Enzymic synthesis of 3-[3-13C]dehydroquinic acid
Martyn
Frederickson
a,
Emily J.
Parker
ab,
John R.
Coggins
c and
Chris
Abell
*a
aUniversity Chemical Laboratory, Lensfield Road, Cambridge, UK CB2 1EW. E-mail: ca26@cam.ac.uk; Fax: 44 1223 336362; Tel: 44 1223 336405
bCentre for Structural Biology, Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand
cDivision of Biochemistry and Molecular Biology, Institute of Biomedical and Life Sciences, University of Glasgow, Glasgow, UK G12 8QQ
First published on 9th September 2003
Abstract
The title compound has been prepared enzymically over four steps from commercially available D-[5-13C]fructose.
Isotopically labelled compounds have been widely exploited in the field of biological chemistry. Molecules specifically labelled with either radioactive or nuclear active elements have allowed the elucidation of specific features of a plethora of enzyme mechanisms and have enabled researchers to determine the precise biochemical origins of particular chemical moieties within numerous biologically active molecules.
Dehydroquinase [3-dehydroquinate dehydratase; E.C. 4.2.1.10] is common to both the shikimate1,2 and quinate3 pathways. It catalyses the reversible interconversion of 3-dehydroquinate (DHQ) 1 and 3-dehydroshikimate (DHS) 2 (Fig. 1). The enzyme occurs in two chemically and biochemically distinct forms (type I and type II) that catalyse the same overall chemical transformation via different mechanisms. Type I enzymes are heat labile protein dimers4 that catalyse a syn elimination5 of water through Schiff's base intermediates6 involving a conserved lysine. Type II dehydroquinases are more thermally robust dodecameric species7 that effect an anti dehydration8via an enzyme-stabilized enolate anion.
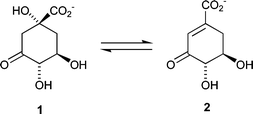 | ||
| Fig. 1 | ||
As part of our long standing interest in the chemistry and biochemistry of dehydroquinase8–12 we required access to quantities of DHQ specifically labelled with 13C at the C(3) carbonyl. Chemical approaches to labelled DHQ using isotopically labelled cyanide or carbon monoxide were viable, however, we felt that synthetic routes were likely to be long and overly complicated by the protecting group manipulation that is so often necessary in the synthesis of sugars and related poly-hydroxylated carbocycles.
Enzymic synthesis, in contrast, appeared to allow a far more direct route to the desired labelled product. The high chemical specificity characteristic of enzymic systems often eliminates the need for protecting groups and thus seemed perfect for the synthesis of the small, highly functionalized water soluble target. As an added bonus, the enzymic synthesis also promised to deliver a series of highly functionalized and specifically labelled intermediates that could prove valuable for a variety of biochemical studies.
Herein we report the synthesis and purification of [3-13C]DHQ over four enzyme-catalysed steps from a commercially available isotopically labelled D-fructose precursor. Each of the four enzymic steps was high yielding (70–80% after purification). Key to the success of this approach was our ability to prepare and purify a series of labile phosphorylated intermediates en route to labelled DHQ. In order to minimize levels of decomposition, purification of all the phosphorylated intermediates was performed as rapidly as possible in a cold room at 4 °C.
![Enzymic synthesis of [3-13C] labelled DHQ.](/image/article/2003/OB/b309666a/b309666a-s1.gif) | ||
| Scheme 1 Enzymic synthesis of [3-13C] labelled DHQ. | ||
We have previously described the use of shikimate pathway enzymes for the preparation of a series of specifically fluorinated13,14 and deuterated15 shikimate pathway intermediates. It therefore seemed reasonable to adopt a similar approach (utilizing the enzymes DAHP synthase and DHQ synthase) to make 13C labelled derivatives (Scheme 1). Central to this strategy was the need to prepare [3-13C] labelled D-erythrose-4-phosphate (E4P) 5.
E4P has been prepared chemically by lead tetraacetate oxidation of D-glucose-6-phosphate.16 It is notoriously difficult to work with, being both sensitive to pH and temperature and unstable with respect to phosphate elimination. In dilute aqueous solution it exists as a mixture of the monomeric aldehyde and its hydrate but at higher concentrations it rapidly converts to a complex mixture of cyclic dimeric forms17–19 which make chromatographic purification difficult. For these reasons we chose not to isolate 5 but to convert it directly to 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) 6 using DAHP synthase. Synthesis of 5 was achieved from commercially available D-[5-13C]fructose203via a procedure involving chemospecific phosphorylation at C(6) followed by the transfer of a C2 unit to D-ribose-5-phosphate (R5P).
The relatively high degree of substrate tolerance of hexokinase together with its ready commercial availability made it the enzyme of choice for the first step. Treatment of 3 (25 mg) with ATP and hexokinase at ambient temperature afforded D-[5-13C]fructose-6-phosphate 4. The conversion was monitored by assaying small aliquots of the reaction mixture for the formation of ADP using a standard pyruvate kinase–lactate dehydrogenase coupled assay system.21 Spectrophotometric analysis (monitoring at 340 nm for the consumption of NADH) showed phosphorylation to be essentially complete after 1 hour. Purification by anion-exchange chromatography (Amersham MonoQ® eluting with an NH4HCO3 gradient) afforded 4 (80%) upon lyophilization.
To catalyse the conversion of 4 to E4P 5 we utilized transketolase, a thiamine pyrophosphate (TPP) dependent enzyme and coupled this to the phosphoenolpyruvate (PEP) utilizing enzyme DAHP synthase.22 Thus a solution of 4, R5P, TPP, transketolase, PEP and DAHP synthase was held at ambient temperature and aliquots of the reaction mixture were analysed spectrophotometrically (monitoring at 260 nm for the consumption of PEP). After around 5 hours PEP consumption had ceased. Purification by anion-exchange chromatography (Amersham MonoQ® eluting with an NH4HCO3 gradient) and lyophilization afforded the desired phosphate 6 (67%), the overall yield representing an average of 82% per enzymic step which was acceptable given the instability of 5.
Synthesis of 1 was readily completed from 5 using the NAD-dependent enzyme DHQ synthase.23 A mixture of 6, NAD and DHQ synthase was held at ambient temperature and assayed spectrophotometrically for the formation of 1 by monitoring the appearance of DHS 2 (234 nm) upon treatment of an aliquot of the reaction mixture with type II dehydroquinase.24 Formation of 1 was found to be essentially complete within 3 hours. Purification by HPLC (BioRad Aminex® HPX-87H) eluting with 50 mM formic acid afforded 3-[3-13C]DHQ 1 (10 mg, 70%) as a colourless gum upon lyophilization. The product was shown to be essentially homogeneous by proton-decoupled 13C NMR spectroscopy; only a single resonance could be observed and was located in the carbonyl region of the spectrum (δC 209.5).
In order to determine the efficiency of the synthesis we followed the overall conversion of 3 to 1 by 13C NMR spectroscopy to trace the position of the 13C label during each of the enzyme catalysed steps. Thus a sample of 3 (in H2O containing 10% 2H2O) was treated sequentially with the four enzymes (in the presence of all of the necessary reagents and cofactors) and 13C NMR spectra recorded at each stage to monitor the resulting interconversion (Fig. 2).
![Proton decoupled 13C NMR spectra (62.9 MHz) following the conversion of d-[5-13C]fructose 3 to 3-[3-13C]dehydroquinate 1. a)
d-[5-13C]fructose 3; b) formation of d-[5-13C]fructose-6-phosphate 4 upon addition of hexokinase; c) formation of [6-13C]DAHP 6 upon addition of transketolase and DAHP synthase; d) formation of 3-[3-13C]DHQ 1 upon addition of DHQ synthase.](/image/article/2003/OB/b309666a/b309666a-f2.gif) | ||
| Fig. 2 Proton decoupled 13C NMR spectra (62.9 MHz) following the conversion of D-[5-13C]fructose 3 to 3-[3-13C]dehydroquinate 1. a) D-[5-13C]fructose 3; b) formation of D-[5-13C]fructose-6-phosphate 4 upon addition of hexokinase; c) formation of [6-13C]DAHP 6 upon addition of transketolase and DAHP synthase; d) formation of 3-[3-13C]DHQ 1 upon addition of DHQ synthase. | ||
D-[5-13C]Fructose 3 appears to exist in solution as a mixture of three interconverting isomeric species (Fig. 2a). Addition of hexokinase resulted in the rapid and relatively clean conversion to the 6-phosphate 4 which exists in solution as an isomeric pair of phosphorus coupled (JC–P 8 Hz) species (Fig. 2b). Upon subsequent treatment with a mixture of transketolase and DAHP synthase there was a simplification of the reaction mixture and a phosphorus coupled (JC–P 5 Hz) signal attributable to DAHP 6 was observed. At this stage the mixture contained only trace quantities of the intermediates 4 and 5 and the starting material 3 had been completely consumed (Fig. 2c). After subsequent treatment with DHQ synthase we observed only a single resonance (Fig. 2d) in the 13C NMR spectrum corresponding to the carbonyl signal (δC 209.5) of 3-[3-13C]DHQ 1.
In summary, we report a short and efficient synthesis of 3-[3-13C]DHQ 1 from commercially available D-[5-13C]fructose 3 over four enzyme-catalysed steps. The purified product has been shown to be essentially homogenous with respect to 13C incorporation. Additionally we have used the 13C label in 3 to monitor the progress of each of the enzymic steps and have shown the overall conversion to be extremely 13C atom efficient.
We thank the BBSRC for postdoctoral support (to M.F. and E.J.P).
Notes and references
- E. Haslam, Shikimic acid: Metabolism and Metabolites, John Wiley and Sons, Chichester, 1993 Search PubMed.
- C. Abell, in Comprehensive Natural Products Chemistry, ed. U. Sankawa, Elsevier, Amsterdam, 1999, vol. 1, p. 573 Search PubMed.
- N. H. Giles, M. E. Case, J. A. Baum, R. F. Geever, L. Huiet, V. B. Patel and B. M. Tyler, Microbiol. Rev., 1985, 49, 338 Search PubMed.
- S. Chaudhuri, K. Duncan, L. D. Graham and J. R. Coggins, Biochem. J., 1991, 275, 1 CAS.
- B. W. Smith, M. J. Turner and E. Haslam, J. Chem. Soc., Chem. Commun., 1970, 842 Search PubMed.
- A. Schneier, C. Kleanthous, R. Deka, J. R. Coggins and C. Abell, J. Am. Chem. Soc., 1991, 113, 9416 CrossRef.
- A. R. Hawkins, W. R. Reinhert and N. H. Giles, Biochem. J., 1982, 203, 769 CAS.
- J. M. Harris, C. González-Bello, C. Kleanthous, A. R. Hawkins, J. R. Coggins and C. Abell, Biochem. J., 1996, 319, 333 CAS.
- M. Frederickson, E. J. Parker, A. R. Hawkins, J. R. Coggins and C. Abell, J. Org. Chem., 1999, 64, 2612 CrossRef CAS.
- A. W. Roszak, D. A. Robinson, T. Krell, I. S. Hunter, M. Frederickson, C. Abell, J. R. Coggins and A. J. Lapthorn, Structure (London), 2002, 10, 493 Search PubMed.
- M. Frederickson, J. R. Coggins and C. Abell, Chem. Commun., 2002, 1886 RSC.
- M. D. Toscano, M. Frederickson, D. P. Evans, J. R. Coggins, C. Abell and C. González-Bello, Org. Biomol. Chem., 2003, 1, 2075 RSC.
- P. J. Duggan, E. Parker, J. Coggins and C. Abell, Bioorg. Med. Chem. Lett., 1995, 5, 2347 CrossRef CAS.
- S. Balasubramanian, G. M. Davies, J. R. Coggins and C. Abell, J. Am. Chem. Soc., 1991, 113, 8945 CrossRef CAS.
- S. Balasubramanian and C. Abell, Tetrahedron Lett., 1991, 32, 963 CrossRef CAS.
- A. S. Sieben, A. S. Perlin and F. J. Simpson, Can. J. Biochem., 1966, 44, 663 CAS.
- P. F. Blackmore, J. F. Williams and J. K. Macleod, FEBS Lett., 1976, 64, 222 CrossRef CAS.
- J. F. Williams, P. F. Blackmore, C. C. Duke and J. K. Macleod, Int. J. Biochem., 1980, 12, 339 Search PubMed.
- C. C. Duke, J. K. Macleod and J. K. Williams, Carbohydr. Res., 1981, 95, 1 CrossRef CAS.
- D-[5-13C]fructose was obtained from Omicron Biochemicals, Inc., 1347 North Ironwood Drive, South Bend, IN 46615-3566, USA (product code: FRU-006).
- C. Blondin, L. Serina, L. Weismuller, A. M. Giles and O. Barzu, Anal. Biochem., 1994, 220, 219 CrossRef CAS.
- C. M. Stephens and R. Bauerle, J. Biol. Chem., 1991, 266, 20810 CAS.
- J. W. Frost, J. L. Bender, J. T. Kadonaga and J. R. Knowles, Biochemistry, 1984, 23, 4470 CrossRef CAS.
- S. Chaudhuri, J. M. Lambert, L. A. McColl and J. R. Coggins, Biochem. J., 1986, 239, 699 CAS.
| This journal is © The Royal Society of Chemistry 2003 |
