Transamidation of thioamides with nucleophilic amines: thioamide N–C(S) activation by ground-state-destabilization†
Abstract
Thioamides are ‘single-atom’ isosteres of amide bonds that have found broad applications in organic synthesis, biochemistry and drug discovery. In this New Talent themed issue, we present a general strategy for activation of N–C(S) thioamide bonds by ground-state-destabilization. This concept is outlined in the context of a full study on transamidation of thioamides with nucleophilic amines, and relies on (1) site-selective N-activation of the thioamide bond to decrease 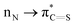 resonance and (2) highly chemoselective nucleophilic acyl addition to the thioamide C
resonance and (2) highly chemoselective nucleophilic acyl addition to the thioamide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond. The follow-up collapse of the tetrahedral intermediate is favored by the electronic properties of the amine leaving group. The ground-state-destabilization concept of thioamides enables weakening of the N–C(S) bond and rationally modifies the properties of valuable thioamide isosteres for the development of new methods in organic synthesis. We fully expect that in analogy to the burgeoning field of destabilized amides introduced by our group in 2015, the thioamide bond ground-state-destabilization activation concept will find broad applications in various facets of chemical science, including metal-free, metal-catalyzed and metal-promoted reaction pathways.
S bond. The follow-up collapse of the tetrahedral intermediate is favored by the electronic properties of the amine leaving group. The ground-state-destabilization concept of thioamides enables weakening of the N–C(S) bond and rationally modifies the properties of valuable thioamide isosteres for the development of new methods in organic synthesis. We fully expect that in analogy to the burgeoning field of destabilized amides introduced by our group in 2015, the thioamide bond ground-state-destabilization activation concept will find broad applications in various facets of chemical science, including metal-free, metal-catalyzed and metal-promoted reaction pathways.

- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...