Alkali and alkaline earth metals in liquid salts for supercapatteries
Abstract
The full oxidation of lithium metal (4Li + O2 ⇌ 2Li2O) offers a mass normalised Gibbs energy change greater than that for the combustion of carbon (C + O2 ⇌ CO2) or any hydrocarbon fuel (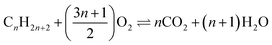 ). This thermodynamic comparison promises a lithium–oxygen (air) battery with a petrol comparable energy density. Similar analyses apply to other abundant alkali and alkaline earth metals (AAEMs) which all feature very high specific charge capacity and the most negative electrode potentials. The success of lithium ion batteries (LIBs) in both research and commercial development confirms these thermodynamic predictions. However, the experimentally demonstrated energy capacities of all AAEM-based batteries are only small fractions of the thermodynamic values. A main cause is that a satisfactory oxygen positive electrode (positrode) is still to be developed, whilst the very few options of AAEM storage positrodes still do not match AAEM negative electrodes (negatrodes) in charge capacity. Another challenge results from the complicated interactions between AAEMs and the currently used organic carbonate electrolytes that not only reduce the negatrode capacity but also exert restrictions on both electron and ion transfers. The flammability of currently used organic electrolytes is another major concern with respect to the safety of AAEM batteries. Herein, we introduce the concept and potential, and review the relevant practices of a promising ionic liquid supercapattery that couples an AAEM negatrode with a supercapacitor positrode to bypass the thermodynamic and kinetic difficulties of an oxygen or AAEM storage positrode. The further discussion aims at the selection of ionic liquid-based electrolytes that can enable the reversible anodic dissolution of AAEMs and a wide potential window for the supercapacitor positrode. The use of molten salt-based electrolytes is also postulated and analysed, not only because of their high ionic conductivity, low cost and unique applications, but also their high temperatures that eliminate dendritic growth on the liquid AAEM negatrode and heat buildup in the cell.
). This thermodynamic comparison promises a lithium–oxygen (air) battery with a petrol comparable energy density. Similar analyses apply to other abundant alkali and alkaline earth metals (AAEMs) which all feature very high specific charge capacity and the most negative electrode potentials. The success of lithium ion batteries (LIBs) in both research and commercial development confirms these thermodynamic predictions. However, the experimentally demonstrated energy capacities of all AAEM-based batteries are only small fractions of the thermodynamic values. A main cause is that a satisfactory oxygen positive electrode (positrode) is still to be developed, whilst the very few options of AAEM storage positrodes still do not match AAEM negative electrodes (negatrodes) in charge capacity. Another challenge results from the complicated interactions between AAEMs and the currently used organic carbonate electrolytes that not only reduce the negatrode capacity but also exert restrictions on both electron and ion transfers. The flammability of currently used organic electrolytes is another major concern with respect to the safety of AAEM batteries. Herein, we introduce the concept and potential, and review the relevant practices of a promising ionic liquid supercapattery that couples an AAEM negatrode with a supercapacitor positrode to bypass the thermodynamic and kinetic difficulties of an oxygen or AAEM storage positrode. The further discussion aims at the selection of ionic liquid-based electrolytes that can enable the reversible anodic dissolution of AAEMs and a wide potential window for the supercapacitor positrode. The use of molten salt-based electrolytes is also postulated and analysed, not only because of their high ionic conductivity, low cost and unique applications, but also their high temperatures that eliminate dendritic growth on the liquid AAEM negatrode and heat buildup in the cell.

- This article is part of the themed collection: RSC Sustainability Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...