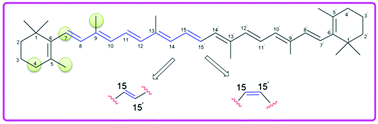
The antioxidant capacity of β-carotene has been studied in terms of H-atom abstraction reactions using quantum chemical methods. These oxidation reactions are studied for the all-trans as well as 15,15′-cis isomers (15Z) of β-carotene, as the latter is only ∼10 kJ mol−1 less stable than the all-trans isomer in the gas phase and about 9 kJ mol−1 less stable in aqueous solution. Hydrogen abstraction from the rotamers obtained through C–C single and double bond rotations has been shown to play an important role in determining the antioxidant capacity of β-carotene. Hydrogen abstraction from the C4 and C5-CH3 positions of the β-ionone rings and the C7 and C9 positions along the polyene chain of β-carotene by the hydroxyl radical have been studied. In the all-trans form the most favorable H-atom abstraction reaction occurs at the C4 position of the terminal regions of the polyene π-system of β-carotene, closely followed by hydrogen abstraction from the C5 methyl position. The H-atom abstraction reactions are more exothermic in water than in the gas phase due to solvation energies for the water product.