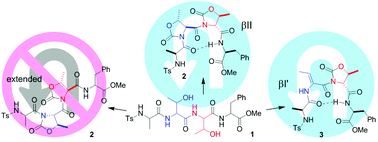
Small and easy-to-do mimetics of β-turns are of great interest to interfere with protein–protein recognition events mediated by β-turn recognition motifs. We propose a straightforward procedure for constraining the conformation of tetrapeptides lacking a pre-formed scaffold. According to the stereochemistry array, N-Ts tetrapeptides including Thr or PhSer (phenylserine) at the positions 2 or 3 gave rise in a single step to the sequences Oxd2-Oxd3 or ΔAbu2-Oxd3 (Oxd, oxazolidin-2-one; ΔAbu, 2,3-dehydro-2-aminobutyric). These pseudo-Pro residues displayed highly constrained ϕ, ψ, and χ dihedral angles, and induced clear β-turns or inverse turns of type I or II, as determined by extensive spectroscopic and computational analyses.