Noninvasive and prospective diagnosis of coronary heart disease with urine using surface-enhanced Raman spectroscopy†
Huinan
Yang
a,
Chang
Zhao
 a,
Rong
Li
b,
Chengxing
Shen
*b,
Xiaoshu
Cai
*a,
Li
Sun
a,
Rong
Li
b,
Chengxing
Shen
*b,
Xiaoshu
Cai
*a,
Li
Sun
 a,
Chengfang
Luo
a and
Yuechao
Yin
a
a,
Chengfang
Luo
a and
Yuechao
Yin
a
aSchool of Energy and Power Engineering, University of Shanghai for Science and Technology, 200093 Shanghai, China. E-mail: usst_caixs@163.com
bXin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 200093 Shanghai, China. E-mail: shencx@sjtu.edu.cn
First published on 16th March 2018
Abstract
A prospective diagnosis method for coronary heart disease (CHD) using human urine based on surface-enhanced Raman spectroscopy (SERS) is proposed, and could provide valuable information for judging whether to perform percutaneous coronary intervention (PCI) in clinics. Here, urine samples from 87 patients with CHD, including patients with PCI before operation (degree of cardiovascular congestion above 70%) and without PCI (degree of cardiovascular congestion under 70%), and 20 healthy humans were measured using SERS. Principal component analysis (PCA) combined with linear discriminant analysis (LDA) was employed to analyze the SERS spectra, revealing that the classification sensitivity and specificity were 90% and 78.9%, respectively, and the absolute value for loading of PC1 at 1509 cm−1 was the largest. Since platelet-derived growth factor-BB (PDGF-BB) is closely related to CHD, PDGF-BB aqueous solutions with various concentrations (1, 0.5, 0.1, 0.05 and 0.01 ppm) and a mixture of healthy human urine and PDGF-BB aqueous solutions were then investigated in this work, and it was found that the Raman peak at 1509 cm−1 may be attributed to PDGF-BB. Moreover, the measured SERS spectra of all the urine samples from the 87 patients with CHD were compared with the clinical data provided by a hospital, and it was revealed that the appearance of a peak at 1509 cm−1 in the SERS spectra was in good agreement with the results of coronary angiography tests when cardiovascular congestion was above 70%. This indicated that the classification sensitivity and specificity were 87.9% and 87.0%, respectively, through identification of the Raman peak at 1509 cm−1.
Introduction
Coronary heart disease (CHD) is a type of cardiovascular disease that originates from angiogenesis of atherosclerotic lesions on coronary arteries, which leads to myocardial ischemia, hypoxia or necrosis. In China, the death rate from cardiovascular disease was the highest among death rates from other diseases in 2014. The death rate from CHD in rural areas was 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 372/100
372/100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and in urban areas was 13
000, and in urban areas was 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 621/100
621/100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in 2014.1 There are various conventional detection methods for CHD, such as an electrocardiogram, multi-slice computed tomography (MSCT), coronary angiography, etc. Electrocardiograms are simple to perform, but have a high misdiagnosis rate. The measurement accuracy of MSCT is higher, but the operation is complex and has a high cost. Coronary angiography, although known as the “gold standard” for CHD diagnosis, is an intrusive detection method. Therefore, it is necessary to develop a non-invasive, prospective diagnosis method for CHD that has high sensitivity and reliability.
000 in 2014.1 There are various conventional detection methods for CHD, such as an electrocardiogram, multi-slice computed tomography (MSCT), coronary angiography, etc. Electrocardiograms are simple to perform, but have a high misdiagnosis rate. The measurement accuracy of MSCT is higher, but the operation is complex and has a high cost. Coronary angiography, although known as the “gold standard” for CHD diagnosis, is an intrusive detection method. Therefore, it is necessary to develop a non-invasive, prospective diagnosis method for CHD that has high sensitivity and reliability.
Raman spectroscopy has been widely applied in the field of bioscience.2–4 Surface-enhanced Raman spectroscopy (SERS) is an advantageous tool due to its high detection sensitivity and is particularly suitable for the investigation of substances with low concentrations.5,6 For instance, Feng et al. determined the plasma composition of patients with nasopharyngeal carcinoma using SERS.7 Mistro et al. studied urine from patients with prostate cancer using SERS.8 In recent years, SERS has been further applied to investigate viruses, bacteria and DNA.9–11
Platelet-derived growth factor (PDGF) is a type of mitotic factor. It stimulates cell migration and the proliferation of vascular smooth muscle,12,13 and participates in the development process of atherosclerosis.14 There are several kinds of dimer of PDGF, such as PDGF-AA, PDGF-BB and PDGF-AB. In previous research, the relationship between PDGF-BB and CHD has been discussed extensively.15 Therefore, PDGF-BB can be identified as a biomarker for CHD diagnosis.
Wang et al. found that the SERS signal of 4-mercaptobenzoic acid (4-MBA), as a Raman reporter, increased due to the induction of aggregation in the presence of PDGF-BB,16 however, the Raman peak of PDGF-BB cannot be directly measured. Zeng et al. investigated PDGF-BB in serum and found that PDGF-BB may play an important role in the formation of carotid atherosclerosis using enzyme linked immunosorbent assay (ELISA).17 Comparatively, most of the proteins in the blood are also reflected in the urine,18 and it is also more convenient to collect urine samples than blood samples. Therefore, urine was chosen as the medium for detecting PDGF-BB herein.
In this work, 87 urine samples from patients with CHD, including patients with percutaneous coronary intervention (PCI) and without PCI, and 20 urine samples from healthy humans were investigated, and principal component analysis (PCA) and linear discriminant analysis (LDA) were employed to evaluate the measured SERS spectra. Then, the PDGF-BB aqueous solutions with different low concentrations (1, 0.5, 0.1, 0.05 and 0.01 ppm) were measured and mixtures of PDGF-BB and urine samples from healthy humans were then studied. All of the experiments were performed using a self-constructed Raman spectrometer and the experimental results were compared with the clinical data from a coronary angiography, provided by a hospital. This revealed that the SERS spectra of human urine with a peak at 1509 cm−1 attributed to PDGF-BB could provide valuable information for CHD diagnosis and decisions on whether to perform PCI.
Experimental section
Self-constructed 785 nm Raman spectrometer
A self-constructed 785 nm Raman spectrometer was constructed with 0.7 cm−1 spectral resolution. A diode laser (50 mw) at 785 nm was employed as the light source, and the incident light transmitted through the bandpass filter (OD6, Edmund) was then focused onto a cuvette. The scattering light was then filtered using an edge filter (OD ≥6, Edmund) to eliminate Rayleigh scattering light. The Raman scattering light was received by a spectrometer (iHR320) and CCD detector (Horiba–Syncerity).19 The detected spectra were recorded with 1200 grooves per mm grating, with an acquisition time of 10 seconds and accumulation four times. The spectra were acquired and transferred to a computer for post-processing. The spectra were recorded in the range 1140–1620 cm−1, four adjacent pixels of the second Savitzky–Golay smoothing method were employed to eliminate noise,20 and the fourth-order polynomial fit was utilized to correct the baseline of the spectra (Labspec5). It is desirable to overcome the manual intervention and single fitting spectral distortion.21 Each sample was measured five times and the average spectra were processed for subsequent analysis. The spectrometer was calibrated using ethanol, and the signal-to-noise ratio (SNR) of the Raman spectrum for ethanol (Fig. S1a†) is 3.44 times that measured using a commercial Raman spectrometer (Fig. S1b†).PDGF-BB aqueous solution samples
PDGF-BB (PEPROTECH) aqueous solutions were prepared with mass fractions of 1, 0.5, 0.1, 0.05 and 0.01 ppm. The detection limit of the normal Raman spectrometer was a mass fraction of about 100 ppm; therefore, SERS was employed here to investigate PDGF-BB.Urine samples
20 urine samples from healthy humans were prepared and 87 urine samples from patients with CHD were divided into two groups, a group with PCI and a group without PCI. The group with PCI consisted of 30 patients’ urine samples, and the average age was 66.5 ± 17.5 years old; the group without PCI consisted of 57 patients’ urine samples and the average age was 64 ± 16 years old. All urine samples were provided by the Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Ethical approval and informed consent was obtained from all patients before conducting the experiments. All experiments were performed in compliance with the relevant laws and institutional guidelines. The protocol of study was approved by the University of Shanghai for Science and Technology and Shanghai Jiao Tong University School of Medicine, respectively. The urine samples were also sterilized before taking them out of the hospital. The urine samples were obtained before performing PCI and all the patients are from similar backgrounds and the same race. The urine samples were collected in the morning at 8 o'clock and then stored at 4 °C for one or two hours before measurement. Sediments were not formed for most of the urine samples, determined with visual observation, however, only the supernatant of the urine samples was taken through centrifugation for the experiments.Silver colloid solution samples
In this work, silver colloid solutions (50 nm) were mixed with the samples to improve the intensity of the Raman signals. They were prepared via the microwave-assisted reduction method with sodium citrate (Sinopharm Chemical Reagent Co., Ltd) as a reducing agent.22Fig. 1 shows the UV-visible absorption spectrum (a) and a transmission electron micrograph image (b) of the silver colloid solution.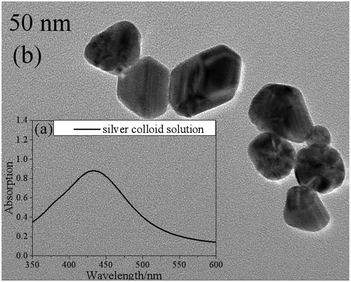 | ||
| Fig. 1 The UV-visible absorption spectrum (a) and a transmission electron micrograph image (b) of the silver colloid solution. | ||
Multivariate analysis
A PCA method was first employed to evaluate the measured SERS spectra (Statistical Product and Service Solutions). PCA is a powerful multivariate statistical method used for spectra analysis. A large amount of spectra information included in the SERS spectra could be reduced to a couple of important parameters (principal components, PCs) with PCA.23 LDA was then applied to the retained PCs to determine the corresponding line to separate these two groups properly. In order to evaluate the performance of such classification, the leave-one-out cross validation (LOOCV) method was used.24Results and discussion
Investigation of the urine samples from patients with CHD and healthy humans
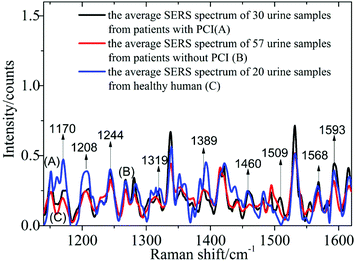
1 ml silver colloid solutions were blended with 0.5 ml urine samples from 87 patients with CHD, including 30 urine samples from patients with PCI and 57 urine samples from patients without PCI, and 20 healthy humans, then prepared in a 10 mm cuvette and then measured using the self-constructed Raman spectrometer.In Fig. 2, spectrum A corresponds to the average spectrum from 30 urine samples from patients with PCI, while spectrum B is the average spectrum from 57 urine samples from patients without PCI, and spectrum C is the average spectrum from 20 urine samples from healthy humans.
Nine peaks could be observed in these three average spectra at 1170, 1208, 1244, 1319, 1389, 1460, 1509, 1568 and 1593 cm−1. Urine serves not only as an ideal source of biomarkers for diseases of the kidney and other tissues of the urogenital system, but also as a potential source for providing information on diseases in other physiological systems.25 It was found that abundant proteins are found in the urine, which is the main medium for excretory product transportation. For example, the Raman characteristic peak at 1170 cm−1 is attributed to the stretching vibration of C–N of collagen and the Raman characteristic peak at 1208 cm−1 is attributed to the ring vibration of phenylalanine.26 Although the spectral shapes of the three groups were similar, the intensity of each of the Raman characteristic peaks was different. The average values and standard deviations of the nine Raman peaks of the urine samples from patients with PCI and without PCI, and from healthy humans, are depicted in Fig. 3.
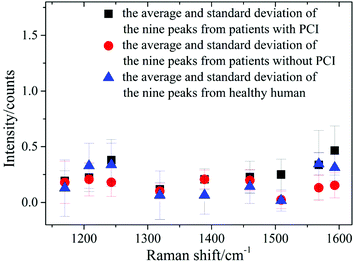 | ||
| Fig. 3 The average and standard deviations of the nine Raman peak intensities of the urine samples from patients with PCI and without PCI, and from healthy humans. | ||
This revealed that only the average value of the peak intensity at 1509 cm−1 approximated to zero, and there was no overlap between the error bars (i.e. standard deviations) for patients with and without PCI, however, the error bars did overlap between the patients without PCI and the healthy humans. Therefore, the identification of the peak at 1509 cm−1 could be a univariate approach to distinguish the Raman spectra of patients with and without PCI, but it is difficult to distinguish patients without PCI and the healthy humans.
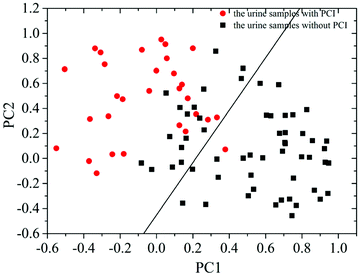
Then, PCA was employed to further analyze the measured SERS spectra of the 87 patients with CHD. The results demonstrated that the summation of variances for the first eight PCs was 100% (1st PC, 29.803%; 2nd PC, 22.551%; 3rd PC, 12.726%; 4th PC, 11.071%; 5th PC, 9.132%; 6th PC, 7.301%; 7th PC, 4.114%; 8th PC, 3.302%). The correlation between the 1st PC (PC1) and 2nd PC (PC2) is shown in Fig. 4. This revealed that the data points for these two groups could be partly separated by the corresponding line calculated using LDA.
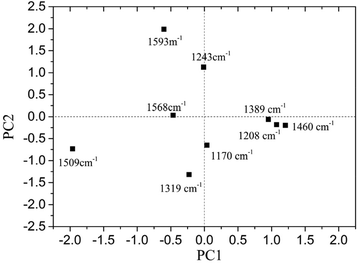
The loading plot of PC1 and PC2 is shown in Fig. 5.
It was found that for PC1, the loadings at 1319, 1509, 1568 and 1593 cm−1 were all negative, while the loadings at 1170, 1208, 1389 and 1460 cm−1 were positive. As for PC2, the loadings at 1243, 1568 and 1593 cm−1 were positive, and the others were all negative. Different peaks provided various contributions to PC1 and PC2. It was obvious that the absolute value for loading of PC1 at 1509 cm−1 was the largest, and it showed a significant influence on PC1.
By analyzing the SERS spectra of 87 urine samples from 30 patients with PCI and 57 patients without PCI based on PCA-LDA and LOOCV, it was revealed that the classification sensitivity (proportion of actual positives that are correctly identified as such) was 90% and the classification specificity (proportion of actual negatives that are correctly identified as such) was 78.9%. The associated error rates were 10% for false negatives and 21.1% for false positives, and the results are shown in Table 1.
| With PCI | Without PCI | Classification efficiency | |
|---|---|---|---|
| Classified with PCI | 27 (TP) | 12 (FP) | 90% (sensitivity) |
| Classified without PCI | 3 (FN) | 45 (TN) | 78.9% (specificity) |
| Total | 30 | 57 |
The concentrations of PDGF-BB in the urine samples from patients with CHD were also investigated by our group using the ELISA method,27 and it was found that the higher the concentration of PDGF-BB was, the more serious the degree of cardiovascular congestion could be. Therefore, the appearance of the Raman characteristic peak at 1509 cm−1 may be attributed to PDGF-BB.
Measurement of PDGF-BB with various concentrations using SERS
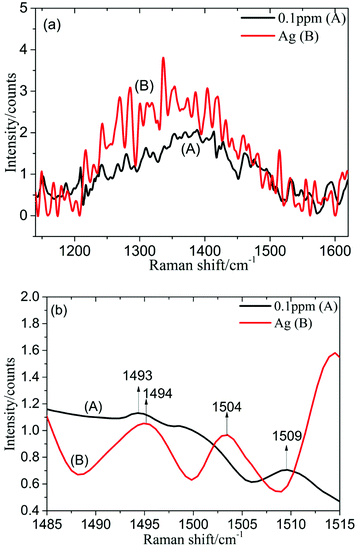
1 ml silver colloid solution was blended with 0.5 ml PDGF-BB aqueous solution with different concentrations (1, 0.5, 0.1, 0.05 and 0.01 ppm), prepared in a 10 mm cuvette and then measured using the self-constructed Raman spectrometer.The spectrum of the silver colloid solution mixed with 0.1 ppm PDGF-BB aqueous solution (spectrum A) and the spectrum of the silver colloid solution (spectrum B) in the spectral range from 1140 to 1620 cm−1 are shown in Fig. 6a. This revealed that it was difficult to distinguish between these two spectra in this spectral range, however, the difference was obvious when the SERS spectral range was chosen to be from 1485 to 1515 cm−1 (Fig. 6b). In contrast with the spectrum of the silver colloid solution (spectrum B), in the spectrum of the silver colloid solution mixed with 0.1 ppm PDGF-BB aqueous solution (spectrum A), the Raman characteristic peak at 1493 cm−1 may be attributed to the reducing agent (the Raman spectrum of sodium citrate is provided in the ESI, Fig. S2†), while the Raman characteristic peak at 1509 cm−1 was attributed to PDGF-BB, and could be caused by the anti-parallel β-sheet.28 For other low concentrations of PDGF-BB aqueous solutions (1, 0.5, 0.05 and 0.01 ppm), the peak at 1509 cm−1 can also be detected (Fig. S3 and S4†). It was found that the limit of detection was 0.01 ppm in this work.
Measurement of the mixture of PDGF-BB with various concentrations and the urine samples from healthy humans
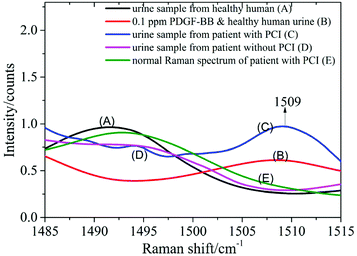
0.5 ml supernatant of urine from healthy humans was mixed with 0.5 ml PDGF-BB aqueous solution with mass fractions of 1, 0.5, 0.1, 0.05 and 0.01 ppm and blended with 1 ml silver colloid solution. The mixture was prepared in a 10 mm cuvette and measured using the Raman spectrometer. Spectrum A was recorded from the urine of healthy humans mixed with the silver colloid solution, and spectrum B resulted from the mixture of the PDGF-BB aqueous solution (0.1 ppm) and the urine samples from healthy humans blended with the silver colloid solution (Fig. 7). This revealed that the peak at 1509 cm−1 can be detected in spectrum B when the PDGF-BB was added.SERS spectra of the urine samples from patients with CHD
The SERS spectra of the urine samples from a patient with PCI (spectrum C) and without PCI (spectrum D) are also shown in Fig. 7. By comparing these two spectra, it was found that the peak at 1509 cm−1 can be detected in the urine sample from the patient with PCI and vice versa. The reason could be that the concentration of PDGF-BB was relatively high in the urine sample from the patient with PCI and it was extremely low in the urine sample from the patient without PCI as well as the healthy human (spectrum A).Measurement of a urine sample from a patient with PCI using a normal Raman spectrometer
0.5 ml supernatant of the same urine sample from the patient with PCI was mixed with 1 ml distilled water and then measured using a Raman spectrometer to obtain the normal Raman spectrum. In Fig. 7, spectrum E represents the normal Raman spectrum of a urine sample from a patient with PCI. This revealed that the concentration of PDGF-BB in the urine sample from the patient with PCI was not high enough to be detected without mixing with the silver colloid solution and the peak at 1509 cm−1 can only be detected in the SERS spectrum of the urine sample from the patient with PCI (spectrum C). The spectra over the spectral range from 1140 to 1620 cm−1 is provided in the ESI (Fig. S5†).Therefore, PDGF-BB could be a biomarker to distinguish patients with PCI and patients without PCI, in other words, to distinguish whether the degree of congestion of the patient's cardiovascular system was serious or not.
The 87 measured SERS spectra of patients with CHD were compared with the coronary angiography clinical data. The coronary angiography clinical data for the left anterior descending branch (LAD), left circumflex artery (LCX), left main coronary artery (LM) and right coronary artery (RCA) for the 87 patients were provided by the hospital.
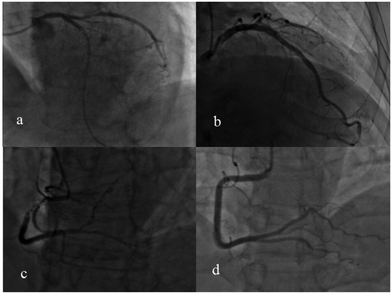
The appearance of the Raman peak at 1509 cm−1 and the degree of cardiovascular congestion for the 30 patients with PCI are listed in Table 2. The + symbol means with PCI, and the − indicates without PCI. This indicated that the degree of blocking of these patients’ blood vessels was very serious and the degree of congestion was measured using a computer-aided quantity analysis method, which was based on the degree of narrowing of the area of the coronary artery lumen. From Table 2, the degree of congestion of a single cardiovascular component was not less than 70%. For example, the images of coronary angiography for patient no. 14, 19 and 20 are shown in Fig. 8. The degree of congestion of the LAD for patient no. 14 was 100% (Fig. 8a); the degree of congestion of the LCX for patient no. 19 was 86.7% (Fig. 8b); the degree of congestion of the RCA for patient no. 20 (Fig. 8c) was 99%. This showed that when the degree of congestion of a single cardiovascular component was not less than 70%, the Raman characteristic peak at 1509 cm−1 attributed to PDGF-BB could be detected directly in the SERS spectra of human urine, in all cases except for patient no. 5. This may be caused by the complete denaturation of PDGF-BB in the urine of patient no. 5.
| Number | 1509 cm−1 | +/− | LAD % | LCX % | LM % | RCA % | Summation % |
|---|---|---|---|---|---|---|---|
| 1 | ✓ | + | 95 | 85 | — | 60 | 240 |
| 2 | ✓ | + | 40 | 60 | — | 90 | 190 |
| 3 | ✓ | + | 50 | 100 | — | 50 | 200 |
| 5 | ✗ | + | 40 | 95 | — | 90 | 225 |
| 7 | ✓ | + | 50 | 90 | 50 | 90 | 280 |
| 8 | ✓ | + | 40 | 90 | 40 | 50 | 220 |
| 11 | ✓ | + | — | — | — | 95 | 95 |
| 12 | ✓ | + | 60 | 90 | — | 50 | 200 |
| 14 | ✓ | + | 100 | — | — | 95 | 195 |
| 19 | ✓ | + | — | 90 | — | 80 | 170 |
| 20 | ✓ | + | — | — | — | 99 | 99 |
| 30 | ✓ | + | — | 90 | — | — | 90 |
| 31 | ✓ | + | — | 90 | — | 60 | 150 |
| 34 | ✓ | + | 70 | — | — | — | 70 |
| 40 | ✓ | + | 90 | 90 | 30 | — | 210 |
| 42 | ✓ | + | — | 80 | — | 50 | 130 |
| 46 | ✓ | + | — | 85 | — | 80 | 165 |
| 47 | ✓ | + | 50 | — | — | 90 | 140 |
| 53 | ✓ | + | 90 | 80 | — | — | 170 |
| 55 | ✓ | + | 95 | 90 | — | — | 185 |
| 62 | ✓ | + | 90 | — | — | — | 90 |
| 63 | ✓ | + | 70 | 60 | — | 60 | 190 |
| 68 | ✓ | + | 30 | 100 | — | 100 | 230 |
| 69 | ✓ | + | 70 | 70 | 20 | 80 | 240 |
| 71 | ✓ | + | 100 | 80 | — | — | 180 |
| 73 | ✓ | + | 60 | — | — | 70 | 130 |
| 74 | ✓ | + | 100 | — | — | — | 100 |
| 76 | ✓ | + | 100 | — | — | — | 100 |
| 79 | ✓ | + | 100 | 90 | 40 | 60 | 290 |
| 82 | ✓ | + | 50 | 70 | — | 50 | 170 |
Through analyzing the clinical data from 57 patients without PCI, it was also found the degree of congestion of a single cardiovascular component was less than 70%. For example, there was no cardiovascular congestion of the RCA, as shown in the image of the coronary angiography for patient no. 65 (Fig. 8d). This validated the fact that the Raman characteristic peak at 1509 cm−1 cannot be detected in the SERS spectra for most of patients whose degree of congestion of a single cardiovascular component was less than 70%. The proportion of these two cases is 87.4%.
However, it should be noted that the Raman characteristic peak at 1509 cm−1 can also be detected in a few cases of patients without PCI and their degree of congestion of a single cardiovascular component was less than 70%, as listed in Tables 3 and 4.
| Number | 27 | 36 | 67 |
|---|---|---|---|
| 1509 cm−1 | ✓ | ✓ | ✓ |
| (+)/(−) | — | — | — |
| LAD% | — | 30 | 40 |
| LCX% | 30 | 40 | 30 |
| LM% | — | — | — |
| RCA% | 50 | — | — |
| LAD + LCA + LM + RCA% | 80 | 70 | 70 |
| Number | 41 | 50 | 51 | 75 |
|---|---|---|---|---|
| 1509 cm−1 | ✓ | ✓ | ✓ | ✓ |
| (+)/(−) | — | — | — | — |
| LAD% | 60 | 30 | 30 | — |
| LCX% | — | — | — | — |
| LM% | — | — | — | — |
| RCA% | — | — | — | 50 |
| LAD + LCA + LM + RCA% | 60 | 30 | 30 | 50 |
As shown in Table 3, for patient no. 27, 36 and 67, the degree of congestion of a single cardiovascular component was less than 70%, but the summation of LAD, LCX, RCA and LM was 80%, 70% and 70%. Therefore, this revealed that the Raman characteristic peak at 1509 cm−1 can also be detected in the SERS spectra of the patients when their total degree of congestion of a single cardiovascular component was larger than 70%, and the proportion of these patients is 3.4%.
As shown in Table 4, for patient no. 41, 50, 51 and 75, the Raman characteristic peak at 1509 cm−1 can be detected in the SERS spectra, although the degree of congestion of a single cardiovascular component was less than 70%, as was their summation (60%, 30%, 30% and 50%). Combined with the CT of the brain and ultrasonography of the neck for these four patients provided by the hospital, it was found that there was a certain degree of cerebral infarction or neck vascular blockage for these patients, which led to the increase of concentration of PDGF-BB in the human body, and the proportion of these patients is 4.6%.
Moreover, the classification results of the degree of congestion above or under 70% by identification of the Raman peak at 1509 cm−1 is shown in Table 5. This revealed that the classification sensitivity and specificity were 87.9% and 87.0%, respectively. The associated error rates were 12.1% for false negatives and 13% for false positives, respectively. It should be noted that antiplatelet medicines like Bayaspirin and clopidogrel were provided to the patients with CHD before conducting coronary angiography. These drugs, as the basic clinical treatment of CHD, have an impact on PDGF-BB. However, the medical treatment with drugs should not influence the experiments performed in this work because the same types and amounts of drugs were taken by every patient with CHD.
| Above 70% | Under 70% | Classification efficiency | |
|---|---|---|---|
| Classified with PCI | 29 (TP) | 7 (FP) | 87.9% (sensitivity) |
| Classified without PCI | 4 (FN) | 47 (TN) | 87.0% (specificity) |
| Total | 33 | 54 |
Conclusion
In summary, 20 urine samples from healthy humans and 87 urine samples from patients with CHD were measured and analyzed via PCA-LDA with a LOOCA method. This revealed that the classification sensitivity and specificity for the two groups of patients (with PCI and without PCI) were 90% and 78.9%, respectively. It was also found that the absolute value for loading of PC1 at 1509 cm−1 was the largest, therefore, the univariate approach could be effective to distinguish these two groups. Moreover, PDGF-BB aqueous solutions with different low concentrations were investigated and a mixture of PDGF-BB and urine samples from healthy humans was also studied. It was found that the peak at 1509 cm−1 could be attributed to PDGF-BB. Finally, the results for 87 urine samples from patients with CHD measured using SERS were compared with clinical data provided by the hospital. Generally speaking, the results measured using SERS matched well with results from coronary angiography tests when the degree of cardiovascular congestion was above 70%. The classification sensitivity and specificity were found to be 87.9% and 87.0%, respectively, through the identification of the Raman peak at 1509 cm−1. The intensities of the spectra were relatively low since the concentrations of PDGF-BB in aqueous solutions or PDGF-BB in the urine were extremely low, and the silver colloid solution without further modification was utilized as the SERS substrate here, however, the properties of the silver colloid solution could be modified to enhance the intensities of the spectra in future work.The developed novel in vitro method offers the advantages of being less time consuming and more straight forward, and does not require preparation of the nanoparticle tag before the measurement. A yes/no answer should be enough for the judgment of whether to perform percutaneous coronary intervention (PCI), since a patient with a degree of cardiovascular congestion above 70% should be treated with PCI in the clinic, and the appearance of a peak at 1509 cm−1 was in good agreement with the results of coronary angiography. Therefore, the clinical application of SERS as a noninvasive and prospective diagnosis tool for CHD comes with great potential and could provide valuable information for the prospective diagnosis of suspected cases of CHD, especially for suggesting whether patients require PCI.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 51676130, 81670321 and 51306123) and the Joint Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20133120120008).Notes and references
- H. Sui, W. Chen and W. Wang, Chin. J. Cardiovasc. Med., 2016, 21(4), 259–261 Search PubMed.
- Z. W. Huang, A. Mcwilliams, H. Lui, D. I. McLean, S. Lam and H. S. Zeng, Int. J. Cancer, 2003, 107, 1047–1052 CrossRef CAS PubMed.
- M. A. Short, W. B. Wang, I. T. Tai and H. S. Zeng, J. Biophotonics, 2016, 9(1–2), 44–48 CrossRef CAS PubMed.
- U. Utzinger, D. L. Heintzelman, A. Mahadevan-Jasen, A. Malpica, M. Follen and R. Richards-Kortum, Soc. Appl. Spectrosc., 2001, 55(8), 955–959 CrossRef CAS.
- D. I. Ellis and R. Goodacre, Analyst, 2006, 131, 875–885 RSC.
- K. Kneipp, H. Kneipp, R. Manoharan, E. Hanlon, I. Itzkan, R. Dasari and M. Feld, Appl. Spectrosc., 1998, 52(12), 1493–1497 CrossRef CAS.
- S. Y. Feng, R. Chen, J. Q. Lin, J. J. Pan, G. N. Chen, Y. Z. Li, M. Cheng, Z. F. Huang, J. S. Chen and H. S. Zeng, Biosens. Bioelectron., 2010, 25, 2414–2419 CrossRef CAS PubMed.
- G. D. Mistro, S. Cervo, E. Mansutti, R. Spizzo, A. Colombatti, P. Belmonte, R. Zucconelli, A. Steffan, V. Sergo and A. Bonifacio, Anal. Bioanal. Chem., 2015, 407, 3271–3275 CrossRef PubMed.
- W. Q. Wang, V. Hynninen, L. Qiu, A. W. Zhang, T. Lemma, N. N. Zhang, H. H. Ge, J. J. Toppari, V. P. Hytönen and J. Wang, Sens. Actuators, B, 2017, 239, 515–525 CrossRef CAS.
- Y. Sun, L. Xu, F. D. Zhang, Z. G. Song, Y. W. Hu, Y. J. Ji, J. Y. Shen, B. Li, H. Z. Lu and H. F. Yang, Biosens. Bioelectron., 2017, 89, 906–912 CrossRef CAS PubMed.
- L. J. He, M. Langlet and V. Stambouli, Appl. Surf. Sci., 2016, 339, 702–710 Search PubMed.
- K. H. Choi, J. E. Kim, N. R. Song, J. E. Son, M. K. Hwang, S. Byun, J. H. Kim, K. W. Lee and H. J. Lee, Cardiovasc. Res., 2010, 85(4), 836–844 CrossRef CAS PubMed.
- J. E. Son, E. Lee, S. K. Jung, J. E. Kim, M. H. Oak, K. W. Lee and H. Y. Lee, Cardiovasc. Res., 2014, 101, 503–512 CrossRef CAS PubMed.
- A. C. Doran, N. Meller and C. A. McNamara, Arterioscler., Thromb., Vasc. Biol., 2008, 28(5), 812–819 CrossRef CAS PubMed.
- Y. Lu and Q. Qin, Tianjin Med. J., 2012, 40(5), 525–527 CAS.
- C. W. Wang and H. T. Chang, Anal. Chem., 2014, 86, 7606–7611 CrossRef CAS PubMed.
- Y. R. Zeng, W. H. Wang, M. Lu and L. J. Yan, Chin. J. Pract. Med., 2014, 41(3), 4–6 Search PubMed.
- Y. H. Gao, Sci. China: Life Sci., 2013, 56(12), 1145–1146 CrossRef PubMed.
- Q. Wang, C. Zhao, H. N. Yang, M. X. Su and X. S. Cai, Laser Optoelectron. Prog., 2016, 53(9), 093001 CrossRef.
- Y. Q. Zhang, W. Dong, B. Zhang and X. P. Wang, Spectrosc. Spectral Anal., 2012, 32(6), 1554–1558 CAS.
- X. W. Feng, Z. L. Zhu, M. J. Shen and P. S. Cong, Comput. Appl. Chem., 2009, 26(6), 759–762 CAS.
- D. Q. Zhang, M. Z. Si, R. M. Liu and Y. B. Su, Spectrosc. Spectral Anal., 2013, 33(4), 996–999 CAS.
- J. Q. Lin, R. Chen, S. Y. Feng, J. J. Pan, Y. Z. Li, G. N. Chen, M. Cheng, Z. F. Huang, Y. Yu and H. S. Zeng, Nanomedicine, 2011, 7, 655–663 CrossRef CAS PubMed.
- J. M. Connolly, K. Davies, A. Kazakeviciute, A. M. Wheatley, P. Dockery, I. Keogh and M. Olivo, Nanotechnol. Biol. Med., 2016, 12, 1593–1601 CrossRef CAS PubMed.
- M. L. Li, M. D. Zhao and Y. H. Gao, Sci. China: Life Sci., 2014, 57(7), 649–656 CrossRef CAS PubMed.
- S. Y. Feng, J. J. Pan, Y. A. Wu, D. Lin, Y. P. Chen, G. Q. Xi, J. Q. Lin and R. Chen, Sci. China: Life Sci., 2011, 41(7), 550–557 Search PubMed.
- R. Li, C. X. Shen, M. Q. Wu and L. Zhao, Mod. Med. J., 2017, 45(6), 766–700 Search PubMed.
- S. Cratic, J. Clements, A. L. Cook, D. T. F. Dryden, D. R. Green, K. Herenans, P. M. Kirwin, M. J. Price and A. Fallow, Biochem. J., 1992, 28, 62–72 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7an02022h |
| This journal is © The Royal Society of Chemistry 2018 |