Role and advances in nonmetal doping of electrocatalysts for the hydrogen evolution reaction: a review
Shenyue
Xu
,
Chenxi
Liu
,
Zefeng
Teng
,
Fahao
Sun
,
Jingqi
Chi
 *,
Xiaobin
Liu
,
Jianping
Lai
*,
Xiaobin
Liu
,
Jianping
Lai
 ,
Fusheng
Liu
* and
Lei
Wang
,
Fusheng
Liu
* and
Lei
Wang
 *
*
Key Laboratory of Eco-chemical Engineering, International Science and Technology Cooperation Base of Eco-chemical Engineering and Green Manufacturing, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, PR China. E-mail: chijingqi@qust.edu.cn; liufusheng63@sina.com; inorchemwl@126.com
First published on 19th May 2025
Abstract
Hydrogen produced by water electrolysis is considered an ideal, safe, and clean energy source. However, the high cost of noble metal-based electrocatalysts and the high overpotential of non-noble metals for the hydrogen evolution reaction (HER) are key bottlenecks limiting the practical application of water splitting technology. To tackle this challenge, extensive efforts have been made to explore strategies for improving the HER activity. This paper systematically reviews the critical roles of nonmetallic doping in enhancing HER catalyst performance, including electronic structure adjustment, active site regulation, and the generation of synergistic effects. Subsequently, we categorized the reported nonmetal-atom-doped HER catalysts by composition and introduced the mechanisms behind their high performance and long-term stability. In addition, this review also provides a comprehensive summary of the synthesis strategies and characterization techniques associated with non-metallic doping, aiming to offer practical guidance for future research and catalyst development in this field. Finally, this review discusses the development of non-metal-doped electrocatalysts, the key challenges in industrial water electrolysis, and the promising application of artificial intelligence (AI) in accelerating catalyst design and system optimization.
1. Introduction
Although fossil fuels have brought tremendous benefits to the development of human society, their associated environmental costs are becoming increasingly prominent.1 This requires us to seek a balance between economic development and environmental sustainability.2,3 In the long term, society needs to gradually reduce its dependence on fossil fuels and explore alternative energy forms and carriers.4–6Hydrogen, as a type of renewable energy, with high calorific value (1.42 × 108 J kg−1) and zero carbon emission, has attracted widespread attention from researchers.7–9 There are various methods to obtain hydrogen, among which water electrolysis is considered to be promising technology with the potential for near-zero net CO2 emissions.9–11 Water electrolysis involves the hydrogen evolution reaction (HER) occurring at the cathode and the oxygen evolution reaction (OER) taking place at the anode.12–14 Although the HER is considered to be kinetically favorable, its catalytic performance can still be limited under certain conditions, particularly at high current densities or in highly concentrated electrolytes, due to issues such as active site saturation, hydrogen bubble accumulation, and inefficient electron transport. Therefore, further optimization of the HER catalytic efficiency remains necessary.15–17 To address this challenge, the use of effective electrocatalysts is necessary to accelerate the HER kinetics and enhance the efficiency of the hydrogen production process. The development of advanced electrocatalysts with optimized properties has become a central focus in water electrolysis research and development.18–20
It is necessary to clarify that the key factors influencing the performance of electrocatalytic materials are the number of active sites on the electrocatalyst surface and the intrinsic activity of each active site.21,22 Since the surface structure of electrocatalysts can be deliberately tuned and engineered, achieving high-performance catalysts requires extensive efforts in structural optimization and material design.23–25 In recent years, a variety of advanced design strategies have been proposed to optimize the surface structure of catalysts and achieve excellent electrocatalytic performance, including atomic doping, interface engineering, defect engineering, strain engineering, and others.26–28 Among these strategies, heteroatom doping is widely regarded as a highly promising method due to its numerous advantages.29–31 Heteroatom doping refers to the introduction of different metal or metalloid atoms into the main structure of the catalyst, enabling the electrocatalytic material to undergo performance-related changes in conductivity, morphology, and band gap energy. Depending on the type of heteroatom, doping can be categorized as metal atom doping, nonmetal atom doping, and metal–nonmetal co-doping.32–34 Among them, nonmetallic atom doping offers advantages such as low cost, excellent stability, uniform distribution, and minimal aggregation. Its impact on catalysts is primarily reflected in the following aspects (Fig. 1):
(1) Modulation of the electronic structure: nonmetal doping to modulate the electronic structure of catalysts is an effective strategy for enhancing the water-splitting performance by adjusting the electronic states, energy band structure, and adsorption characteristics.35,36
(2) Regulating active sites: nonmetal doping can enhance the catalytic reaction kinetics by increasing the density of active sites and enhancing the intrinsic activity of catalytic centers.37,38
(3) Enhancing electrical conductivity: the enhancement of electrical conductivity can accelerate the rate of electron transport in the electrocatalysis process, thereby improving the intrinsic activity of the catalyst.39,40
(4) Synergistic effects: the synergistic effect between the doping atoms and the catalyst backbone can effectively regulate the electronic structure of the active center and increase the concentration of active sites, thereby significantly improving the intrinsic activity of the electrocatalytic process.41,42
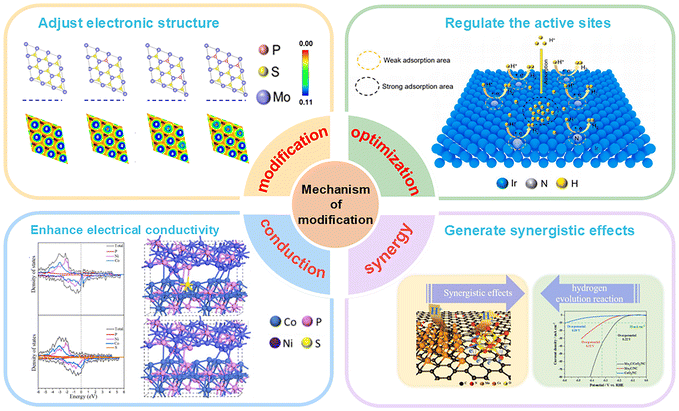 | ||
| Fig. 1 Schematic representation of the role of nonmetal doping in the HER.43–46 Reprinted with permission from ref. 43. Copyright 2021, American Chemical Society. Reprinted with permission from ref. 44. Copyright 2024, Elsevier. Reprinted with permission from ref. 45. Copyright 2022, Elsevier. Reprinted with permission from ref. 46. Copyright 2023, Elsevier. | ||
The application of nonmetal-doped catalysts in the HER has witnessed significant progress in recent years (Fig. 2). This review systematically summarizes recent advances in nonmetallic doping strategies for enhancing the electrocatalytic performance of HER catalysts. First, six mainstream doping methods, including hydrothermal synthesis, calcination, and electrochemical deposition, are introduced, with a comparative analysis of their respective advantages and limitations. Next, four primary mechanisms through which nonmetallic doping improves the catalytic performance are discussed: regulation of the electronic structure, optimization of active sites, enhancement of electrical conductivity, and the construction of synergistic interfacial effects. The review also highlights typical characterization techniques applicable to nonmetal-doped systems, such as HRTEM, EDS, Cs-corrected TEM, XRD, XPS, XANES, and EXAFS. Subsequently, the catalytic behavior and regulatory features of representative nonmetallic dopants, including phosphorus (P), sulfur (S), nitrogen (N), boron (B), and halogens, are analyzed in detail through a survey of specific research studies. Finally, the review discusses existing challenges in the field and proposes future directions, including the application of artificial intelligence (AI) to accelerate catalyst design and optimization, the promotion of industrial-scale green hydrogen production, and the use of abundant seawater as a sustainable electrolyte. It is hoped that this work will serve as a valuable reference and inspiration for the design and application of nonmetal-doped electrocatalysts.
 | ||
| Fig. 2 Research progress for nonmetal-doped materials as HER electrocatalysts.47–53 Reprinted with permission from ref. 47. Copyright 2018, Wiley. Reprinted with permission from ref. 48. Copyright 2019, Elsevier. Reprinted with permission from ref. 49. Copyright 2020, Wiley. Reprinted with permission from ref. 50. Copyright 2021, Wiley. Reprinted with permission from ref. 51. Copyright 2022, Wiley. Reprinted with permission from ref. 52. Copyright 2023, Elsevier. Reprinted with permission from ref. 53. Copyright 2024, American Chemical Society. | ||
2. Mechanisms of water splitting
In the water electrolysis system for hydrogen production, the electrolyser is composed of the cathode, anode, and electrolyte. The overall electrochemical water splitting process comprises two half-reactions: the HER occurring at the cathode and the OER taking place at the anode. The specific chemical reaction pathways for water electrolysis are influenced by the prevailing reaction conditions:Overall water splitting reaction:
| H2O → H2 + 1/2O2 | (1) |
In acidic electrolyte:
Anode OER:
| H2O − 2e− → 1/2O2 + 2H+ | (2) |
Cathodic HER:
| 2H+ + 2e− → H2 | (3) |
In alkaline electrolyte:
Anode OER:
| 2OH− − 2e− → 1/2O2 + H2O | (4) |
Cathodic HER:
| 2H2O + 2e− → H2 + 2OH− | (5) |
2.1 HER
The HER occurs at the cathode of the electrolytic cell, where water is reduced to produce hydrogen.54 Generally, the HER is divided into two types:In acidic media, the reduction reaction of H+/H2O that occurs on the electrode surface goes through three consecutive steps, and the specific reactions are as follows.
| H+ + e− + * → H* (Volmer step) |
| H+ + e− + H* → H2 + * (Heyrovsky step) |
| H* + H* → H2 + 2* (Tafel step) |
In an acidic medium, the reactions occurring on the catalyst surface involve two prominent mechanistic pathways: the Volmer–Tafel mechanism and the Volmer–Heyrovsky mechanism. In the Volmer step, H+ is first firmly adsorbed on the surface of the cathode, generating adsorbed hydrogen species (H*). Then, when the concentration of H+ is relatively low, H+, H*, and electrons (e−) can combine to form molecular hydrogen (H2) through the Heyrovsky step. Alternatively, when the coverage of adsorbed H* on the cathode surface is sufficiently high, the combination of two adjacent H* species can also produce H2via the Tafel step.
The reaction equation in an alkaline electrolyte is as follows:
| H2O + e− → H* + OH− (Volmer step) |
| H2O + e− + H* → H2 + OH− + * (Heyrovsky step) |
| H* + H* → H2 + 2* (Tafel step) |
In alkaline and neutral media, the Volmer and Heyrovsky processes involved in the HER differ from those under acidic conditions. In the absence of H+ ions, the Volmer step involves the generation of adsorbed H* and OH− on the cathode surface. Subsequently, a chemical reaction occurs among H*, H2O, and e− to produce H2 and OH−, which corresponds to the Heyrovsky step. Additionally, the Tafel step, involving the combination of two adsorbed H* species, is similar to the mechanism in acidic media.
Regardless of whether the conditions are alkaline, neutral, or acidic, the HER proceeds through either the two-step Volmer–Heyrovsky mechanism or the Volmer–Tafel mechanism.
3. Advanced methods for nonmetallic doping
At present, the methods for introducing nonmetallic atom doping are mainly: hydrothermal, solvothermal, calcination, ion exchange, plasma treatment, and electrodeposition. These methods are shown in Fig. 3.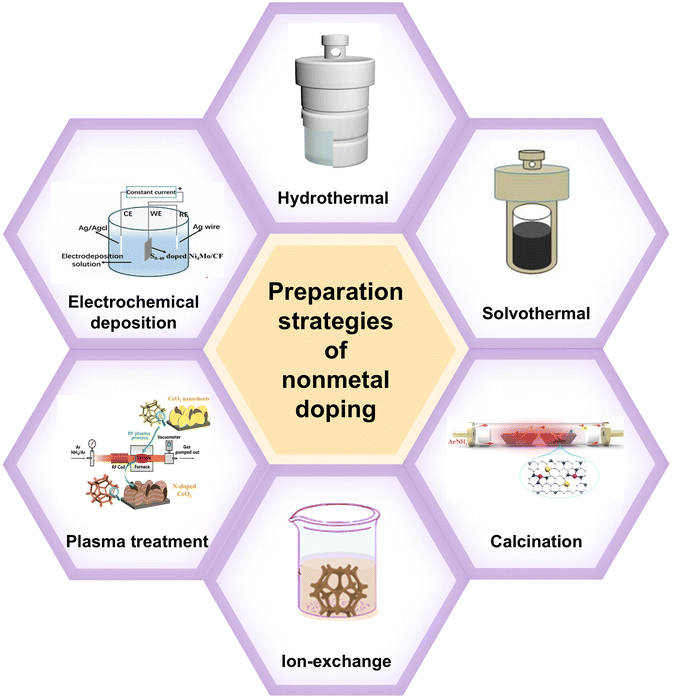 | ||
| Fig. 3 Common methods of introducing nonmetallic doping.55–60 Reprinted with permission from ref. 55. Copyright 2022, Elsevier. Reprinted with permission from ref. 56. Copyright 2022, Wiley. Reprinted with permission from ref. 57. Copyright 2021, American Chemical Society. Reprinted with permission from ref. 58. Copyright 2023, Royal Society of Chemistry. Reprinted with permission from ref. 59. Copyright 2024, MDPI. Reprinted with permission from ref. 60. Copyright 2021, Wiley. | ||
3.1. Hydrothermal method
The hydrothermal method is a synthetic approach in which water is employed as both the medium and reactant, with the process taking place in a sealed container under appropriate temperature and pressure conditions.61 The hydrothermal method offers several advantages, including simple operation, suitability for large-scale production, and excellent tunability in terms of morphology, crystallinity, and structural characteristics.62 The hydrothermal process can be classified into three categories based on the reaction temperature: low-temperature hydrothermal method (<100 °C), medium-temperature hydrothermal method (100–300 °C), and high-temperature hydrothermal method (>300 °C). In the medium-temperature heating process, a tetrafluoroethylene (Teflon) lining is typically used in the reactor, which provides low internal pressure, good sealing, and low synthesis cost.63 The key to obtaining high-performance catalysts via the hydrothermal method is the selection of suitable precursors and appropriate reaction conditions (such as temperature, concentration, duration, and pH value).64 With this method, Zhao et al.65 directly grew oxygen-doped MoS2 nanosheets on carbon paper substrates. Then, through a short H2O2 etching process, they obtained MoS2 with dual defects of O doping and S vacancies (O–MoS2−x). The O-doping increased the active site density and also favored the formation of S vacancies. Moreover, the synergistic effect of O-doping and S vacancies enhanced the HER activity by accelerating the reaction kinetics and improving charge transport.3.2. Solvothermal method
The solvothermal method is developed based on the hydrothermal approach, which is a synthetic technique for reacting a desired precursor mixture in a closed system under appropriate temperature and pressure conditions, utilizing organic compounds or non-aqueous solvents as the reaction medium.66,67 The key distinction from the hydrothermal method is that the solvent used in the solvothermal process is an organic solvent rather than water. This method is not only easy to operate but also enables rapid reactions. However, it is necessary to ensure that the operating temperature is higher than the critical temperature of the solvent, and the required pressure is also relatively high. Common organic solvents include EtOH, EG, IPA, PEG, t-BuOH, and DMF.68 It is well-established that the morphology, crystallinity, and structure of catalysts can be tuned by adjusting parameters such as pH, temperature, and the choice of solvent.62 In recent years, the solvothermal method has been widely employed to prepare various nonmetal-doped electrocatalysts with outstanding catalytic performance. For example, Li et al.56 synthesized a composite material (N–MoS2/N-CNTs) composed of N-doped MoS2 (N–MoS2) nanosheets anchored on N-doped carbon nanotubes (N-CNTs) via a one-step solvothermal method. The incorporation of N atoms can expand the interlayer spacing of MoS2 and replace S atoms to form more structural defects, exposing more catalytic active sites. Meanwhile, N doping also increases the defects of CNTs, which benefits the interface coupling between MoS2 and CNTs, thereby greatly improving the HER catalytic performance.3.3. Calcination method
Under an inert gas atmosphere, certain upstream compounds (e.g., NaH2PO2, (NH4)2CO3, H2S) that rapidly decompose into reactive gaseous species can be utilized, as these active gases will fully react with downstream precursors.69 This process is primarily dependent on the heating temperature and duration, allowing the crystal structure of the material to be regulated by controlling these two key parameters. Based on the above considerations, Yin et al.70 introduced P dopant into the PtZn alloy by calcination, which induced lattice tensile strain in Pt and electronic interactions between P and Zn, thereby optimizing the HER activity. Additionally, their study revealed that as the calcination temperature increased, the P content in the samples also increased. The sample with optimal P content exhibited the highest catalytic performance for the HER.3.4. Ion-exchange method
The ion exchange method facilitates the replacement of ions in a liquid phase and in solid materials. Particularly effective for creating hollow transition metal oxide nanostructures, this approach offers significant advantages, such as a large specific surface area and abundant electron transfer channels, which together provide ideal structural conditions for electrochemical reactions. However, the ion exchange method has certain limitations, including slow reaction kinetics and specific requirements for the solubility product constant (Ksp).14,62 Despite these challenges, recent research has actively leveraged ion exchange to develop doped materials with enhanced properties. For instance, Tian et al.45 employed ion exchange to construct a core–shell heterostructure of S–Co2P@Ni2P, achieving interfacial S doping by carefully controlling the reaction time. This S doping promotes electron transfer from Co2P to Ni2P, resulting in electron-rich nickel and electron-deficient cobalt at the interface, thereby enhancing the catalytic activity.3.5. Plasma treatment method
Plasma treatment typically employs nonmetallic gases such as N2 and NH3 to generate reactive nonmetallic species. These species are subsequently activated and dissociated into highly reactive forms, which then interact with metallic substrates to achieve effective nonmetal doping. Plasma treatment is a nonmetallic doping method characterized by high efficiency and environmental friendliness. This method not only significantly reduces the reaction time, sometimes to just a few minutes, but can also be performed at room temperature.71,72 Chen and his colleagues73 prepared N-doped yttrium–nickel–cobalt phosphide (N–YNiCoP/PNCF) by radio frequency plasma phosphorization under a nitrogen atmosphere. The YNiCo layered double hydroxide (LDH) precursor was transformed into the N-doped YNiCo phosphide (N–YNiCoP) structure. The incorporation of N dopants and the creation of multiphase heterointerfaces can expose a large number of active sites for catalysis. Additionally, the hydrophilic nature of these interfaces facilitates the transfer and diffusion of electrolytes and reaction product bubbles at the electrode–electrolyte interface.3.6. Electrochemical deposition method
The electrochemical deposition method refers to technology for forming a coating through the migration of positively and negatively charged ions in an electrolyte solution under the influence of an external electric field, coupled with redox reactions involving the gain and loss of electrons at the electrode surface. Electrochemical deposition stands out as a simple, efficient, and cost-effective strategy for synthesizing doped materials for hydrogen production via water electrolysis. Compared to other electrocatalyst synthesis techniques, it offers several advantages, including mild reaction conditions, minimal equipment requirements, and straightforward operation, making it highly suitable for being scalable. It is worth noting that the electrochemical deposition process can also be carried out under ambient temperature and pressure conditions. This versatility provides the prerequisites for the large-scale industrial application of this method. Most importantly, the electrochemical deposition approach is a binder-free method, which enables the direct fabrication of electrodes with inherently high electrocatalytic activity.74,75 For instance, Pham and co-workers76 synthesized S-doped Ni–P nanospheres at different deposition frequencies. The optimal doping of S elements was found to enhance the intrinsic electrocatalytic activity of Ni–P, endowing the catalyst with bifunctional activity for water electrolysis.To facilitate a more direct comparison of the advantages and disadvantages of various methods, relevant studies have been reviewed and are summarized in Table 1.
| Synthesis methods | Advantages | Weaknesses |
|---|---|---|
| Hydrothermal | Simple operation, suitable for large-scale production | Long reaction time, high temperature and high pressure conditions |
| The morphology, crystallinity, and structure are adjustable | ||
| Solvothermal | Simple technique | High temperature and high pressure conditions |
| Fast reaction kinetics | High risk factor | |
| The morphology, crystallinity, and structure are adjustable | ||
| Calcination | Low cost | Prone to carbonization |
| Simple technique | High deposition temperature | |
| Wide application range | ||
| Controllable product structure | ||
| Ion-exchange | Moderate reaction conditions | Sluggish reaction kinetics stringent solubility product requirements |
| Excellent controllability | ||
| Versatile applicability | ||
| Plasma treatment | Rapid and efficient | High equipment investment |
| Moderate reaction conditions | High energy consumption | |
| Environmentally friendly | ||
| Electrochemical deposition | Simple and efficient | Limited in depositing certain materials |
| Equipment requirements are relatively simple | Challenging to achieve uniform film thickness | |
| Moderate reaction conditions |
4. The effect of nonmetal doping on HER electrocatalysts
The mechanisms of nonmetallic doping modification in HER catalysts can be broadly classified into four categories: (1) doping introduces impurity states and alters the electronic band structure, which can optimize the adsorption/desorption of reaction intermediates; (2) doping can modulate the activity and density of active sites, thereby optimizing the reaction kinetics; (3) doping can improve conductivity, thus enhancing the intrinsic catalytic activity; (4) the interplay between doped species and the host matrix can generate cooperative effects, such as charge transfer and electronic sensitization, to boost the overall catalytic performance.These four regulatory mechanisms can often work in concert, where one or more aspects cooperate to synergistically enhance the intrinsic activity of the HER catalyst. The delicate interplay between these factors underpins the rational design of highly efficient nonmetallic doped HER electrocatalysts.
4.1. Adjust the electronic structure
Nonmetal doping plays a crucial role in modifying the electronic structure of catalysts by introducing new energy levels or altering the energy band structure.77 This leads to a redistribution and adjustment of electrons within the catalyst. For instance, the incorporation of nonmetal atoms like N, S, or P creates additional energy levels, allowing for the fine-tuning of the catalyst's Fermi level position and electron density. Consequently, this manipulation of the electronic structure influences the charge transfer kinetics and the interaction between the active center and reactant molecules. By modulating the electronic structure, nonmetal doping can effectively enhance the catalyst's adsorption capacity and reduce the reaction activation energy, ultimately improving the catalyst's reaction activity.50,78 Inspired by this concept, researchers have directed their attention towards the development of electrocatalysts with superior electrocatalytic properties by incorporating nonmetal dopants. For instance, Wang et al.43 enhanced the catalytic activity of MoS2 by doping it with phosphorus. The low electronegativity of P atoms induced electron redistribution among nearby Mo and S atoms, resulting in a more uniform charge gradient. In this study, the hydrogen adsorption free energy of medium-concentration P-doped MoS2 (MP-MoS2) was found to be optimal, approximately 0.16 eV, exhibiting a volcano-like trend that effectively balanced strong adsorption and rapid desorption. Furthermore, P doping increased the interlayer spacing of MoS2 from 0.64 nm to 0.80 nm, which enlarged the ion transport channels and facilitated ion adsorption. Notably, after 30 h of continuous operation, the performance of doped MoS2 remained virtually unchanged, demonstrating exceptional stability.4.2. Regulate active sites
Active sites refer to specific locations on the catalyst surface that interact with reactants to facilitate catalytic reactions. Incorporating nonmetallic elements into electrocatalysts can influence these active sites in two core ways: first, increasing the number of active sites raises their overall density, thereby enhancing the material's electrocatalytic activity.79 Second, introducing nonmetallic dopants allows for precise modification of these sites, improving catalytic properties and increasing efficiency in electrochemical reactions.80 For example, Tong et al.81 introduced Co–S and Fe–S bonds into the oxygen vacancies within the FeCo2O4 spinel structure through S doping. This modification enabled the catalyst to expose more active sites for reactions, as S doping not only filled the vacancies but also provided a better chemical environment at these sites, enhancing their ability to adsorb reactants and improving the efficiency of the catalytic reaction. As a basic concept in catalysis research, the Sabatier principle suggests that the adsorption strength of catalytic active sites should be balanced—strong enough to facilitate effective adsorption of reactants but weak enough to enable timely desorption. In this field, noble metal-based catalysts with excellent hydrogen evolution activity often suffer from excessively strong adsorption strength. To address this, Kang and colleagues44 introduced N doping into Ir-based catalysts to create two distinct types of active sites: Ir sites near N atoms exhibit weaker hydrogen adsorption energy, while those farther from N retain stronger adsorption energy. This “strong–weak” synergy enhances hydrogen's efficient adsorption and rapid desorption, boosting the catalytic performance.4.3. Enhance the electrical conductivity
Conductivity refers to the ability of electrons to migrate within a material, which directly impacts the efficiency of electron transfer from the electrode to the active sites during electrocatalytic processes.82 Nonmetal doping plays a crucial role in optimizing electron transfer pathways by altering the material's band structure, Fermi level, and electron density of states (DOS), thereby enhancing its conductivity. Recently, Li's team83 demonstrated a significant improvement in catalyst conductivity using a molten salt chemical-assisted strategy combined with N doping, leading to enhanced HER performance. In their study, N doped into the carbon substrate was categorized into various types, with graphitic N contributing electrons to the carbon layer and pyridinic N enhancing the electron-attracting ability of neighboring carbon atoms. Similarly, Shi et al.84 developed a three-dimensional conductive network by integrating N-doped carbon substrates with carbon nanotubes (CNTs), which markedly improved the catalyst's electron transfer efficiency. By optimizing electron transfer pathways, these strategies effectively reduce electron migration impedance during the HER process, resulting in enhanced overall catalytic performance.4.4. Generate synergistic effects
The synergy between nonmetallic doping and other modification strategies, such as vacancy engineering and heterostructure construction, has demonstrated significant potential for enhancing the electrocatalytic performance. Notably, nonmetallic doping can induce the formation of vacancies, particularly anion vacancies, by modulating the local bonding environment or introducing lattice strain. These vacancies contribute to charge redistribution and the exposure of additional active sites.85,86 For example, Wang et al.87 systematically investigated the mechanism by which nitrogen doping enhanced the catalytic performance of CoS. Due to the high electronegativity of nitrogen, its introduction reduces the electron density of neighboring Co2+, thereby facilitating the formation of Co3+ species and cobalt vacancies. Under the synergistic effect of nitrogen doping and Co vacancies, the originally inert sulfur atoms experience an upward shift in their 3p orbital energy level, leading to stronger orbital overlap with the 1s orbital of H. This significantly lowers ΔGH*, enabling the S atoms adjacent to Co vacancies to serve as new active sites for the HER, and thus markedly enhances the HER catalytic activity of N-doped CoxS. When coupled with heterostructures, the introduction of doping further facilitates interfacial charge transfer and promotes favorable electronic interactions, thereby synergistically optimizing the intrinsic activity, electrical conductivity, and structural stability of the catalyst.88,89 Sun et al.90 proposed a synergistic mechanism combining S-doping-induced interfacial coupling and built-in electric field effects. The electron-donating nature of the doped carbon support drives spontaneous electron transfer toward the Pt nanoparticles, generating electron-rich Pt active sites. This electronic reconstruction significantly optimizes the adsorption/desorption energy barriers of H*, thereby fundamentally enhancing the kinetics of the HER.5. Characterization methods for nonmetal-doped HER catalysts
5.1 Morphology characterization methods
5.2 Structural characterization methods
To provide a clearer overview of the functions and distinguishing features of the characterization methods, the relevant content is systematically summarized in Table 2.
| Methods | Available information | Feature description |
|---|---|---|
| HRTEM | Lattice fringes | Observation of structural changes |
| Structural distortion | ||
| EDS | Elemental distribution | No valence state information Often combined with TEM |
| Semi-quantitative analysis | ||
| Cs-corrected TEM | Location of doped nonmetallic elements | ABF is suitable for light elements |
| HAADF provides Z-contrast | ||
| XRD | Lattice parameter changes | Suitable for detecting whether atoms cause structural changes |
| XPS | Presence, valence, and bonding state of doping elements | Surface sensitive |
| XANES | Electronic states of dopant atoms | Depth analysis of local structure and valence state |
| EXAFS/WT | Coordination environment |
6. Nonmetal-doped HER electrocatalysts
The development of highly active HER electrocatalysts is essential for achieving efficient hydrogen production through water electrolysis.103 Among cathode HER catalysts, platinum group metals (PGMs) like Pt, Ru, and Ir are the most effective due to their moderate d-orbital electron density, which overlaps effectively with the 1s orbital of hydrogen atoms—an interaction that is highly favorable for the HER process.104 However, these noble metals are scarce and costly, posing challenges for the large-scale production of hydrogen.105,106 To mitigate costs, attention has increasingly focused on high-performance, low-loading noble metal catalysts and non-noble metal electrocatalysts.24,107Nonmetallic atoms typically exhibit higher electronegativity than metallic atoms. Consequently, doping with nonmetallic elements can induce more pronounced modifications in the electronic structure and chemical properties of the host materials. Such doping can adjust the catalyst's electronic configuration by altering its surface chemical state, thereby increasing the exposure of active sites, enhancing conductivity, and optimizing hydrogen adsorption energy—factors that may significantly boost the electrocatalyst's catalytic activity.108,109 Furthermore, the introduction of nonmetallic doping is relatively straightforward. Based on these observations, we have summarized recent advancements in the synthesis of efficient water-splitting catalysts via nonmetallic doping methods, such as P doping, S doping, N doping, B doping, and halogen doping (Fig. 4).
 | ||
| Fig. 4 Types of non-metal doping discussed in this article.55,110–123 Reprinted with permission from ref. 110. Copyright 2022, Wiley. Reprinted with permission from ref. 111. Copyright 2022, Elsevier. Reprinted with permission from ref. 112. Copyright 2025, Wiley. Reprinted with permission from ref. 113. Copyright 2024, Wiley. Reprinted with permission from ref. 114. Copyright 2021, Springer. Reprinted with permission from ref. 55. Copyright 2022, Elsevier. Reprinted with permission from ref. 115. Copyright 2021, Wiley. Reprinted with permission from ref. 116. Copyright 2022, Wiley. Reprinted with permission from ref. 117. Copyright 2023, Wiley. Reprinted with permission from ref. 118. Copyright 2022, American Chemical Society. Reprinted with permission from ref. 119. Copyright 2020, Elsevier. Reprinted with permission from ref. 120. Copyright 2022, Wiley. Reprinted with permission from ref. 121. Copyright 2022, Wiley. Reprinted with permission from ref. 122. Copyright 2021, Elsevier. Reprinted with permission from ref. 123. Copyright 2020, Wiley. | ||
6.1. P-doped electrocatalysts for the HER
The large atomic size and excellent electron-donating capability of P atoms make them appealing as dopants, effectively enhancing the electrocatalytic activity by altering the electrocatalyst's electronic properties. To enhance intrinsic catalytic activity and accelerate reaction kinetics, P doping is employed to modulate the local charge density, optimize surface charge distribution, and supply a significant number of active surface sites, thereby boosting the efficiency of water electrolysis.124,125 Additionally, compared to transition metal doping, P doping offers a more advantageous approach for enhancing the catalytic performance by leveraging the in situ adjustment of the electronic structure through P atoms while preserving the host material's intrinsic properties. Therefore, P doped into electrocatalytic materials usually endows the electrocatalyst with low overpotential and high conductivity.126In recent years, P doping has become a widely adopted strategy to enhance the performance of new high-efficiency electrocatalytic materials, significantly advancing the development of low-loaded PGM catalysts. Furthermore, the unique atomicity and electronegativity of PGM and P create synergistic effects that further boost catalytic activity. This synergy increases the density of active sites and enhances electron transfer efficiency within the catalyst, ultimately improving its catalytic performance. Based on this, our team127 synthesized a new nanocatalyst, Ru/P–MoB, through a microwave quasi-solid-state method. In this work, strong interactions between Ru nanoclusters and the support are established through Ru–Mo and Ru–P bonds, providing abundant active sites to accelerate reaction kinetics. P doping lowers the work function and facilitates electron transfer from the bulk phase to the surface (Fig. 5(a)), creating an electron-rich state for ruthenium at the interface (Fig. 5(b)). This electron-rich interface proved beneficial for water molecule adsorption/dissociation and optimized the adsorption free energy of intermediate H species (Fig. 5(c)). As a result, Ru/P–MoB demonstrated not only efficient H2 production with a low overpotential in alkaline pure water, alkaline seawater, and acidic media, but also exhibited excellent activity and performance in anion exchange membrane electrolyzed water, showcasing remarkable stability. In addition, Cho et al.128 synthesized a catalyst, Ru/P–TiO2, in which Ru clusters were supported on a P-doped, defective TiO2 substrate. The P doping process partially replaces Ti4+ sites in TiO2, leading to the formation of Ti–O–P bonds and the creation of abundant oxygen vacancies on the surface. Notably, the mass activity of the Ru/P–TiO2 catalyst synthesized in this study was 34.3 times higher than that of Pt/C and 18.7 times higher than Ru/TiO2 (Fig. 5(d)). This enhancement is attributed to the Ru/P–(R)TiO2-VO surface, where the alkaline HER involves water dissociation, strong adsorption of H on Ru sites, hydrogen spillover from Ru sites to P sites, and efficient hydrogen desorption on P sites (Fig. 5(e)). The synergistic effect of P doping and oxygen vacancies enhances water adsorption and lowers the energy barrier for water decomposition (Fig. 5(f)), significantly enhancing hydrogen production as it transfers from Ru sites to surface P sites, thereby markedly improving HER activity. Similarly, Li's team129 developed a novel P-doped Pt3Co electrocatalyst. Upon P doping, negatively charged P sites are generated, enabling effective capture and storage of protons (H+) (Fig. 5(g)). These P sites function as “proton reservoirs” during catalysis, providing a steady supply of protons for the HER. Through a short-path hydrogen spillover effect, P sites facilitate rapid proton transfer to catalytic Pt sites, thereby reducing energy loss along the reaction pathway (Fig. 5(h)). Additionally, the incorporation of P lowers the electronic density of states of Pt near the Fermi level, which promotes the electrochemical desorption of hydrogen (Fig. 5(i)). Consequently, the efficiency of hydrogen generation is significantly enhanced, as demonstrated by the P–Pt3Co/NC catalyst, which delivers an overpotential of just 219 mV at 1.5 A cm−2 under acidic conditions, while exhibiting a HER activity that is 4.7 times greater than that of Pt3Co. In the study conducted by Luo et al.,130 the impact of controlling the doping amount of P on ruthenium-based catalysts was explored. It was found that the P doping content should be carefully controlled, as both too low and too high doping amounts had undesirable effects. However, an appropriate P doping content can effectively regulate the surface electronic structure of the catalyst, leading to a more significant enhancement in the catalytic activity of hydrogen oxidation and reactions in alkaline electrolytes (Fig. 5(j)).
 | ||
| Fig. 5 (a) Working function of P–MoB and comparison samples. (b) Plane-averaged and charge density difference (inset) of Ru/P–MoB. (c) Water dissociation kinetic barriers of Ru/P–MoB and a control group. Reprinted with permission from ref. 127. Copyright 2023, Wiley. (d) Mass activities of Ru/P–TiO2, etc. (e) Free energy diagrams for hydrogen spillover on Ru/P–(R)TiO2-VO (black for Ru, red for P atoms). (f) Kinetic barriers for water dissociation at different active sites. Reprinted with permission from ref. 128. Copyright 2022, Wiley. (g) Hydrogen adsorption energies on P–Pt3Co and Pt3Co. (h) Schematic of hydrogen spillover on P–Pt3Co. (i) PDOS plots of P–Pt3Co and Pt3Co. Reprinted with permission from ref. 129. Copyright 2023, American Chemical Society. (j) Performance comparison of samples with different P doping contents. Reprinted with permission from ref. 130. Copyright 2020, American Chemical Society. | ||
The development of non-noble metal catalysts as cost-effective alternatives has attracted substantial attention; however, matching the catalytic performance of noble metal systems continues to pose a major challenge. Among the various strategies employed, P doping has emerged as a popular and effective approach. Recent advancements in this field have been significant. For instance, Xu et al.131 conducted a study where they incorporated P into the cobalt molybdate lattice, resulting in the formation of a Co(OH)2–CoMoO4/P–CoMoO4 structure on the surface of cobalt molybdate (Fig. 6(a)). The introduction of P through doping was employed to modulate the electronic structure and surface active sites of the material, leading to enhanced catalyst activity and stability. The study found that as the phosphating temperature increased, the degree of amorphization increased. The optimized catalyst exhibited the lowest overpotential of 44 mV for the HER to achieve a current density of 10 mA cm−2 (Fig. 6(b)). Similarly, Waterhouse et al.132 synthesized a novel Fe–P-CMO catalyst with a sea urchin-like structure. P doping introduced numerous oxygen vacancies (OV) and formed a Fe2O3/P–CoMoO4 heterojunction on the material's surface, enhancing electronic conductivity and increasing the density of reactive active sites (Fig. 6(c)). During the HER process, the P-doped CoMoO4 in Fe–P-CMO undergoes surface reconstruction, resulting in the in situ generation of Co(OH)2 on its surface, forming a Co(OH)2/P–CoMoO4 heterostructure, as shown in Fig. 6(d) and (e). This reconstruction exposes a greater number of active sites. As a result, the activity of CoMoO4 was significantly improved, facilitating the efficient reduction of H+. Remarkably, Fe–P-CMO exhibited outstanding performance in 1.0 M KOH electrolyte, requiring low overpotentials of only 68 and 151 mV to achieve current densities of 100 and 300 mA cm−2, respectively, surpassing the performance of the benchmark Pt/C catalyst (Fig. 6(f)). Recently, Zhou's team133 successfully demonstrated the effectiveness of P-doped W2C nanoparticles anchored on graphene (P@W2C–C) as efficient electrocatalysts for the HER, utilizing a wind-driven triboelectric nanogenerator (TENG). The P@W2C–C catalyst exhibited an impressively low overpotential of only 179 mV and a Tafel slope of 87.8 mV dec−1 (Fig. 6(g)), which correlated well with the low power output of the TENG. In this study, P doping altered the electronic structure of W2C, shifting its d-band center closer to the ideal position (Fig. 6(h) and (i)) and optimizing the hydrogen adsorption free energy (ΔGH*). This optimization not only balances hydrogen adsorption and desorption but also lowers the reaction energy barrier (Fig. 6(j)), further accelerating the HER and significantly enhancing the catalytic activity.
 | ||
| Fig. 6 (a) Illustration of the synthesis process for P-CMO/NF. (b) HER polarization curves of P-CMO/NF-400 and comparison samples. Reprinted with permission from ref. 131. Copyright 2022, Elsevier. XRD patterns of (c) Fe–P-CMO and P-CMO; and (d) Fe–P-CMO after reaction. (e) Raman analysis of Fe–P-CMO prior to and following the reaction. (f) HER polarization curves of different catalysts in 1.0 M KOH. Reprinted with permission from ref. 132. Copyright 2024, Elsevier. (g) HER performance comparison of P@W2C–C and a control. (h and i) DOS for P@W2C–C and W2C–C. (j) ΔG diagrams for H2 generation from H2O on P@W2C–C, Si@W2C–C, and W2C–C (inset: preferred configurations of intermediates adsorbed on P@W2C–C). Reprinted with permission from ref. 133. Copyright 2024, Elsevier. | ||
As a representative 2D transition metal dichalcogenide (TMDs), the modification of MoS2 by heteroatom doping has been attracting much attention. Compared with pristine MoS2, the introduction of P atoms can enhance the electronic conductivity and HER catalytic activity of MoS2. Based on their findings, Ye and colleagues134 successfully synthesized P-doped MoS2 (P–MoS2) using a one-step air calcination method. They observed that P doping significantly improved the HER performance of MoS2 nanosheets, achieving a remarkably low onset potential of approximately 30 mV and a Tafel slope of 48 mV dec−1 (Fig. 7(a) and (b)). Interestingly, they discovered that the edge sites of P–MoS2 exhibited higher activity compared to the basal plane. Through in situ transport measurements, they determined that the enhanced HER performance was primarily attributed to an increase in intrinsic catalytic activity rather than a modulation of charge transport (Fig. 7(c) and (d)). Subsequent DFT calculations confirmed that the edge sites of P–MoS2 were energetically more favorable for the hydrogen reaction (Fig. 7(e) and (f)). Similarly, our team135 successfully synthesized MoS2 nanoflowers with P doping, S vacancies, and a crystalline–amorphous heterostructure using a P-assisted rapid calcination method. The synergistic effect of these multiple strategies resulted in MoS2–P2 with outstanding stability at both low and high current densities throughout the entire pH range (Fig. 7(g) and (h)). This significant finding provides valuable insights for the rational design of edge-dominant P–MoS2 catalysts. In addition, Kang et al.136 successfully synthesized P-doped biphasic MoS2 (P-BMS) nanosheets as electrocatalysts through a one-step hydrothermal method. The doping of P atoms can modify the electronic structure of MoS2 (Fig. 7(i) and (j)), optimize its electrocatalytic reaction kinetics (Fig. 7(k)), and enhance its conductivity and structural stability. As a result, the optimized P-BMS electrocatalyst exhibited low overpotentials of 60 and 72 mV, respectively, at 10 mA cm−2 in 0.5 M H2SO4 and 1.0 M KOH electrolytes, and smaller Tafel slopes of 76 and 80 mV dec−1, respectively.
 | ||
| Fig. 7 (a) LSV and (b) Tafel plots for plane and edge sites of MoS2 and P–MoS2 in 0.5 M H2SO4. Electrochemical (orange) and electronic (violet) signals of a MoS2 nanosheet before (c) and after (d) P doping for the HER. Minimum energy paths and critical HER structures on (e) basal plane and (f) armchair edge of P–MoS2. Reprinted with permission from ref. 134. Copyright 2023, American Chemical Society. Durability of MoS2–P2 in different electrolytes, with HER polarization curves pre- and post-cycling in (g) 1.0 M KOH and (h) 0.5 M H2SO4. Reprinted with permission from ref. 135. Copyright 2022, Springer. XPS spectra of (i) Mo 3d and (j) S 2p. (k) Adsorption energy diagram for MoS2 and P-BMS at different sites. Reprinted with permission from ref. 136. Copyright 2023, Wiley. | ||
At present, while scientific researchers have successfully synthesized some high-performance catalysts through various approaches (Table 3), the understanding of P-doped catalysts still requires further improvement. The P-doped surface is susceptible to oxidation, which can potentially affect the nature of the active sites, reduce electrical conductivity, diminish catalytic activity, and lead to poor air stability.
| Electrocatalyst | Electrolyte | η 10 (mV) | TOF (s−1) | η (mV) | Stability | Ref. |
|---|---|---|---|---|---|---|
| P–Pt1Ni2NH/NGA | 1.0 M KOH | 15 | 13.5 | 100 | 20 h | 137 |
| P0.3–CuMn2O4 | 1.0 M KOH | 103 | 1.3 | 260 | 3000 cycles | 138 |
| P–Os | 1.0 M KOH | 32 | 0.28 | 100 | 100 h | 139 |
| PtP NDs | 0.5 M H2SO4 | 13.3 | 0.3 | 50 | 5000 cycles | 110 |
| P,Mo–Ru@PC | 1.0 M KOH | 21 | 10 h | 140 | ||
| P–CoNS@CuPD | 1.0 M KOH | 166 | 10 h | 141 | ||
| P–PtNi networks | 1.0 M KOH + 0.5 M NaCl | 12 | 24.5 | 200 | 120 h | 142 |
| 1.0 M KOH | 23 | 16.4 | 200 | |||
| P/Cr60–NiMoO4 | 1.0 M KOH | 35.7 | 120 h | 143 | ||
| P–Co3O4 | 1.0 M KOH | 80 | 60 h | 144 | ||
| P/FeCo-NC | 0.5 M H2SO4 | 38 | 0.6 | 50 | 10 h | 112 |
| P@MNTC | 1.0 M KOH | 120 | 25 h | 145 | ||
| P–CoS2/MoS2/MoO2 | 1.0 M KOH | 85 | 0.87 | 200 | 146 | |
| P-doped MoS2/CFC | 0.5 M H2SO4 | 162 | 24 h | 147 |
6.2. S-doped electrocatalysts for the HER
S is a representative chalcogen element, which can typically be introduced as a dopant into the crystal lattice of the primary catalyst through a sulfurization reaction. Studies have demonstrated that S doping can tune the electronic structure, engineer lattice defects, and increase the active surface area of the catalytic materials.148 These adjustments enable the catalysts to achieve superior electrical conductivity, outstanding electrochemical activity, enhanced durability, and greater chemical stability.MoXn exhibits several advantages such as low cost, high stability, and abundant edge positions, which serve as active sites for the HER. S doping effectively adjusts the electronic structure of MoXn and optimizes ΔGH*. Building upon this, Zhang and his colleagues149 achieved ultrathin S-doped molybdenum compound nanosheets by employing an in situ topological conversion method on MoS2 ultrathin nanosheets grown on carbon cloth. Examples include S–Mo2C, S–MoP, S–MoN, and S–MoO3 (Fig. 8(a)). Notably, during the high-temperature phase transformation, MoS2 nanosheets served as both the Mo source and S supplier, with the released S species being in situ doped into the synthesized Mo-based composites. The precisely engineered porous nanosheet structure offered abundant exposed active sites, improved electronic conductivity, and facilitated efficient mass and charge transfer, thus boosting interfacial electrocatalytic activity. Crucially, experimental and theoretical calculations demonstrated the synergistic effect between the S dopant and MoXn, inducing an optimized electronic state in S–MoXn (Fig. 8(b)). This resulted in favorable energy for proton adsorption and hydrogen desorption on the S–MoXn surface. Consequently, 2D S–MoXn/CC exhibited enhanced HER activity compared to MoXn/CC. Notably, the optimized S–MoP/CC electrode displayed low overpotentials of 75, 75, and 127 mV at 10 mA cm−2 in acidic, alkaline, and neutral media, respectively, while maintaining stable performance for 30 h during continuous operation. Furthermore, in the study conducted by An et al.,114 a chemical conversion method was employed to transform 1T phase MoS2 into S-doped 2H-MoTe2 (Fig. 8(c)). The remarkable research findings revealed that S-doped 2H-MoTe2, with tellurium vacancies, exhibited exceptional electrocatalytic performance for H2 production. Specifically, it reached an impressive current density of 100 mA cm−2 at an overpotential of 217 mV, with negligible decay after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 8(d)). This improvement is attributed to the combined effects of S doping and Te vacancies, which increase the negative charge at active sites, making them more favorable for H* adsorption and facilitating the HER process, thereby boosting catalytic efficiency (Fig. 8(e)). Similarly, in their study, Zhao et al.150 achieved the successful synthesis of the S–Mo5N6 catalyst by carefully controlling the ammoniation process of MoS2. Through a combination of experiments and DFT simulations, it was found that during hydrogen adsorption, electrons transfer from N atoms to S atoms (Fig. 8(f)). This electron transfer reduces the adsorption strength of S atoms on protons, thereby optimizing the ΔGH* value (Fig. 8(g)). As a result, when tested in 0.5 M H2SO4, this S-doped Mo5N6 catalyst exhibited exceptional performance in the HER. It achieved a current density of 10 mA cm−2 with a low overpotential of 56 mV and a Tafel slope of 37.9 mV dec−1 (Fig. 8(h) and (i)). These findings highlight the significant contribution of S doping in enhancing the electrocatalytic activity of Mo5N6 for an efficient HER.
000 cycles (Fig. 8(d)). This improvement is attributed to the combined effects of S doping and Te vacancies, which increase the negative charge at active sites, making them more favorable for H* adsorption and facilitating the HER process, thereby boosting catalytic efficiency (Fig. 8(e)). Similarly, in their study, Zhao et al.150 achieved the successful synthesis of the S–Mo5N6 catalyst by carefully controlling the ammoniation process of MoS2. Through a combination of experiments and DFT simulations, it was found that during hydrogen adsorption, electrons transfer from N atoms to S atoms (Fig. 8(f)). This electron transfer reduces the adsorption strength of S atoms on protons, thereby optimizing the ΔGH* value (Fig. 8(g)). As a result, when tested in 0.5 M H2SO4, this S-doped Mo5N6 catalyst exhibited exceptional performance in the HER. It achieved a current density of 10 mA cm−2 with a low overpotential of 56 mV and a Tafel slope of 37.9 mV dec−1 (Fig. 8(h) and (i)). These findings highlight the significant contribution of S doping in enhancing the electrocatalytic activity of Mo5N6 for an efficient HER.
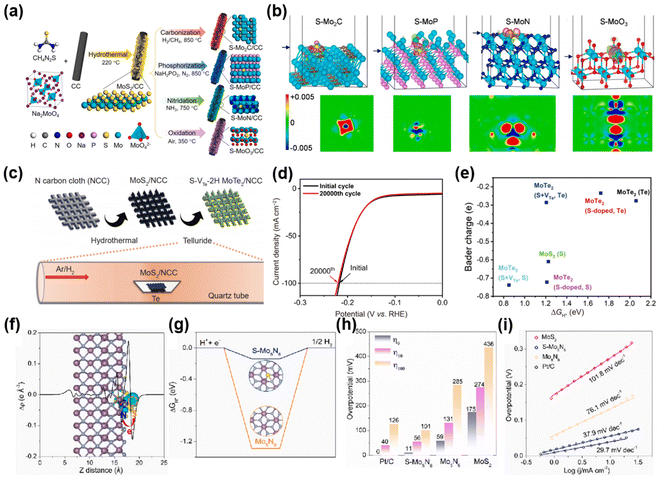 | ||
Fig. 8 (a) Preparation schematic and (b) differential charge density of S–MoXn/CC and its comparison samples. Reprinted with permission from ref. 149. Copyright 2023, Elsevier. (c) Synthesis path diagram of S–VTe-2H MoTe2/NCC. (d) Polarization curves of S–VTe-2H MoTe2/NCC before and after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. (e) Relationship between HER free energy change and Bader charge. Reprinted with permission from ref. 114. Copyright 2021, Springer. S–Mo5N6 and comparison samples: (f) differential charge density, (g) free energy diagram for H adsorption. (h) Overpotentials corresponding to different current densities. (i) Tafel plots. Reprinted with permission from ref. 150. Copyright 2022, Elsevier. 000 cycles. (e) Relationship between HER free energy change and Bader charge. Reprinted with permission from ref. 114. Copyright 2021, Springer. S–Mo5N6 and comparison samples: (f) differential charge density, (g) free energy diagram for H adsorption. (h) Overpotentials corresponding to different current densities. (i) Tafel plots. Reprinted with permission from ref. 150. Copyright 2022, Elsevier. | ||
Iron-based oxide electrocatalysts have garnered significant attention in recent research due to their affordability, non-polluting nature, and excellent durability. However, enhancing their catalytic activity has proved to be a challenge due to their poor conductivity. In a recent study, Liu et al.151 successfully developed a novel catalyst, S–Fe2O3 nanosheet array supported on iron foam (S–Fe2O3/IF), through a simple and convenient two-step synthesis process. The catalyst exhibited improved performance in alkaline media for the HER. It demonstrated a low overpotential of 134 mV, enabling a current density of 10 mA cm−2, and displayed a small Tafel slope of 76 mV dec−1 (Fig. 9(a) and (b)). Furthermore, DFT calculations reveal that S doping significantly reduces the adsorption free energy of water molecules (ΔGH2O*), indicating that S–Fe2O3/IF more effectively promotes water adsorption and the Volmer reaction (Fig. 9(c)). Additionally, S doping optimizes ΔGH*, with a near-zero ΔGH* value suggesting that S–Fe2O3 is particularly well-suited for catalyzing the HER (Fig. 9(d)). These results offer novel insights for the development of iron oxide electrocatalysts with enhanced performance. Additionally, S doping has been utilized to enhance the efficiency of precious metal catalysts. A notable example is the work by Xia and his team,152 who developed a novel method for synthesizing network-like aerogels composed of nanowires in an aqueous solution, leading to the successful production of S-doped gold–lead–cobalt (S–AuPbPt) alloy aerogels. Within this alloy aerogel, S doping modifies the electronic structure of Pt, specifically lowering the d-band center (Fig. 9(e)). This shift reduces the adsorption strength of hydrogen intermediates (H*) on the Pt surface, allowing adsorbed H* to more readily combine to form H2 and rapidly release it into the reaction system. As a result, the S–AuPbPt alloy nanowire network catalyst exhibited an intrinsic HER activity approximately 15.4 times higher than that of Pt/C, demonstrating its outstanding performance (Fig. 9(f)).
 | ||
| Fig. 9 Electrochemical properties of S–Fe2O3 and its comparative sample: (a) LSV and (b) Tafel plot of HER. (c) ΔGH2O* and (d) ΔGH* plots of Fe2O3 and S–Fe2O3. Reprinted with permission from ref. 151. Copyright 2022, American Chemical Society. (e) TDOS of Pt4 on S–AuPbPt and AuPbPt surfaces. (f) TOF value at an overpotential of 0.05 V (vs. RHE) of S–AuPbPt (red) and Pt/C (black). Reprinted with permission from ref. 152. Copyright 2020, Royal Society of Chemistry. | ||
Although researchers from various countries have achieved significant progress in the research and development of S-doped catalytic materials (Table 4), the underlying mechanisms of these S-doped catalysts still require further exploration. At present, most transition metal sulfides can exhibit excellent HER activity under acidic or alkaline conditions. However, there are relatively few studies on their HER performance under neutral pH conditions. A possible reason for this is the difficulty in adsorbing water molecules to produce adsorbed hydrogen intermediates under neutral conditions. Nonetheless, hydrogen production under neutral conditions is particularly important for industrial applications.
| Electrocatalyst | Electrolyte | J (mA cm−2) | η (mV) | Stability | Ref. |
|---|---|---|---|---|---|
| Pt/S–TiN NTs | 0.5 M H2SO4 | 10 | 12 | 25 h | 153 |
| 1.0 M KOH | 10 | 25 | 25 h | ||
| 1.0 M PBS | 10 | 39 | 25 h | ||
| S–PtCo@MC | 0.5 M H2SO4 | 10 | 23 | 30 h | 154 |
| Pt@S–NiFe LDH | 1.0 M KOH | 300 | 132 | 200h | 155 |
| S–Co2P@Ni2P | 1.0 M KOH | 100 | 103 | 20 h | 45 |
| S–WP2-5 | 0.5 M H2SO4 | 10 | 115 | 24 h | 156 |
| M–CoSe1.28S0.72 | 0.5 M H2SO4 | 10 | 67 | 1000 h | 157 |
| NFS-6 | 1.0 M KOH | 10 | 171 | 20 h | 158 |
| S–CoMoO-12.4 | 1.0 M KOH | 10 | 105 | 40 h | 159 |
| S–MoO2-2 | 0.5 M H2SO4 | 10 | 176 | 10 h | 160 |
| MoSe1.44S0.56 | 0.5 M H2SO4 | 10 | 167 | 24 h | 55 |
6.3. N-doped electrocatalysts for the HER
There are 3 single electrons and 1 lone pair of electrons in the valence electron shell of the N atom, which means the N atom has multiple bonding types. N atoms have a small atomic radius, and they can be embedded into the interstitial positions of the catalyst easily without affecting the overall conductivity. In addition, the high electronegativity of the N atom causes the surrounding atoms to be polarized easily. The vacant orbitals of the N atom facilitate electron transfer during the Tafel and Volmer steps, thereby enhancing the catalytic activity for hydrogen production.148N doping can adjust the electron density and d-band center of the active site, thereby enhancing the HER activity.161 Generally, we prepare electrocatalysts by doping N into the host lattice. Two common methods are as follows: (a) plasma treatment, also known as ammonification directly—it is a method of high-temperature treatment of precursors under NH3 conditions; (b) the ion exchange method involves reacting the precursor with N-containing reagents (such as urea) in the solid phase. N atom doping can not only play a part in improving the HER performance of metal compounds, but also improve the HER performance of nonmetals such as carbon.
For metal compounds, N doping not only maintains the metallic character of the host catalyst, but it also improves the electrical conductivity and enhances charge transfer in some cases. Additionally, N doping can alter the surface crystal structure to create more reactive sites and lower the hydrogen adsorption energy. Inspired by this concept, researchers have directed their attention towards the development of electrocatalysts with superior electrocatalytic properties by incorporating N dopants. For instance, Huang et al.162 synthesized a N-doped Ni5P4@CoP/CFP nanocatalyst, which exhibited a remarkable HER performance. In acidic, alkaline, and neutral media, the N–Ni5P4@CoP/CFP catalyst achieved HER overpotentials as low as 55, 56, and 59 mV, respectively, at a current density of 10 mA cm−2 (Fig. 10(a)–(c)). The DFT calculation results indicated that the integration of N atoms into Ni5P4 modified the coordination environment of Ni, thereby adjusting the distribution of the interface electronic field (Fig. 10(d)). This adjustment enhances charge transfer from CoP to N–Ni5P4, boosts the catalyst's electron transfer efficiency, and creates nucleophilic P sites in N–Ni5P4 with balanced hydrogen adsorption and desorption, thereby accelerating the HER (Fig. 10(e)). Furthermore, Sun et al.116 conducted a study on the rational design and preparation of a self-supporting N-doped Ni2P (N–Ni2P/NF) catalyst on NF using a simple NH4H2PO2-assisted gas–solid reaction process. The catalyst exhibited remarkable catalytic activity and stability for the HER, demonstrating a low overpotential of 50 mV at 10 mA cm−2, a small Tafel slope of 45 mV dec−1, and long-term stability. DFT calculations were employed to investigate the catalyst. The incorporation of N causes electrons to transfer from nickel and P to N, forming new positive and negative charge centers, while significantly reducing the energy barriers for water adsorption and decomposition, accelerating the first step of the Volmer process of the HER (Fig. 10(f)). An optimized ΔGH* achieves a favorable balance between hydrogen adsorption and desorption, thereby facilitating the HER process (Fig. 10(g)). In addition, N doping significantly increases the specific surface area of the Ni2P/NF catalyst, facilitating the full exposure of active sites (Fig. 10(h) and (i)). Similarly, Zhang and his colleagues163 successfully synthesized N–Co9S8/Ni3S2/NF using an electrodeposition method, showcasing remarkable activity for the HER (Fig. 10(j)). After N doping, the d-band center of the Co9S8/Ni3S2 catalyst shifts from −1.547 eV to −0.811 eV, indicating enhanced electron interactions with adsorbates and improved HER performance (Fig. 10(k)). Specifically, the water adsorption free energy (ΔGH2O) at both cobalt and nickel sites is significantly lowered, facilitating water adsorption and dissociation processes (Fig. 10(l)). Additionally, the ΔGH* value at the cobalt site near N approaches zero after doping, demonstrating that N doping markedly enhances the catalyst's intrinsic HER activity.
 | ||
| Fig. 10 LSV curves of N–Ni5P4@CoP/CFP and reference catalysts measured in (a) 0.5 M H2SO4, (b) 1.0 M KOH, and (c) 1.0 M PBS. (d) Charge density difference and (e) Gibbs free energy diagram of ΔGH* of N–Ni5P4@CoP. Reprinted with permission from ref. 162. Copyright 2022, Elsevier. (f) The energy barriers associated with the kinetic process of water dissociation on N–Ni2P/NF and Ni2P/NF. (g) ΔGH* on various samples at equilibrium potential. N2 adsorption/desorption isotherms of (h) Ni2P/NF and (i) N–Ni2P/NF. Reprinted with permission from ref. 116. Copyright 2022, Wiley. (j) LSV curves of N–Co9S8–Ni3S2 and contrasting catalysts. (k) The d-band of the DOS for Co9S8/Ni3S2 and N–Co9S8–Ni3S2. (l) Water adsorption energy diagram for Co9S8/Ni3S2 and N–Co9S8/Ni3S2. Reprinted with permission from ref. 163. Copyright 2023, Wiley. | ||
The widespread use of carbon-based materials in HER research can be attributed to their low cost, tunable structures, and robust stability over a wide pH range. Incorporating heteroatoms into carbon materials has proved effective at activating their intrinsically inert surfaces and enhancing catalytic performance.164 Building on this strategy, Xi et al.165 synthesized PtMo-NC, where the N-doped carbon with a high surface area and abundant nitrogen sites effectively immobilized metal atoms and prevented aggregation during high-temperature calcination, resulting in uniformly dispersed Pt and enhanced atomic utilization (Fig. 11(a) and (b)). Similarly, Sun et al.166 synthesized Ir@NC using a simple impregnation followed by a calcination–reduction method. This process formed strong covalent Ir–N bonds, which effectively inhibited Ir corrosion and aggregation in acidic environments. In Ir@NC, the N atoms doped in the carbon support regulated the electronic structure of iridium, resulting in a lower valence state for Ir compared to that of Ir@C (Fig. 11(c)), which contributed to the superior HER performance of Ir@NC over Ir@C. High entropy alloys (HEAs) possess unique electronic structures and exceptional stability, making them promising candidates for HER catalysis. Using a low-temperature thermal reduction method, Hu's team167 achieved the uniform dispersion of ultrasmall (sub-2 nm) PtRuCoNiCu HEA nanoparticles on hierarchical N-doped carbon nanocages (hNCNCs). This configuration benefits from electron transfer from pyridinic N in the hNCNC support to the metals (Fig. 11(d)), which strengthens the metal–support interactions and significantly enhances the catalyst's stability during prolonged HER operation. Recently, significant progress has been made in the research of non-precious metal HEAs. Yin et al.168 successfully synthesized HEA catalysts coated with a N-doped graphene layer of controllable thickness (CuNiFeCoCrTi@NC NPs). The alloy core exhibits strong hydrogen adsorption, which impacts the H* desorption step, while the N-doped graphene layer has weaker hydrogen adsorption, affecting the adsorption step. Consequently, although the alloy and graphene layer individually show suboptimal catalytic performance, the ΔGH* value is effectively modulated when the alloy is coated with the N-doped graphene layer, enhancing the overall catalytic efficiency (Fig. 11(e)). Furthermore, Xiao et al.169 synthesized a highly efficient HER catalyst featuring an urchin-like N-doped carbon structure encapsulating a MoO2/Mo3P/Mo2C triple-interface heterojunction. The catalyst exhibited exceptional performance, with an overpotential of merely 69 mV at a current density of 10 mA cm−2 and demonstrated remarkable long-term stability (Fig. 11(f) and (g)). This outstanding performance can be attributed to the synergistic effect between the unique heterostructure and the encapsulated N-doped carbon, highlighting the importance of their combined influence for enhancing the catalytic activity.
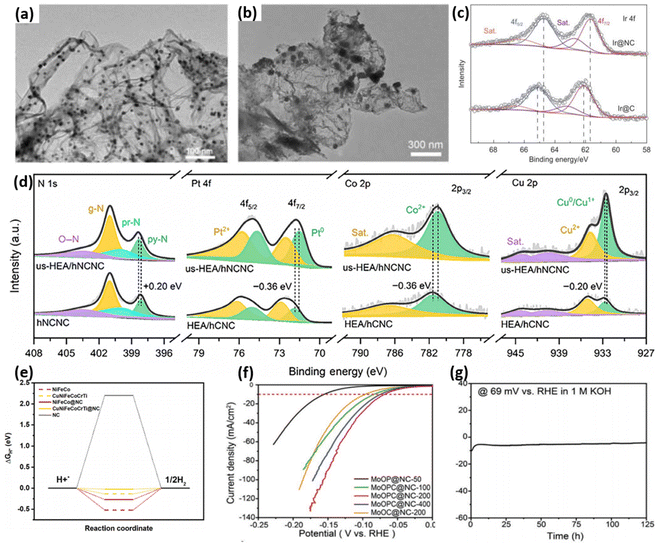 | ||
| Fig. 11 TEM images of (a) Pt-NC and (b) Pt–C after Ar/H2 pyrolysis. Reprinted with permission from ref. 165. Copyright 2023, Wiley. (c) Ir 4f spectra of Ir@NC and comparison samples. Reprinted with permission from ref. 166. Copyright 2024, Elsevier. (d) N 1s, Pt 4f, Co 2p, and Cu 2p spectra of hNCNCs and control samples. Reprinted with permission from ref. 167. Copyright 2024, Springer. (e) ΔGH* diagram of CuNiFeCoCrTi@NC and comparison samples. Reprinted with permission from ref. 168. Copyright 2024, Elsevier. HER performance evaluated in 1.0 M KOH. (f) Polarization curves; (g) the stability evaluation of MoOPC@NC-200 over an extended period. Reprinted with permission from ref. 169. Copyright 2023, Wiley. | ||
In addition, several researchers have achieved significant results through N doping, see Table 5 for details.
| Electrocatalyst | Electrolyte | J (mA cm−2) | η (mV) | Stability | Ref. |
|---|---|---|---|---|---|
| LaCeOx@NGr/Ru1 | 1.0 M KOH | 10 | 22 | 30 h | 170 |
| N-fcc-Ir-NSs | 0.5 M H2SO4 | 10 | 19 | 72 h | 44 |
| N–MoO2/Cu | 1.0 M KOH | 1000 | 363 | 50 h | 171 |
| N–FeS2 | 1.0 M KOH | 10 | 126 | 20 h | 172 |
| MoCoSex@NC | 0.5 M H2SO4 | 10 | 60 | 12 h | 173 |
| 4%Mo-Co3O4/NC | 1.0 M KOH | 10 | 91 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
174 |
| MoP@NC-250 | 1.0 M KOH | 10 | 96 | 35 h | 161 |
| MoSe2@NC | 1.0 M KOH | 10 | 175 | 12 h | 175 |
| 0.5 M H2SO4 | 10 | 183 | 12 h | ||
| Mo2C@NC@Pt | 0.5 M H2SO4 | 10 | 27 | 10 h | 176 |
| 1.0 M KOH | 10 | 47 | 10 h | ||
| 1.0 M PBS | 10 | 25 | 10 h | ||
| Mo2C@NC-160 | 1.0 M KOH | 10 | 90 | 12 h | 177 |
| MoC@NC | 0.5 M H2SO4 | 10 | 171 | 70 h | 178 |
6.4. B-doped electrocatalysts for the HER
Boron (B), with its electron-deficient nature and three valence electrons, exhibits distinct advantages as a doping element. Compared to P, B has a lower electronegativity of 2.04, which allows it to interact more readily with a single electron from oxygen in water. This interaction facilitates the rapid release of a proton during the HER process. By effectively participating in the HER, B enhances the catalytic activity and efficiency of the system, contributing to improved performance in various electrochemical applications. Recently, our team52 developed an effective B-doped strategy, leading to the successful synthesis of a novel B-doped Ni4Mo nanoarray (Bx–Ni4Mo/NF, X = B doping level). By introducing electron-deficient B atoms, they effectively tuned the electronic structure of Ni4Mo, optimized the H* adsorption affinity, and enhanced H2O dissociation (Fig. 12(a)–(d)). The presence of B atoms also facilitated electronic interactions with molybdenum, resulting in improved stabilization of Mo atoms. Furthermore, the formation of B–metal bonds reduced the oxygen affinity of Mo, weakening OH− adsorption and preventing Mo dissolution during the alkaline HER (Fig. 12(e) and (f)). The B4.7–Ni4Mo/NF catalyst exhibited exceptional catalytic performance in the alkaline HER, displaying a low overpotential of only 135.2 mV at 1.0 A cm−2 and a low Tafel slope of 37.2 mV dec−1 (Fig. 12(g) and (h)). Additionally, B4.7–Ni4Mo/NF achieved a current density of 1.0 A cm−2 at 1.73 V in a 60 °C electrolyser, demonstrating its high efficiency. Notably, the catalyst maintained stable operation at this current density for an impressive duration of 800 h (Fig. 12(i)). Similarly, B doping can be employed to enhance the electrocatalytic performance of noble metal-based catalysts. A notable example is our group's study,179 which applied the B doping method to strengthen the SMSI effect in Rh@NC nanoparticles. Through a combination of experimental and theoretical investigations, it has been demonstrated that B doping optimizes the electronic structure and water adsorption energy, effectively activating the SMSI between Rh and the NC support (Fig. 12(j)–(m)). Consequently, the B–Rh@NC catalyst synthesized exhibited remarkable electrocatalytic activity for the HER over a broad pH range, while maintaining impressive durability (Fig. 12(n)–(p)). In addition to the aforementioned studies, several other research works involving B doping are summarized in Table 6. | ||
| Fig. 12 XPS spectra of (a) Mo 3d and (b) Ni 2p, (c) free energy diagram of H2O activation, (d) ΔGH*, (e) Mo dissolution energy, (f) Mo dissolved in the electrolyte after the electrode worked for 48 h for B4.7–Ni4Mo/NF and comparison samples. HER performance of B4.7–Ni4Mo/NF and control samples in 1.0 M KOH: (g) HER polarization curve; (h) Tafel slope. (i) Stability test results for B4.7–Ni4Mo/NF//IrO2/NF in an AEM electrolyser. Reprinted with permission from ref. 52. Copyright 2023, Elsevier. (j) Bader charge and (k) charge density difference analysis of B–Rh@CN. (l) ELF of B–Rh@CN. (m) Water dissociation free energy of B–Rh@CN and comparison samples. The HER performance test of B–Rh@CN and its comparison sample in (n) 0.5 M H2SO4, (o) 1.0 M PBS and (p) 1.0 M KOH. Reprinted with permission from ref. 179. Copyright 2023, Elsevier. | ||
| Electrocatalyst | Electrolyte | J (mA cm−2) | η (mV) | Stability | Ref. |
|---|---|---|---|---|---|
| B–Ru@CNT | 1.0 M KOH | 10 | 17 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
180 |
| 0.5 M H2SO4 | 10 | 62 | 5000 cycles | ||
| B–Ir NSs/NF | 1.0 M KOH | 10 | 18 | 24 h | 181 |
| M–B–Ni2P | 1.0 M KOH | 100 | 217 | 100 h | 182 |
| B-ZIS@TiO2 | 1.0 M KOH | 10 | 237 | 18 h | 122 |
| 6B-Fe7S8/FeS2 | 1.0 M KOH | 10 | 113 | 30 h | 121 |
| Ni3Fe@BC-500 °C | 1.0 M KOH | 10 | 330 | 20 h | 183 |
| B–CoP | 1.0 M KOH | 100 | 112 | 20 h | 184 |
| NiCo@BC (500 °C) | 1.0 M KOH | 10 | 209 | 200 h | 185 |
6.5. Halogen element-doped electrocatalysts for the HER
Doping elements used to improve HER catalysts should possess certain advantages, such as the ability to adjust the electronic structure, increase active sites, inhibit surface oxidation, adjust acid–base properties, and improve catalyst stability. In addition to the commonly used elements mentioned earlier, such as P, N, S, and B, researchers have also been exploring other types of elements.Halogen ions have been extensively studied for their ability to improve the selectivity and activity of the carbon dioxide reduction reaction (CO2RR) by forming unique nanostructures and promoting adsorption/desorption of intermediates.186 Recently, researchers have started applying halogen doping in the development of HER catalysts. For instance, our team187 synthesized a halogen (X = Cl, Br, I) modified Ru/RuP2 heterojunction structure using the microwave phosphide plasma method in just 1 min. The introduction of halogen elements increased the electrochemically active surface area. Experimental and DFT calculations demonstrated that the presence of halogen elements facilitated the dissociation of water molecules and optimized the hydrogen binding energy, thereby improving the electrocatalytic kinetics. Compared to I and Cl doping, the Br-doped catalyst demonstrates optimal electronegativity (Fig. 13(a)). Consequently, the Br–Ru/RuP2 catalyst significantly reduced the energy barrier for breaking H–OH bonds, leading to accelerated water dissociation (Fig. 13(b)). Moreover, when H is adsorbed in the Br–Ru/RuP2 system, the center of Ru's d band is appropriately located, resulting in a moderate strength of the Ru–H bond (Fig. 13(c)). According to Sabatier's principle, achieving the “just right” adsorption energy is crucial for optimizing all chemical reactions. Therefore, Br–Ru/RuP2 exhibited optimal electrocatalytic performance, demonstrating a low overpotential of 34 mV to achieve a current density of 10 mA cm−2 and a small Tafel slope of 27 mV dec−1 in alkaline electrolytes (Fig. 13(d) and (e)). These indicated the high efficiency and effectiveness of Br doping at enhancing the HER catalytic activity. In the study conducted by our team,188 we employed F doping to effectively narrow the work function difference between the Ru metal and the F–FeCoOOH support (Fig. 13(f)). This strategic adjustment facilitated the creation of a favorable interface between the metal and the support, enabling the efficient spillover of H species from Ru to F–FeCoOOH (Fig. 13(g)). By coordinating the hydrogen-rich and hydrogen-depleted components within the catalyst, the HER performance of Ru-based catalysts in alkaline seawater was significantly enhanced (Fig. 13(h)). Recent studies on halogen doping have proliferated, and a summary of these works is provided in Table 7.
 | ||
| Fig. 13 (a) Bader charge of Ru; (b) Gibbs free energy diagrams for HER via hydrogen spillover; (c) PDOS of Ru for Ru/Br–RuP2 and comparison sample; (d) LSV and (e) Tafel curves of X (Cl, Br, I)–Ru/RuP2 and Pt/C in 1.0 M KOH at 5 mV s−1. Reprinted with permission from ref. 187. Copyright 2023, Wiley. (f) Work functions; (g) Cφvs. η; (h) HER polarization curves for Ru/F–FeCoOOH and comparison sample. Reprinted with permission from ref. 188. Copyright 2023, Elsevier. | ||
| Electrocatalyst | Electrolyte | J (mA cm−2) | η (mV) | Stability | Ref. |
|---|---|---|---|---|---|
| 3F-FeP | 1.0 M KOH | 1000 | 369 | 100 h | 189 |
| F–Pt NCs | 1.0 M KOH | 500 | 274 | 24 h | 190 |
| Ru@F–Ni3N | 1.0 M KOH | 100 | 115 | 30 h | 118 |
| F–CoP–Vp-2 | 1.0 M PBS | 10 | 108 | 20 h | 191 |
| Co–I–N/G | 0.5 M H2SO4 | 10 | 52 | 5000 cycles | 57 |
| I-G-20 | 0.5 M H2SO4 | 10 | 245 | 30 h | 192 |
| ISA-CoPn/CoP-2 | 0.5 M H2SO4 | 10 | 44 | 50 h | 193 |
| Ni12P5−xBrx | 1.0 M KOH | 10 | 18 | 125 h | 194 |
7. Discussion
Although substantial progress has been achieved in this field, further improvements are still required to meet the standards for large-scale industrial applications (Fig. 14). | ||
| Fig. 14 Schematic illustration of ideal nonmetal-doped HER electrocatalysts and future perspectives. | ||
The authors believe that for the future development of hydrogen production by electrolysis of water, we should focus on the following aspects:
1. In-depth understanding of the mechanism: nonmetal atom doping can enhance catalyst activity and stability by modifying the electronic structure, adjusting active sites, and inducing synergistic effects within the catalytic material. However, the precise mechanisms underlying these enhancements require further investigation and systematic study. In situ characterization techniques and first-principles calculations provide critical insights into how doping enhances catalyst performance and the key elements of the reaction mechanism, offering essential support for a deeper understanding and optimization of the reaction process.
Specifically, regulation of the electronic structure can be visualized using in situ XPS and synchrotron radiation, allowing direct comparison of surface electronic structure changes before and after doping. In situ infrared and Raman spectroscopy enable real-time detection of chemical bond changes, monitoring of active site surface states, and tracking of reaction intermediates. By observing reaction rates at varying temperatures, these methods also allow for the derivation of activation energies, providing a comprehensive view of the catalytic process. DFT calculations can simulate and model the catalyst's microstructure, while experimental characterization results provide a means to validate and explore the catalyst's reaction mechanism further. By combining theoretical calculations with experimental data, an effective structure–activity relationship (SAR) framework can be established to predict the catalytic activity of various materials and structural configurations in water electrolysis for hydrogen production. This approach holds significant potential in the rapidly advancing field of AI. If machine learning (ML) can process large-scale datasets derived from both DFT calculations and experimental data, while integrating and learning from past experiments, it could predict the catalytic activity and reaction mechanisms of new catalysts, thus greatly accelerating the catalyst design process.195,196 The integration of machine learning with established computational methods and experiments can streamline the screening and development of new catalysts, improving overall R&D efficiency and aiding the discovery of cost-effective alternative materials to reduce or replace precious metal catalysts, thereby substantially lowering costs. This is crucial for advancing the practical implementation and commercialization of water electrolysis technology for hydrogen production. Moreover, detailed mechanistic studies have clarified the impact of various operational conditions on catalyst performance, enabling the design of more adaptable catalysts tailored to the specific demands of different applications.
2. Exploring prospects for industrial application: to promote the industrial application of water electrolysis for hydrogen production, the development of catalyst synthesis strategies that are both highly efficient and scalable remains a critical challenge. This endeavor requires the optimization of synthesis parameters to meet the demands of large-scale production while maintaining high catalytic activity. Furthermore, achieving a comprehensive balance between catalytic performance, long-term stability, economic feasibility, and environmental sustainability—particularly in terms of reducing pollutant emissions and greenhouse gas generation—is essential. Among various approaches, nonmetallic doping has emerged as a promising strategy due to its compatibility with existing industrial processes. Nevertheless, several nonmetal doping synthesis routes still encounter significant limitations when applied to large-scale manufacturing, and further research is needed to establish robust and scalable methods suitable for practical deployment.
To accelerate the technological transformation of water electrolysis, it is essential to establish an evaluation framework that closely aligns with industrial scenarios. Currently, electrolysers are widely employed in laboratory settings to simulate industrial production environments for assessing the potential application of electrocatalysts. However, most existing studies remain focused on activity and stability tests, with limited exploration of underlying reaction mechanisms, thereby constraining their ability to inform large-scale process design. With the rapid advancement of computational technologies, digital twin (DT) frameworks offer a promising approach to bridge this gap.197 By integrating AI with multi-physics simulations, such as current distribution, heat transfer, and gas bubble dynamics within electrolysers, DT enables precise optimization of both electrolyser design (e.g., plate and flow channel architecture) and operational parameters (e.g., temperature gradient control). This approach not only reduces energy loss but also allows for predictive modeling of performance changes under scale-up conditions. Looking forward, the industrialization of water electrolysis-based hydrogen production will require the dismantling of disciplinary boundaries and the advancement of cross-disciplinary collaboration. In particular, the integration of materials science, fluid mechanics, and data science will be critical for addressing technical bottlenecks across the entire value chain, from catalyst synthesis to reactor design and system-level optimization.
3. Shift the focus towards seawater electrolysis: we are aware that the majority of the Earth's water resources are in the form of seawater, and many regions face scarcity of freshwater supplies. Developing direct seawater electrolysis for hydrogen production can effectively circumvent these limitations. However, it should be noted that seawater has a much more complex composition than fresh water, making it challenging to develop electrocatalysts that can operate stably in marine environments.198,199 In cathodic reactions, the high concentration of calcium and magnesium ions in seawater tends to form insoluble precipitates on the cathode surface, leading to blockage and deactivation of active sites. Currently, research on the preparation of catalysts for direct seawater electrolysis using nonmetal atom doping is still limited. Future studies should focus more on whether nonmetal atom doping can improve the catalyst's surface microenvironment to prevent the deposition of calcium and magnesium ions from seawater on the cathode surface, thereby developing catalysts that can stably operate in natural seawater.
8. Summary
Designing efficient, low-cost, stable, and environmentally friendly electrocatalysts for hydrogen production through water electrolysis is of great significance for addressing the global energy crisis and advancing the development of a green hydrogen energy system. This review systematically summarizes nonmetal atom doping strategies from both theoretical and practical perspectives, aiming to provide a comprehensive reference for further fundamental research and engineering applications in this field.This article first summarizes six mainstream nonmetallic doping methods, namely, the hydrothermal method, the solvothermal method, the calcination method, the ion exchange method, plasma treatment, and electrochemical deposition. For each approach, a brief introduction is provided, along with representative research examples and a discussion of its advantages and limitations, offering a clear and intuitive basis for the rational selection of synthesis strategies in future research.
To gain a deeper understanding of how nonmetallic doping influences electrocatalytic performance, this article outlines four key mechanisms: regulation of the electronic structure, optimization of surface active sites, enhancement of overall electrical conductivity, and induction of interfacial synergistic effects between metal centers and supports. These mechanisms are interrelated and collectively contribute to improved reaction kinetics and long-term stability in the HER. In parallel, this review summarizes commonly used experimental techniques for characterizing nonmetallic doping systems, including HRTEM, XPS, and XAS, which provide crucial insights into the structural and electronic effects underpinning the doping process.
From the perspective of practical applications, this review systematically compares the regulatory characteristics and representative advances of various nonmetallic dopants, including P, S, N, B, and halogens, in HER catalyst systems in recent years. For instance, P doping enhances electronic coupling between metal centers and supports, while optimizing the hydrogen adsorption free energy. S doping facilitates modulation of the d-band center of transition metals and improves overall conductivity. N doping effectively alters the electron distribution and tailors the structure of active sites by introducing lone-pair electrons. In contrast, B and halogen dopants demonstrate promising capabilities in regulating water dissociation pathways and tuning surface adsorption selectivity, respectively. This comprehensive summary and comparative analysis not only elucidates the mechanisms by which nonmetallic dopants influence catalytic performance but also provides a clearer theoretical framework and methodological reference for researchers working in related fields.
Looking ahead, considering the current limitations of nonmetallic doping strategies, such as insufficient mechanistic understanding, a lack of scalable synthesis methods, and limited integration with industrial hydrogen production, we propose the incorporation of AI-assisted data analysis and predictive modeling to accelerate the inverse design of structure–performance relationships and facilitate high-throughput catalyst screening. This approach is expected to enhance catalyst design efficiency and support the transition toward industrial-scale production. Moreover, in line with the principles of green development, this review advocates for the use of seawater as an alternative to pure water in electrolysis processes. This strategy not only addresses the global scarcity of freshwater resources but also holds promise for maintaining a high HER catalytic performance, offering a viable pathway toward the establishment of a sustainable clean energy system.
Data availability
The data are available upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (52072197, 52174283, and 22301156), the Natural Science Foundation of Shandong Province (ZR2021QE165), the Qingdao Natural Science Foundation (24-4-4-zrjj-16-jch), and the Shandong Province “Double-Hundred Talent Plan” (WST2020003).References
- D. Welsby, J. Price, S. Pye and P. Ekins, Unextractable fossil fuels in a 1.5 °C world, Nature, 2021, 597, 230–234 CrossRef CAS PubMed.
- K. K. Zander, R. Nepal and S. T. Garnett, Assessing good governance principles of renewable energy megaprojects, J. Cleaner Prod., 2024, 477, 143848 CrossRef.
- A. Sartbaeva, V. Kuznetsov, S. A. Wells and P. Edwards, Hydrogen nexus in a sustainable energy future, Energy Environ. Sci., 2008, 1, 79–85 RSC.
- S. Al, COP28: the science is clear-fossil fuels must go, Nature, 2023, 624, 225 CrossRef PubMed.
- J.-I. Nishizawa, A method to avoid dangers caused by fossil fuels, Proc. IEEE, 2008, 96, 1559–1561 Search PubMed.
- M. S. A. S. Shah, G. Y. Jang, K. Zhang and J. H. Park, Transition metal carbide–based nanostructures for electrochemical hydrogen and oxygen evolution reactions, EcoEnergy, 2023, 1, 344–374 CrossRef.
- P.-A. Le, V. D. Trung, P. L. Nguyen, T. V. B. Phung, J. Natsuki and T. Natsuki, The current status of hydrogen energy: an overview, RSC Adv., 2023, 13, 28262–28287 RSC.
- F. Yu, H. Zhou, Y. Huang, J. Sun, F. Qin, J. Bao, W. A. Goddard III, S. Chen and Z. Ren, High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting, Nat. Commun., 2018, 9, 2551 CrossRef PubMed.
- L. Kong, L. Li, G. Cai, C. Liu, P. Ma, Y. Bian and T. Ma, Techno-economic analysis of hydrogen energy for renewable energy power smoothing, Int. J. Hydrogen Energy, 2021, 46, 2847–2861 CrossRef CAS.
- Q. Zhu, Y. Qu, D. Liu, K. W. Ng and H. Pan, Two-dimensional layered materials: high-efficient electrocatalysts for hydrogen evolution reaction, ACS Appl. Nano Mater., 2020, 3, 6270–6296 CrossRef CAS.
- S. Jiao, X. Fu, S. Wang and Y. Zhao, Perfecting electrocatalysts via imperfections: towards the large-scale deployment of water electrolysis technology, Energy Environ. Sci., 2021, 14, 1722–1770 RSC.
- X. Wang, X. Zhai, Q. Yu, X. Liu, X. Meng, X. Wang and L. Wang, Strategies of designing electrocatalysts for seawater splitting, J. Solid State Chem., 2022, 306, 122799 CrossRef CAS.
- C. Fan, Z. Zang and X. Zhang, Non-metal doping regulation in transition metal and their compounds for electrocatalytic water splitting, Int. J. Hydrogen Energy, 2024, 56, 1273–1283 CrossRef CAS.
- L. Tian, Z. Li, X. Xu and C. Zhang, Advances in noble metal (Ru, Rh, and Ir) doping for boosting water splitting electrocatalysis, J. Mater. Chem. A, 2021, 9, 13459–13470 RSC.
- L. Su, J. Chen, F. Yang, P. Li, Y. Jin, W. Luo and S. Chen, Electric-double-layer origin of the kinetic pH effect of hydrogen electrocatalysis revealed by a universal hydroxide adsorption-dependent inflection-point behavior, J. Am. Chem. Soc., 2023, 145, 12051–12058 CrossRef CAS PubMed.
- R. Li, P. Ren, P. Yang, Y. Li, D. Wang, X. Lu, H. Zhang, F. Meng, X. Peng and B. Yuan, VOX-doped CoP catalysts with synergistic dual-active configuration for boosting hydrogen evolution kinetics, Nano Energy, 2024, 126, 109613 CrossRef CAS.
- S. Li, Z. Xin, Y. Luo, J. Pan, G. Liao, Q. Li, Y. Sun, Z. Feng and R. Tan, Recent advances in the development of single atom catalysts for oxygen evolution reaction, Int. J. Hydrogen Energy, 2024, 82, 1081–1100 CrossRef CAS.
- X. Liu, J. Chi, H. Mao and L. Wang, Principles of designing electrocatalyst to boost reactivity for seawater splitting, Adv. Energy Mater., 2023, 13, 2301438 CrossRef CAS.
- L. K. Putri, B.-J. Ng, R. Y. Z. Yeo, W.-J. Ong, A. R. Mohamed and S.-P. Chai, Engineering nickel phosphides for electrocatalytic hydrogen evolution: A doping perspective, Chem. Eng. J., 2023, 461, 141845 CrossRef CAS.
- Z. Li, X. Liu, Q. Yu, X. Qu, J. Wan, Z. Xiao, J. Chi and L. Wang, Recent advances in design of hydrogen evolution reaction electrocatalysts at high current density: A review, Chin. J. Catal., 2024, 63, 33–60 CrossRef CAS.
- L. Zavala-Sanchez, X. Portier, F. Maugé, L. Dubau and L. Oliviero, Active sites for HDS on (Pt, Co) MoS2 catalysts are also active for HER reaction: A proof of concept, Catal. Today, 2024, 115020 Search PubMed.
- H. Xue, T. Yang, Z. Zhang, Y. Zhang, Z. Geng and Y. He, Stimulate the hidden catalysis potential and exposure of nickel site in NiSe@CNTs result in ultra-high HER/OER activity and stability, Appl. Catal., B, 2023, 330, 122641 CrossRef CAS.
- Q. Yu, X. Liu, G. Liu, X. Wang, Z. Li, B. Li, Z. Wu and L. Wang, Constructing three-phase heterojunction with 1D/3D hierarchical structure as efficient trifunctional electrocatalyst in alkaline seawater, Adv. Funct. Mater., 2022, 32, 2205767 CrossRef CAS.
- X. Wang, W. Zhang, Q. Yu, X. Liu, Q. Liang, X. Meng, X. Wang and L. Wang, Fe-doped CoNiP@N-doped carbon nanosheet arrays for hydrazine oxidation assisting energy-saving seawater splitting, Chem. Eng. J., 2022, 446, 136987 CrossRef CAS.
- L. Song, J. Chi, J. Tang, X. Liu, Z. Xiao, Z. Wu and L. Wang, Anode design principles for efficient seawater electrolysis and inhibition of chloride oxidation, Chin. J. Catal., 2024, 66, 53–75 CrossRef CAS.
- B. Wang, F. Yang and L. Feng, Recent advances in Co–based electrocatalysts for hydrogen evolution reaction, Small, 2023, 19, 2302866 CrossRef CAS PubMed.
- J. Chi, J. Tang, X. Liu, Z. Xiao, J. Lai and L. Wang, Research progress of anionic vacancies in electrocatalysts for oxygen evolution reaction, Chin. J. Catal., 2024, 66, 110–138 CrossRef.
- C. Huang, J. Zhou, D. Duan, Q. Zhou, J. Wang, B. Peng, L. Yu and Y. Yu, Roles of heteroatoms in electrocatalysts for alkaline water splitting: A review focusing on the reaction mechanism, Chin. J. Catal., 2022, 43, 2091–2110 CrossRef CAS.
- X. Yang, Y. Liu, R. Guo and J. Xiao, Ru doping boosts electrocatalytic water splitting, Dalton Trans., 2022, 51, 11208–11225 RSC.
- T. Liang, J. Xie, A. Wang, D. Ma, Z. Mao, J. Wang and H. Li, Prediction of HER electrocatalyst with enhanced performance based on atoms-doped black phosphorene: A first-principles study, Appl. Surf. Sci., 2022, 604, 154508 CrossRef CAS.
- Y. Tang, C. Yang, M. Sheng, X. Yin and W. Que, Synergistically Coupling phosphorus-doped molybdenum carbide with Mxene as a highly efficient and stable electrocatalyst for hydrogen evolution reaction, ACS Sustainable Chem. Eng., 2020, 8, 12990–12998 CrossRef CAS.
- Y. Wan, J. Xu and R. Lv, Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions, Mater. Today, 2019, 27, 69–90 CrossRef CAS.
- S. Li, Z. Luo, S. Wang and H. Cheng, Atomic structure and HER performance of doped MoS2: A mini-review, Electrochem. Commun., 2023, 107563 CrossRef CAS.
- L. Hou, H. Jang, X. Gu, X. Cui, J. Tang, J. Cho and X. Liu, Design strategies of ruthenium–based materials toward alkaline hydrogen evolution reaction, EcoEnergy, 2023, 1, 16–44 CrossRef.
- S. Zhou, C. Yang, L. Guo, R. A. Soomro, M. Niu, Z. Yang, R. Du, D. Wang, F. Fu and B. Xu, Synergism of electronic structure regulation and interface engineering for boosting hydrogen evolution reaction on S-Scheme FeS2/S-ZnSnO3 heterostructure, Appl. Surf. Sci., 2023, 625, 157192 CrossRef CAS.
- Y. Luo, Y. Zhang, J. Zhu, X. Tian, G. Liu, Z. Feng, L. Pan, X. Liu, N. Han and R. Tan, Material engineering strategies for efficient hydrogen evolution reaction catalysts, Small Methods, 2024, 8, 2400158 CrossRef CAS PubMed.
- J. Shi, T. Chen and X. Sun, The effect of heteroatom doping on the active metal site of CoS2 for hydrogen evolution reaction, RSC Adv., 2022, 12, 17257–17263 RSC.
- J.-J. Lv, Y. Li, S. Wu, H. Fang, L.-L. Li, R.-B. Song, J. Ma and J.-J. Zhu, Oxygen species on nitrogen-doped carbon nanosheets as efficient active sites for multiple electrocatalysis, ACS Appl. Mater. Interfaces, 2018, 10, 11678–11688 CrossRef CAS PubMed.
- H. Zhao, J.-T. Ren and Z.-Y. Yuan, Microenvironment engineering of gas-involving energy electrocatalysis and device applications, Coord. Chem. Rev., 2024, 514, 215901 CrossRef CAS.
- J. Tian, C. Yang, R. Hao, F. Li, Z. Liu, W. Chen, Y. Lv and C. Lin, Fabrication of phosphorus-mediated MoS2 nanosheets on carbon cloth for enhanced hydrogen evolution reaction, Int. J. Hydrogen Energy, 2022, 47, 17871–17878 CrossRef CAS.
- S. Xu, J. Chi, T. Cui, Z. Li, F. Liu, J. Lai and L. Wang, Substitutional phosphorus doping stimulates strong metal-support interactions for anion exchange membrane based alkaline seawater electrolysis, Nano Energy, 2024, 126, 109698 CrossRef CAS.
- Y. Fan, Y. Sun, X. Zhang and J. Guo, Synergistic effect between sulfur and CoFe alloys embedded in N-doped carbon nanosheets for efficient hydrogen evolution under neutral condition, Chem. Eng. J., 2021, 426, 131922 CrossRef CAS.
- C. Peng, L. Song, L. Wang, F. Yang, J. Ding, F. Huang and Y. Wang, Effect of surface charge distribution of phosphorus-doped MoS2 on hydrogen evolution reaction, ACS Appl. Energy Mater., 2021, 4, 4887–4896 CrossRef CAS.
- Q. Lin, Y. Liu, J. Li, K. Feng, J. Zhong, H. Huang, M. Shao, Z. Fan, F. Liao, Y. Liu and Z. Kang, Enhanced Acidic hydrogen evolution reaction kinetics via Nitrogen-Doped iridium nanosheet with optimized hydrogen adsorption energy, Chem. Eng. J., 2024, 153214 CrossRef CAS.
- W. Yuan, T. Jiang, X. Fang, Y. Fan, S. Qian, Y. Gao, N. Cheng, H. Xue and J. Tian, Interface engineering of S-doped Co2P@Ni2P core-shell heterostructures for efficient and energy-saving water splitting, Chem. Eng. J., 2022, 439, 135743 CrossRef CAS.
- L. Tian, H. Liu, X. Yi, X. Wang, L. Pang and J. Li, Synergistic effects of Mo2C/CeO2 nanoparticles anchored on nitrogen-doped carbon as an efficiently electrocatalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2023, 48, 23831–23841 CrossRef CAS.
- T. Kou, T. Smart, B. Yao, I. Chen, D. Thota, Y. Ping and Y. Li, Theoretical and experimental insight into the effect of nitrogen doping on hydrogen evolution activity of Ni3S2 in alkaline medium, Adv. Energy Mater., 2018, 8, 1703538 CrossRef.
- J. Lu, Z. Tang, L. Luo, S. Yin, P. K. Shen and P. Tsiakaras, Worm-like S-doped RhNi alloys as highly efficient electrocatalysts for hydrogen evolution reaction, Appl. Catal., B, 2019, 255, 117737 CrossRef CAS.
- X. Liu, F. Liu, J. Yu, G. Xiong, L. Zhao, Y. Sang, S. Zuo, J. Zhang, H. Liu and W. Zhou, Charge redistribution caused by S, P synergistically active Ru endows an ultrahigh hydrogen evolution activity of S–doped RuP embedded in N, P, S–doped carbon, Adv. Sci., 2020, 7, 2001526 CrossRef CAS PubMed.
- W. Xu, G. Fan, S. Zhu, Y. Liang, Z. Cui, Z. Li, H. Jiang, S. Wu and F. Cheng, Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction, Adv. Funct. Mater., 2021, 31, 2107333 CrossRef CAS.
- J. Cai, J. Yang, X. Xie, J. Ding, L. Liu, W. Tian, Y. Liu, Z. Tang, B. Liu and S. Lu, Carbon Doping Triggered Efficient Electrochemical Hydrogen Evolution of Cross–Linked Porous Ru–MoO2 Via Solid–Phase Reaction Strategy, Energy Environ. Mater., 2023, 6, e12424 CrossRef CAS.
- P. Liu, Y. Shi, X. Zhang, J. Yin, D. Zhang, T. Wang, J. Fei, T. Zhan, G. Li and J. Lai, Interstitial boron-doped Ni4Mo nanoarray catalyst boosts stable hydrogen evolution reaction, Appl. Catal., B, 2024, 341, 123332 CrossRef CAS.
- K. Fan, L. Zong, J. Liu, C. H. Chuang, M. Dong, Y. Zou, Y. Xu, H. Q. Fu, L. Zhang and L. Wang, In Situ Reconstruction to Surface Sulfide Adsorbed Metal Scaffold for Enhanced Electrocatalytic Hydrogen Evolution Activity, Adv. Energy Mater., 2024, 2400052 CrossRef CAS.
- J. Wang, X. Yue, Y. Yang, S. Sirisomboonchai, P. Wang, X. Ma, A. Abudula and G. Guan, Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review, J. Alloys Compd., 2020, 819, 153346 CrossRef CAS.
- H. Li, L. Zhu, C. Li, Z. Wu, H. Li, Q. Chen, Y. Huang, X. Zhu and Y. Sun, S-doping induced phase engineering of MoSe2 for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2022, 47, 30371–30377 CrossRef CAS.
- H. Li, F. Xie, R. Snyders, C. Bittencourt and W. Li, Structural Engineering of Nitrogen-Doped MoS2 Anchored on Nitrogen-Doped Carbon Nanotubes towards Enhanced Hydrogen Evolution Reaction, ChemElectroChem, 2022, 9, e202200420 CrossRef CAS.
- J. Liu, D. Wang, K. Huang, J. Dong, J. Liao, S. Dai, X. Tang, M. Yan, H. Gong and J. Liu, Iodine-doping-induced electronic structure tuning of atomic cobalt for enhanced hydrogen evolution electrocatalysis, ACS Nano, 2021, 15, 18125–18134 CrossRef CAS PubMed.
- X. Zhang, K. Zhao, H. Li, Y. Li, W. Yang, J. Liu and D. Li, Plasma-assisted synthesis of hierarchical defect N-doped iron-cobalt sulfide@Co foam as an efficient bifunctional electrocatalyst for overall water splitting, New J. Chem., 2023, 47, 7613–7621 RSC.
- Z. Wang, T. Li and Q. Wang, Plasma-Engineered CeOX Nanosheet Array with Nitrogen-Doping and Porous Architecture for Efficient Electrocatalysis, Nanomaterials, 2024, 14, 185 CrossRef CAS PubMed.
- S. Feng, J. Wang, W. Wang, X. Wang, Y. Zhang, C. Chen, A. Ju, J. Pan and R. Xu, The Ni–Mo–S Catalyst@Copper Foams with Excellent Stability and 1.5 V Drive Electrolytic Water, Adv. Mater. Interfaces, 2021, 8, 2100500 CrossRef CAS.
- Q. Jing, J. Zhu, X. Wei, Y. Lin, X. Wang and Z. Wu, An acid-base molecular assembly strategy toward N-doped Mo2C@C nanowires with mesoporous Mo2C cores and ultrathin carbon shells for efficient hydrogen evolution, J. Colloid Interface Sci., 2021, 602, 520–533 CrossRef CAS PubMed.
- A. H. Al-Naggar, N. M. Shinde, J.-S. Kim and R. S. Mane, Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts, Coord. Chem. Rev., 2023, 474, 214864 CrossRef CAS.
- T. Cui, J. Chi, J. Zhu, X. Sun, J. Lai, Z. Li and L. Wang, Tuning the size and chemisorption of FeP4 by trace Ru doping for hydrazine-assisted hydrogen evolution in seawater at large–current–density, Appl. Catal., B, 2022, 319, 121950 CrossRef CAS.
- Q. Zhu, M. Shao, S. H. Yu, X. Wang, Z. Tang, B. Chen, H. Cheng, Z. Lu, D. Chua and H. Pan, One-pot synthesis of Co-doped VSe2 nanosheets for enhanced hydrogen evolution reaction, ACS Appl. Energy Mater., 2018, 2, 644–653 CrossRef.
- H. Zhao, H. Zhang, R. Huang, J. Wang, J. Cai, J. Hu, Z. Chen, Y. Li and H. Li, Fabrication of MoS2 with Dual Defects of O-Doping and S-Vacancies for High-Efficiency Hydrogen Production, Electrocatalysis, 2024, 15, 20–28 CrossRef CAS.
- H. Bai, X. Li, F. Guo, B. Zhang, Q. Yang, L. Zhang, Y. Song and Y. Huang, Comparative study on the growth mechanism of multi-shaped PbS via hydro-and solvothermal methods, Opt. Mater. Express, 2019, 9, 932–943 CrossRef CAS.
- Q. Hu, G. Li, Z. Han, Z. Wang, X. Huang, H. Yang, Q. Zhang, J. Liu and C. He, Nonmetal doping as a robust route for boosting the hydrogen evolution of metal–based electrocatalysts, Chem. – Eur. J., 2020, 26, 3930–3942 CrossRef CAS PubMed.
- A. Zhang, Y. Liang, H. Zhang, Z. Geng and J. Zeng, Doping regulation in transition metal compounds for electrocatalysis, Chem. Soc. Rev., 2021, 50, 9817–9844 RSC.
- L. Song, L. Guo, J. Mao, Z. Li, J. Zhu, J. Lai, J. Chi and L. Wang, Boosting Hydrogen Adsorption via Manipulating the d-Band Center of Ferroferric Oxide for Anion Exchange Membrane-Based Seawater Electrolysis, ACS Catal., 2024, 14, 6981–6991 CrossRef CAS.
- T. Yu, K. Tan, J. Wu, Y. Zou and S. Yin, Bifunctional interstitial phosphorous doping strategy boosts platinum-zinc alloy for efficient ammonia oxidation reaction and hydrogen evolution reaction, Nano Res., 2024, 17, 1182–1189 CrossRef CAS.
- N. Chen, W. Zhang, J. Zeng, L. He, D. Li and Q. Gao, Plasma-Engineered MoP with nitrogen doping: Electron localization toward efficient alkaline hydrogen evolution, Appl. Catal., B, 2020, 268, 118441 CrossRef CAS.
- Q. Qi, D. Shao, Y. Zhou, Q. Wang and X.-Y. Yu, Plasma-induced implanting of active species in metal-organic frameworks for efficient hydrogen evolution reaction, J. Mater. Chem. A, 2023, 11, 15663–15669 RSC.
- G. Chen, H. Xiang, Y. Guo, J. Huang, W. Chen, Z. Chen, T. Li and K. Ostrikov, Yttrium–and nitrogen–doped NiCo phosphide nanosheets for high–efficiency water electrolysis, Carbon Energy, 2024, e522 CrossRef CAS.
- J. Yu, S. Zhang, Y. Liu, Y. Tong, Y. Zhong, B. He, E. Hu, J. Zhang and Z. Chen, Construction of Sulfur Doped Nickel and Copper Interfaces for Hydrogen Evolution Reaction, ACS Mater. Lett., 2024, 6, 4719–4727 CrossRef CAS.
- Q. Wu, J. Wang, X. Wang, J. Wei, J. Wang, C. Zhang, R. Xu and L. Yang, Synergistic Effect of P and Co Dual Doping Endows CuNi with High–Performance Hydrogen Evolution Reaction, Small, 2024, 2402615 CrossRef CAS PubMed.
- M. A. Ashraf, Y. Yang, D. Zhang and B. T. Pham, Bifunctional and binder-free S-doped Ni-P nanospheres electrocatalyst fabricated by pulse electrochemical deposition method for overall water splitting, J. Colloid Interface Sci., 2020, 577, 265–278 CrossRef CAS PubMed.
- H. Wang, W. Fan, S. Yang, G. Gong, S. Chen, L. Jiao, F. You and J. Qi, Deeply understanding electrocatalytic oxygen evolution reaction from the perspective of defect structures, Chem. Eng. J., 2024, 156124 CrossRef CAS.
- P. Zhou, R. Li, J. Lv, G. Zhao, X. Zhang, X. Huang, Y. Lu and G. Wang, Optimizing the electronic structure of CoNx via coupling with N-doped carbon for efficient electrochemical hydrogen evolution, J. Colloid Interface Sci., 2022, 628, 350–358 CrossRef CAS PubMed.
- Y. Chen, Y. Wang, B. Liu, C. Zhang, D. Sun, H. Liu and W. Zhou, Room-temperature sulfur doped NiMoO4 with enhanced conductivity and catalytic activity for efficient hydrogen evolution reaction in alkaline media, J. Colloid Interface Sci., 2024, 664, 469–477 CrossRef CAS PubMed.
- L. Wang, C. Zhang, Z. Cao, G. Zeng, J. Liu and S. Ye, Dual Modulation of Bulk Electronic Structure and Surficial Active Sites in Sea Urchin–Like MoO2 Nanoreactors Promoting Electrocatalytic Hydrogen Evolution, Adv. Funct. Mater., 2024, 34, 2406670 CrossRef CAS.
- L. Bo, X. Ji, W. Shi, Y. Zhang, L. Xia, Y. Shen, Y. Yao and J. Tong, Active sites engineering construction of spinel cobalt oxide based excellent bifunctional electrocatalyst for water splitting by modifying oxygen vacancy with S dopant, Sep. Purif. Technol., 2023, 322, 124355 CrossRef CAS.
- L. Sun, M. Gao, Z. Jing, Z. Cheng, D. Zheng, H. Xu, Q. Zhou and J. Lin, 1 T-Phase Enriched P doped WS2 nanosphere for highly efficient electrochemical hydrogen evolution reaction, Chem. Eng. J., 2022, 429, 132187 CrossRef CAS.
- M. Zeng, R. Luo, X. Deng, Y. Song, W. Hao, J. Fan, Q. Bi and G. Li, Molten-salt-chemistry-assisted synthesis of layered N-doped tungsten carbide with enhanced electrical conductivity for efficient electrocatalytic hydrogen evolution, Int. J. Hydrogen Energy, 2024, 92, 1021–1029 CrossRef CAS.
- Y. Wen, S. Xu, P. Wang, X. Shao, X. Sun, J. Hu and X.-R. Shi, Bimetallic FeCo phosphide nanoparticles anchored on N-doped carbon foam for wide pH hydrogen evolution reaction, J. Alloys Compd., 2023, 931, 167570 CrossRef CAS.
- D. Chen, Y. Zou and S. Wang, Surface chemical-functionalization of ultrathin two-dimensional nanomaterials for electrocatalysis, Mater. Today Energy, 2019, 12, 250–268 CrossRef.
- Y.-l. Wang, G.-q. Sun, L.-h. Chen, Z.-k. Du, X.-y. Li, C.-f. Ye, J.-p. Liu and B.-l. Su, Engineering dual defective graphenes to synergistically improve electrocatalytic hydrogen evolution, Appl. Surf. Sci., 2021, 566, 150712 CrossRef CAS.
- P. Zeng, Y. Meng, Z. Liu, G. Q. Sun, X. Y. Li, X. Y. Yang, C. F. Ye, Y. Li, J. P. Liu, L. H. Chen, B. L. Su and Y. L. Wang, N–Doping Coupled with Co–Vacancies Activating Sulfur Atoms and Narrowing Bandgap for CoS Toward Synergistically Accelerating Hydrogen Evolution, Small, 2023, 19, 2301279 CrossRef CAS PubMed.
- J. Wang, Z. Zhao, C. Shen, H. Liu, X. Pang, M. Gao, J. Mu, F. Cao and G. Li, Ni/NiO heterostructures encapsulated in oxygen-doped graphene as multifunctional electrocatalysts for the HER, UOR and HMF oxidation reaction, Catal. Sci. Technol., 2021, 11, 2480–2490 RSC.
- F. Jia, X. Zou, X. Wei, W. Bao, T. Ai, W. Li and Y. Guo, Synergistic effect of P doping and Mo-Ni-based heterostructure electrocatalyst for overall water splitting, Materials, 2023, 16, 3411 CrossRef CAS PubMed.
- Y. Li, J. Luo, P. Liu, L. Zhang, W. Song, Y. Wei, Z. Zhao, X. Zhang, J. Liu and Y. Sun, Sulfur doping activated metal-support interaction drives Pt nanoparticles to achieve acid-base hydrogen evolution reaction, J. Mater. Chem. A, 2025, 13, 11518–11529 RSC.
- L. Huang, Q. Wang, H. Liu, Y. Wu, Y. Yang, G. Ma, Z. Lei and S. Ren, N+ irradiation regulates surface defects and doping towards efficient hydrogen evolution reaction on Sb2Te3, Appl. Surf. Sci., 2023, 609, 155347 CrossRef CAS.
- W. Lu, X. Li, F. Wei, K. Cheng, W. Li, Y. Zhou, W. Zheng, L. Pan and G. Zhang, In situ transformed Ni, S-codoped CoO from amorphous Co-Ni sulfide as an efficient electrocatalyst for hydrogen evolution in alkaline media, ACS Sustainable Chem. Eng., 2019, 7, 12501–12509 CAS.
- L. Xu, X. Zhou, X. Xu, L. Ma, J. Luo and L. Zhang, Triethylenetetramine-assisted hydrothermal synthesis of sulfur-doped few-layer MoSe2/nitrogenated graphene hybrids and their catalytic activity for hydrogen evolution reaction, Adv. Powder Technol., 2016, 27, 1560–1567 CrossRef CAS.
- W. Lin, B. Zhang, J. Jiang, E. Liu, J. Sha and L. Ma, Anionic and cationic co-substitutions of S into vertically aligned WTe2 nanosheets as catalysis for hydrogen evolution under alkaline conditions, ACS Appl. Nano Mater., 2022, 5, 7123–7131 CrossRef CAS.
- M. Zhou, X. Jiang, W. Kong, H. Li, F. Lu, X. Zhou and Y. Zhang, Synergistic effect of dual-doped carbon on Mo2C nanocrystals facilitates alkaline hydrogen evolution, Nano-Micro Lett., 2023, 15, 166 CrossRef CAS PubMed.
- J. Gázquez, G. Sánchez-Santolino, N. Biškup, M. A. Roldán, M. Cabero, S. J. Pennycook and M. Varela, Applications of STEM-EELS to complex oxides, Mater. Sci. Semicond., 2017, 65, 49–63 CrossRef.
- M. Jiang, J. Xu, Y. Chen, L. Wang, Q. Zhou, P. Munroe, L. Li, Z. H. Xie and S. Peng, Interstitial Doping in Ultrafine Nanocrystals for Efficient and Durable Water Splitting, Angew. Chem., Int. Ed., 2025, 64, e202424195 CrossRef CAS PubMed.
- Y. Long, L. Yang, M. Xi, Y. Zhao, H. Zhang, T. Liu, A. Chen, X. An, G. Hu and Z. Ni, Modulating the Local Charge Distribution of Single–Atomic Ru Sites for an Efficient Hydrogen Evolution Reaction, Carbon Energy, 2025, e690 CrossRef.
- J. Wang, Y. Dong, X. Zheng, H. Ling, L. Xie, Z. Xiang and W. He, P non-metal doping induced surface charge redistribution of VS2 for strengthening hydrogen evolution activity, Appl. Surf. Sci., 2025, 694, 162834 CrossRef CAS.
- F. Li, H. Lin, H. Yu, P. Du, Y. Wang and J. Cao, Vacancy formation mechanism and synergy with doping in NiS2-based electrocatalysts for benzyl alcohol oxidation and hydrogen evolution, Inorg. Chem. Front., 2025, 12, 1839–1849 RSC.
- R. Li, H. Zhao, L. Wang, Q. Zhou, X. Yang, L. Jiang, X. Luo, J. Yu, J. Wei and S. Mu, Strengthened d-p orbital hybridization and hydrogen diffusion in a hollow N-doped porous carbon/Ru cluster catalyst system for hydrogen evolution reactions, Chem. Sci., 2025, 16, 4383–4391 RSC.
- K. Fan, L. Zong, J. Liu, C. H. Chuang, M. Dong, Y. Zou, Y. Xu, H. Q. Fu, L. Zhang, L. Wang, M. Zhou, T. Zhan, P. Liu and H. Zhao, In situ reconstruction to surface sulfide adsorbed metal scaffold for enhanced electrocatalytic hydrogen evolution activity, Adv. Energy Mater., 2024, 14, 2400052 CrossRef CAS.
- M. Humayun, M. Israr, A. Khan and M. Bououdina, State-of-the-art single-atom catalysts in electrocatalysis: from fundamentals to applications, Nano Energy, 2023, 113, 108570 CrossRef CAS.
- M. Zeng and Y. Li, Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction, J. Mater. Chem. A, 2015, 3, 14942–14962 RSC.
- P. Pathak and L. Singh, Transition Metal-Based Electrocatalysts: Applications in Green Hydrogen Production and Storage, ACS Publications, 2023, pp. 21–42 Search PubMed.
- S. Anantharaj, S. Chatterjee, K. C. Swaathini, T. S. Amarnath, E. Subhashini, D. K. Pattanayak and S. Kundu, Stainless steel scrubber: a cost efficient catalytic electrode for full water splitting in alkaline medium, ACS Sustainable Chem. Eng., 2018, 6, 2498–2509 CrossRef CAS.
- X. Wang, X. Liu, S. Wu, K. Liu, X. Meng, B. Li, J. Lai, L. Wang and S. Feng, Phosphorus vacancies enriched cobalt phosphide embedded in nitrogen doped carbon matrix enabling seawater splitting at ampere-level current density, Nano Energy, 2023, 109, 108292 CrossRef CAS.
- C. Chen, J. Li, Z. Lv, M. Wang and J. Dang, Recent strategies to improve the catalytic activity of pristine MOFs and their derived catalysts in electrochemical water splitting, Int. J. Hydrogen Energy, 2023, 48, 30435–30463 CrossRef CAS.
- W. Yu, Y. Gao, Z. Chen, Y. Zhao, Z. Wu and L. Wang, Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides, Chin. J. Catal., 2021, 42, 1876–1902 CrossRef CAS.
- K. Guo, D. Fan, J. Bao, Y. Li and D. Xu, Atomic-level phosphorus-doped ultrathin Pt nanodendrites as efficient electrocatalysts, Adv. Funct. Mater., 2022, 32, 2208057 CrossRef CAS.
- M. Maleki, A. S. Rouhaghdam, G. B. Darband, D. Han, M. Chehelamirani and S. Shanmugam, Binder-free P-doped Ni-Se nanostructure electrode toward highly active and stable hydrogen production in wide pH range and seawater, J. Electroanal. Chem., 2022, 916, 116379 CrossRef CAS.
- X. Tao, Y. Liu, R. Lu, J. Liu, H. I. Wang, J. Yang, M. Bonn, K. Müllen and Y. Zhou, Phosphorus-Enhanced Bimetallic Single-Atom Catalysts for Hydrogen Evolution, Adv. Energy Mater., 2025, 15, 2404167 CrossRef CAS.
- L. M. Cao, L. H. Yu, H. B. Huang, C. J. Gao, X. Huang, X. F. Zhang, X. H. Zhang, Z. Y. Du and C. T. He, A Molecular Engineered Strategy to Remolding Architecture of RuP2 Nanoclusters for Sustainable Hydrogen Evolution, Adv. Funct. Mater., 2024, 34, 2411111 CrossRef CAS.
- Y. Wang, Y. Shen, X. Xiao, L. Dai, S. Yao and C. An, Topology conversion of 1T MoS2 to S-doped 2H-MoTe2 nanosheets with Te vacancies for enhanced electrocatalytic hydrogen evolution, Sci. China Mater., 2021, 64, 2202–2211 CrossRef CAS.
- S. L. Zhang, X. F. Lu, Z. P. Wu, D. Luan and X. W. Lou, Engineering Platinum–Cobalt Nano–alloys in Porous Nitrogen–Doped Carbon Nanotubes for Highly Efficient Electrocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2021, 60, 19068–19073 CrossRef CAS PubMed.
- C. Yang, Z. Wang, Z. Li, Y. Pan, L. Jiang, C. Li, C. Wang and Q. Sun, Nitrogen disturbance awakening the intrinsic activity of nickel phosphide for boosted hydrogen evolution reaction, ChemSusChem, 2022, 15, e202200072 CrossRef CAS PubMed.
- Z. Lu, H. Yang, G. Qi, Q. Liu, L. Feng, H. Zhang, J. Luo and X. Liu, Efficient and Stable pH–Universal Water Electrolysis Catalyzed by N–Doped Hollow Carbon Confined RuIrOX Nanocrystals, Small, 2024, 20, 2308841 CrossRef CAS PubMed.
- Q. Fu, L. Lin, T. Wu, Q. Zhang, X. Wang, L. Xu, J. Zhong, L. Gu, Z. Zhang and P. Xu, Electronegativity Enhanced Strong Metal-Support Interaction in Ru@F-Ni3N for Enhanced Alkaline Hydrogen Evolution, ACS Appl. Mater. Interfaces, 2022, 14, 36688–36699 CrossRef CAS PubMed.
- X.-Y. Zhang, Y.-R. Zhu, Y. Chen, S.-Y. Dou, X.-Y. Chen, B. Dong, B.-Y. Guo, D.-P. Liu, C.-G. Liu and Y.-M. Chai, Hydrogen evolution under large-current-density based on fluorine-doped cobalt-iron phosphides, Chem. Eng. J., 2020, 399, 125831 CrossRef CAS.
- H. Song, J. Yu, Z. Tang, B. Yang and S. Lu, Halogen–doped carbon dots on amorphous cobalt phosphide as robust electrocatalysts for overall water splitting, Adv. Energy Mater., 2022, 12, 2102573 CrossRef CAS.
- J. Wu, Q. Zhang, K. Shen, R. Zhao, W. Zhong, C. Yang, H. Xiang, X. Li and N. Yang, Modulating interband energy separation of boron–doped Fe7S8/FeS2 electrocatalysts to boost alkaline hydrogen evolution reaction, Adv. Funct. Mater., 2022, 32, 2107802 CrossRef CAS.
- X. Wu, Y. Wang, Y. Si, C. Ma, J. Yu, Y.-T. Liu and B. Ding, Boron-induced sulfur vacancies in ZnIn2S4 nanosheets coupled to TiO2 nanofibers enhance the hydrogen evolution performance, Compos. Commun., 2021, 27, 100903 CrossRef.
- E. Cao, Z. Chen, H. Wu, P. Yu, Y. Wang, F. Xiao, S. Chen, S. Du, Y. Xie and Y. Wu, Boron–induced electronic–structure reformation of CoP nanoparticles drives enhanced pH–universal hydrogen evolution, Angew. Chem., Int. Ed., 2020, 59, 4154–4160 CrossRef CAS PubMed.
- F. Jiao, J. Li, J. Wang, Y. Lin, Y. Gong and X. Jing, Regulating the electronic structure of CoMoO4 microrod by phosphorus doping: an efficient electrocatalyst for the hydrogen evolution reaction, Dalton Trans., 2020, 49, 13152–13159 RSC.
- Q. Wang, K. Cui, J. Li, Y. Wu, Y. Yang, X. Zhou, G. Ma, Z. Yang, Z. Lei and S. Ren, Phosphorus-doped CoTe2/C nanoparticles create new Co-P active sites to promote the hydrogen evolution reaction, Nanoscale, 2020, 12, 9171–9177 RSC.
- J. Ding, Z. Peng, Z. Wang, C. Zeng, Y. Feng, M. Yang, G. Hu, J. Luo and X. Liu, Phosphorus-tungsten dual-doping boosts acidic overall seawater splitting performance over RuOX nanocrystals, J. Mater. Chem. A, 2024, 12, 28023–28031 RSC.
- P. Yang, F. Liu, X. Zang, L. Xin, W. Xiao, G. Xu, H. Li, Z. Li, T. Ma and J. Wang, Microwave Quasi–Solid State to Construct Strong Metal–Support Interactions with Interfacial Electron–Enriched Ru for Anion Exchange Membrane Electrolysis, Adv. Energy Mater., 2024, 14, 2303384 CrossRef CAS.
- S. Zhou, H. Jang, Q. Qin, L. Hou, M. G. Kim, S. Liu, X. Liu and J. Cho, Boosting hydrogen evolution reaction by phase engineering and phosphorus doping on Ru/P–TiO2, Angew. Chem., 2022, 134, e202212196 CrossRef.
- C. Li, C. Tian, H. Tang, M. Liu, L. Zheng, F. Huang, G.-R. Li and Q. Li, Efficient Hydrogen Evolution Promoted by Short Pathway-Hydrogen Spillover Over P-Doped Pt3Co, ACS Energy Lett., 2023, 8, 5161–5169 CrossRef CAS.
- Y. Zhao, X. Wang, G. Cheng and W. Luo, Phosphorus-induced activation of ruthenium for boosting hydrogen oxidation and evolution electrocatalysis, ACS Catal., 2020, 10, 11751–11757 CrossRef CAS.
- J. Wang, J. Hu, C. Liang, L. Chang, Y. Du, X. Han, J. Sun and P. Xu, Surface reconstruction of phosphorus-doped cobalt molybdate microarrays in electrochemical water splitting, Chem. Eng. J., 2022, 446, 137094 CrossRef CAS.
- B. Wang, X. Chen, Y. He, Q. Liu, X. Zhang, Z. Luo, J. V. Kennedy, J. Li, D. Qian, J. Liu and G. Waterhouse, Fe2O3/P-doped CoMoO4 electrocatalyst delivers efficient overall water splitting in alkaline media, Appl. Catal., B, 2024, 346, 123741 CrossRef CAS.
- Y. Wang, J. Chen, L. Gong, J. Tang, X. Wang, H. Guo and X. Zhou, P-doped W2C nanoparticles for hydrogen evolution reaction powered by a wind-driven triboelectric nanogenerator, Nano Energy, 2024, 121, 109242 CrossRef CAS.
- W. Wang, Y. Song, C. Ke, Y. Li, Y. Liu, C. Ma, Z. Wu, J. Qi, K. Bao, L. Wang, J. Wu, S. Jiang, J. Zhao, C. Lee, Y. Chen, G. Luo, Q. He and R. Ye, Filling the gap between heteroatom doping and edge enrichment of 2D electrocatalysts for enhanced hydrogen evolution, ACS Nano, 2023, 17, 1287–1297 CrossRef CAS PubMed.
- Y. Shi, D. Zhang, H. Miao, X. Wu, Z. Wang, T. Zhan, J. Lai and L. Wang, Amorphous/2H-MoS2 nanoflowers with P doping and S vacancies to achieve efficient pH-universal hydrogen evolution at high current density, Sci. China: Chem., 2022, 65, 1829–1837 CrossRef CAS.
- Y. Qian, J. Yu, Z. Lyu, Q. Zhang, T. H. Lee, H. Pang and D. J. Kang, Durable hierarchical phosphorus–doped biphase MoS2 electrocatalysts with enhanced H* adsorption, Carbon Energy, 2024, 6, e376 CrossRef CAS.
- J. Yang, J. Wang, R. Hübner, X. Tao, Y. Ren, Z. Zheng and W. Liu, Highly Open Phosphorized PtNi Nanohexapod/N–doped Graphene Aerogel for High–Performance Alkaline Hydrogen Evolution, Adv. Energy Mater., 2024, 2404684 Search PubMed.
- M. D. Albaqami, M. U. Nisa, S. Mohammad, J. H. Shah, A. U. R. Baloch, A. G. Abid and S. Saleem, Phosphorus-Doped CuMn2O4 Nanoflakes: A Highly Performed Electrocatalyst for Oxygen Evolution Reaction and Hydrogen Evolution Reaction in an Alkaline Environment, Energy Fuels, 2024, 38, 15533–15542 CrossRef CAS.
- Q. Li, X. Fu, H. Li, Z. Xiao, G. Xu, D. Chen, C. Li, W. Jin, T. Ma and Z. Wu, Strong d–p Orbital Hybridization of Os–P via Ultrafast Microwave Plasma Assistance for Anion Exchange Membrane Electrolysis, Adv. Funct. Mater., 2024, 34, 2408517 CrossRef CAS.
- C. Li, H. Jang, S. Liu, M. G. Kim, L. Hou, X. Liu and J. Cho, P and Mo dual doped Ru ultrasmall nanoclusters embedded in P–doped porous carbon toward efficient hydrogen evolution reaction, Adv. Energy Mater., 2022, 12, 2200029 CrossRef CAS.
- S. Zhuang, Y. Tang, X. Tai, Q. Huang, P. Wan, Y. Chen, Y. Sun, J. Pan and X. J. Yang, Hydrogen and electricity co-generation from hydrazine-assisted water electrolysis on hierarchical porous heteroatoms-doped CoCu catalysts, Appl. Catal., B, 2022, 306, 121132 CrossRef CAS.
- Y. X. Xiao, J. Ying, J. B. Chen, X. Yang, G. Tian, J. H. Li, C. Janiak and X. Y. Yang, Phosphorous Incorporated PtNi Networks with Synergistic Directional Electron Transfer for Efficient and Durable Seawater Hydrogen Production, Adv. Funct. Mater., 2024, 2418264 Search PubMed.
- Y. Li, H. Guo, Y. Zhang and R. Song, Cooperative phosphorus and chromium doping induced electronic and morphological dual modulation in NiMoO4 hydrate for energy-efficient urea-assisted hydrogen production, Appl. Catal., B, 2024, 341, 123296 CrossRef CAS.
- D. Song, J. Sun, L. Sun, S. Zhai, G. W. Ho, H. Wu and W. Q. Deng, Acidic media regulated hierarchical cobalt compounds with phosphorous doping as water splitting electrocatalysts, Adv. Energy Mater., 2021, 11, 2100358 CrossRef CAS.
- W. Ma, D. Liu, F. Gao, Z. Lv, X. Lv, Y. Li, Y. You and J. Dang, P–doped MoS2/Ni2P/Ti3C2TX heterostructures for efficient hydrogen evolution reaction in alkaline media, J. Am. Ceram., 2022, 105, 6096–6104 CrossRef CAS.
- Z. Yang, H. Zhao, X. Dai, F. Nie, Z. Ren, X. Yin, Y. Gan, B. Wu, Y. Cao and X. Zhang, Phosphorus-doping induced electronic modulation of CoS2-MoS2 hollow spheres on MoO2 film-Mo foil for synergistically boosting alkaline hydrogen evolution reaction, Int. J. Hydrogen Energy, 2021, 46, 33388–33396 CrossRef CAS.
- T. Wu and H. Meng, Introducing phosphorus atoms into MoS2 nanosheets through a vapor-phase hydrothermal process for the hydrogen evolution reaction, Dalton Trans., 2024, 53, 5808–5815 RSC.
- H. Wang, Y. Liang, S. Liu, X. Mu, H. Yu, K. Deng, Z. Wang, Y. Xu and L. Wang, Electronic and active site engineering in Rh metallene via phosphorus and sulfur dual-doping for electrocatalytic sulfion recycling and hydrogen generation, Inorg. Chem. Front., 2023, 10, 5686–5693 RSC.
- M. Hu, B. Liu, H. Chen, X. Xu, P. Jing, X. Guo, R. Yang, X. Wang, R. Gao and J. Zhang, Universal construction of sulfur doped molybdenum-based nanosheets for enhanced hydrogen evolution in a wide pH range, Appl. Catal., B, 2023, 322, 122131 CrossRef CAS.
- J. Chen, X. Liu, H. Wang, C.-L. Chiang, P. Hou, J. Li, H. Jin, S. Liu, X. Meng, Y.-G. Lin, J.-M. Lee and Q. Zhao, Sulfur-induced electronic optimization of Mo5N6 for hydrogen evolution through topochemical substitution, Chem. Eng. J., 2023, 466, 143221 CrossRef CAS.
- Q. Hao, L. Zhang, Y. Gao, D. Cao, J. Zhao, Y. Li, C. Liu, Z. Qiao, W. He and H. Liu, Construction of Fe2O3 nanosheet arrays by sulfur doping toward efficient alkaline hydrogen evolution, ACS Appl. Energy Mater., 2022, 5, 1793–1800 CrossRef CAS.
- X. Zhang, S. Wang, C. Wu, H. Li, Y. Cao, S. Li and H. Xia, Synthesis of S-doped AuPbPt alloy nanowire-networks as superior catalysts towards the ORR and HER, J. Mater. Chem. A, 2020, 8, 23906–23918 RSC.
- J. Yan, Z. Xi, L. Cong, K. Lv, R. Xin, B. Cao, B. Liu, J. He and J. Zhang, Synergy of Platinum Single Atoms and Platinum Atomic Clusters on Sulfur–Doped Titanium Nitride Nanotubes for Enhanced Hydrogen Evolution Reaction, Small, 2022, 18, 2205603 CrossRef CAS PubMed.
- Z. Tian, W. Wang, C. Dong, X. Deng and G.-H. Wang, A general and scalable approach to sulfur-doped mono-/bi-/trimetallic nanoparticles confined in mesoporous carbon, ACS Nano, 2023, 17, 3889–3900 CrossRef CAS PubMed.
- H. Lei, Q. Wan, S. Tan, Z. Wang and W. Mai, Pt–Quantum–Dot–Modified Sulfur–Doped NiFe Layered Double Hydroxide for High–Current–Density Alkaline Water Splitting at Industrial Temperature, Adv. Mater., 2023, 35, 2208209 CrossRef CAS PubMed.
- W. Liu, Z. Xiao, S. Chandrasekaran, D. Fan, W. Li, H. Lu and Y. Liu, Insights into the effect of sulfur incorporation into tungsten diphosphide for improved hydrogen evolution reaction, ACS Appl. Mater. Interfaces, 2022, 14, 16157–16164 CrossRef CAS PubMed.
- X.-L. Zhang, P.-C. Yu, X.-Z. Su, S.-J. Hu, L. Shi, Y.-H. Wang, P.-P. Yang, F.-Y. Gao, Z.-Z. Wu and L.-P. Chi, Efficient acidic hydrogen evolution in proton exchange membrane electrolyzers over a sulfur-doped marcasite-type electrocatalyst, Sci. Adv., 2023, 9, eadh2885 CrossRef CAS PubMed.
- S. Zhang, Y. Ji, S. Wang, P. Zhang, D. Shi, F. Lu and B. Zhang, Sulfur doping induces internal polarization field in NiFe-LDH for bifunctioanl HER/OER and overall water/simulated seawater splitting, J. Alloys Compd., 2024, 1002, 175323 CrossRef CAS.
- J. Dong, S. Chen, C. Lv, M. G. Humphrey, C. Zhang and Z. Huang, Sulfur-doped cobalt molybdenum oxide with a hydrangea-like structure for bi-functionally efficient overall water splitting, J. Mater. Chem. A, 2024, 12, 6983–6995 RSC.
- S. Geng, Y. Liu, Y. S. Yu, W. Yang and H. Li, Engineering defects and adjusting electronic structure on S doped MoO2 nanosheets toward highly active hydrogen evolution reaction, Nano Res., 2020, 13, 121–126 CrossRef CAS.
- J. Li, H. Huang, X. Cao, H.-H. Wu, K. Pan, Q. Zhang, N. Wu and X. Liu, Template-free fabrication of MoP nanoparticles encapsulated in N-doped hollow carbon spheres for efficient alkaline hydrogen evolution, Chem. Eng. J., 2021, 416, 127677 CrossRef CAS.
- S. Zhang, C. Zhang, X. Zheng, G. Su, H. Wang and M. Huang, Integrating electrophilic and nucleophilic dual sites on heterogeneous bimetallic phosphide via enhancing interfacial electronic field to boost hydrazine oxidation and hydrogen evolution, Appl. Catal., B, 2023, 324, 122207 CrossRef CAS.
- H. Xie, Y. Feng, X. He, Y. Zhu, Z. Li, H. Liu, S. Zeng, Q. Qian and G. Zhang, Construction of nitrogen–doped biphasic transition–metal sulfide nanosheet electrode for energy–efficient hydrogen production via urea electrolysis, Small, 2023, 19, 2207425 CrossRef CAS PubMed.
- J. Tong, Y. Li, L. Bo, W. Li, T. Li, Q. Zhang, D. Kong, H. Wang and C. Li, CoP/N-doped carbon hollow spheres anchored on electrospinning core-shell N-doped carbon nanofibers as efficient electrocatalysts for water splitting, ACS Sustainable Chem. Eng., 2019, 7, 17432–17442 CrossRef CAS.
- H. Zhang, F. Wan, X. Li, X. Chen, S. Xiong and B. Xi, Ultrafine PtMo Nanocrystals Confined on N–Doped Carbon Toward Efficient pH–Universal Hydrogen Evolution Reaction, Adv. Funct. Mater., 2023, 33, 2306340 CrossRef CAS.
- X.-a. Wang, Y.-s. Gong, Z.-k. Liu, P.-s. Wu, L.-x. Zhang and J.-k. Sun, Ir nanoclusters on ZIF-8-derived nitrogen-doped carbon frameworks to give a highly efficient hydrogen evolution reaction, New Carbon Mater., 2024, 39, 164–172 CrossRef CAS.
- M. Jia, J. Jiang, J. Tian, X. Wang, L. Yang, Q. Wu and Z. Hu, Ultrasmall high-entropy alloy nanoparticles on hierarchical N-doped carbon nanocages for tremendous electrocatalytic hydrogen evolution, Nano Res., 2024, 17, 9518–9524 CrossRef CAS.
- Q. Gao, Z. Wang, W. Gao and H. Yin, NiFeCo-based high entropy alloys nanoparticles coated with N-doped graphene layers as hydrogen evolution catalyst in alkaline solution, Chem. Eng. J., 2024, 489, 151370 CrossRef CAS.
- J. Xiao, S. Zhang, Y. Sun, X. Liu, G. He, H. Liu, J. Khan, Y. Zhu, Y. Su and S. Wang, Urchin–Like Structured MoO2/Mo3P/Mo2C Triple–Interface Heterojunction Encapsulated within Nitrogen–Doped Carbon for Enhanced Hydrogen Evolution Reaction, Small, 2023, 19, 2206472 CrossRef CAS PubMed.
- V. Dao, H. Choi, S. Yadav, J. D. Jiménez, C. Kim, T. Van Nguyen, K. Chen, P. Uthirakumar, Q. Van Le and S. D. Senanayake, LaCeOX coupled N-doped graphene/Ru single-atoms as a binary-site catalyst for efficient hydrogen evolution based on hydrogen spillover, Appl. Catal., B, 2024, 343, 123452 CrossRef CAS.
- S. Wang, S. Chen, C. Wen, L. Dong, C. Tan, B. Li, M. Fan, H. He and Z. Chen, Nitrogen-doped MoO2/Cu heterojunction electrocatalyst for highly efficient alkaline hydrogen evolution reaction, Int. J. Hydrogen Energy, 2024, 66, 103–109 CrossRef CAS.
- J. Ye, Y. Zang, Q. Wang, Y. Zhang, D. Sun, L. Zhang, G. Wang, X. Zheng and J. Zhu, Nitrogen doped FeS2 nanoparticles for efficient and stable hydrogen evolution reaction, J. Energy Chem., 2021, 56, 283–289 CrossRef CAS.
- Y.-N. Zhou, Y. Ma, L. Feng, J. Zhao, Z. Tong, B. Dong, Y.-R. Zhu, L. Wang, C.-G. Liu and Y.-M. Chai, Optimized Mo-doped cobalt selenides coupled carbon nanospheres for efficient hydrogen evolution, Appl. Surf. Sci., 2020, 531, 147404 CrossRef CAS.
- X. Zhao, F. Yin, X. He, B. Chen and G. Li, Efficient overall water splitting over a Mo(IV)-doped Co3O4/NC electrocatalyst, Int. J. Hydrogen Energy, 2021, 46, 20905–20918 CrossRef CAS.
- L. Zhang, X. Cao, C. Feng, W. Zhang, Z. Wang, S. Feng, Z. Huang, X. Lu and F. Dai, Interfacial Mo-N-C Bond Endowed Hydrogen Evolution Reaction on MoSe2@N-Doped Carbon Hollow Nanoflowers, Inorg. Chem., 2021, 60, 12377–12385 CrossRef CAS PubMed.
- J.-Q. Chi, J.-Y. Xie, W.-W. Zhang, B. Dong, J.-F. Qin, X.-Y. Zhang, J.-H. Lin, Y.-M. Chai and C.-G. Liu, N-doped sandwich-structured Mo2C@C@Pt interface with ultralow Pt loading for pH-universal hydrogen evolution reaction, ACS Appl. Mater. Interfaces, 2019, 11, 4047–4056 CrossRef CAS PubMed.
- J. Li, C. Zhou, J. Mu, E.-C. Yang and X.-J. Zhao, In situ synthesis of molybdenum carbide/N-doped carbon hybrids as an efficient hydrogen-evolution electrocatalyst, RSC Adv., 2018, 8, 17202–17208 RSC.
- Y. Li, Q. Huang, H. Wu, L. Cai, Y. Du, S. Liu, Z. Sheng and M. Wu, N-doped Mo2C nanoblock for efficient hydrogen evolution reaction, J. Solid State Electrochem., 2019, 23, 2043–2050 CrossRef CAS.
- Q. Yu, Y. Fu, J. Zhao, B. Li, X. Wang, X. Liu and L. Wang, Boron doping activate strong metal-support interaction for electrocatalytic hydrogen evolution reaction in full pH range, Appl. Catal., B, 2023, 324, 122297 CrossRef CAS.
- X. Sun, W. Li, J. Chen, X. Yang, B. Wu, Z. Wang, B. Li and H. Zhang, Boron-induced activation of Ru nanoparticles anchored on carbon nanotubes for the enhanced pH-independent hydrogen evolution reaction, J. Colloid Interface Sci., 2022, 616, 338–346 CrossRef CAS PubMed.
- Z. Duan, T. Ren, Y. Xu, Z. Wang, H. Yu, K. Deng, L. Wang and H. Wang, Boron-doped iridium nanosheets array for energy-saving hydrogen production by hydrazine-assisted water electrolysis, Int. J. Hydrogen Energy, 2023, 48, 37045–37052 CrossRef CAS.
- F.-G. Wang, X. Liu, X.-Q. Yuan, Y. Wu, H.-Y. Zhao, J.-F. Yu, B. Liu, Y.-M. Chai and B. Dong, Constructing porous boron doped nickel phosphide (Ni2P) rod arrays with optimized electron coordination for alkaline hydrogen evolution, J. Alloys Compd., 2022, 927, 166938 CrossRef CAS.
- Y. B. Adegbemiga, N. Ullah, M. Xie, S. Hussain, C. J. Oluigbo, W. Yaseen, A. J. Kumar, Y. Xu and J. Xie, Ni3Fe nanoparticles enclosed by B-doped carbon for efficient bifunctional performances of oxygen and hydrogen evolution reactions, J. Alloys Compd., 2020, 835, 155267 CrossRef CAS.
- Y. Gao, S. Qian, H. Wang, W. Yuan, Y. Fan, N. Cheng, H. Xue, T. Jiang and J. Tian, Boron-doping on the surface mediated low-valence Co centers in cobalt phosphide for improved electrocatalytic hydrogen evolution, Appl. Catal., B, 2023, 320, 122014 CrossRef CAS.
- B. A. Yusuf, M. Xie, W. Yaseen, C. J. Oluigbo, W. Wei, Y. Xu and J. Xie, Highly stable ultrafine boron–doped NiCo@carbon nanoparticles as a robust electrocatalyst for the hydrogen evolution reaction, ChemElectroChem, 2021, 8, 1337–1348 CrossRef.
- W. Ma, S. Xie, T. Liu, Q. Fan, J. Ye, F. Sun, Z. Jiang, Q. Zhang, J. Cheng and Y. Wang, Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C-C coupling over fluorine-modified copper, Nat. Catal., 2020, 3, 478–487 CrossRef CAS.
- Z. Wu, Q. Li, G. Xu, W. Jin, W. Xiao, Z. Li, T. Ma, S. Feng and L. Wang, Microwave Phosphine–Plasma–Assisted Ultrafast Synthesis of Halogen–Doped Ru/RuP2 with Surface Intermediate Adsorption Modulation for Efficient Alkaline Hydrogen Evolution Reaction, Adv. Mater., 2024, 36, 2311018 CrossRef CAS PubMed.
- J. Zhu, J. Chi, X. Wang, T. Cui, L. Guo, B. Dong, X. Liu and L. Wang, Boosting hydrogen evolution reaction activity of Ru anchored binary oxyhydroxide by F-doping in alkaline seawater, Nano Energy, 2024, 121, 109249 CrossRef CAS.
- X.-Y. Zhang, F.-T. Li, J. Zhao, B. Dong, F.-L. Wang, Z.-X. Wu, L. Wang, Y.-M. Chai and C.-G. Liu, Tailoring the d-band centers of FeP nanobelt arrays by fluorine doping for enhanced hydrogen evolution at high current density, Fuel, 2022, 316, 123206 CrossRef CAS.
- H. Hu, L. Zhou, F. Duan, H. Zhu, H. Gu, S. Lu and M. Du, Hydrogen bonding mediated spillover enabling superior alkaline industrial-level current density hydrogen evolution, J. Mater. Chem. A, 2022, 10, 23294–23303 RSC.
- K. Xu, Y. Sun, X. Li, Z. Zhao, Y. Zhang, C. Li and H. J. Fan, Fluorine-induced dual defects in cobalt phosphide nanosheets enhance hydrogen evolution reaction activity, ACS Mater. Lett., 2020, 2, 736–743 CrossRef CAS.
- K. Chu, F. Wang, X.-l. Zhao, X.-p. Wei, X.-w. Wang and Y. Tian, One-step and low-temperature synthesis of iodine-doped graphene and its multifunctional applications for hydrogen evolution reaction and electrochemical sensing, Electrochim. Acta, 2017, 246, 1155–1162 CrossRef CAS.
- H. Wang, C. Zhang, D. Zhang, L. Jiang, Y. Gao, T. Zhuang and Z. Lv, I Single-Atom Doped P-Rich CoPn Nanocluster@CoP with Enhanced HER, Small, 2024, 20, e2403170 CrossRef PubMed.
- T. Xu, D. Jiao, L. Zhang, H. Zhang, L. Zheng, D. J. Singh, J. Zhao, W. Zheng and X. Cui, Br-induced P-poor defective nickel phosphide for highly efficient overall water splitting, Appl. Catal., B, 2022, 316, 121686 CrossRef CAS.
- X. Tian, S. Zhou, H. Hao, H. Ruan, R. R. Gaddam, R. C. Dutta, T. Zhu, H. Wang, B. Wu and N. P. Brandon, Machine learning and density functional theory for catalyst and process design in hydrogen production, Chain, 2024, 1, 150–166 Search PubMed.
- T. Liu, X. Zhao, X. Liu, W. Xiao, Z. Luo, W. Wang, Y. Zhang and J.-C. Liu, Understanding the hydrogen evolution reaction activity of doped single-atom catalysts on two-dimensional GaPS4 by DFT and machine learning, J. Energy Chem., 2023, 81, 93–100 CrossRef CAS.
- Z. Feng, I. Eiubovi, Y. Shao, Z. Fan and R. Tan, Review of digital twin technology applications in hydrogen energy, Chain, 2024, 1, 54–74 Search PubMed.
- J. Ding, H. Yang, H. Zhang, Z. Wang, Q. Liu, L. Feng, G. Hu, J. Luo and X. Liu, Dealloyed NiTiZrAg as an efficient electrocatalyst for hydrogen evolution in alkaline seawater, Int. J. Hydrogen Energy, 2024, 53, 318–324 CrossRef CAS.
- M. Yang, J. Ding, Z. Wang, J. Zhang, Z. Peng and X. Liu, NiMo-based alloy and its sulfides for energy-saving hydrogen production via sulfion oxidation assisted alkaline seawater splitting, Chin. Chem. Lett., 2025, 110861 CrossRef.
| This journal is © the Partner Organisations 2025 |
