Linyitian Wang, Huize Guo, Jiaxin Luo, Wenxu Zhen, Shiping Wang, Zailai Xie and Chunfa Xu
Org. Biomol. Chem., 2025, Advance Article
DOI:
10.1039/D5OB00509D,
Paper
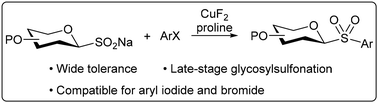
Glycosyl aryl sulfones exhibit diverse biological activities. Herein, we developed a copper-promoted coupling strategy using glycosyl sodium sulfinates and aryl iodides or bromides, enabling efficient synthesis of carbohydrate-based sulfone with broad functional group compatibility. This method offers a versatile approach for the late-stage modification of bioactive molecules.