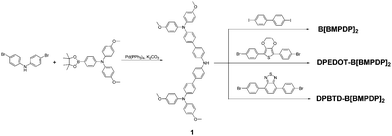
Efficient, symmetric oligomer hole transporting materials with different cores for high performance perovskite solar cells†
Hyeju
Choi
a,
Sojin
Park
a,
Moon-Sung
Kang
b and
Jaejung
Ko
*a
aDepartment of Advanced Material Chemistry, Korea University Sejong Campus, Sejong-ro 2511, Sejong City 339-700, Republic of Korea. E-mail: jko@korea.ac.kr; Fax: +82-41-867-1331; Tel: +82-41-860-1337
bDepartment of Environmental Engineering, Sangmyung University, 300 Anseo-dong, Dongnam-gu, Cheonan-si, Chungnam 330-720, Republic of Korea
First published on 25th August 2015
Abstract
Novel symmetric oligomer hole transporting materials (HTMs) incorporating 3,4-ethylenedioxythiophene (EDOT) and 2,1,3-benzothiadiazole (BTD) cores have been synthesized and tested for high performance perovskite solar cells. A maximum energy conversion efficiency of 14.23% has been achieved by employing DPEDOT-B[BMPDP]2 with the electron donating EDOT unit as the core, which is comparable to that of the traditional spiro-OMeTAD (14.55%).
Organometal halide perovskites (CH3NH3PbX3, X = Cl, Br, I) possess special features, such as a direct band gap and intense light harvesting ability.1 Thereby they can be utilized as both efficient light absorbers and electron–hole transporting materials for thin-film organic–inorganic hybrid solar cells.2 Therefore, organometal halide perovskite solar cells have been recently receiving great attention owing to their outstanding features such as high efficiency and low cost.3 In the perovskite solar cells, hole transporting materials (HTMs) play a key role in determining the photovoltaic performance. In this regard, the development of cost-effective and efficient HTMs is urgently needed. The most effective HTM for the hybrid solar cells is 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenyl-amine)-9,9′-spirobifluorene (spiro-OMeTAD) in terms of the efficiency and stability.4 However, spiro-OMeTAD is quite expensive owing to the difficulty in its purification. For commercial purposes, the exploration of alternative HTMs with high performance and cheap synthetic price is strongly required. Recently, impressive photovoltaic performance has been achieved using small molecule HTMs, such as azomethine-,5 3,4-ethylenedioxythiophene-,6 pyrene-,7 linear π-conjugated-,8 butadiene-,9spiro-OMeTAD derivative-,10 carbazole derivative-,11 tetrathiafulvalene-,12 tetraphenyl-benzidine-,13 oligothiophene derivative-,14 and spiro-thiophene derivative-15 based HTMs showing efficiencies in the range of 10–15%. Recently, we16 have reported quinolizino acridine-, star-shaped triphenylamino-, and triazine based HTMs, exhibiting high conversion efficiencies of ∼14%. Nevertheless, most of the small molecule HTMs are still expensive, with low conversion efficiency and low stability compared to spiro-OMeTAD. Therefore, the development of cheap HTMs with operation stability is still very important.
Herein, we report three types of hole transporting materials by incorporating an electron donating unit or an electron-withdrawing unit into tetraphenyl benzidine or substituting four triphenylamines into the phenyl unit. The molecular structures of the three HTMs are shown in Fig. 1.
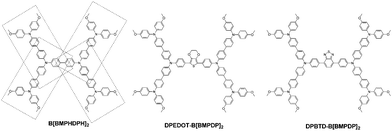 | ||
| Fig. 1 Chemical structures of B[BMPDP]2, DPEDOT-B[BMPDP]2 and DPBTD-B[BMPDP]2 moieties studied in the present work. | ||
The synthetic scheme for the preparation of B[BMPDP]2, DPEDOT-B[BMPDP]2 and DPBTD-B[BMPDP]2 is shown in Scheme 1. The primary step for their syntheses is the Suzuki coupling reaction17 of bis(4-bromophenyl)amine and N-bis(methoxyphenyl)-N-(4-(4,4,5,5,-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)amine. The final two HTMs were synthesized by the Buchwald–Hartwig amination18 of 1 and dihalo derivatives.
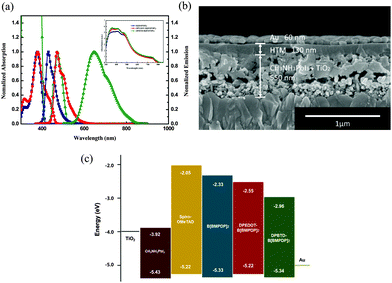
The UV-vis absorption spectra of the three HTMs recorded in chlorobenzene are displayed in Fig. 2a. As summarized in Table S1 (ESI†), the UV-vis light absorption peaks of B[BMPDP]2, DPEDOT-B[BMPDP]2 and DPBTD-B[BMPDP]2 were observed at 376, 377, and 473 nm, respectively. The λabs of DPBTD-B[BMPDP]2 is more red shifted than that of DPEDOT-B[BMPDP]2. This bathochromic shift seems to be attributed to an intramolecular charge transfer in DPBTD-B[BMPDP]2. The fluorescence spectra of B[BMPDP]2 and DPEDOT-B[BMPDP]2 exhibit an emission at 429 and 472 nm with a small Stokes shift of 53–95 nm compared with a large Stokes shift of 175 nm in DPBTD-B[BMPDP]2, demonstrating that a small structural change in the excited state occurs in the two former compounds. The inset of Fig. 2a shows the absorption spectra of the perovskite-coated mesoporous TiO2 films with the three HTMs. Three HTM-coated films exhibit an enhanced absorption band from 350 to 580 nm.
The cross-sectional field-emission scanning electron microscopy (FE-SEM) image of the prepared perovskite solar cell is displayed in Fig. 2b, showing a well-defined multi-layer structure with clear interfaces. The thickness of the TiO2 scaffold film incorporated with perovskite, HTM layer and Au is 550 nm and 130 nm and 60 nm, respectively. Fig. 2c shows a comparative diagram of the energy levels of the corresponding components in the device. The HOMO levels of B[BMPDP]2, DPEDOT-B[BMPDP]2 and DPBTD-B[BMPDP]2 are measured to be −5.33, −5.22 and −5.34 eV, respectively, which are well matched with CH3NH3PbI3 (−5.43 eV).
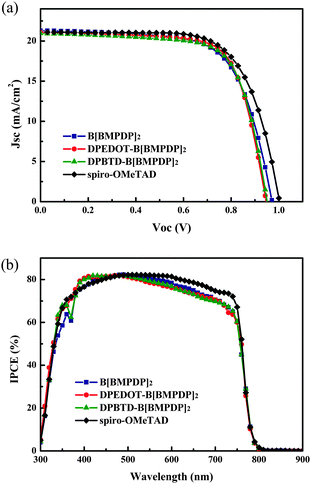
Fig. 3a shows the photocurrent–voltage (J–V) curves of the perovskite solar cells employing the HTMs. For reference, the device with spiro-OMeTAD as the HTM is fabricated. The detailed photovoltaic parameters of the devices are also summarized in Table 1. We have fabricated 30 solar cells for each HTM and the histogram of energy conversion efficiencies for the cells is shown in Fig. S3 (ESI†). The energy conversion efficiencies of the cells employing three HTMs were shown to be almost comparable with that of the spiro-OMeTAD based reference cell. The average efficiencies for B[BMPDP]2, DPEDOT-B[BMPDP]2, and DPBTD-B[BMPDP]2 and spiro-OMeTAD were observed to be 12.37, 13.12, 13.09, and 13.43%, respectively.
| HTM | J sc (mA cm−2) | V oc (V) | FF | η (%) | |
|---|---|---|---|---|---|
| Performances of devices were measured with 0.16 cm2 working area. | |||||
| B[BMPDP]2 | Average | 20.69 | 0.938 | 0.637 | 12.37 |
| Best | 21.29 | 0.972 | 0.667 | 13.81 | |
| DPEDOT-B[BMPDP]2 | Average | 20.87 | 0.955 | 0.660 | 13.12 |
| Best | 21.07 | 0.947 | 0.713 | 14.23 | |
| DPBTD-B[BMPDP]2 | Average | 20.69 | 0.965 | 0.657 | 13.09 |
| Best | 20.96 | 0.951 | 0.702 | 13.99 | |
| spiro-OMeTAD | Average | 20.82 | 0.939 | 0.688 | 13.43 |
| Best | 21.17 | 1.003 | 0.685 | 14.55 | |
The highest energy conversion efficiency (14.23%) was achieved by employing the DPEDOT-B[BMPDP]2 under one sun illumination. The incident photon-to-current efficiency (IPCE) spectra of the solar cells fabricated with three HTMs are shown in Fig. 3b. The integrated photocurrent densities of the cells employing B[BMPDP]2, DPEDOT-B[BMPDP]2, and DPBTD-B[BMPDP]2 were calculated to be 19.54, 19.92 and 19.76 mA cm−2, respectively, which are well consistent with the measured photocurrent densities as summarized in Table 1. The DPBTD-B[BMPDP]2 based cell shows a slightly high open circuit voltage (Voc) compared with those of other two cells, which can be rationalized by considering that the EHOMO for DPBTD-B[BMPDP]2 was about 10–120 mV lower than the value for the other two cells. The similar and slight differences of Jsc value of three cells in different wavelength ranges may be related to their low series resistance (Rs) due to their packing patterns. From the slope of the J–V curve around Voc, the Rs values of B[BMPDP]2, DPEDOT-B[BMPDP]2, and DPBTD-B[BMPDP]2 based cells are calculated to be 26.4, 22.6 and 23.8 Ω cm−2, respectively, in which the low value of Rs in DPEDOT-B[BMPDP]2 leads to slightly improved photocurrent density. The high fill factors in the DPEDOT-B[BMPDP]2 based cell followed a trend identical to the measured value in mobility. The hole mobilities of the HTMs were measured from the space charge limitation of current (SCLC) in the J–V characteristics. The hole mobilities evaluated by the Mott–Gurney law19 in B[BMPDP]2, DPEDOT-B[BMPDP]2, DPBTD-B[BMPDP]2 and spiro-OMeTAD are 7.04 × 10−5, 8.48 × 10−5, 8.05 × 10−5, 1.01 × 10−4 cm2 V−1 s−1, respectively. The high hole mobilities of DPEDOT-B[BMPDP]2 and DPBTD-B[BMPDP]2 based cells relative to that of the B[BMPDP]2 based one led to an improved fill factor.
Electrochemical impedance spectroscopy measurements have also been carried out to analyse the recombination properties of the HTMs. The Nyquist plots of the devices with B[BMPDP]2, DPEDOT-B[BMPDP]2, and DPBTD-B[BMPDP]2 in the dark over different forward biases (600–1000 mV) are shown in Fig. S5 (ESI†). The magnitude of the main arc is dominated by the recombination resistance (Rrec), which drops with the increasing forward bias voltage. The Rrec values were shown to be significantly increased under identical bias conditions upon introducing the EDOT and BTD core units into HTMs, demonstrating the decrease in the charge recombination. These results are also well consistent with the Voc values evaluated from the J–V curves.
Fig. 4 shows the photovoltaic performance during the long-term aging test of the two devices. After 200 h of aging, the initial efficiency of 12.55% of the DPEDOT-B[BMPDP]2 based cell decreased to 11.01%, giving a 12.55% reduction. On the other hand, the initial efficiency of 13.55% of the spiro-OMeTAD based cell also decreased to 12.57%, giving a 7.23% reduction. The relative stability of the DPEDOT-B[BMPDP]2 based cell may be attributable to an interfacial tight packing due to a planar and sterically bulky configuration.
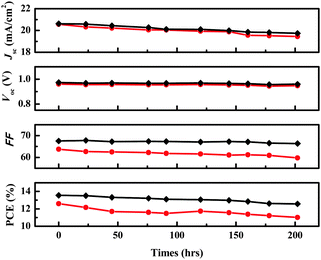 | ||
| Fig. 4 Time-course changes in the performance parameters of the solar cells with DPEDOT-B[BMPDP]2 (●) and spiro-OMeTAD (◆) as the HTMs under inert conditions at room temperature. | ||
In summary, we have designed and synthesized three novel HTMs with 3,4-ethylenedioxythiophene (EDOT) and 2,1,3-benzothiadiazole (BTD) as the core units. The photo-physical properties and photovoltaic performance are quite sensitive to the structural configuration. The DPEDOT-B[BMPDP]2 based cell affords an overall conversion efficiency of 13.12%, showing a comparable photovoltaic performance to the spiro-OMeTAD based cell (13.43%). We believe that the development of efficient and stable hole transporting materials comparable to the spiro-OMeTAD is possible through meticulous molecular design, and studies directed towards this goal are now in progress.
This work was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2014R1A2A2A03004716) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2015R1A1A1A05001486).
Notes and references
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395 CrossRef CAS PubMed; L. Etgar, P. Gao, Z. Xue, Q. Peng, A. K. Chandiran, B. Liu, M. d. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2012, 134, 17396 CrossRef PubMed.
- L. Etgar, P. Gao, Z. Xue, Q. Peng, A. K. Chandiran, B. Liu, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2012, 134, 17396 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 Search PubMed; J.-H. Yum, C.-R. Lee, J.-W. Lee, S.-W. Park and N.-G. Park, Nanoscale, 2011, 3, 4088 RSC.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316 CrossRef CAS PubMed; D. Liu and T. L. Kelly, Nat. Photonics, 2014, 8, 133 CrossRef PubMed.
- M. L. Petrus, T. Bein, T. J. Dingemans and P. Docampo, J. Mater. Chem. A, 2015, 3, 12159 CAS.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem., Int. Ed., 2014, 53, 4085 CrossRef CAS PubMed.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, J. Am. Chem. Soc., 2013, 135, 19087 CrossRef CAS PubMed.
- J. Wang, S. Wang, X. Li, L. Zhu, Q. Meng, Y. Xiao and D. Li, Chem. Commun., 2014, 50, 5829 RSC.
- S. Lv, L. Han, J. Xiao, L. Zhu, J. Shi, H. Wei, Y. Xu, J. Dong, X. Xu, D. Li, S. Wang, Y. Luo, Q. Meng and X. Li, Chem. Commun., 2014, 50, 6931 RSC.
- N. J. Jeon, H. G. Lee, Y. C. Kim, J. Seo, J. H. Noh, J. Lee and S. I. Seok, J. Am. Chem. Soc., 2014, 136, 7837 CrossRef CAS PubMed.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629 CrossRef CAS PubMed; S. D. Sung, M. S. Kang, I. T. Choi, H. M. Kim, H. Kim, M. P. Hong, H. K. Kim and W. I. Lee, Chem. Commun., 2014, 50, 14161 RSC.
- J. Liu, Y. Wu, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963 CAS.
- Y. Song, S. Lv, X. Liu, X. Li, S. Wang, H. Wei, D. Li, Y. Xiao and Q. Meng, Chem. Commun., 2014, 50, 15239 RSC.
- L. Zheng, Y.-H. Chung, Y. Ma, L. Zhang, L. Xiao, Z. Chen, S. Wang, B. Qu and Q. Gong, Chem. Commun., 2014, 50, 11196 RSC.
- S. Ma, H. Zhang, N. Zhao, Y. Cheng, M. Wang, Y. Shen and G. Tu, J. Mater. Chem. A, 2015, 3, 12139 CAS.
- P. Qin, S. Peak, M. I. Dar, N. Pellet, J. Ko, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2014, 136, 8516 CrossRef CAS PubMed; K. Do, H. Choi, K. Lim, H. Jo, J. W. Cho, M. K. Nazeeruddin and J. Ko, Chem. Commun., 2014, 50, 10971 RSC; H. Choi, S. Park, S. Paek, P. Ekanayake, M. K. Nazeeruddin and J. Ko, J. Mater. Chem. A, 2014, 2, 19136 Search PubMed; H. Choi, S. Paek, N. Lim, Y. H. Lee, M. K. Nazeeruddin and J. Ko, Chem. – Eur. J., 2014, 20, 10894 CrossRef PubMed.
- C.-H. Huang, N. D. McClenaghan, A. Kuhn, J. W. Hofstraat and D. M. Bassani, Org. Lett., 2005, 7, 3409 CrossRef CAS PubMed; K. J. Hoffmann, E. Bakken, E. J. Samuelsen and P. H. J. Carlsen, Synth. Met., 2000, 113, 39 CrossRef.
- N. Cho, H. Choi, D. Kim, K. Song, M.-S. Kang, S. O. Kang and J. Ko, Tetrahedron, 2009, 65, 6236 CrossRef CAS PubMed.
- V. D. Mihailetchi, H. Xie, B. de Boer, L. J. A. Koster and P. W. M. Blom, Adv. Funct. Mater., 2006, 16, 699 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc05814g |
| This journal is © The Royal Society of Chemistry 2015 |