Synthesis of β-enaminodicarbonyl derivatives in the titanium(IV) chloride-promoted reactions of β-dicarbonyl compounds with nitriles†
Shuchen Pei,
Chenchen Xue,
Li Hai and
Yong Wu*
Key Laboratory of Drug Targeting of Education Ministry, West China School of Pharmacy, West China Hospital, Sichuan University, No. 37, GuoXue Road, Chengdu 610041, P.R. China. E-mail: wyong@scu.edu.cn; Fax: +862885503666
First published on 18th August 2014
Abstract
Titanium(IV) chloride selectively promoted the nucleophilic attack of ethyl acetoacetate with nitriles to give enaminoketoesters, which were valuable intermediates for the syntheses of 2,3,4-substituted heterocyclics. Moreover a plausible mechanism for this transformation was given.
Introduction
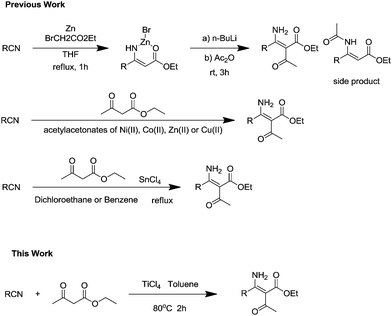
It is believed that β-enaminodicarbonyl derivatives have many potential applications as biologically active compounds and as precursors of substituted β-amino acids, heterocyclic systems, and other classes of valuable compounds.1 As a consequence, much attention has been paid to the development of an appropriate method for the synthesis of β-enaminodicarbonyl derivatives. Conceptually, many of these β-enaminodicarbonyl derivatives should be available by the addition of appropriate carbon nucleophiles to nitriles. In the past few years, promising progress has been made in this area. Lee and coworkers2,3 demonstrated that the Blaise reaction, the addition of zinc enolates derived from a-halo esters to nitriles, proceeded via a zinc bromide complex of a β-enamino ester. However, this reaction was quite limited due to the competitive amino-acylated side product, as show in Scheme 1. So far, the most popular route to β-enaminodicarbonyl derivatives is the reaction of β-ketoesters with nitrile by using catalytic amounts (1–5 mol%) of acetylacetonates of Ni(II), Co(II), Zn(II) or Cu(II),4−9 whereas tin(IV) chloride also promotes C–C bond formation.10−13 Therefore, in view of higher yield and for environmental concern, the development of a direct synthetic method for β-enaminodicarbonyl derivatives in the presence of Lewis acids would be highly desirable. In this paper we reported a direct titanium(IV) chloride-promoted method for β-dicarbonyl compounds with nitriles providing β-enaminodicarbonyl derivatives and their conversion to 3,4,5-substituted heterocyclic.Results and discussion
Our study began with the C–C bonds formation of nitriles with ethyl acetoacetate. Initially we examined direct benzonitrile and acetoacetate in the presence of different kinds of Lewis acid as a catalytic in toluene at 110 °C. To our surprise, the screening of different Lewis acid (Table 1, entries 1–8) led to the discovery that TiCl4 was an effective catalytic, forming the product in an encouraging yield of 65% (Table 1, entry 7). Other additives such as SnCl4 (Table 1, entry 3) and I2 (Table 1, entry 4) also promoted exclusive generation of the product, but the yields are lower than when TiCl4 used. However, FeCl3 (Table 1, entry 1), ZnCl2 (Table 1, entry 2), CoCl2 (Table 1, entry 5), HgCl2 (Table 1, entry 6), CuBr2 (Table 1, entry 8) did not activate the reaction efficiently affording the product. With the aim of improving the desired product yield, the equivalent of TiCl4 was examined, and a stoichiometric amount of TiCl4 gave the best yield compared with others (Table 1, entries 9–12). Increase or decrease the equivalent of TiCl4 would reduce the reaction yield. Given that temperature might play an important role in this reaction, we tested the temperature from 50 °C to 100 °C (Table 1, entries 13–18). It was found out that this reaction could provide the most efficient yield of 82% at 80 °C. At higher temperatures the by-products would show up, however, the remaining ingredients were the key factors affecting the yield at lower temperatures. Interestingly, the choice of solvent exerted great influence on the reaction yield (Table 1, entries 19–22). Evaluation of various organic solvents in the presence of TiCl4 revealed that toluene provided the best yield. Polar aprotic solvents, such as DMF and DMSO did not effectively improve the reaction yields. Running the reaction in polar protic solvent such as ethanol only afforded a small amount of desired product.‡| Entry | Catalytic (mol) | Temperature | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a The reaction were carried out with 1 (1 mmol), 2 (1.2 mmol), solvent (2 mL).b Isolated yield based on 1a.c No reaction. | ||||
| 1 | FeCl3 (1.5) | 110 °C | Toluene | NRc |
| 2 | ZnCl2 (1.5) | 110 °C | Toluene | NRc |
| 3 | SnCl4 (1.5) | 110 °C | Toluene | 57 |
| 4 | I2 (1.5) | 110 °C | Toluene | 23 |
| 5 | CoCl2 (1.5) | 110 °C | Toluene | NRc |
| 6 | HgCl2 (1.5) | 110 °C | Toluene | NRc |
| 7 | TiCl4 (1.5) | 110 °C | Toluene | 65 |
| 8 | CuBr2 (1.5) | 110 °C | Toluene | NRc |
| 9 | TiCl4 (0.5) | 110 °C | Toluene | 45 |
| 10 | TiCl4 (1.0) | 110 °C | Toluene | 72 |
| 11 | TiCl4 (2.0) | 110 °C | Toluene | 40 |
| 12 | TiCl4 (3.0) | 110 °C | Toluene | 18 |
| 13 | TiCl4 (1.0) | 50 °C | Toluene | NRc |
| 14 | TiCl4 (1.0) | 60 °C | Toluene | 25 |
| 15 | TiCl4 (1.0) | 70 °C | Toluene | 66 |
| 16 | TiCl4 (1.0) | 80 °C | Toluene | 82 |
| 17 | TiCl4 (1.0) | 90 °C | Toluene | 80 |
| 18 | TiCl4 (1.0) | 100 °C | Toluene | 75 |
| 19 | TiCl4 (1.0) | 80 °C | DMSO | 52 |
| 20 | TiCl4 (1.0) | 80 °C | DMF | NRc |
| 21 | TiCl4 (1.0) | 80 °C | EtOH | 30 |
| 22 | TiCl4 (1.0) | 40 °C | DCM | 45 |
With these optimized conditions in hand, we proceeded to investigate the substrates of nitriles. Aromatic nitriles (Table 2, entries 1–8) as well as aliphatic nitriles such as benzyl cyanide acetonitrile, and 3-bromopropanenitrile (Table 2, entries 9–12) were converted to their corresponding β-enaminodicarbonyl derivatives 1a–14n in moderate to excellent yield. Electron-deficient aryl nitriles gave the expected β-enaminodicarbonyl products in good-to-excellent yield, which was much better than electron-rich one (Table 2, entries 2–7). Many synthetically important functional groups, such as alkoxy, alkyl, nitro and halogen were well-tolerated under the optimal conditions. Moreover, adjacent heteroatom substituted aryl nitriles reacted with ethyl acetoacetate under our conditions to give the products in higher yield, up to 85% (Table 2, entries 8). In the case of other active β-dicarbonyl compounds such as acetyl acetone and diethyl malonate, the yield was also desirable (Table 2, entries 13 and 14). Therefore, these results clearly demonstrated that titanium(IV) chloride serves as a useful Lewis acid catalyst for the reaction of nucleophilic attack of ethyl acetoacetate with nitriles.
| Entry | R1 | R2 | R3 | Yieldb (%) | |
|---|---|---|---|---|---|
| a The reaction were carried out with 1 (1 mmol), 2 (1.2 mmol), solvent (2 mL).b Isolated yield. | |||||
| 1 | a | Ph | CH3 | OC2H5 | 82 |
| 2 | b | 2-NO2C6H4 | CH3 | OC2H5 | 88 |
| 3 | c | 4-NO2C6H4 | CH3 | OC2H5 | 92 |
| 4 | d | 2-MeOC6H4 | CH3 | OC2H5 | 74 |
| 5 | e | 2,4-Dimethoxy phenyl | CH3 | OC2H5 | 56 |
| 6 | f | 2-ClC6H4 | CH3 | OC2H5 | 68 |
| 7 | g | 2-Methoxy-3-pyridyl | CH3 | OC2H5 | 70 |
| 8 | h | 2-Furly | CH3 | OC2H5 | 85 |
| 9 | i | Benzyl | CH3 | OC2H5 | 76 |
| 10 | j | Styryl | CH3 | OC2H5 | 78 |
| 11 | k | Me | CH3 | OC2H5 | 70 |
| 12 | l | BrCH2CH2 | CH3 | OC2H5 | 65 |
| 13 | m | Ph | CH3 | CH3 | 60 |
| 14 | n | Ph | OC2H5 | OC2H5 | 80 |
Finally, application of this new metal-promoted method in the synthesis of heterocyclic was tested. The resulting β-enaminodicarbonyl derivatives were transformed to pyrazoles, isoxazoles and isothiazoles, which are important pharmacophores in various biologically active compounds.14 As show in Scheme 2, reaction of 3 with hydrazine hydrate, hydroxylamine hydrochloride and phosphorus pentasulfide in appropriate solvent afforded the corresponding 3,4,5-trisubstituted pyrazoles, isoxazoles and isothiazoles in good to excellent yields. These encouraging results indicated that the present method provided an efficient approach for the preparation of diverse 3,4,5-trisubstituted heterocyclic.
A plausible reaction mechanism for titanium(IV) chloride-promoted reactions of β-dicarbonyl compounds was presented in Scheme 3. The first step of the mechanism involves the formation of a Ti-enolate by interaction of TiCl4 with ethyl acetoacetate and in this step the HCl will go out first. Then corresponding Ti-enolate that formed will attack on the nitrile to generate a N–Ti–O cyclic intermediate 6, which is intercepted by the Ti-enolate to produce the final product 3.
Conclusions
In conclusion, we have successfully developed the metal-promoted reaction of β-dicarbonyl compounds with nitriles using the readily available reagent TiCl4. The reaction could be carried out under mild conditions and was compatible with many functional groups. This reaction provides a straightforward, practically useful way to prepare various β-enaminodicarbonyl derivatives.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (NSFC) (grant numbers 81102324 and 81373259).Notes and references
- (a) D. N. McGregor, U. Corbin, J. E. Swigor and L. C. Cheney, Tetrahedron, 1969, 25, 389–395 CrossRef CAS; (b) G. Dannhardt, A. Bauer and U. Nowe, J. Prakt. Chem./Chem.-Ztg., 1998, 340, 256 CrossRef CAS PubMed; (c) D. L. Boger, T. Ishizaki, J. R. J. Wysocki, S. A. Munk, P. A. Kitos and O. Suntornwat, J. Am. Chem. Soc., 1989, 111, 6461 CrossRef CAS; (d) I. O. Edafiogho, C. N. Hinko, H. Chang, J. A. Moore, D. Mulzac, J. M. Nicholson and K. R. Scott, J. Med. Chem., 1992, 35, 2798 CrossRef CAS; (e) K. R. Scott, I. O. Edafiogho, E. L. Richardson, V. A. Farrar, J. A. Moore, E. I. Tietz, C. N. Hinko, H. Chang, A. El-Assadi and J. M. Nicholson, J. Med. Chem., 1993, 36, 1947 CrossRef CAS; (f) K. R. Scott, G. O. Rankin, J. P. Stables, M. S. Alexander, I. O. Edafiogho, V. A. Farrar, K. R. Kolen, J. A. Moore, L. D. Sims and A. D. Tonnu, J. Med. Chem., 1995, 38, 4033 CrossRef CAS; (g) A. Alberola, L. A. Calvo, A. G. Ortega, M. C. S. Ruíz and P. Yustos, J. Org. Chem., 1999, 64, 9493 CrossRef CAS; (h) M. N. Eberlin and C. Kascheres, J. Org. Chem., 1988, 53, 2084 CrossRef CAS; (i) F. Al-Omran and A. A. El-Khair, J. Heterocycl. Chem., 2005, 42, 307 CrossRef CAS PubMed; (j) E. Bejan, H. Aït-Haddou, J. C. Daran and G. G. A. Balavoine, Eur. J. Org. Chem., 1998, 2907 CrossRef CAS.
- Y. S. Chun, K. K. Lee, Y. O. Ko, H. Shin and S.-g. Lee, Chem. Commun., 2008, 5098–5100 RSC.
- Y. O. Ko, Y. S. Chun, C.-L. Park, Y. Kim, H. Shin, S. Ahn, J. Hong and S.-g. Lee, Org. Biomol. Chem., 2009, 7, 1132–1136 CAS.
- B. Corain, M. Basato and A. C. Veronese, J. Mol. Catal., 1993, 81, 133 CrossRef CAS.
- A. C. Veronese, V. Gandolfi, M. Basato and B. Corain, J. Chem. Res., Synop., 1988, 246 CAS.
- B. Croxtall, E. G. Hope and A. M. Stuart, Chem. Commun., 2003, 2430–2431 RSC.
- R. Maggi, G. Bosica, S. Gherardi, C. Oro and G. Sartori, Green Chem., 2005, 7, 182–184 RSC.
- M. Basato, B. Corain, A. C. Veronese, F. D'Angeli, G. Valle and G. Zanottil, J. Org. Chem., 1984, 49, 4696–4700 CrossRef CAS.
- M. Basato, E. Faggin, C. Tubaro and A. C. Veronese, Polyhedron, 2009, 28, 1229–1234 CrossRef CAS PubMed.
- X. Zhou, M. P. Arend, W. Min and L. A. Flippin, PCT Int. Appl., 2009089547, 16 July 2009.
- M. Manferdini, C. F. Morelli and A. C. Veronese, Tetrahedron, 2002, 58, 1005–1010 CrossRef CAS.
- A. C. Veronese, C. F. Morelli and M. Basato, Tetrahedron, 2002, 58, 9709–9712 CrossRef CAS.
- F. Scavo and P. Helquist, Tetrahedron Lett., 1985, 22, 2603–2606 CrossRef.
- (a) T. D. Penning, J. J. Talley, S. R. Bertenshaw, J. S. Carter, P. W. Collins, S. Docter, M. J. Graneto, L. F. Lee, J. W. Malecha, J. M. Miyashiro, R. S. Rogers, D. J. Rogier, S. S. Yu, G. D. Anderson, E. G. Burton, J. N. Cogburn, S. A. Gregory, C. M. Koboldt, W. E. Perkins, K. Seibert, A. W. Veenhuizen, Y. Y. Zhang and P. C. Isakson, J. Med. Chem., 1997, 40, 1347 CrossRef CAS PubMed; (b) N. K. Terrett, A. S. Bell, D. Brown and P. Ellis, Bioorg. Med. Chem. Lett., 1996, 6, 1819 CrossRef; (c) M. J. Genin, C. Biles, B. J. Keiser, S. M. Poppe, S. M. Swaney, W. G. Taroley, Y. Yagi and D. L. Romero, J. Med. Chem., 2000, 43, 1034 CrossRef CAS PubMed; (d) A. Guzmán-Pérez, R. T. Webster, M. C. Allen, J. A. Brown, A. R. Buchholz, E. R. Cook, W. W. Day, E. S. Hamnaka, S. P. Kennedy, D. R. Knight, P. J. Kowalcyk, R. B. Marala, C. J. Mularski, W. A. Novomisle, R. B. Ruggeri, W. R. Tracy and R. J. Hill, Bioorg. Med. Chem. Lett., 2001, 11, 803 CrossRef; (e) W. T. Ashton, R. M. Sisco, H. Dong, K. A. Lyons, H. He, G. A. Doss, B. Leiting, R. A. Patel, J. K. Wu, F. Marsilio, N. A. Thornberry and A. E. Weber, Bioorg. Med. Chem. Lett., 2005, 15, 2253 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Copies of the 1H NMR and 13C NMR spectra are required for all key intermediates and final products; additional information as needed. See DOI: 10.1039/c4ra05100a |
| ‡ A representative procedure for the synthesis of 3a: To a solution of the benzonitrile (1 mmol), TiCl4 (1 mmol) and ethyl acetacetate (1.2 mmol) were added at room temperature with stirring. The mixture was refluxed with stirring for 2 h. After cooling to room temperature, saturated sodium carbonate solution was added, and the mixture was extracted with EtOAc. The combined organic phases were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel. |
| This journal is © The Royal Society of Chemistry 2014 |