Concise approach to pyrrolizino[1,2-b]indoles from indole-derived donor–acceptor cyclopropanes†
Elena V. Villemsonab,
Ekaterina M. Budyninaa,
Olga A. Ivanovaab,
Dmitriy A. Skvortsovab,
Igor V. Trushkov*ab and
Mikhail Ya. Melnikova
aDepartment of Chemistry, M. V. Lomonosov Moscow State University, Leninskie Gory, 1-3, Moscow, Russian Federation 119991. E-mail: itrushkov@mail.ru
bLaboratory of Chemical Synthesis, Federal Research Center of Pediatric Hematology, Oncology and Immunology, Samory Mashela st., 1, Moscow, Russian Federation 117997
First published on 23rd June 2016
Abstract
Developed here is a direct three-step approach to a previously unexplored pyrrolizino[1,2-b]indole system based on a sequence of three synthetic steps: (a) one-pot azide ion promoted ring opening of the indole-derived donor–acceptor cyclopropane and Krapcho dealkoxycarbonylation; (b) Vilsmeier–Haack formylation; (c) tandem Staudinger/intramolecular aza-Wittig reaction and reductive cyclization realized in a one pot fashion.
Finding creative strategies for assembling diverse polycyclic indolo[b]-fused carbo- and heterocycles1,2 is a challenging task in synthetic and medicinal chemistry. The subset of donor–acceptor (D–A) cyclopropanes3 containing the indole moiety as the donor group4 is well suited for this purpose. By combining multifaceted reactivity of the activated cyclopropane and the indole core, these compounds offer tremendous synthetic potential for the construction of fused indoles with versatile scaffold diversity: cyclopenta-,5 cyclohepta-6 and cycloocta[b]indoles,7 carbazoles,7,8 indolo[3,2-b]carbazoles,8a cyclopenta[c]carbazoles,8c pentaleno[x,y-b]indoles,7,9 as well as indolo[b]-fused bicyclo[n.2.m] compounds.10
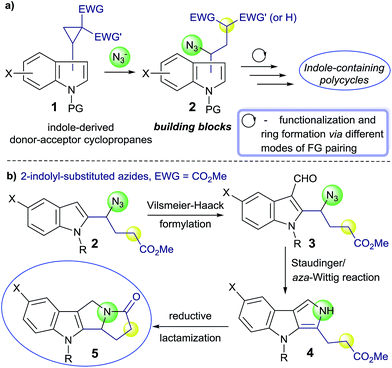
Recently, we have shown that nucleophilic ring opening of D–A cyclopropanes with the azide ion opens an avenue to the synthetically valuable γ-azidocarbonyl compounds that are not easily accessible via alternative approaches.11 These polyfunctional compounds can be further utilized in the manifold transformations due to the presence of donor substituent, electron-withdrawing group, azide moiety and activated CH-acidic fragment. Our synthetic strategy directed to the indole-based polycyclic systems of potential pharmacological value is based on the use of such azides obtained from the indole-derived D–A cyclopropanes and consists of intramolecular reactions between the indole moiety, on the one hand, and other functional groups, on the another hand (Scheme 1a). Preliminary modification of both the indole ring and functional group(s), all of them can be included into the overall process of the indole-containing polycycle formation, greatly diversifies the resulting polycyclic products.
 | ||
| Scheme 1 Indole-derived azides 2 as building blocks in the indole-based polycyclic compounds construction. | ||
We set out to use this general strategy in the synthesis of pyrrolizino[1,2-b]indoles as their framework includes pyrrolo[3,4-b]indole motif which is a key structural feature associated with diverse bioactive compounds.12 To the best of our knowledge, this tetracyclic system is unexplored yet due to lack of methods for its synthesis. Currently, the only reported preparation of this heterocyclic scaffold was based on the transannular cyclization of difficult accessible hexahydroazocino[4,3-b]indolones.13
In this context, we hypothesized that D–A cyclopropanes bearing 2-indolyl moiety may be appropriate starting compounds for the preparation of pyrrolizino[1,2-b]indoles 5. These cyclopropanes were readily available from the corresponding 2-formylindoles14 and revealed high reactivity in diverse cycloadditions,10a annulations,5c,15 cyclodimerizations,8a isomerizations.16 Moreover, easy modification of the indole ring at the C(3) atom enables the introduction of the appropriate functionality and functional group pairing to produce the required rings. Herein, we present an efficient approach to pyrrolizino[1,2-b]indoles via Vilsmeier–Haack formylation of the indole ring in azides 2 affording aldehydes 3 followed by one pot procedure including tandem Staudinger/aza-Wittig reaction and the reductive cyclization of products 4 (Scheme 1b).
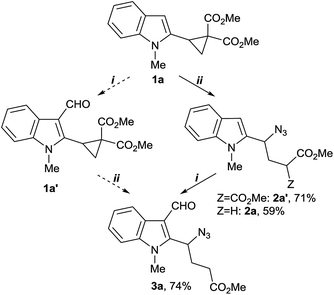
We began with a study of two approaches to the key intermediate 3 using cyclopropane 1a as a model compound (Scheme 2). The first one involves the Vilsmeier–Haack formylation followed by three-membered ring opening with sodium azide/Krapcho dealkoxycarbonylation via methodology developed recently.10 Alternatively, we explored a reverse sequence wherein the ring opening and dealkoxycarbonylation precedes the Vilsmeier–Haack reaction.
We found that the formylation of indole 1a with the Vilsmeier reagent failed to provide the required aldehyde in reasonable yield. After 48 h at room temperature, only 6% conversion of 1a to aldehyde 1a′ was achieved. Efforts to improve the reaction efficiency were not successful: even moderate heating (40–45 °C) was accompanied by the formation of intractable polymeric mixture, and the target 1a′ was obtained in 10% yield only. Three-membered ring opening of the acid-susceptible cyclopropanes 1a,a′ is suggested responsible for the low efficiency of this reaction.
Fortunately, the alternative approach was more successful. Cyclopropane 1a underwent the efficient ring opening with the azide ion under reaction conditions similar to those applied for nucleophilic ring opening of other D–A cyclopropanes11 (Scheme 2). It is noteworthy, that 2-indolyl-substituted cyclopropane 1a was found to have lower reactivity than D–A cyclopropanes bearing 3-indolyl group as the donor. Namely, full conversion of cyclopropane 1a into azide 2a′ required heating at 75 °C for 4 h, while 3-indolyl-substituted D–A cyclopropanes produced the corresponding azides in 86–91% isolated yield after 3–4 h at 50 °C.11
Then, one pot synthesis of 4-azido-4-indolylbutyrate 2a from donor–acceptor cyclopropane 1a was tested. The first step of three-membered ring opening with the azide ion was carried out under established conditions. Next, water and LiCl (6 equivalents of both) were added to the reaction mixture which then was heated at 110 °C (Scheme 2). This procedure allowed an efficient access to 4-azido-4-(2-indolyl)butyrate 2a in reasonable yield. The subsequent Vilsmeier–Haack formylation of 2a with slight excess of POCl3 in dimethylformamide proceeded efficiently under mild conditions (0–50 °C, 4 h) resulting in 3-formylindole 3a.
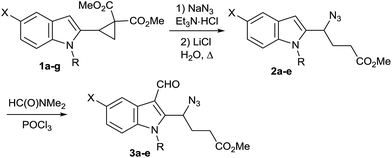
Next, we applied the optimized reaction conditions to the synthesis of a series of γ-azido-γ-(indol-2-yl)butyrates 2 and 3 from the corresponding indole-substituted cyclopropanes 1a–g (Table 1). In general, azides 2 were obtained in moderate to good yields. It is noteworthy that the use of microwave irradiation allows one to shorten reaction time significantly without decrease of the yield. Herewith, substituents at the C(5) atom as well as alkyl group at the N(1) atom of the indole nucleus have little influence on the efficiency of the nucleophilic cyclopropane ring opening. However, N-tosyl derivative 1f did not produce azide 2f under the reaction conditions optimized for N-alkylindoles. This may be attributed to the electron-withdrawing effect of the tosyl group that decreases the polarization of the cyclopropane C(1)–C(2) bond and, as a result, hampers the ring opening. Attempts to solve this problem by use of harsher reaction conditions (130 °C) were unsuccessful due to significant tarring of the reaction mixture. N-Boc protected indole 1g was found to be unstable under the typical reaction conditions yielding oligomerization products.
| Entry | 1–3 | R | X | Conditions, T [°C]/t [h] | Yieldb, % | ||
|---|---|---|---|---|---|---|---|
| (a) | (b) | 2 | 3 | ||||
| a Reaction conditions. Azide 2 synthesis: (a) 0.5 M solution of 1 (1 equiv.), NaN3 (2 equiv.), Et3N·HCl (2 equiv.), DMF, 75–90 °C, 4–5 h; (b) H2O (6 equiv.), LiCl (6 equiv.), 110–115 °C, 16–25 h. Vilsmeier–Haack formylation: 5 M solution of 2 (1 equiv.), DMF (4.4 equiv.), POCl3 (1.1 equiv.), 0–50 °C, 4 h.b Isolated yield.c PMB = p-methoxybenzyl.d Azide was obtained in trace amounts. | |||||||
| 1 | a | Me | H | 75/4 | 110/25 | 59 | 74 |
| 2 | b | PMBc | H | 90/4 | 110/16 | 61 | 76 |
| 3 | c | Me | Cl | 80/4 | 110/22 | 63 | 71 |
| 4 | d | Bn | F | 85/5 | 110/20 | 72 | 62 |
| 5 | e | Bn | OMe | 90/4 | 115/19 | 70 | 70 |
| 6 | f | Ts | H | 90/5 | — | —d | — |
| 7 | g | Boc | H | 85/4 | — | —d | — |
Thereby, we investigated the formylation of γ-azidoesters 2 and found that substrates possessing both electron-withdrawing and electron-releasing substituents (halogen, methoxy) at the 5-position of the indole ring as well as different N-protecting groups (methyl, benzyl, p-methoxybenzyl) afford the corresponding aldehydes 3 with similar efficiency (Table 1).
Unfortunately, the similar acylation of 1a using POCl3 in N,N-dimethylacetamide to the corresponding 3-acetylindole was unsuccessful. A moderate heating (up to 85 °C) did not increase the yield of the target product but initiated the polymerization process. Analogously, attempts of indole 1a acylation with acetyl chloride under diverse conditions failed to afford the desired product.
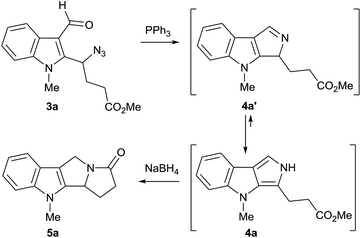
The presence of the formyl and azide moieties in compounds 3 allows to prepare a new nitrogen-containing ring annulated to the C(2)–C(3) bond of the indole framework via the combination of these functionalities.17 Indeed, the treatment of formylazide 3a with Ph3P induced a domino process consisting of Staudinger and aza-Wittig reactions. According to 1H and 13C NMR data, the product of this condensation exists in 2H,4H-pyrrolo[3,4-b]indole tautomeric form 4a which is significantly more stable than 3H,4H-tautomer 4a′ (by 22.0 kJ mol−1 according to DFT calculations at B3LYP/6-311G** level).14,18 However, our attempts to isolate this compound by column chromatography on silica gel or neutral alumina were unsuccessful due to its lability. When we treated the crude product with methanolic NaBH4, 4a was easily transformed into pyrrolizino[1,2-b]indole 5a via the reduction of the imine moiety in the tautomeric form 4a′ followed by spontaneous amidative ring closure (Scheme 3). This is the first demonstration of the reactivity of pyrrolo[3,4-b]indoles in their 3H,4H-tautomeric form with non-aromatic pyrrole ring. The further extension of this ability of 4a to react as imine 4a′ has promising prospects for the development of new synthetic sequences for pyrrolo[3,4-b]indoles and related [c]-fused pyrroles. Thus, the facile Staudinger/aza-Wittig domino reaction combined with the reductive cyclization of pyrrolo[3,4-b]indoles 4 to pyrrolizino[1,2-b]indoles 5, provides an efficient way of appending the pyrrolizine moiety to the indole scaffold in a one-pot fashion.
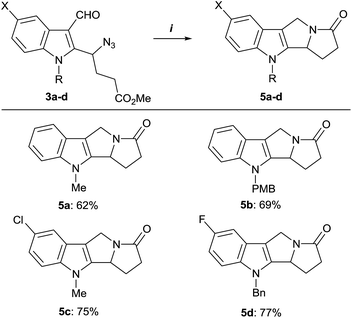
With the optimized conditions in hand, we investigated the scope of this Staudinger/intramolecular aza-Wittig reaction/reductive cyclization one pot protocol (Scheme 4). A number of substituted γ-azido-γ-(2-indolyl)butyrates 3b–e were subjected to this reaction sequence to obtain the corresponding tetracyclic pyrrolizino[1,2-b]indoles 5b–e. The one-pot method allowed to avoid the isolation of unstable pyrrolo[3,4-b]indoles 4 and produced the target compounds 5 in reasonable yields and good purity. The variation of substituents in the indole ring of azides 3 had no significant influence on the reaction efficiency, except for 5-methoxy-substituted azide 3e. Unfortunately, in that case the complex mixture of unidentified products was obtained. Structural assignments for the target compounds 5 were made by means of 1D and 2D NMR experiments.
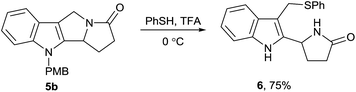
On the next step of this study, we investigated dealkylation of N-alkylpyrrolizino[1,2-b]indole 5 encouraged by the abundance of N-unsubstituted compounds among the indole-based drugs. To probe the dealkylation, we selected (4-methoxybenzyl)-substituted indole 5b as a number of procedures were reported for the removal of para-methoxybenzyl group. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone19 was found to produce a complex mixture of products in this case. Therefore, we tried the treatment of 5b with a large excess of trifluoroacetic acid (TFA) and thiophenol at 0 °C.20 Unexpectedly, we found that under these conditions N-debenzylation was accompanied by pyrrolidine ring opening resulting in pyrrolidone 6 (Scheme 5), the structure of which was established by a combination of 1D and 2D NMR data. This pyrrolidone was evidently formed via protonation of the amide oxygen followed by nucleophilic attack on the benzylic carbon atom inducing C–N bond cleavage.21
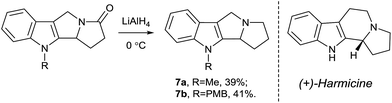
Another important modification of the obtained pyrrolizino[1,2-b]indoles 5 is the reduction of the amide moiety affording the corresponding amines 7 which are structural analogues of the related indolizinoindoles, such as antileishmanial and analgesic alkaloid harmicine22 or cytotoxic and antimicrobial fascaplysin.23 We found that the reduction of substrates 5a,b with LiAlH4 produced the target amines 7a,b albeit in moderate yields (Scheme 6).
The cytotoxicity of a series of the synthesized pyrrolizino[1,2-b]indoles was evaluated against HEK-293, VA13, MCF-7, and A549 cell lines. Compounds 5a and 7a were found to be almost inactive while 5d and 7b demonstrated cytotoxicity of ca. 10 μM against all studied cells. The obtained results are summarized in Table 2.
| Compound | R | X | IC50, μM | |||
|---|---|---|---|---|---|---|
| HEK-293 | VA13 | MCF-7 | A549 | |||
| 5a | Me | H | >100 | 90.5 | >100 | 100.6 |
| 5b | PMB | H | 24.3 | 30.0 | 25.2 | 23.2 |
| 5c | Me | Cl | 35.9 | 39.3 | 40.9 | 41.0 |
| 5d | Bn | F | 11.2 | 12.4 | 13.4 | 11.2 |
| 7a | Me | H | 90.1 | 45.7 | >100 | 98.3 |
| 7b | PMB | H | 5.2 | 7.5 | 11.2 | 8.7 |
| Etoposide | 0.1 | 0.6 | 0.6 | 0.4 | ||
Conclusions
In conclusion, we have developed the first preparative approach to pyrrolizino[1,2-b]indole scaffold based on the indole-derived donor–acceptor cyclopropanes ring-opening with sodium azide. A series of tetracyclic pyrrolizino[1,2-b]indoles were rapidly constructed in moderate to good yields from these cyclopropanes in three synthetic steps only. The synthesized pyrrolizino[1,2-b]indoles demonstrated moderate cytotoxicity towards HEK-293, VA13, MCF-7, A549 cells with IC50 up to 5 μM. The developed methodology broadens the utilization of the indole-derived cyclopropane nucleophilic ring-opening in the assembly of indole-fused polycyclic systems with diverse structural features.Acknowledgements
We thank Russian Science Foundation (grant 14-13-01178) for financial support of this work.Notes and references
- For some reviews, see: (a) W. Zi, Z. Zuo and D. Ma, Acc. Chem. Res., 2015, 48, 702 CrossRef CAS PubMed; (b) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (c) H. J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef.
- For recent syntheses, see: (a) S. P. Nikumbh, A. Raghunadh, T. S. Rao, V. N. Murthy, S. C. Joseph, Y. L. N. Murthy and M. Pal, RSC Adv., 2016, 6, 23489 RSC; (b) P. Purohit, A. K. Pandey, B. Kumar and P. M. S. Chauhan, RSC Adv., 2016, 6, 21165 RSC; (c) Z. Wang, X. Xu, Z. Gu, W. Feng, H. Qian, Z. Li, X. Sun and O. Kwon, Chem. Commun., 2016, 52, 2811 RSC; (d) D. Sole, M.-L. Bennasar, T. Roca and M. Valldosera, Eur. J. Org. Chem., 2016, 1355 CrossRef CAS; (e) Z. Chai, J.-N. Chen, Z. Liu, X.-F. Li, P.-J. Yang, J.-P. Hu and G. Yang, Org. Biomol. Chem., 2016, 14, 1024 RSC; (f) Y.-P. Han, X.-R. Song, Y.-F. Qiu, H.-R. Zhang, L.-H. Li, D.-P. Jin, X.-Q. Sun, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2016, 18, 940 CrossRef CAS PubMed; (g) Z. Zhang, F. Xiao, B. Huang, J. Hu, B. Fu and Z. Zhang, Org. Lett., 2016, 18, 908 CrossRef CAS PubMed; (h) A. Kamlah, F. Lirk and F. Bracher, Tetrahedron, 2016, 72, 837 CrossRef CAS; (i) C. Liu, L. Zhou, W. Huang, M. Wang and Y. Gu, Tetrahedron, 2016, 72, 563 CrossRef CAS; (j) D. Anumandla, A. Acharya and C. S. Jeffrey, Org. Lett., 2016, 18, 476 CrossRef CAS PubMed.
- For some recent reviews, see: (a) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC; (b) F. de Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912 RSC; (c) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 CrossRef CAS PubMed; (d) M. A. Cavitt, K. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804 RSC.
- I. V. Trushkov, Isr. J. Chem. DOI:10.1002/ijch.201500069.
- (a) E. R. Rakhmankulov, K. L. Ivanov, E. M. Budynina, O. A. Ivanova, A. O. Chagarovskiy, D. A. Skvortsov, G. V. Latyshev, I. V. Trushkov and M. Y. Melnikov, Org. Lett., 2015, 17, 770 CrossRef CAS PubMed; (b) O. A. Ivanova, E. M. Budynina, D. A. Skvortsov, I. V. Trushkov and M. Y. Melnikov, Synlett, 2014, 25, 2289 CrossRef CAS; (c) Y. A. Volkova, E. M. Budynina, A. E. Kaplun, O. A. Ivanova, A. O. Chagarovskiy, D. A. Skvortsov, V. B. Rybakov, I. V. Trushkov and M. Y. Melnikov, Chem.–Eur. J., 2013, 19, 6586 CrossRef CAS PubMed.
- P. J. Gritsch, E. Stempel and T. Gaich, Org. Lett., 2013, 15, 5472 CrossRef CAS PubMed.
- A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1388 CrossRef CAS PubMed.
- (a) O. A. Ivanova, E. M. Budynina, V. N. Khrustalev, D. A. Skvortsov, I. V. Trushkov and M. Y. Melnikov, Chem.–Eur. J., 2016, 22, 1223 CrossRef CAS PubMed; (b) H. K. Grover, T. P. Lebold and M. A. Kerr, Org. Lett., 2011, 13, 220 CrossRef CAS PubMed; (c) C. Venkatesh, H. Ila, H. Junjappa, S. Mathur and V. Hush, J. Org. Chem., 2002, 67, 9477 CrossRef CAS PubMed.
- O. A. Ivanova, E. M. Budynina, A. O. Chagarovskiy, E. R. Rakhmankulov, I. V. Trushkov, A. V. Semeykin, N. L. Shimanovskii and M. Y. Melnikov, Chem.–Eur. J., 2011, 17, 11738 CrossRef CAS PubMed.
- (a) O. A. Ivanova, E. M. Budynina, A. O. Chagarovskiy, A. E. Kaplun, I. V. Trushkov and M. Y. Melnikov, Adv. Synth. Catal., 2011, 353, 1125 CrossRef CAS; (b) D. A. Dias and M. A. Kerr, Org. Lett., 2009, 11, 3694 CrossRef CAS PubMed.
- K. L. Ivanov, E. V. Villemson, E. M. Budynina, O. A. Ivanova, I. V. Trushkov and M. Y. Melnikov, Chem.–Eur. J., 2015, 21, 4975 CrossRef CAS PubMed.
- (a) A. V. Ivachtchenko, E. B. Frolov, O. D. Mitkin, S. E. Tkachenko and A. V. Khvat, Chem. Heterocycl. Compd., 2010, 46, 170 CrossRef CAS; (b) D. Page, H. Yang, W. Brown, C. Walpole, M. Fleurent, M. Fyfe, F. Gaudreault and S. St-Onge, Bioorg. Med. Chem. Lett., 2007, 17, 6183 CrossRef CAS PubMed; (c) D. Hamprecht, F. Micheli, G. Tedesco, D. Donati, M. Petrone, S. Terreni and M. Wood, Bioorg. Med. Chem., 2007, 17, 424 CrossRef CAS PubMed.
- J. D. Street, M. Harris, D. I. Bishop, F. Heatley, R. L. Beddoes, O. S. Mills and J. A. Joule, J. Chem. Soc., Perkin Trans. 1, 1987, 1599 RSC.
- For details, see ESI.†.
- K. Sapeta and M. A. Kerr, Org. Lett., 2009, 11, 2081 CrossRef CAS PubMed.
- P.-L. Zhu, Z. Zhang, X.-Y. Tang, I. Marek and M. Shi, ChemCatChem, 2015, 7, 595 CrossRef CAS.
- (a) E. T. Pelkey, J. Jiang and G. W. Gribble, J. Indian Chem. Soc., 2013, 90, 1525 CAS; (b) E. T. Pelkey, L. Chang and G. W. Gribble, Chem. Commun., 1996, 1909 RSC; (c) C.-K. Sha and J.-F. Yang, Tetrahedron, 1992, 48, 10645 CrossRef CAS.
- See also: (a) C.-K. Sha, K.-S. Chuang and S.-J. Wey, J. Chem. Soc., Perkin Trans. 1, 1987, 977 RSC; (b) C.-K. Sha, K.-S. Chuang and J.-J. Young, J. Chem. Soc., Chem. Commun., 1984, 1552 CAS.
- Y. Miki, H. Hachiken, Y. Kashima, W. Sugimura and N. Yanase, Heterocycles, 1998, 48, 1 CrossRef CAS.
- F. Arioli, M. Perez, F. Subrizi, N. Llor, J. Bosch and M. Amat, J. Org. Chem., 2014, 79, 7740 CrossRef CAS PubMed.
- For related reactions wherein –NHC(O)R moiety serves as leaving group, see: (a) M. G. Uchuskin, N. V. Molodtsova, S. A. Lysenko, V. N. Strel’nikov, I. V. Trushkov and A. V. Butin, Eur. J. Org. Chem., 2014, 2508 CrossRef CAS; (b) W. Li, Y. Ye, J. Zhang and R. Fan, Tetrahedron Lett., 2009, 50, 5536 CrossRef CAS.
- (a) C. S. Lood and A. M. P. Koskinen, Chem. Heterocycl. Compd., 2015, 50, 1367 CrossRef CAS; (b) I. Chakraborty and S. Jana, Synthesis, 2013, 45, 3325 CrossRef CAS.
- (a) S. Manda, S. Sharma, A. Wani, P. Joshi, V. Kumar, S. K. Guru, S. S. Bharate, S. Bhushan, R. A. Vishwakarma, A. Kumar and S. B. Bharate, Eur. J. Med. Chem., 2016, 107, 1 CrossRef CAS PubMed; (b) E. Ampofo, T. Später, I. Müller, H. Eichler, M. D. Menger and M. W. Laschke, Mar. Drugs, 2015, 13, 6774 CrossRef CAS PubMed; (c) G. Hamilton, Mar. Drugs, 2014, 12, 1377 CrossRef PubMed; (d) S. B. Bharate, S. Manda, N. Mupparapu, N. Battini and R. A. Vishwakarma, Mini-Rev. Med. Chem., 2012, 12, 650 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, results of quantum chemical calculations, and copies of NMR spectra for all new products. See DOI: 10.1039/c6ra11233a |
| This journal is © The Royal Society of Chemistry 2016 |