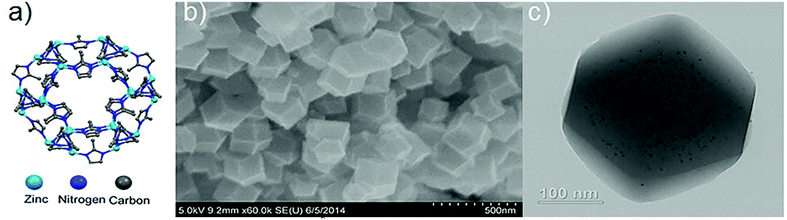
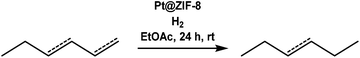
Pt@ZIF-8 composite for the regioselective hydrogenation of terminal unsaturations in 1,3-dienes and alkynes†
Casey J.
Stephenson
a,
Joseph T.
Hupp
*a and
Omar K.
Farha
*ab
aDepartment of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208, USA. E-mail: j-hupp@northwestern.edu; o-farha@northwestern.edu
bDepartment of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
First published on 6th March 2015
Abstract
Pt@ZIF-8 composites with crystallite sizes of ca. 150 nm were synthesized. The composite was used as a catalyst for the regioselective hydrogenation of trans-1,3-hexadiene to 3-hexene. 1-Hexyne was hydrogenated to 1-hexene and hexane while 3-hexyne did not react. In all cases, the Pt@ZIF-8 composite had higher selectivity than Pt/C.
As atom economical reactions become increasingly important to the chemical industry, catalysts that can perform reactions with not only high activity, but also with good selectivity are sought after. Regioselective hydrogenations, in particular, are critical reactions in the fine chemical and pharmaceutical industries where molecules are more elaborate.1 The connectivity and sterics of the substrate in question are generally the most determinant factors with regard to regioselectivity of a hydrogenation.2,3 The steric environment of the catalyst can also have a dramatic effect on the regioselectivity of a reaction.2a,4
Zeolitic imidazolate frameworks5 (ZIFs), a subset of metal–organic frameworks (MOFs),6 are metal imidazole based materials that share many of the same features as zeolites. ZIFs are generally composed of a divalent metal (e.g. Zn2+ or Co2+)7 and imidazolate based ligands. Many are microporous and much like zeolites, find use in size and shape selective applications.8 Recently, encapsulation of catalytically active nanoparticles within ZIFs and MOFs has resulted in catalysts for interesting reactions.9–11
One of the popular methods of encapsulating nanoparticles in MOFs and ZIFs is incipient wetness impregnation,10 which entails dissolution of a metal-cation-containing precursor in a liquid phase followed by removal of solvent and reduction of the metal precursor, generally under hydrogen. However, this method generally produces a distribution of nanoparticles sizes with some on the surface of the framework,11b resulting in non-selective catalysis. For high reaction selectivity, it is imperative that nanoparticles are completely enshrouded by the host framework. Lately, researchers have utilized linkers with functional group (such as NH2 groups) to enhance the selectivity of the encapsulation. During impregnation, the metal precursor coordinates to the functional group,10 which is followed by reduction of the metal precursors. This method produces a more even distribution of nanoparticles while decreasing the fraction of nanoparticles formed on the MOF external.
A more general approach to encapsulating various shape, size, and composition of nanoparticles within ZIF-8 has been reported Lu et al.12 ZIF-8, which is composed of 2-methylimidazole and Zn2+, has a sodalite topology with an aperture of 3.4 Å and a large pore diameter of 11.6 Å. Our team relied on the coating of the nanoparticles with polyvinylpyrrolidone (PVP) which then added during the synthesis of the MOF materials12 a procedure which has since been adapted by other groups for use other MOFs as well.13 Pt nanoparticles encapsulated in the sterically confined environment of ZIF-8 performed well in both size selective and regioselective hydrogenations of olefins.12cis-Cyclooctene was not hydrogenated while linear terminal alkenes such as 1-hexene were hydrogenated. The Pt@ZIF-8 catalyzed conversion of 1-hexene to hexane, however, occurred in low yield. It is possible that the low product yield reflects slow diffusive transport of the alkene substrate through the ca. 600 nm diameter particles of the ZIF/catalyst composite. For ZIF-8, comparatively slow transport of molecular permeants of kinetic diameter greater than 3.4 Å (such as 1-hexene) is not unexpected as the “hinged” Zn-imidazolate-Zn units defining the edges of the 3.4 Å aperture must swing open to admit the molecules.8f,i,j We hypothesized that decreasing the ZIF crystallite size would result in enhanced conversion of 1-hexene without affecting the regioselectivity of the reaction. Thus, a decrease in crystallite size would both diminish distances for diffusive transport and increase (for a given mass of composite) the number of entry points into the composite, as the number will scale as the external surface area of the crystallites. In the limit of spherical crystallites, the external surface area (for a given mass of composite) will increase inversely with crystallite diameter. Herein, we report the synthesis of Pt@ZIF-8 composite with crystallite diameters of about 150 nm for the regioselective hydrogenation of terminal alkynes and alkenes. Pt@ZIF-8 with 2.7 nm Pt nanoparticles and crystallite sizes of 150 nm was synthesized by adapting our previously reported method.12 We obtained the Pt@ZIF-8 with crystallite size of 150 nm by increasing the methanolic concentration of both 2-methylimidazole and zinc nitrate hexahydrate from 25 mM to 100 mM. Separately, we prepared a solution of 2.7 nm PVP coated Pt nanoparticles and added the equivalent of 1 wt% Pt to the solution. The mixture was allowed to stand for 24 h before isolating the grey powder via centrifugation. The composite was washed several times with methanol to remove unreacted precursor before being dried overnight on a Schlenk line. The composites were characterized by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) as shown in Fig. 1. The Pt content of the composites as determined by ICP-AES was ca. 1 wt% Pt.
We found that the best method for determining whether Pt nanoparticles are on the surface of the ZIF is by attempting size selective hydrogenation, where the examined substrates include one that is too large to pass through ZIF apertures. (Elsewhere it has been shown that the maximum viable substrate size for aperture-based permeation of ZIF-8 is ca. 5.8 Å.)8a,i,j Size selective hydrogenations were performed with Pt@ZIF-8, using 2 mmol of cis-cyclooctene (kinetic diameter: 5.5 Å), in 4 mL total solvent with 3.6 mL ethyl acetate (EtOAc) under 1 bar of H2 for 24 h using undecane as an internal standard. EtOAc was chosen as a solvent since solvent can influence the selectivity of a reaction by binding to the reactive sites of a nanoparticle and EtOAc is expected to have a limited effect on reaction selectivity.14 Also, as a result of its kinetic diameter (4.8 Å),15 EtOAc should be largely hindered from entering the ZIF at room temperature. The composite was activated for hydrogenation reactions by first heating at 150 °C under vacuum. The composite was reduced under a H2 atmosphere for 2 h at the same temperature. Presumably, there would be a large local concentration of H2 within the pores of the ZIF and around the Pt nanoparticles which should enhance the catalyst performance.16 After 24 h, the reaction was purged with N2 and an aliquot was analysed by GC-TOF. No cyclooctane was observed in the GC chromatogram (Table 1, entry 1). We also attempted hydrogenations using 1,3,5-trimethylbenzene with both Pt@ZIF and Pt/C under identical reaction conditions as with cis-cyclooctene (Table 1, entries 3 and 4). As with cis-cyclooctene, 1,3,5-trimethylbenzene was hydrogenated in 5% conversion to predominantly 1,3,5-trimethylcyclohexane when using Pt/C, but did not react at all when using Pt@ZIF-8 as a catalyst. These observations are in agreement with the previously reported results from our team and others.12,13
| Entrya | Catalyst | Substrate(s) | Conv. (%) | Product(s) | Select (%) |
|---|---|---|---|---|---|
a General reaction conditions: reactions were carried out using Pt@ZIF-8 or Pt/C (0.1 mmol based on Pt) and substrate (2 mmol) in EtOAc using undecane as an internal standard in 4 mL total volume under 1 bar H2 for 24 h at room temp.
b From ref. 12.
c One![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) one mixture of 1-hexene and 1-hexyne.
d 1,3,5-Trimethylcyclohexane or 1,3,5-trimethylcyclohexene. one mixture of 1-hexene and 1-hexyne.
d 1,3,5-Trimethylcyclohexane or 1,3,5-trimethylcyclohexene.
|
|||||
| 1 | Pt@ZIF-8 |
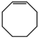
|
0 | — | — |
| 2b | Pt/C | 7.6 | Cyclooctane | 100 | |
| 3 | Pt@ZIF-8 |
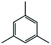
|
0 | — | — |
| 4 | Pt/C | 5 | -aned | 90 | |
| -ened | 10 | ||||
| 5 | Pt@ZIF-8 |
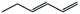
|
60 | 3-Hexene | 95 |
| 6 | Pt/C | 100 | 3-Hexene | 20 | |
| 1-Hexene | |||||
| n-Hexane | 80 | ||||
| 7 | Pt@ZIF-8 |

|
0 | — | — |
| 8 | Pt/C | 80 | 15 | ||
| n-Hexane | 85 | ||||
| 9 | Pt@ZIF-8 |
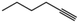
|
40 | 1-Hexene | 80 |
| n-Hexane | 20 | ||||
| 10 | Pt/C | 100 | 1-Hexene | 5 | |
| n-Hexane | 95 | ||||
| 11c | Pt@ZIF-8 |

|
40 | 1-Hexene | 85 |
| n-Hexane | 15 | ||||
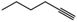
| 12c | Pt/C |
|
90 | 1-Hexene | 30 |
| n-Hexane | 70 | ||||
| 13 | Pt@ZIF-8 |

|
0 | — | — |
| 14 | Pt/C | 100 | 3-Hexene | 5 | |
| n-Hexane | 95 | ||||
Next, we performed hydrogenations with linear alkenes 1,3-hexadiene and 3-hexene. In our previous work,12 we observed that Pt@ZIF-8 could regioselectively hydrogenate terminal olefins, but we did not attempt selective hydrogenation of a multiply unsaturated substrate. We can now report that the hydrogenation of 1,3-hexadiene produces 3-hexene in 60% yield after 24 h (Table 1, entries 5 and 6). No n-hexane or 1-hexene is observed in GC traces, indicating that only the terminal olefin is catalytically hydrogenated. Conversely, the hydrogenation of 1,3-hexadiene when performed with Pt/C yielded n-hexane in 80% yield, together with some 1-hexene and 3-hexene. Many factors govern which unsaturated site(s) will react preferentially, but one of the most important is the sterics of the substrate. Generally, terminal olefins react orders of magnitude more rapidly than internal olefins since the terminal olefins are more accessible to the catalyst; thus, comparative kinetics may play a role.3 In order to determine whether the regioselectivity we observe is due to inherent reactivity differences, rather than ZIF-controlled accessibility (or inaccessibility) of specific sites to the enshrouded catalyst, we performed a hydrogenation of 1,3-hexadiene until the terminal olefinic site was completely hydrogenated. After 4 days of reaction time, only 3-hexene remained. This result supports the notion that the ZIF-defined steric environment about the nanoparticle plays an important role in regioselectivity. If the regioselectivity that was obtained after 24 h was strictly the result of kinetics, hexane or 1-hexene would be observed after 4 days reaction time. Expectedly, the hydrogenation of 3-hexene with Pt@ZIF-8 yielded no product after 24 h (Table 1, entry 5). When Pt/C was used as a catalyst, hexane was obtained in 80% yield (Table 1, entry 6).
Considering our results in which terminal olefins were hydrogenated within Pt@ZIF-8 exclusively, we were interested in investigating whether the selectivity would extend to alkynes, which are sterically more accessible than trans-alkenes. We performed hydrogenation reactions with 1-hexyne and 3-hexyne with Pt@ZIF-8 and Pt/C (Table 1, entries 9–14). With Pt@ZIF-8, we obtained 1-hexene in 32% yield and n-hexane in 8% yield (Table 1, entries 9 and 10). In contrast, hydrogenation of 1-hexyne over Pt/C produced n-hexane in 95% yield, together with 5% 1-hexene (i.e. no alkyne remained). To determine whether 1-hexyne would preferentially react over 1-hexene within Pt@ZIF-8, we performed a hydrogenation experiment using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of 1-hexyne and 1-hexene as the substrate feed (Table 1, entries 11 and 12). Again, we observed a preference for the hydrogenation of the alkyne to the alkene as n-hexane is obtained in only 15% yield. Pt/C produced predominantly n-hexane and a small amount of 1-hexene. The preferential hydrogenation of alkynes over alkenes has been reported in the literature and is attributed to the greater affinity (stronger binding) of an alkyne versus an alkane to Pt atoms.17,18
1 molar ratio of 1-hexyne and 1-hexene as the substrate feed (Table 1, entries 11 and 12). Again, we observed a preference for the hydrogenation of the alkyne to the alkene as n-hexane is obtained in only 15% yield. Pt/C produced predominantly n-hexane and a small amount of 1-hexene. The preferential hydrogenation of alkynes over alkenes has been reported in the literature and is attributed to the greater affinity (stronger binding) of an alkyne versus an alkane to Pt atoms.17,18
Conclusions
A Pt@ZIF-8 composite has been synthesized with crystallite diameters of ca. 150 nm. The observed increase in yield for hydrogenation of linear alkenes with 150 nm crystallites compared with our previously reported Pt@ZIF-8 composites with 600 nm crystallite points to a reduction in substrate mass-transport limitations and an increase in external surface area (for a given quantity of Pt@ZIF-8) for the 150 nm version of the catalytic composite. 150 nm diameter Pt@ZIF-8 performed well in size-selective hydrogenations whereby linear alkenes and alkynes were hydrogenated while large cyclic substrates went unreacted. These findings support the contention that the Pt nanoparticles are fully enshrouded by the ZIF. In comparative studies, Pt@ZIF-8 readily hydrogenated terminal alkenes and alkynes while internal sites of unsaturation went unreacted. Exhaustive exposure of 1,3-hexadiene to Pt@ZIF-8 and H2 yielded 3-hexene, to the exclusion of n-hexane and 1-hexene. These observations indicate that the observed high regioselectivity is not a kinetic result, and the confined environment of the ZIF plays an important part in dictating the regioselectivity of the product. These results demonstrate that control of ZIF crystallite size in conjunction with encapsulation of a range of reactive nanoparticles within a confined environment is a promising strategy for discovering new heterogeneous catalysts for selective organic transformations.The encapsulation of reactive nanoparticles within frameworks with different pore and channel sizes could enable us to perform regioselective transformations of more elaborate substrates that are more relevant to the chemical world.
Acknowledgements
O.K.F and J.T.H gratefully acknowledged the financial support from National Science Foundation (DMR-1334928). Acquisition of data on trace analysis and GC instruments used in the IMSERC facility of Northwestern University was made possible by support from Northwestern University and grant CHE-0923236 from the National Science Foundation, respectively. C.J.S would like to acknowledge Dr Benjamin Klahr and Cassandra Whitford for recording SEM and TEM data, respectively. C.J.S. would also like to acknowledge Dr Neil Schweitzer for helpful discussion relating to catalytic results. This work made use of the EPIC facility (NUANCE Center-Northwestern University), which has received support from the MRSEC program (NSF DMR-1121262) at the Materials Research Center; the Nanoscale Science and Engineering Center (NSF EEC-0647560) at the International Institute for Nanotechnology; and the State of Illinois, through the International Institute for Nanotechnology.Notes and references
- (a) R. L. Augustine, Catal. Today, 1997, 37, 419 CrossRef CAS; (b) M. J. Burk, J. G. Allen and W. F. Kiesman, J. Am. Chem. Soc., 1998, 120, 657 CrossRef CAS; (c) T. J. A. Graham, T. H. Poole, C. N. Reese and B. C. Goess, J. Org. Chem., 2011, 76, 4132 CrossRef CAS PubMed.
- (a) S. E. Sen, S. M. Smith and K. A. Sullivan, Tetrahedron, 1999, 55, 12657 CrossRef CAS; (b) Y. A. Ryndin, C. C. Santini, D. Prat and J. M. Basset, J. Catal., 2000, 190, 364 CrossRef CAS.
- H. Pines, in The Chemistry of Catalytic Hydrocarbon Conversions, ed. H. Pines, Academic Press, New York, 1981, pp. 156–184 Search PubMed.
- J. M. Thomas, Angew. Chem., Int. Ed., 1999, 38, 3588 CrossRef.
- (a) A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2009, 43, 58 CrossRef PubMed; (b) J. P. Zhang, Y. B. Zhang, J. B. Lin and X. M. Chen, Chem. Rev., 2011, 112, 1001 CrossRef PubMed.
- (a) D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502 CrossRef CAS PubMed; (b) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (c) J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213 RSC; (d) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC; (e) O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166 CrossRef CAS PubMed; (f) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (g) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS PubMed.
- Y. Q. Tian, C. X. Cai, Y. Ji, X. Z. You, S. M. Peng and G. H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1384 CrossRef CAS.
- (a) G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832 CrossRef CAS PubMed; (b) P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., Int. Ed., 2010, 49, 9640 CrossRef CAS PubMed; (c) C. Chizallet, S. Lazare, D. Bazer-Bachi, F. Bonnier, V. Lecocq, E. Soyer, A. A. Quoineaud and N. Bats, J. Am. Chem. Soc., 2010, 132, 12365 CrossRef CAS PubMed; (d) J. C. Tan, T. D. Bennett and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9938 CrossRef CAS PubMed; (e) D. Fairen-Jimenez, S. A. Moggach, M. T. Wharmby, P. A. Wright, S. Parsons and T. Düren, J. Am. Chem. Soc., 2011, 133, 8900 CrossRef CAS PubMed; (f) O. Karagiaridi, W. Bury, A. A. Sarjeant, C. L. Stern, O. K. Farha and J. T. Hupp, Chem. Sci., 2012, 3, 3256 RSC; (g) L. T. L. Nguyen, K. K. A. Le, H. X. Truong and N. T. S. Phan, Catal. Sci. Technol., 2012, 2, 521 RSC; (h) O. Karagiaridi, M. B. Lalonde, W. Bury, A. A. Sarjeant, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 18790 CrossRef CAS PubMed; (i) D. Fairen-Jimenez, R. Galvelis, A. Torrisi, A. D. Gellan, M. T. Wharmby, P. A. Wright, C. Mellot-Draznieks and T. Duren, Dalton Trans., 2012, 41, 10752 RSC; (j) D. Peralta, G. Chaplais, A. Simon-Masseron, K. Barthelet, C. Chizallet, A. A. Quoineaud and G. D. Pirngruber, J. Am. Chem. Soc., 2012, 134, 8115 CrossRef CAS PubMed.
- A. Aijaz and Q. Xu, J. Phys. Chem. Lett., 2014, 5, 1400 CrossRef CAS.
- (a) Z. Guo, C. Xiao, R. V. Maligal-Ganesh, L. Zhou, T. W. Goh, X. Li, D. Tesfagaber, A. Thiel and W. Huang, ACS Catal., 2014, 4, 1340 CrossRef CAS; (b) X. Li, Z. Guo, C. Xiao, T. W. Goh, D. Tesfagaber and W. Huang, ACS Catal., 2014, 4, 3490 CrossRef CAS.
- (a) Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468 RSC; (b) C. Rosler and R. A. Fischer, CrystEngComm, 2015, 17, 199 RSC.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310 CrossRef CAS PubMed.
- (a) P. Wang, J. Zhao, X. Li, Y. Yang, Q. Yang and C. Li, Chem. Commun., 2013, 49, 3330 RSC; (b) Y. Huang, Y. Zhang, X. Chen, D. Wu, Z. Yi and R. Cao, Chem. Commun., 2014, 50, 10115 RSC; (c) K. Na, K. M. Choi, O. M. Yaghi and G. A. Somorjai, Nano Lett., 2014, 14, 5979 CrossRef CAS PubMed; (d) M. Zhang, Y. Yang, C. Li, Q. Liu, C. T. Williams and C. Liang, Catal. Sci. Technol., 2014, 4, 329 RSC; (e) W. Zhang, G. Lu, C. Cui, Y. Liu, S. Li, W. Yan, C. Xing, Y. R. Chi, Y. Yang and F. Huo, Adv. Mater., 2014, 26, 4056 CrossRef CAS PubMed.
- R. L. Augustine and P. Techasauvapak, J. Mol. Catal., 1994, 87, 95 CrossRef CAS.
- M. E. van Leeuwen, Fluid Phase Equilib., 1994, 99, 1 CrossRef CAS.
- R. L. Augustine and R. W. Warner, J. Org. Chem., 1981, 46, 2614 CrossRef CAS.
- (a) J. Sheridan, J. Chem. Soc., 1945, 0, 305 RSC; (b) G. C. Bond and P. B. Wells, J. Catal., 1966, 5, 65 CrossRef CAS; (c) R. S. Mann and K. C. Khulbe, J. Catal., 1970, 17, 46 CrossRef CAS; (d) S. Tanaka, A. Yasuda, H. Yamamoto and H. Nozaki, J. Am. Chem. Soc., 1975, 97, 3252 CrossRef CAS; (e) B. Bridier and J. Pérez-Ramírez, J. Catal., 2011, 284, 165 CrossRef CAS PubMed.
- In a large excess of H2, the alkyne will be fully hydrogenated rapidly to the alkane with no selectivity towards the alkene. In contrast, at low H2 pressures the alkyne will be consumed preferentially and both alkene and alkanes be observed with higher selectivity to alkene.
Footnote |
| † Electronic supplementary information (ESI) available: details of synthetic procedure, catalytic experiments, and GC-TOF chromatographs are presented in the ESI. See DOI: 10.1039/c5qi00010f |
| This journal is © the Partner Organisations 2015 |