Light-induced synthesis of photoluminescent carbon nanoparticles for Fe3+ sensing and photocatalytic hydrogen evolution†
Pengju
Yang
ab,
Jianghong
Zhao
*a,
Jian
Wang
ab,
Baoyue
Cao
ab,
Li
Li
a and
Zhenping
Zhu
*a
aState Key Laboratory of Coal Conversion Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, 030001, P. R. China. E-mail: zjh_sx@sxicc.ac.cn; zpzhu@sxicc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100039, P. R. China
First published on 13th November 2014
Abstract
We report a new method to prepare photoluminescent carbon nanoparticles (CNPs) by a light-induced process. The obtained CNPs are sensitive to the specific detection of Fe3+ with a detection limit of 0.55 ppm. CNPs also show excellent photocatalytic hydrogen production under xenon lamp irradiation. The hydrogen evolution rate is up to 4.89 μmol h−1 over 2.1 mg CNPs.
Carbon nanomaterials have attracted much attention owing to their unique properties and potential applications, including catalysis, adsorption, separation, and electrode materials, among others.1,2 Recently, carbon nanomaterials with photoluminescent properties have been found and they have attracted much interest.3–5 Compared to traditional semiconductor quantum dots and organic dyes, photoluminescent carbon nanomaterials (PCNs) are superior in high aqueous solubility, robust chemical inertness, easy functionalization, good photo-stability, low toxicity and excellent biocompatibility.6 PCNs have exhibited potential applications in biological labeling, bioimaging, disease detection, drug delivery, sensing and energy-related applications.6–9
To date, numerous approaches have been developed to prepare these versatile materials, including electrochemical synthesis, combustion, hydrothermal, acidic oxidation, microwave, ultrasonic, solution chemistry, arc discharge, laser ablation, plasma treatment and so on.6,10 However, almost all of these techniques usually require high temperature and a relatively complicated process.
Here we report a new and simple method for the synthesis of photoluminescent CNPs with glucose as the raw material by light-induced processes at room temperature and ambient pressure. Data show that the obtained CNPs are sensitive to the specific detection of Fe3+. A detection limit as low as 0.55 ppm for Fe3+ was obtained, which is promising for biological applications. It is worth noting that CNPs also show excellent photocatalytic hydrogen evolution. The hydrogen evolution rate of CNPs alone reaches 2.11 μmol h−1. The hydrogen evolution rate was further improved to 4.89 μmol h−1 when noble metal Pt was used as a co-catalyst.
The transmission electron microscopy (TEM) image (Fig. 1a) shows that the obtained CNPs are well-dispersed onion-like nanospheres with nearly homogeneous sizes. The average diameter of the CNPs is about 40 nm. Dynamic light scattering (DLS) was also used to measure the size distribution of CNPs, which however exhibited the larger sizes of aggregated CNPs (Fig. S1†) owing to the inherent principles of this method.11,12 The high-resolution TEM (HRTEM) image (Fig. 1b) shows the short-range ordered structure of onion-like nanocarbons with concentric graphitic layers, whose interlayer spacings are around 0.38 nm. The value is slightly larger than ideal graphite (0.334 nm), consistent with X-ray diffraction (XRD) results (Fig. S2†).13,14
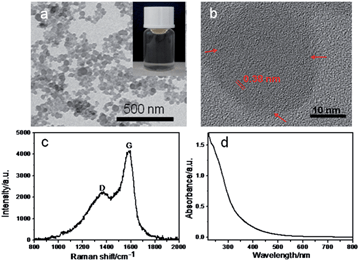 | ||
| Fig. 1 (a) TEM (the inset is an optical image of the CNPs) and (b) HRTEM images, (c) Raman spectrum and (d) UV-visible adsorption spectrum of the CNPs. | ||
The Raman spectrum provided compelling evidence for the different types of ordered and disordered bonding environments of the sp2 and sp3 hybridized carbon.15Fig. 1c shows that the peak at 1588 cm−1 is the G band corresponding to the E2g mode of the graphite and is related to the vibration of sp2-bonded carbon atoms, whereas the D band located at 1378 cm−1 is disordered because of the scattering at the sp3-bonded carbon edges.15 The co-existence of the two structures is confirmed, which is consistent with the HRTEM characterization. Fig. 1d depicts the typical UV-visible absorption spectrum of CNPs. A typical absorption peak at 230 nm was observed, which is assigned to the π–π* transition of aromatic sp2 domains. In addition, a shoulder at around 320 nm is ascribed to the n–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, which is in agreement with previous reports.16
O bond, which is in agreement with previous reports.16
The X-ray photoelectron spectroscopy (XPS) measurement shows the existence of two elements, C and O (Fig. 2a). Fig. 2b shows the deconvolution of the C1s spectrum of CNPs, four types of carbon bonds: 284.5, 285.5, 287.2 and 288.6 eV, which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C
C, C–C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /
/![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C–H, C–O–C, and C
C–H, C–O–C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, respectively.17 The Fourier transform infrared spectroscopy (FTIR) spectrum was recorded to gain further structural insights into CNPs. As depicted in Fig. 2c, the presence of various functional groups including OH (3430 cm−1), C
O species, respectively.17 The Fourier transform infrared spectroscopy (FTIR) spectrum was recorded to gain further structural insights into CNPs. As depicted in Fig. 2c, the presence of various functional groups including OH (3430 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1760 cm−1) C
O (1760 cm−1) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1620 cm−1), C–OH (1190 cm−1), C–O–C (1098 cm−1), C–H (1411 cm−1) is confirmed, which agrees well with XPS results.18–20 Elemental analysis of CNPs shows that the carbon, hydrogen and oxygen composition was 61.32 wt%, 5.595 wt% and 33.085 wt% respectively. Fig. 2d shows the photoluminescence (PL) spectra of CNPs. A unique phenomenon of excitation dependent photoluminescence was observed, similar to previous reports.21–25 The zeta potential of CNPs in aqueous solution is −9.21 mV, owing to the carboxylic groups on the surface, which indicates that CNPs are highly dispersed and stable in aqueous systems (Fig. S3†).26
C (1620 cm−1), C–OH (1190 cm−1), C–O–C (1098 cm−1), C–H (1411 cm−1) is confirmed, which agrees well with XPS results.18–20 Elemental analysis of CNPs shows that the carbon, hydrogen and oxygen composition was 61.32 wt%, 5.595 wt% and 33.085 wt% respectively. Fig. 2d shows the photoluminescence (PL) spectra of CNPs. A unique phenomenon of excitation dependent photoluminescence was observed, similar to previous reports.21–25 The zeta potential of CNPs in aqueous solution is −9.21 mV, owing to the carboxylic groups on the surface, which indicates that CNPs are highly dispersed and stable in aqueous systems (Fig. S3†).26
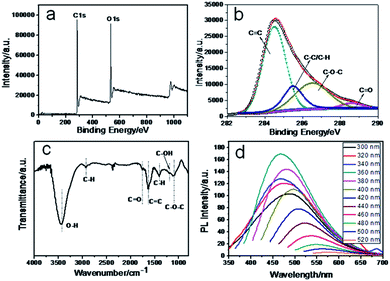 | ||
| Fig. 2 (a) Full range XPS spectrum, (b) XPS high resolution scan of the C1s, (c) FTIR and (d) PL spectrum of the CNPs. | ||
Taking into account the special coordinate interaction between Fe3+ ions and phenolic hydroxyl groups, we speculate that CNPs may be employed to optically detect Fe3+ ions.27Fig. 3a shows the PL change of CNPs with and without addition of Fe3+ ions. The PL intensity was almost quenched when 137.5 ppm Fe3+ ions were added. Fig. S4† shows the dependence of (I0 − I)/I0 on the concentrations of Fe3+ ions, where I0 and I are CNP fluorescence intensities at 468 nm in the absence and presence of Fe3+ ions, respectively. It can be seen that the detection limit for Fe3+ ions reaches 0.55 ppm. Selectivity is a very important parameter to evaluate the performance of the sensing system. Therefore, we investigated the effect of representative metal ions (Mg2+, Ag+, Cd2+,Cr3+, Mg2+, Ca2+, Na+, K+, Co2+, Zn2+, Mn2+, Ni+ and Cu2+) on the CNP fluorescence quenching at 10 ppm concentration. As shown in Fig. 3b, most of those metal ions did not induce obvious fluorescence quenching, whereas the PL intensity of CNPs resulted in the strongest quenching effect in the presence of Fe3+. The selectivity diversity of different metals may be attributed to the difference of interaction between CNPs and metal ions.28,29
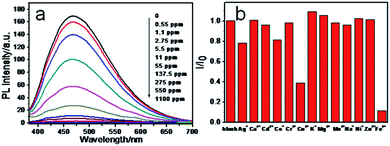 | ||
| Fig. 3 (a) Fluorescence quenching of the CNPs in the presence of Fe3+ ions and (b) fluorescence quenching of the CNPs after addition of different metal ions. | ||
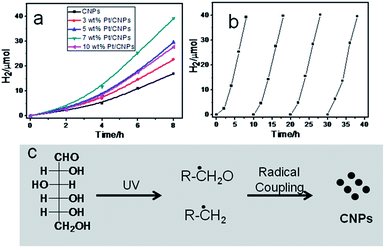
Recently, some reports have confirmed that photoluminescent CNPs show excellent photocatalytic activity for hydrogen evolution.30,31Fig. 4a shows the photocatalytic hydrogen evolution on the CNPs and Pt/CNPs. The hydrogen evolution rate reached 2.11 μmol h−1 over single CNPs under a 300 W xenon lamp, without any modification and co-catalyst. When noble metal Pt was employed as a co-catalyst, the hydrogen evolution rate was further increased. The highest hydrogen evolution rate of 4.89 μmol h−1 was achieved when 7 wt% Pt was employed as the co-catalyst. On further increasing the amount of noble metal Pt, the photocatalytic activity becomes low, which suggests the existence of an optimal value of the co-catalyst (Fig. S5†). Stability is another key parameter to evaluate the photocatalytic performance of photocatalysts. In our case, 7 wt% Pt/CNPs were employed to study their stability. As shown in Fig. 4b, the photocatalytic hydrogen evolution rates of the 7 wt% Pt/CNPs showed a negligible change even after four cycles of testing, showing the good photo-stability of CNPs.
To uncover the formation mechanism of CNPs, we performed a blank experiment without light irradiation. TEM results show that no CNPs were obtained. Previous reports have proven that carbohydrates can be decomposed into other compounds by a radical process after UV irradiation and carbon radicals were detected using EPR spectra.32,33 Phillips reported that photodecomposition of glucose takes place directly due to adsorption of the light itself.34 Fig. S6† shows the UV-visible adsorption spectrum of glucose aqueous solution. It can be seen that glucose molecules show obvious adsorption in the ultraviolet region. Based on preliminary data, the possible formation mechanism of CNPs might be that glucose molecules are decomposed into carbon-related radicals after UV irradiation. Then, these carbon-related radical building blocks formed CNPs by C–C coupling polymerization (as shown in Fig. 4c).
Conclusions
In summary, we develop a new and simple method to prepare CNPs by the light-induced process. The results show that the obtained CNPs are sensitive to the detection of Fe3+ ions with a 0.55 ppm detection limit. It was found that the obtained CNPs can be used as an excellent photocatalyst. The highest photocatalytic hydrogen evolution rate of CNPs reaches 4.89 μmol h−1 under xenon lamp irradiation. This finding will provide a new possibility to prepare CNPs by photo-polymerization of photo-active organic molecules under light irradiation.Acknowledgements
We acknowledge the financial support from the Nature Science Foundation of China (no. 21173250), the Knowledge Innovation Project of Chinese Academy of Sciences (no. KGCX2-EW-311), the Youth Innovation Foundation of Institute of Coal Chemistry, and the Chinese Academy of Sciences (2011SQNRC19).Notes and references
- M. S. Mauter and M. Elimelech, Environ. Sci. Technol., 2008, 42, 5843 CrossRef CAS.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, Chem. Soc. Rev., 2013, 42, 2824 RSC.
- L. Cao, M. J. Meziani, S. Sahu and Y. P. Sun, Acc. Chem. Res., 2013, 46, 171 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- W. Li, Z. Zhang, B. Kong, S. Feng, J. Wang, L. Wang, J. Yang, F. Zhang, P. Wu and D. Zhao, Angew. Chem., Int. Ed., 2013, 52, 8151 CrossRef CAS PubMed.
- H. T. Li, Z. H. Kang, Y. Liu and S. T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- Z. Zhang, J. Zhang, N. Chen and L. Qu, Energy Environ. Sci., 2012, 5, 8869 CAS.
- Y. F. Wang and A. G. Hu, J. Mater. Chem. C, 2014, 2, 6921 RSC.
- J. H. Shen, Y. H. Zhu, X. L. Yang and C. Z. Li, Chem. Commun., 2012, 48, 3686 RSC.
- L. L. Li, G. H. Wu, G. H. Yang, J. Peng, J. W. Zhao and J. J. Zhu, Nanoscale, 2013, 5, 4015 RSC.
- V. Filipe, A. Hawe and W. Jiskoot, Pharm. Res., 2010, 27, 796 CrossRef CAS PubMed.
- H. Kato, A. Nakamura, K. Takahashi and S. Kinugasa, Nanomaterials, 2012, 2, 15 CrossRef CAS PubMed.
- W. Lian, H. Song, X. Chen, L. Li, J. Huo, M. Zhao and G. Wang, Carbon, 2008, 46, 525 CrossRef CAS PubMed.
- C. R. Houska and B. E. Warren, J. Appl. Phys., 1954, 25, 1503 CrossRef CAS PubMed.
- A. C. Ferrari and D. M. Basko, Nat. Nanotechnol., 2013, 8, 235 CrossRef CAS PubMed.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS PubMed.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102 CrossRef CAS PubMed.
- Y. Deng, X. Chen, F. Wang, X. Zhang, D. Zhao and D. Shen, Nanoscale, 2014, 6, 10388 RSC.
- X. M. Sun and Y. D. Li, Angew. Chem., Int. Ed., 2004, 43, 597 CrossRef PubMed.
- L. Zhao, F. Di, D. Wang, L.-H. Guo, Y. Yang, B. Wan and H. Zhang, Nanoscale, 2013, 5, 2655 RSC.
- D. Y. Pan, J. C. Zhang, Z. Li, C. Wu, X. M. Yan and M. H. Wu, Chem. Commun., 2010, 46, 3681 RSC.
- H. Z. Zheng, Q. L. Wang, Y. J. Long, H. J. Zhang, X. X. Huang and R. Zhu, Chem. Commun., 2011, 47, 10650 RSC.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, Carbon, 2013, 60, 421 CrossRef CAS PubMed.
- Y. Shin, J. Lee, J. Yang, J. Park, K. Lee, S. Kim, Y. Park and H. Lee, Small, 2014, 10, 866 CrossRef CAS.
- Y. Dong, J. Lin, Y. Chen, F. Fu, Y. Chi and G. Chen, Nanoscale, 2014, 6, 7410 RSC.
- Z. L. Wu, M. X. Gao, T. T. Wang, X. Y. Wan, L. L. Zheng and C. Z. Huang, Nanoscale, 2014, 6, 3868 RSC.
- D. Wang, L. Wang, X. Dong, Z. Shi and J. Jin, Carbon, 2012, 50, 2147 CrossRef CAS PubMed.
- W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2012, 84, 5351 CrossRef CAS PubMed.
- C. Shen, Y. Sun, J. Wang and Y. Lu, Nanoscale, 2014, 6, 9139 RSC.
- L. Cao, S. Sahu, P. Anilkumar, C. E. Bunker, J. Xu, K. A. S. Fernando, P. Wang, E. A. Guliants, K. N. Tackett II and Y.-P. Sun, J. Am. Chem. Soc., 2011, 133, 4754 CrossRef CAS PubMed.
- T.-F. Yeh, C.-Y. Teng, S.-J. Chen and H. Teng, Adv. Mater., 2014, 26, 3297 CrossRef CAS PubMed.
- N. D. Yordanov and E. Georgieva, Spectrochim. Acta, Part A, 2004, 60, 1307 CrossRef PubMed.
- G. O. Phillips and T. Rickards, J. Chem. Soc. B, 1969, 455, 10.1039/j29690000455.
- G. O. Phillips and G. J. Moody, J. Chem. Soc., 1960, 3398, 10.1039/jr9600003398.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta05155f |
| This journal is © The Royal Society of Chemistry 2015 |