations, respectively, tentatively assigned to nucleic acids (DNA/RNA)16,23 due to the fact that the absorbance values of the fatty acid moieties (ca. 3000–2800 cm−1 in Fig. 6) are low in both KLN205 and mESCs with respect to the MSFs and this will be discussed later. Significant increments in the IR peaks at 963, 910 and 852 cm−1, which are due to sugar–phosphate skeletal motions in nucleic acids23,27 as well as a positive signal at around 1716 cm−1 due to the DNA base pair vibration23,27,28 were detected in the difference spectra as well. These altogether suggest the existence of a less compact, a higher amount and/or a conformational change of DNA in both the cancer and mouse embryonic stem cells in comparison with the normal cells. These observations are consistent with the former studies.16,29–31 They reported that a very compact DNA has a low spectral absorbance due to the loss of Beer law linearity while a less densely packed DNA gives rise to a high absorbance value because of the increased nucleic acid synthesis activity. In our work, the detection of the positive bands at around 1120 and 992 cm−1, tentatively assigned to the RNA ribose ring and uracil,27,28 can be explained by an increased amount of RNA molecules in the KLN205 (highest) and mESC cells. A higher absorption at these wavenumbers associated with the nucleic acids for the cancer cells and mouse embryonic stem cells may be indicative of cellular events such as cell cycle phases, and accelerated cell growth and division. Thus, the observation of spectral alterations in the phosphate region of nucleic acids clearly demonstrates the potential difference in the nucleic acid abundance, differentiation and conformation among the cancer, normal and embryonic stem cells. Additionally, the detection of pronounced positive bands located around 1024 (shoulder) and 1042 cm−1 is a strong indicative of a higher amount of polymeric sugars like glycogen in the KLN205 and mESC cells with respect to the MSFs (for band assignments see ref. 23 and 29).
Similarity of the spectral differences for the KLN205 and mESC cells can be straightforwardly visualized in Fig. 4A. Overall, this indicates that the cellular constitutions of these cell lines are quite similar in terms of their structure, composition and concentration. However, these cells are completely different from the MSFs. Although the difference spectra of the KLN205 and mESC cells seem to be very similar at first glance, their subtle variations could be detected when the mean absorbance spectrum of the KLN205 cells was subtracted from the mean absorbance spectrum of the mESCs, represented by ΔA = (mESCs) − (KLN205) (Fig. 4B). This indicates the sensitivity of the FTIR approach to discriminate to some extent between the cancer cells and mouse embryonic stem cells. For instance, strong positive peaks at 1738, 1724 and 1710 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands) are observed for the mESCs. The splitting and shifting of the ester carbonyl stretching bands at around 1750–1715 cm−1 are usually assigned to triglycerides and cholesterol esters32 probably due to the different forms of carbonyl–water interactions.33 Besides these, the strong positive bands at 1464 cm−1 (scissoring mode of the CH2/CH3 groups) and 1178 cm−1 (C–O–C stretching mode) for the mESCs are attributed to the ester moieties.32 These altogether suggest that the contents of the lipid esters such as phospholipids, triglycerides and cholesterol esters are abundant in the mESCs in comparison with the cancer cells (lowest in the KLN205 cells among other cell types). Previous studies have also reported a low content of cholesterol in some malignant tissues.34,35 Herein, our FTIR data imply that lipid metabolism differs among the cancer, normal and mouse embryonic stem cells. Thus, the variation of the lipid mechanism will be discussed more in detail in the next section. It is worth noting that the observation of negative peaks at 1587 and 1394 cm−1 for the mESCs indicates a high amount of anionic carboxylate groups most likely associated with the cellular metabolites in the cancer cells. In the protein amide I region, the observation of the peaks at 1652(+), 1645(−) and 1638(+) clearly demonstrates that the mESCs have high ordered protein secondary structures (α-helix and β-sheets) than the cancer cells. Moreover, the detection of high intensities for the phosphate bands located at 1240 (less or non H-bonded) and 1221 (H-bonded) cm−1 as well as at 1090 and 968 cm−1 suggests that the mESCs harbour a high content of phospholipids (at least two populations of phosphate groups which are differently H-bonded). Since the IR signals of fatty acid acyl chains are higher in the mESCs than KLN205 (Fig. 6), these bands associated with the phosphate groups are tentatively attributed to the cell membrane phospholipids. Multiple weak positive bands positioned at 1140, 1064 and 1052 cm−1 imply a slight increase in the amounts of glycogen and some kinds of polysaccharides29 in the embryonic stem cells with respect to the cancer cells. A negative band at 990 and 1120 cm−1 attributed most likely to RNA27,28 was also detected in the difference spectrum, indicating a low amount of RNA in the mESCs when compared to the cancer cells. The observation of multiple negative and positive peaks below 900 cm−1 indicates the existence of strong changes in the N-type sugar conformations in the nucleic acid backbone between the mESC and cancer cells.
O stretching bands) are observed for the mESCs. The splitting and shifting of the ester carbonyl stretching bands at around 1750–1715 cm−1 are usually assigned to triglycerides and cholesterol esters32 probably due to the different forms of carbonyl–water interactions.33 Besides these, the strong positive bands at 1464 cm−1 (scissoring mode of the CH2/CH3 groups) and 1178 cm−1 (C–O–C stretching mode) for the mESCs are attributed to the ester moieties.32 These altogether suggest that the contents of the lipid esters such as phospholipids, triglycerides and cholesterol esters are abundant in the mESCs in comparison with the cancer cells (lowest in the KLN205 cells among other cell types). Previous studies have also reported a low content of cholesterol in some malignant tissues.34,35 Herein, our FTIR data imply that lipid metabolism differs among the cancer, normal and mouse embryonic stem cells. Thus, the variation of the lipid mechanism will be discussed more in detail in the next section. It is worth noting that the observation of negative peaks at 1587 and 1394 cm−1 for the mESCs indicates a high amount of anionic carboxylate groups most likely associated with the cellular metabolites in the cancer cells. In the protein amide I region, the observation of the peaks at 1652(+), 1645(−) and 1638(+) clearly demonstrates that the mESCs have high ordered protein secondary structures (α-helix and β-sheets) than the cancer cells. Moreover, the detection of high intensities for the phosphate bands located at 1240 (less or non H-bonded) and 1221 (H-bonded) cm−1 as well as at 1090 and 968 cm−1 suggests that the mESCs harbour a high content of phospholipids (at least two populations of phosphate groups which are differently H-bonded). Since the IR signals of fatty acid acyl chains are higher in the mESCs than KLN205 (Fig. 6), these bands associated with the phosphate groups are tentatively attributed to the cell membrane phospholipids. Multiple weak positive bands positioned at 1140, 1064 and 1052 cm−1 imply a slight increase in the amounts of glycogen and some kinds of polysaccharides29 in the embryonic stem cells with respect to the cancer cells. A negative band at 990 and 1120 cm−1 attributed most likely to RNA27,28 was also detected in the difference spectrum, indicating a low amount of RNA in the mESCs when compared to the cancer cells. The observation of multiple negative and positive peaks below 900 cm−1 indicates the existence of strong changes in the N-type sugar conformations in the nucleic acid backbone between the mESC and cancer cells.
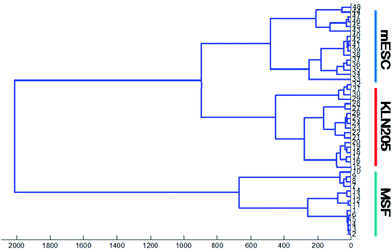
Hierarchical cluster analysis (HCA)
In the current study, HCA is performed for the spectral regions of 1800–800 cm−1 in order to classify and determine the similarity of the FTIR spectra of the cancer, mESC and normal cells. Cluster analysis presents the cluster members with respect to the biochemical similarities with a maximum heterogeneity level in the spectral regions of interest. A high heterogeneity value indicates the existence of significant biochemical alterations among all types of cell lines. It is obvious that the normal, mESC and cancer cell lines probed by FTIR were successfully discriminated using hierarchical classification.Fig. 5 plotted for the absorbance spectra in the spectral range of 1800–800 cm−1 (lipid esters, proteins, nucleic acids and sugar regions) shows that the FTIR spectra of the cancer cells and mESCs harbor significant similarities (first group) whereas the normal cells are clustered separately as a second group, which was distinctly separated from others. Proximity between the cancer cells and mESCs implies that these cells are quite similar in terms of biochemical variations such as lipid esters, proteins, nucleic acids as well as carbohydrates. However, the normal cells are well distinguished from the cancer cells and mESCs. This result is in line with our previous findings mentioned above.
Comparison among the mouse embryonic stem cells, somatic cells and cancer cells
The comparison of the biochemical features among the embryonic stem cells, normal cells and cancer cells is important for determining the cellular behaviors and biological processes. The use of skin cells as a model of “normal” cells and “lung” cells as a model for cancer cells may appear as a limitation. The problem is that there is no perfect model as the normal cells are very difficult to grow in vitro and, when they grow, they lack an environment (physical contact with other cells and chemical messengers provided by neighboring cells). In turn, they adopt a phenotype that is far from the “in vivo” phenotype. On the other hands, the cancer cells can proliferate much more easily in vitro and their phenotype is less affected by the absence of an adequate environment. The rationale for using skin fibroblasts is that these cells are able to grow in cultures without being too much affected by the environment. They are therefore a better model for normal cells which exhibit similar cellular properties in terms of genetic, molecular and biochemical compositions.In the present study, the IR spectral signatures of cellular macromolecules could provide novel knowledge on the close behavior and relationship between the ESCs and cancer cells that are dramatically distinguished from the normal cells. Thus, variations in the mechanisms of lipids, proteins, carbohydrates and nucleic acids will be discussed more in detail below.
Lipid region
Lipids are involved in many biological processes and oncogenic signaling pathways, thus, probing of lipids is crucial for understanding the molecular mechanisms of tumorigenesis. Fig. 6 illustrates the FTIR spectra showing the saturated and unsaturated lipid C–H stretching modes in the region of 3025–2800 cm−1 for the mESC, MSF and KLN205 cells. The olefinic (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) lipid groups (∼3008 cm−1) are used to investigate the unsaturated acyl chains of lipids (having double bonds) while the anti-symmetric and symmetric stretching vibrations of the CH3 and CH2 groups existing in the acyl chains conventionally serve for the analysis of the physical properties (dynamics, flexibility, ordering/disordering state) of the lipid acyl chains.
CH) lipid groups (∼3008 cm−1) are used to investigate the unsaturated acyl chains of lipids (having double bonds) while the anti-symmetric and symmetric stretching vibrations of the CH3 and CH2 groups existing in the acyl chains conventionally serve for the analysis of the physical properties (dynamics, flexibility, ordering/disordering state) of the lipid acyl chains.
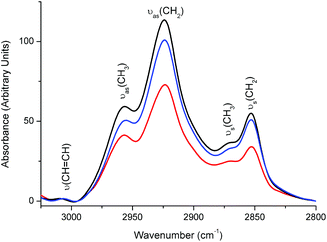 | ||
Fig. 6 The mean absorbance FTIR spectra of the mESC ( ), MSF (—) and KLN205 ( ), MSF (—) and KLN205 ( ) cells, representing the lipid C–H stretching region. ) cells, representing the lipid C–H stretching region. | ||
As described in Materials and methods, all spectra were normalized for an equal area in the amide II band so that the protein concentration is constant. Keeping the amino acid concentration constant, we can compare the lipid changes among the cell lines. The detection of the overall changes in the CH3 and CH2 groups corresponds to lipid alterations relative to the protein concentration. Accordingly, the absorbance values of the bands for both CH3 and CH2 groups associated with the lipid acyl chains have the lowest value in the case of the cancer cell lines (Fig. 6), indicating a lower population of lipid molecules with respect to the MSFs (highest population) and mESCs. This result was also supported by a decrease in the absorbance of the lipid CH2 scissoring mode at around 1464 cm−1 (lowest in the KLN205 cells, Fig. 4A and B). A decrease in the saturation can be due to a decrease in their biosynthesis or an enhancement in the lipid degradation due to lipid peroxidation and hypoxia upon carcinogenesis-induced oxidative stress. Compared to the normal cells (MSFs), cancer cells exhibit significant metabolic alterations with respect to several critical nutrients and substrates. Lipid peroxidation and the generation of reactive oxygen species (ROS) are common biochemical aspects in the cancer cells. Under hypoxic conditions, the ROS can react with polyunsaturated fatty acids and induce lipid peroxidation.36
The chain length in lipids can be simply obtained by calculating the ratio between CH3 and CH2 modes.16,37 In the present study, the integrated area underneath the νas(CH3) band (at ∼2958 cm−1) was divided by the integrated area underneath the νas(CH2) band (at ∼2923 cm−1). Based on our FTIR data, the value for the νas(CH3)/νas(CH2) ratio was found to be 0.313 ± 0.002 for the cancer cells, 0.226 ± 0.004 for the mESCs and 0.240 ± 0.014 for the normal cells. A high value of this ratio in the KLN205 and cancer cell lines shows the existence of short chain fatty acids, a high content of branched fatty acids and thin cell membranes which may be associated with the accelerated cell growth and cell division. On the other hand, the values of this ratio were quite close between the mESCs and MSFs (but lowest in the mESCs), suggesting a thick cell membrane with long chain fatty acids when compared to the cancer cells. This suggests that the normal cells and mESCs resemble in terms of lipid structures to some extent, but their lipid mechanisms are completely different from the cancer cells. Thus, a further investigation of the lipid composition is essential to clarify the early mechanism of tumorigenesis.
The physicochemical properties of the cell plasma membrane such as membrane fluidity play a key role in cellular processes. The νas(CH2)/νs(CH2) ratio can be used to detect the biophysical properties of the cell membrane like disordering and the freedom of motion in lipid acyl chains.38,39 This ratio was found to be slightly higher in the cancer cells (2.791 ± 0.125), indicating relatively lower disordering and slightly less motional freedom, in comparison with the mESCs (2.525 ± 0.044) and MSFs (2.646 ± 0.106). This is perhaps because of having the short chain fatty acids and a high content of branched fatty acids in the cancer cells. Alternatively, these findings may be associated with an increase in the number of trans conformers of lipid molecules in the KLN205 cells, keeping the fatty acid packing tight. FTIR spectroscopy cannot discriminate the plasma membrane lipids from the free lipids found inside the cell. Here, IR signals could also arise from the storage lipids within the cell (intracellular lipid drops) that can overweigh the IR signals of the plasma membrane lipids.
It is evident from the FTIR spectral profile of lipid specific markers that the lipid metabolism is dramatically disturbed in the cancer cells. The FTIR data revealed significant modifications in the content, composition, packing and conformational structure of the cellular lipids (both intracellular lipid particles and membrane lipids), pointing out a pivotal role of lipids in the cancer mechanism. These altogether suggest that the synthesis and regulation of the lipid metabolism is certainly different among the KLN205, mESC and MSF cells. Since the cell membrane is made up of a complex structure of lipids that is closely associated with the cell characteristics, the identification of changes occurring in the lipid structures can provide knowledge on important issues such as cancer formation, metastasis, cell motility and cell differentiation. Therefore, the lipid profiles of the cells are essential in understanding the dynamic behavior of the cells.
Protein region
The amide I and amide II bands are used to reveal the changes in the structure and conformation of the cellular proteins (Fig. 3 and 4). The protein amide I band (1700–1600 cm−1), which is the most intense protein absorption region in the spectrum, corresponds mainly to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes of the protein backbone. Thus, it is the most useful IR band for the analysis of protein secondary structures (α-helix, β-sheets, turns and random coils) and their conformations. The amide II band (1600–1500 cm−1) arises from the N–H bending, C–N and C–C stretching vibrations of the protein backbone.22,24,25 In the present work, all spectra were scaled for equal intensity in the amide II peak so that the protein amount was kept constant in the KLN205, mESCs and MSFs.
O stretching modes of the protein backbone. Thus, it is the most useful IR band for the analysis of protein secondary structures (α-helix, β-sheets, turns and random coils) and their conformations. The amide II band (1600–1500 cm−1) arises from the N–H bending, C–N and C–C stretching vibrations of the protein backbone.22,24,25 In the present work, all spectra were scaled for equal intensity in the amide II peak so that the protein amount was kept constant in the KLN205, mESCs and MSFs.
Discrepancies in the protein metabolisms can be revealed by following the amide I profile. The mESC, KLN205 and MSF cells comprise mainly of α-helices revealed by an amide I maximum at around 1651 cm−1 (Fig. 3). However, the amount of regular protein structures is high in the normal cells when compared to the mESC and cancer cells (Fig. 4A) while the mESCs have highly ordered protein secondary structures (α-helix and β-sheets) than the cancer cells (Fig. 4B) (for band assignments see ref. 24 and 25). Based on the FTIR data, it is suggested that the protein metabolism is also altered in the mechanism of tumorigenesis. The cancer cells harbour a relatively high amount of proteins that involve a high content of unordered structures with respect to the normal and mESC cells.
Fingerprint region
The vibrations of functional groups characterizing several cellular components are superimposed in the IR spectral region below 1300 cm−1, termed as the fingerprint region. The IR signals detected below 1300 cm−1 originate mainly from the phosphate groups associated with nucleic acids, phospholipids, phosphorylated proteins, ATP and inorganic phosphate metabolites. The C–O stretching vibrations of carbohydrates in the form of glycogen and other polymeric sugars as well as the CO groups in the ribose backbone of nucleic acids are also sensed in this region (for band assignments see ref. 23, 29 and 40). Alterations in the content and structure of these cellular biochemicals among the KLN205, mESC and MSF cells can be sensitively resolved in the second derivative spectra. Fig. 7 demonstrates significant changes in the band positions and intensities of major cellular molecules for the KLN205, mESC and MSF cells. Since nucleic acid and lipid changes were explicitly mentioned in the above sections, herein we will focus on the spectral region of carbohydrates.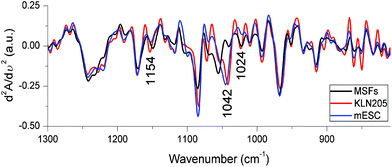 | ||
| Fig. 7 The FTIR second derivative spectra obtained from the mean absorbance spectra shown in Fig. 3. | ||
The observation of essential FTIR spectral variations in the region of 1180–1000 cm−1 reflects predominantly variations in the content and structure of carbohydrates (glycogen) among the probed cell lines. The triplet of bands absorbing at around 1150, 1042 and 1024 cm−1, due to glycogen,29,40 undergo a large shift in the band position and intensity changes as is clearly observed in the second derivative spectra (Fig. 7). This result is in accord with the observation in the difference spectra represented in Fig. 4A that the IR signal absorbing at ∼1042 cm−1 is high in both mESC (highest) and KLN205 cell lines compared to the normal cells. To support this phenomenon and to predict the approximate amount of glycogen as quantitative knowledge, the intensity of the glycogen band absorbing at 1042 cm−1 was calculated from the mean absorbance spectra shown in Fig. 3. Here, the spectra were already normalized to the same amount of protein (to the amide II band) as described in Materials and methods. Based on our data, the lowest glycogen level was detected in the normal cells (59.86 ± 4.74) while the highest glycogen amount was found in the mESCs (mESCs = 83.05 ± 3.07 and cancer cells = 75.17 ± 1.72). The level of glycogen of the cells is of great importance for determining stem cell's behavior under different growth conditions. Previous studies have reported that the level of glycogen synthesis in undifferentiated human embryonic stem cells (hESCs) was higher than that of the human cancer cell lines41 as well as was relatively higher than the glycogen levels in the differentiated hESCs.42 In this regard, a high level of glycogen was also detected in various types of cancers.10,43 There is a significant linkage between the energy metabolism and carbohydrates (glycogen). Thus, the observation of a high glycogen level indicates the accumulation of carbohydrate containing metabolites in the cell, signifying the energy metabolism. This might be associated with synthesis and cell proliferation. The accumulation of glycogen is one of the important strategies developed by cancer cells against stress conditions and improves the survival of the cancer cells. Thus, we suggest that targeting glycogen metabolism is a promising strategy to overcome the drug resistance in cancer therapy.
Conclusion
The aim of the present research was the discrimination and comparison of the normal (MSF), cancer (KLN205) and mouse embryonic stem (mESC) cells using ATR-FTIR spectroscopy. This technique provided cost-effective and label-free deciphering of biomolecular variations among these cells at short notice (a few minutes) in comparison with other traditional bioassay approaches. Using only a drop of cell (very few μl) with minimal sample handling procedures, FTIR data allowed the rapid and sensitive monitoring of the spectral features assigned to lipids, proteins, carbohydrates (glycogen), nucleic acids and other small metabolites in the probed cells. Based on the HCA results and spectral changes in these cellular components, our findings revealed the existence of significant similarities and differences among the normal, cancer and embryonic stem cells. The findings collected in the FTIR absorbance spectra and their second derivative spectra are in good agreement with the observations in the difference spectra. The variations in the major cellular macromolecules are better visualized when the bands of interest discussed above are integrated.Based on the FTIR data, undoubtedly, the cancer and embryonic stem cells are very similar but highly different from the normal cells. We found a global reduction in the fatty acids, triglycerides and/or cholesterol esters in both cancer cells and mESCs in comparison with the normal cells. However, a global increment in the content of various cellular macromolecules (glycogen, irregular protein secondary structures, both DNA and RNA and anionic metabolites) and a less compact and/or a conformational change of DNA were observed in both cancer cells and mESCs with respect to the normal cells.
Indeed, the mESC and cancer cells are more similar than they are different. However, they significantly differ in the lipid amount, acyl chain length as well as in the content and conformations of carbohydrates (or glycoconjugates), protein secondary structures and nucleic acids. The cancer cells harbor a high amount of anionic carboxylate groups most likely belonging to negatively charged oligomeric sugars like sialic acids. However, the embryonic stem cells have more lipids, glycogen and ordered protein secondary structures (α-helices) than those of the cancer cells.
In conclusion, to our knowledge, this is the first study to show the biochemical similarities and differences among the embryonic stem cells, somatic and cancer cells using ATR-FTIR spectroscopy. At present, our results provided a holistic assessment in the molecular mechanisms between carcinogenesis and embryogenesis as well as indicated the power of ATR-FTIR spectroscopy to compare these cell lines. Herein, the FTIR technique reveals that glycogen and lipids are promising biomarkers. In this regard, our data associated with the biosynthesis and regulation of the glycogen and lipid metabolisms are highly valuable due to their key roles in the cancer microenvironment and cancer cell pathophysiology. Accordingly, these differences in the cellular components of the somatic, cancer and embryonic stem cells might be new targets to find new strategies for understanding the cancer metabolism further and for cancer therapy.
Abbreviations
| mESCs | Mouse embryonic stem cells |
| MSFs | Mouse skin fibroblast cells |
| KLN205 | Mouse lung squamous carcinoma cells |
| IR | Infrared |
| FTIR spectroscopy | Fourier transform infrared spectroscopy |
| ATR | Attenuated total reflection |
| ν as | Antisymmetric stretching |
| ν s | Symmetric stretching |
| δ | Bending |
| γ | Wagging vibrations. |
Conflicts of interest
The authors declare no conflict of interest.References
- A. G. Smith, Embryo-derived stem cells: of mice and men, Annu. Rev. Cell Dev. Biol., 2001, 17, 435–462, DOI:10.1146/annurev.cellbio.17.1.435.
- P. H. Lerou and G. Q. Daley, Therapeutic potential of embryonic stem cells, Blood Rev., 2005, 19, 321–331, DOI:10.1016/j.blre.2005.01.005.
- G. I. Evan and K. H. Vousden, Proliferation, cell cycle and apoptosis in cancer, Nature, 2001, 411, 342–348, DOI:10.1038/35077213.
- M. Tarrado-Castellarnau, P. de Atauri and M. Cascante, Oncogenic regulation of tumor metabolic reprogramming, Oncotarget, 2016, 7, 62726–62753, DOI:10.18632/oncotarget.10911.
- D. F. Quail and J. A. Joyce, Microenvironmental regulation of tumor progression and metastasis, Nat. Med., 2013, 19, 1423–1437, DOI:10.1038/nm.3394.
- H. Aktug, E. Acikgoz, A. Uysal, F. Oltulu, G. Oktem, G. Yigitturk, K. Demir, A. Yavasoglu and V. Bozok Cetintas, Comparison of cell cycle components, apoptosis and cytoskeleton-related molecules and therapeutic effects of flavopiridol and geldanamycin on the mouse fibroblast, lung cancer and embryonic stem cells, Tumor Biol., 2016, 37(9), 12423–12440, DOI:10.1007/s13277-016-5108-9.
- X. Wang, Lipid signaling, Curr. Opin. Plant Biol., 2004, 7, 329–336, DOI:10.1016/j.pbi.2004.03.012.
- K. M. Eyster, The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist, Adv. Physiol. Educ., 2007, 31, 5–16, DOI:10.1152/advan.00088.2006.
- P. Saavedra-García, K. Nichols, Z. Mahmud, L. Y. Fan and E. W. Lam, Unravelling the role of fatty acid metabolism in cancer through the FOXO3-FOXM1 axis, Mol. Cell. Endocrinol., 2018, 462(Pt B), 82–92, DOI:10.1016/j.mce.2017.01.012.
- C. E. Zois and A. L. Harris, Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy, J. Mol. Med., 2016, 94, 137–154, DOI:10.1007/s00109-015-1377-9.
- A. Mignolet, A. Derenne, M. Smolina, B. R. Wood and E. Goormaghtigh, FTIR spectral signature of anticancer drugs. Can drug mode of action be identified?, Biochim. Biophys. Acta, Proteins Proteomics, 2016, 1864, DOI:10.1016/j.bbapap.2015.08.010.
- A. Salman, R. K. Sahu, E. Bernshtain, U. Zelig, J. Goldstein and S. Walfisch, et al., Probing cell proliferation in the human colon using vibrational spectroscopy: a novel use of FTIR-microspectroscopy, Vib. Spectrosc., 2004, 34, 301–308, DOI:10.1016/j.vibspec.2004.01.009.
- J. R. Mourant, Y. R. Yamada, S. Carpenter, L. R. Dominique and J. P. Freyer, FTIR spectroscopy demonstrates biochemical differences in mammalian cell cultures at different growth stages, Biophys. J., 2003, 85, 1938–1947, DOI:10.1016/S0006-3495(03)74621-9.
- D. E. Bedolla, S. Kenig, E. Mitri, P. Storici and L. Vaccari, Further insights into the assessment of cell cycle phases by FTIR microspectroscopy, Vib. Spectrosc., 2014, 75, 127–135, DOI:10.1016/j.vibspec.2014.08.007.
- A. Derenne, V. Van Hemelryck, D. Lamoral-Theys, R. Kiss and E. Goormaghtigh, FTIR spectroscopy: A new valuable tool to classify the effects of polyphenolic compounds on cancer cells., Biochim. Biophys. Acta, 2013, 1832, 46–56, DOI:10.1016/j.bbadis.2012.10.010.
- S. Kumar, T. S. Shabi and E. Goormaghtigh, A FTIR imaging characterization of fibroblasts stimulated by various breast cancer cell lines, PLoS One, 2014, 9, e111137, DOI:10.1371/journal.pone.0111137.
- A. Derenne, M. Verdonck and E. Goormaghtigh, The effect of anticancer drugs on seven cell lines monitored by FTIR spectroscopy, Analyst, 2012, 137, 3255–3264 RSC . http://pubs.rsc.org/en/content/articlepdf/2012/AN/C2AN35116A.
- G. I. Dovbeshko, V. I. Chegel, N. Y. Gridina, O. P. Repnytska, Y. M. Shirshov and V. P. Tryndiak, et al., Surface enhanced IR absorption of nucleic acids from tumor cells: FTIR reflectance study, Biopolymers, 2002, 67, 470–486, DOI:10.1002/bip.10165.
- C. Aksoy and F. Severcan, Role of Vibrational Spectroscopy in Stem Cell Research, Spectrosc. Int. J., 2012, 27(3), 167–184, DOI:10.1155/2012/513286.
- D. Ami, T. Neri, A. Natalello, P. Mereghetti, S. M. Doglia and M. Zanoni, et al., Embryonic stem cell differentiation studied by FT-IR spectroscopy, Biochim. Biophys. Acta, 2008, 1783, 98–106, DOI:10.1016/j.bbamcr.2007.08.003.
- W. Tanthanuch, K. Thumanu, C. Lorthongpanich, R. Parnpai and P. Heraud, Neural differentiation of mouse embryonic stem cells studied by FTIR spectroscopy, J. Mol. Struct., 2010, 967, 189–195, DOI:10.1016/j.molstruc.2010.01.007.
- S. Y. Venyaminov and N. N. Kalnin, Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. II. Amide absorption bands of polypeptides and fibrous proteins in alpha-, beta-, and random coil conformations, Biopolymers, 1990, 30, 1259–1271, DOI:10.1002/bip.360301310.
- Z. Movasaghi, S. Rehman and I. ur Rehman, Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues, Appl. Spectrosc. Rev., 2008, 43, 134–179, DOI:10.1080/05704920701829043.
- E. Goormaghtigh, V. Cabiaux and J. M. Ruysschaert, Determination of soluble and membrane protein structure by Fourier transform infrared spectroscopy. I. Assignments and model compounds, in Subcell. Biochem, ed. H. J. Hilderson and G. B. Ralston, Plenum Press, New York and London, 1994, vol. 23, pp. 329–362. http://www.ncbi.nlm.nih.gov/pubmed/7855877 (accessed November 05, 2012) Search PubMed.
- H. Fabian and W. Mäntele, Infrared spectroscopy of proteins, in Handb. Vib. Spectrosc, ed. J. M. Chalmers and P. R. Griffiths, John Wiley & Sons, Ltd, Chichester, UK, 2002, pp. 1–27, DOI:10.1002/0470027320.
- A. Barth, The infrared absorption of amino acid side chains, Prog. Biophys. Mol. Biol., 2000, 74, 141–173 CrossRef CAS PubMed . http://www4.ncsu.edu/~franzen/public_html/Poland/AMU_course/ftir/side_chains.pdf (accessed November 06, 2012).
- B. R. Wood, The importance of hydration and DNA conformation in interpreting infrared spectra of cells and tissues, Chem. Soc. Rev., 2016, 45, 1980–1998, 10.1039/c5cs00511f.
- G. I. Dovbeshko, N. Y. Gridina, E. B. Kruglova and O. P. Pashchuk, FTIR spectroscopy studies of nucleic acid damage, Talanta, 2000, 53, 233–246 CrossRef CAS PubMed . http://www.sciencedirect.com/science/article/pii/S0039914000004628 (accessed November 12, 2013).
- M. Diem, S. Boydston-White and L. Chiriboga, Infrared Spectroscopy of Cells and Tissues: Shining Light onto a novel Subject, Appl. Spectrosc., 1999, 53, 148A–161A CrossRef CAS . http://www.osapublishing.org/abstract.cfm?uri=as-53-4-148A (accessed May 10, 2016).
- S. Boydston-White, M. Romeo, T. Chernenko, A. Regina, M. Miljković and M. Diem, Cell-cycle-dependent variations in FTIR micro-spectra of single proliferating HeLa cells: principal component and artificial neural network analysis, Biochim. Biophys. Acta, 2006, 1758, 908–914, DOI:10.1016/j.bbamem.2006.04.018.
- D. R. Whelan, K. R. Bambery, L. Puskar, D. McNaughton and B. R. Wood, Quantification of DNA in simple eukaryotic cells using Fourier transform infrared spectroscopy, J. Biophotonics, 2013, 6(10), 775–784, DOI:10.1002/jbio.201200112.
- T. P. Wrobel, L. Mateuszuk, S. Chlopicki, K. Malek, M. Baranska and G. K. Hansson, et al., Imaging of lipids in atherosclerotic lesion in aorta from ApoE/LDLR−/− mice by FT-IR spectroscopy and Hierarchical Cluster Analysis, Analyst, 2011, 136, 5247, 10.1039/c1an15311k.
- G. Güler, R. M. Gärtner, C. Ziegler and W. Mäntele, Lipid-Protein Interactions in the Regulated Betaine Symporter BetP Probed by Infrared Spectroscopy, J. Biol. Chem., 2016, 291, 4295–4307, DOI:10.1074/jbc.M114.621979.
- K. Ali, Y. Lu, U. Das, R. K. Sharma, S. Wiebe and K. Meguro, et al., Biomolecular diagnosis of human glioblastoma multiforme using Synchrotron mid-infrared spectromicroscopy, Int. J. Mol. Med., 2010, 26, 11–16 CAS . http://www.ncbi.nlm.nih.gov/pubmed/20514416 (accessed June 13, 2016).
- K. Yano, S. Ohoshima, Y. Gotou, K. Kumaido, T. Moriguchi and H. Katayama, Direct measurement of human lung cancerous and noncancerous tissues by fourier transform infrared microscopy: can an infrared microscope be used as a clinical tool?, Anal. Biochem., 2000, 287, 218–225, DOI:10.1006/abio.2000.4872.
- G. Barrera, Oxidative stress and lipid peroxidation products in cancer progression and therapy, ISRN Oncol., 2012, 2012, 137289, DOI:10.5402/2012/137289.
- A. Derenne, T. Claessens, C. Conus and E. Goormaghtigh, Infrared Spectroscopy of Membrane Lipids, in Encycl. Biophys, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 1074–1081, DOI:10.1007/978-3-642-16712-6_558.
- E. Staniszewska, K. Malek and M. Baranska, Rapid approach to analyze biochemical variation in rat organs by ATR FTIR spectroscopy, Spectrochim. Acta, Part A, 2014, 118, 981–986, DOI:10.1016/j.saa.2013.09.131.
- A. M. Melin, A. Perromat and G. Deleris, Fourier-transform infrared spectroscopy: a pharmacotoxicologic tool for in vivo monitoring radical aggression, Can. J. Physiol. Pharmacol., 2001, 79, 158–165 CrossRef CAS PubMed . http://www.ncbi.nlm.nih.gov/pubmed/11233564 (accessed February 15, 2017).
- V. Zohdi, D. R. Whelan, B. R. Wood, J. T. Pearson, K. R. Bambery and M. J. Black, Importance of tissue preparation methods in FTIR micro-spectroscopical analysis of biological tissues: “traps for new users”, PLoS One, 2015, 10, e0116491, DOI:10.1371/journal.pone.0116491.
- R. J. Chen, G. Zhang, S. H. Garfield, Y.-J. Shi, K. G. Chen and P. G. Robey, et al., Variations in Glycogen Synthesis in Human Pluripotent Stem Cells with Altered Pluripotent States, PLoS One, 2015, 10, e0142554, DOI:10.1371/journal.pone.0142554.
- P. Heraud, E. S. Ng, S. Caine, Q. C. Yu, C. Hirst and R. Mayberry, et al., Fourier transform infrared microspectroscopy identifies early lineage commitment in differentiating human embryonic stem cells, Stem Cell Res., 2010, 4, 140–147, DOI:10.1016/j.scr.2009.11.002.
- K. Yano, S. Ohoshima, Y. Shimizu, T. Moriguchi and H. Katayama, Evaluation of glycogen level in human lung carcinoma tissues by an infrared spectroscopic method, Cancer Lett., 1996, 110, 29–34, DOI:10.1016/S0304-3835(96)04450-3.
| This journal is © The Royal Society of Chemistry 2018 |