Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A minimalist fluorescent probe for differentiating Cys, Hcy and GSH in live cells†
Huatang
Zhang
ab,
Ruochuan
Liu
a,
Jie
Liu
a,
Lin
Li
c,
Ping
Wang
a,
Shao Q.
Yao
d,
Zhengtao
Xu
*a and
Hongyan
Sun
 *ab
*ab
aDepartment of Biology and Chemistry, City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong, China. E-mail: hongysun@cityu.edu.hk; zhengtao@cityu.edu.hk
bKey Laboratory of Biochip Technology, Biotech and Health Centre, Shenzhen Research Institute of City University of Hong Kong, Shenzhen, 518057, PR China
cKey Laboratory of Flexible Electronics & Institute of Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing, 211816, China
dDepartment of Chemistry, National University of Singapore, 117543, Singapore
First published on 22nd September 2015
Abstract
A simple molecule, tetrafluoroterephthalonitrile (4F-2CN), was discovered to be an efficient fluorescent probe for detecting biological thiol species. The probe responded to Cys and emitted strong green fluorescence, whereas it reacted with Hcy/GSH and generated blue fluorescence. Addition of CTAB (cetyl trimethylammonium bromide) was observed to alter the fluorescence color of the reaction product of 4F-2CN and Hcy (from blue to green), but no alteration of the fluorescence color occurred for Cys and GSH. For the very first time, cell imaging experiments showed that the three commonly occurring thiols (Cys/Hcy/GSH) could be differentiated using a single fluorescent probe. In addition, the reaction product of 4F-2CN and Cys exhibits two-photon properties, offering a potentially useful tool for tissue imaging studies. To the best of our knowledge, 4F-2CN is currently the smallest fluorescent probe for thiol detection. We envision that this new and versatile probe will be a useful tool for further elucidating the roles of thiols in biology.
Introduction
Small molecule thiols play crucial roles in maintaining cellular redox environments and mitigating damage from free radicals and toxins.1 Three low-molecular-weight thiols are commonly found in biological systems, namely cysteine (Cys), homocysteine (Hcy) and glutathione (GSH). These thiols are closely involved in regulating various physiological and pathological processes. For example, Cys is an essential amino acid for protein synthesis. Abnormal levels of Cys are related to slow growth, edema, lethargy, liver damage, etc.2 Hcy elevation in plasma, on the other hand, is a risk factor for cardiovascular disease, Alzheimer's disease and osteoporosis,3 while GSH is closely linked to leucocyte loss, cancer, HIV infection, etc.4 The important biological roles of thiols have thus spurred strong interest in developing useful chemical tools for detecting thiols.The use of fluorescent probes, owing to their simplicity and non-invasiveness, has become a popular approach for thiol detection in living cells.5 Over the last decade, a large number of fluorescent probes have been developed for detecting thiols.6 Most of these probes are based on thiol-selective chemical reactions, including Michael addition reactions,7 nucleophilic substitution,8 cyclization reactions between aldehyde and aminothiols,9 cleavage reactions of 2,4-dinitrobenzenesulfonyl (DNBS) with thiols,10 disulfide exchange reaction,11 and others.12
Similarities among the structures and the reaction activities of Cys, Hcy and GSH have posed substantial difficulties for discriminating one thiol species from another. Despite this challenge, several fluorescent probes that allow for the selective detection of Cys or GSH have been reported, e.g., the Strongin group's seminal work on the selective detection of Cys/Hcy over GSH using a cyclization reaction between Cys/Hcy and acrylate.13 Yang and coworkers also designed a GSH-selective probe by employing the specific thiol–halogen reaction between chlorinated BODIPY and GSH.14 Further effort has been devoted recently to develop single probe systems for simultaneous detection of two or three thiol species.15 For example, Guo and coworkers have developed a chlorinated coumarin–hemicyanine probe for the simultaneous detection of Cys and GSH. Nevertheless, single probes that are able to fully discriminate the three thiols from each other are still quite rare. In this study, we report a remarkably simple but versatile probe 4F-2CN (Fig. 1), which is capable of simultaneously detecting Cys and Hcy/GSH using dual emission channels. Moreover, the fluorescence color for Hcy and the probe can be altered by adding a surfactant called CTAB, thereby allowing all three thiols to be differentiated from each other.
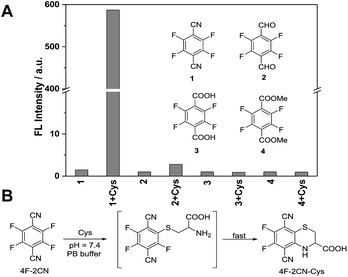 | ||
| Fig. 1 (A) Fluorescence response of 4F-2CN and its analogues after incubation with Cys in PB buffer (Ex/Em at 420 nm/500 nm). (B) Proposed reaction mechanism of 4F-2CN and Cys. | ||
Results and discussion
In a screening experiment, we identified a commercial compound, 2,3,5,6-tetrafluoroterephthalonitrile (4F-2CN), which was shown to react with Cys and produce bright green fluorescence in PB buffer (10 mM phosphate buffer, pH = 7.4) (Fig. 1A). It was noted that when the CN groups were altered to other electron withdrawing groups like CHO/COOMe, no obvious fluorescence was observed with these analogues. TLC experiments also showed that no reaction occurred between the analogues and Cys under PBS/DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after 2 h (Fig. S1†). The possible reason for the different reactivity of these compounds could be due to the difference in the electron-withdrawing abilities of CN, CHO and COOMe. With non-thiol amino acids (e.g., histidine and lysine), no reaction with 4F-2CN occurred as indicated by fluorescence assays and mass spectrometry analysis.
1 after 2 h (Fig. S1†). The possible reason for the different reactivity of these compounds could be due to the difference in the electron-withdrawing abilities of CN, CHO and COOMe. With non-thiol amino acids (e.g., histidine and lysine), no reaction with 4F-2CN occurred as indicated by fluorescence assays and mass spectrometry analysis.
We characterized the reaction product of 4F-2CN and Cys. 4F-2CN was mixed with Cys in a DMF solution to give the product 4F-2CN–Cys which was characterized using 1H, 13C and 19F NMR, ESI-MS, FT-IR and X-ray crystallography of single crystals (CCDC 1402255; Fig. S2–S8, Tables S1–S3†). We hypothesize that the formation of 4F-2CN–Cys was initiated by the Cys thiol group replacing a fluoro group on 4F-2CN in an aromatic nucleophilic substitution, and the subsequent cyclization was facilitated by the six-membered ring configuration afforded by the Cys substrate (Fig. 1B). The strong fluorescence from the cyclized product can be ascribed largely to the electron donation from the amino and sulfide moieties onto the aromatic core. We also determined the quantum yield of 4F-2CN–Cys to be 0.35 (Table S4†), indicating that it is a good fluorophore.
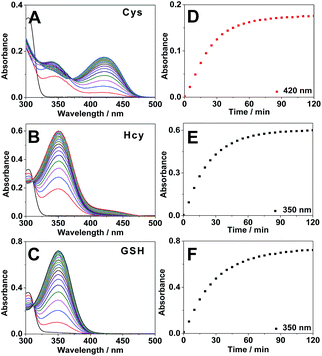
Next we carried out time-dependent absorbance experiments with the probe. 4F-2CN alone in PB buffer did not show any absorption in the range of 340–500 nm (Fig. S9†). Upon addition of Cys, a new absorption peak at 420 nm was immediately observed, and the absorption signal reached equilibrium after around 2 h (Fig. 2A and D). For Hcy and GSH, an absorption peak at 350 nm could be observed, and it also plateaued after around 2 h (Fig. 2B, C, E and F).
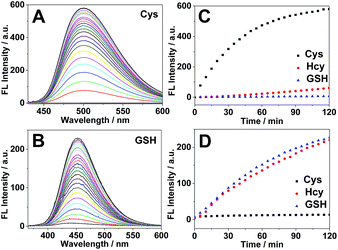
Subsequently we carried out the time-dependent fluorescence experiments of 4F-2CN with Cys, Hcy and GSH respectively. The fluorescence signal was first measured at an excitation wavelength of 420 nm. The probe itself showed negligible fluorescence. Upon addition of Cys, a new emission peak at 500 nm was readily observed and reached equilibrium in around 2 h (Fig. 3A). In comparison with Cys, Hcy displayed only a slight fluorescence increment and GSH did not show a noticeable signal (Fig. 3C). The fluorescence increments of 4F-2CN at 500 nm for Cys, Hcy and GSH are 400-, 30- and 4.5-fold respectively. The results indicated that 4F-2CN could be used to discriminate Cys from Hcy/GSH when excited at 420 nm. Furthermore, we performed titration experiments of 4F-2CN with the addition of different concentrations of Cys. The results indicated that the fluorescence intensity was nearly linear to the Cys concentration in the range of 0–20 μM (Fig. S12†). The detection limit of the probe for Cys was determined to be as low as 20 nM based on the 3σ/slope method. Moreover, an 8.7-fold fluorescence increase was observed when the Cys concentration was 1 μM. These results demonstrated that 4F-2CN could serve as a highly sensitive probe for detecting Cys.
The fluorescence response of 4F-2CN with Cys/Hcy/GSH was then investigated using an excitation wavelength of 350 nm. Similar to the previous study, the probe itself displayed negligible fluorescence in this channel. Upon addition of GSH or Hcy, a new fluorescence emission peak at 450 nm appeared (Fig. 3B and S10†). On the other hand, Cys did not show an obvious fluorescence increment at 450 nm (Fig. 3D). The fluorescence increments at 450 nm for Hcy, GSH and Cys are 29-, 26- and 2.7-fold respectively. The results demonstrated that the probe was able to differentiate GSH/Hcy from Cys when excited at 350 nm. Concentration-dependent experiments with the probe indicated that the fluorescence signal at 450 nm increased with increased concentrations of Hcy or GSH (Fig. S13 and S14†). The detection limits of Hcy and GSH were determined to be 2.27 and 0.24 μM respectively. It is noticed that the LOD of Cys was about ten times lower than that of Hcy/GSH. The higher sensitivity observed for Cys arises from the strong fluorescence of the reaction product of Cys and 4F-2CN. The enhanced fluorescence in the case of Cys can be attributed to the electron-donating amino group, as well as the rigid cyclized structure in the product. In addition, the reaction with Cys proceeded faster than that with Hcy/GSH (Fig. 3C and D), contributing to the higher sensitivity for Cys observed within the given length of time.
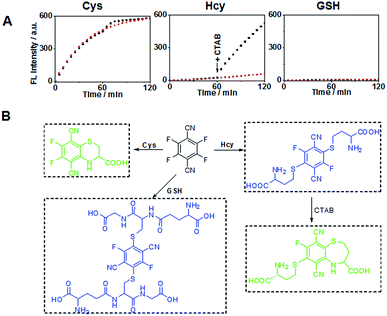
Previous studies have shown that the surfactant CTAB can help to form micelles and facilitate the intramolecular ring formation.16 In our study, it was found that the fluorescence properties of the reaction between 4F-2CN and Hcy could be altered by adding CTAB. As shown in Fig. 4A, a weak fluorescence signal was observed at 500 nm when 4F-2CN was incubated with Hcy for 1 h. After CTAB was added, a gradual fluorescence increase could be readily observed at 500 nm. On the other hand, the reactivity of 4F-2CN with Cys and GSH remained almost unchanged after the addition of CTAB. From these data, we can draw the conclusion that 4F-2CN displayed distinct reactivity patterns towards Cys, Hcy and GSH by using CTAB. The probe can be potentially used to differentiate these three highly similar thiol species. We further characterized the reaction products of 4F-2CN with Hcy and GSH using HPLC, ESI-MS and NMR. Experimental data revealed that disubstituted products were produced in the reaction of 4F-2CN and Hcy/GSH (Fig. 4B and S15–S24†). Addition of CTAB will facilitate the cyclization of 4F-2CN and Hcy, but not the cyclization of 4F-2CN and GSH (Fig. S18†).
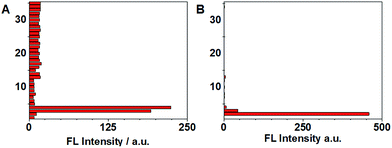
Selectivity experiments are instrumental to ascertain the biological applications of the probe, such as cell imaging experiments. The selectivity experiments were performed by incubating the probe with various biological analytes, including 20 natural amino acids, Hcy, GSH, H2S, RNS, ROS and metal ions. The fluorescence intensities were then measured at two different emission wavelengths. As shown in Fig. 5A, the fluorescence intensity at 450 nm showed that only GSH/Hcy induced significant fluorescence changes whereas the other biological analytes gave marginal increments. On the other hand, the fluorescence intensity for Cys at 500 nm displayed a substantial increment; Hcy showed small increment and the other samples induced very little increment (Fig. 5B). These results indicated that the probe is highly selective toward thiols.
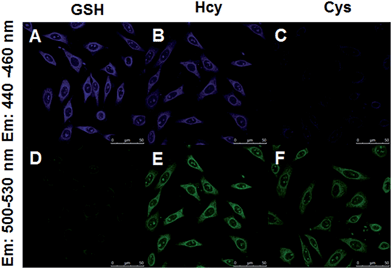
Encouraged by the above results, we moved forward to study the capability of 4F-2CN to image Cys/Hcy/GSH in living cells. HeLa cells were firstly treated with CTAB. N-Ethylmaleimide (NEM), a common thiol depletion reagent was then added. Cys, Hcy and GSH were subsequently added to the medium respectively. 4F-2CN was finally added to the medium and incubated for 20 min. Confocal imaging results showed that cells with the addition of Cys displayed green fluorescence (Fig. 6C and F). GSH treated cells, on the other hand, gave off bright blue fluorescence (Fig. 6A and D). Cells treated with Hcy showed both blue and green fluorescence (Fig. 6B and E). The green fluorescence is attributed to the cyclized product of the reaction between 4F-CN and Hcy. These results together unambiguously proved that 4F-2CN could be used to differentiate between Cys, Hcy and GSH via dual emission channels. We also performed MTT assays to examine the cytotoxicity of 4F-2CN. As shown in Fig S26,† the probe showed relatively low toxicity at 10 μM (this is the concentration we used for the cell imaging studies in this work). Higher toxicity was observed with higher concentrations. Thus it is recommended to keep the probe concentration equal to or below 10 μM.
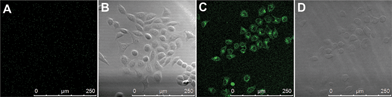
In addition, we found that 4F-2CN–Cys possesses two-photon properties when excited at 860 nm (Fig. S27†), whereas no two-photon signal from the reaction product of 4F-2CN and Hcy/GSH was observed. Further cell imaging experiments showed that living cells displayed fluorescence under two-photon excitation after 4F-2CN was added (Fig. 7). This study indicated that 4F-2CN might serve as a useful two-photon probe for selective detection of Cys in tissue imaging studies. The reason that 4F-2CN–Cys has two-photon properties could be attributed to the following two factors. First, 4F-2CN–Cys has strong electron withdrawing (CN) and electron donating (NH) groups. In general, adding strong electron donors and acceptors to the conjugated π system can enhance two-photon signals.17 For 4F-2CN–Hcy and 4F-2CN–GSH, the electron donating ability of the thiol group is less effective compared with that of the amine moiety. Second, it was observed that a rigid conformation can also enhance two-photon properties.17 4F-2CN–Cys forms a cyclized product, which increases the rigidity of the product's conformation, whereas 4F-2CN–Hcy and 4F-2CN–GSH do not.
Finally, we examined the fluorescence response of 4F-2CN with three sets of thiol mixtures, Cys/Hcy, Cys/GSH and Hcy/GSH. As shown in Fig. S28,† the detection of Cys is highly sensitive. It can be selectively detected even in the presence of 10 eq. of Hcy or GSH (λex = 420 nm). On the other hand, in the Cys/Hcy and Cys/GSH mixtures, Hcy (as well as GSH) can also be detected without much interference from Cys in the mixture (λex = 350 nm). As for the Hcy/GSH mixture, the two thiols cannot be differentiated when excited at 350 nm. However, selective detection of Hcy can be achieved through the addition of CTAB, and the Hcy signal was not interfered with in the presence of GSH.
Conclusion
In this study, we have discovered a novel and remarkably simple probe, 4F-2CN, which could undergo selective reactions with thiols under physiological conditions. Importantly, 4F-2CN displayed distinct reaction profiles for Cys, Hcy and GSH with the use of CTAB. Our bioimaging experiments proved, for the very first time, that Cys, Hcy and GSH could be differentiated using a single fluorescent probe. It should be noted that the probe can be subjected to further chemical modification and produce derivatives with different photophysical properties. The research work for synthesizing different derivatives of 4F-2CN is currently in progress. Interestingly 4F-2CN–Cys was found to possess two-photon properties, whereas the reaction product of 4F-2CN and Hcy/GSH does not possess any two-photon properties. Cell imaging experiments showed that two-photon fluorescence could be observed with the addition of 4F-2CN. The results indicated that the probe could serve as a useful tool for the selective detection of Cys in tissue imaging studies. Given the small size and excellent properties of 4F-2CN, we envision that this new and versatile probe will be a useful tool for further elucidating the roles of thiols in biology.Acknowledgements
For financial support we would like to thank the Hong Kong Early Career Scheme Grant (No. 21300714), the National Science Foundation of China (No. 21202137 and 21572190) and the City University of Hong Kong Grant (No. 9667091 and 9610304).Notes and references
- (a) G. I. Giles, Curr. Pharm. Des., 2006, 34, 4427 CrossRef; (b) N. Brandes, S. Schmitt and U. Jakob, Antioxid. Redox Signaling, 2009, 11, 997 CrossRef CAS PubMed; (c) C. K. Sen, Curr. Top. Cell. Regul., 2000, 36, 1 CAS; (d) T. P. Dalton, H. G. Shertzer and A. Puga, Annu. Rev. Pharmacol. Toxicol., 1999, 39, 67 CrossRef CAS PubMed.
- (a) S. Shahrokhian, Anal. Chem., 2001, 73, 5972 CrossRef CAS PubMed; (b) M. T. Heafield, S. Fearn, G. B. Steventon, R. H. Waring, A. C. Williams and S. G. Sturman, Neurosci. Lett., 1990, 110, 216 CrossRef CAS PubMed.
- (a) P. Sachdev, R. Parslow, C. Salonikas, O. Lux, W. Wen, R. Kumar, D. Naidoo, H. Christensen and A. Jorm, Arch. Neurol., 2004, 61, 1369 CrossRef PubMed; (b) P. Sachdev, J. Neurol. Sci., 2004, 226, 25 CrossRef CAS PubMed; (c) B. Hooshmand, T. Polvikoski, M. Kivipelto, M. Tanskanen, L. Myllykangas, T. Erkinjuntti, M. Mäkelä, M. Oinas, A. Paetau, P. Scheltens, E. C. van Straaten, R. Sulkava and A. Solomon, Brain, 2013, 136, 2707 CrossRef PubMed.
- (a) S. C. Lu, Mol. Aspects Med., 2009, 30, 42 CrossRef CAS PubMed; (b) D. M. Townsend, K. D. Tew and H. Tapiero, Biomed. Pharmacother., 2003, 57, 145 CrossRef CAS PubMed.
- (a) X. Chen, X. Tian, I. Shin and J. Yoon, Chem. Soc. Rev., 2011, 40, 4783 RSC; (b) J. Wang, J. Karpus, B. S. Zhao, Z. Luo, P. R. Chen and C. He, Angew. Chem., Int. Ed., 2012, 51, 9652 CrossRef CAS PubMed; (c) M. Vendrell, D. Zhai, J. C. Er and Y. T. Chang, Chem. Rev., 2012, 112, 4391 CrossRef CAS PubMed; (d) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973 CrossRef CAS PubMed; (e) Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688 CrossRef CAS PubMed.
- (a) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC; (b) Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52 RSC; (c) H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019 RSC; (d) X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590 CrossRef CAS PubMed.
- (a) L. Yi, H. Li, L. Sun, L. Liu, C. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2009, 48, 4034 CrossRef CAS PubMed; (b) X. Chen, S. Ko, M. J. Kim, I. Shin and J. Yoon, Chem. Commun., 2010, 46, 2751 RSC; (c) H. Zhang, P. Wang, Y. Yang and H. Sun, Chem. Commun., 2012, 48, 10672 RSC.
- (a) S.-Y. Lim, K.-H. Hong, D. I. Kim, H. Kwon and H.-J. Kim, J. Am. Chem. Soc., 2014, 136, 7018 CrossRef CAS PubMed; (b) J. Liu, Y.-Q. Sun, H. Zhang, Y. Huo, Y. Shi and W. Guo, Chem. Sci., 2014, 5, 3183 RSC.
- (a) H. Lin, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 45, 5904 Search PubMed; (b) Z. Yang, N. Zhao, Y. Sun, F. Miao, Y. Liu, X. Liu, Y. Zhang, W. Ai, G. Song, X. Shen, X. Yu, J. Sun and W.-Y. Wong, Chem. Commun., 2012, 48, 3442 RSC.
- (a) H. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki and N. Itoh, Angew. Chem., Int. Ed., 2005, 44, 2922 CrossRef CAS PubMed; (b) J. Bouffard, Y. Kim, T. M. Swager, R. Weissleder and S. A. Hilderbrand, Org. Lett., 2008, 10, 37 CrossRef CAS PubMed; (c) H. Zhang, C. Zhang, R. Liu, L. Yi and H. Sun, Chem. Commun., 2015, 51, 2029 RSC.
- (a) C. S. Lim, G. Masanta, H. J. Kim, J. H. Han, H. M. Kim and B. R. Cho, J. Am. Chem. Soc., 2011, 133, 11132 CrossRef CAS PubMed; (b) M. H. Lee, J. H. Han, P.-S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316 CrossRef CAS PubMed.
- (a) T. Zou, C. T. Lum, S. S. Chui and C. M. Che, Angew. Chem., Int. Ed., 2013, 52, 2930 CrossRef CAS PubMed; (b) Y. Bao, Q. Li, B. Liu, F. Du, J. Tian, H. Wang, Y. Wang and R. Bai, Chem. Commun., 2012, 48, 118 RSC; (c) S. R. Malwal, A. Labade, A. S. Andhalkar, K. Sengupta and H. Chakrapani, Chem. Commun., 2014, 50, 11533 RSC.
- X.-F. Yang, Y.-X. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690 CrossRef CAS PubMed.
- L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928 CrossRef CAS PubMed.
- (a) J. Liu, Y.-Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574 CrossRef CAS PubMed; (b) X.-F. Yang, Q. Huang, Y. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177 RSC.
- Y. Guo, X. Yang, L. Hakuma, A. Barve, J. O. Escobedo, M. Lowry and R. M. Strongin, Sensors, 2012, 12, 5940 CrossRef CAS PubMed.
- (a) M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244 CrossRef CAS PubMed; (b) G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245 CrossRef CAS PubMed; (c) H. M. Kim and B. R. Cho, Chem. Commun., 2009, 153 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, spectroscopic properties, NMR, mass spectra and confocal imaging. CCDC 1402255. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc02431e |
| This journal is © The Royal Society of Chemistry 2016 |