Open Access Article
Open Access ArticleSpiers memorial lecture: Experimental discovery of asymmetric bilayers, and a recent asymmetry example
Gerald W.
Feigenson
Biochemistry and Biophysics, Cornell University, USA. E-mail: gwf3@cornell.edu
First published on 2nd April 2025
Abstract
This introductory Faraday Discussions lecture considers the wide range of subjects that involve asymmetric bilayer membranes. The Discussions organizers have chosen four themes that describe the current interest in asymmetric bilayers. The main part of this introductory lecture starts with the discovery from over 50 years ago that the plasma membrane of mature, human erythrocytes has different composition and properties in each leaflet of the plasma membrane. We also comment on what is newly recognized in the asymmetric membrane field. The asymmetric bilayer is a remarkable state of matter. Newly recognized, we can describe it as a “new state of matter”. Like other biological matter, it evolved to be in its particular form. There is new appreciation for the properties of the asymmetric bilayer, and there has been progress in understanding these emerging properties. Asymmetric lipid bilayers have a remarkable range of characteristics that involve the physically and chemically special connection of the two different monolayer leaflets. Much of this work is an explanation of how the “van Deenen researchers”, over 50 years ago, quantitatively measured the phosphatidylcholine, sphingomyelin, and phosphatidylethanolamine content of the erythrocyte plasma membrane exoplasmic leaflet. Their use of surface chemistry principles was important in proper quantitation, and this introductory lecture explains these research findings in detail. We also present a detailed discussion of one remarkable example of a newly-discovered behavior of asymmetric bilayers, which we term “induced order”. This discovery has an absolute requirement for microscopy image data to reveal the superposition of induced order with the thermodynamic order of liquid-ordered + disordered phase separation.
1. Introduction
This introductory Faraday Discussions lecture considers the surprisingly wide range of subjects that involve asymmetric bilayer membranes. The Discussions organizers have chosen four themes that capture the current interest in asymmetric bilayers, and I will return to these themes towards the end. I will start the main part of this discussion with the discovery over 50 years ago that the plasma membrane of mature, human erythrocytes, referred to as red blood cells or here as “rbc”, have different compositions and properties in each leaflet of their plasma membrane. I will also comment on what is newly recognized in the asymmetric membrane field.This year, 2025, is the 100-year anniversary of Gorter and Grendel's proposal that biological membranes are lipid bilayers: “We propose to demonstrate in this paper that the chromocytes of different animals are covered by a layer of lipoids just two molecules thick”.1 Then, in about a decade after most researchers had accepted this central role for the bilayer in plasma membranes (PM) of living cells, the plasma membrane would already be found to have different phospholipid concentrations in its two bilayer leaflets, and therefore different properties in the two leaflets. We now describe this kind of bilayer as “asymmetric”, and thus different from the symmetric bilayers that had been the focus of so much earlier study.
Many interesting questions involve what is increasingly understood to be the remarkable state of matter of an asymmetric bilayer. Like other biological matter, it evolved to be in its particular form as a bilayer of oriented amphiphilic molecules having polar/charged moieties in water, with nonpolar moieties packed together out of the water. What special capabilities did evolution pack into the asymmetric bilayer? We are here for these Faraday Discussions because there is new appreciation for the properties of the asymmetric bilayer. There has been progress in understanding these emerging properties, as we will see in the papers presented in this discussion. Asymmetric lipid bilayers have a remarkable range of recently appreciated characteristics that involve the physically and chemically special connection of the two monolayer leaflets. We are only now recognizing asymmetric bilayers as a new state of matter, with new properties that are very much in the process of being revealed.2–4 Although I focus here on cell plasma membranes, in this discussion meeting we will explore different kinds of asymmetric bilayers that are not in cells, and a range of their applications to new questions.
When I started graduate school in 1969, I knew of two famous centers for study of lipid bilayers, one with Laurens L. M. van Deenen5 at its center in Utrecht, and one with Thomas Thompson at its center at the UVa in Charlottesville.6 I started reading papers from both groups. I soon encountered the name of Dennis Chapman7,8 (1927–1999). In the 1960s his interest in spectroscopy and crystallography was turning toward surface chemistry, and a meeting with L. L. M. van Deenen led to Chapman's new focus on phospholipid phases, which he recognized as having the liquid-crystalline type of order.9 Chapman investigated the influence of hydrocarbon chain lengths, double bonds, headgroup types, and cholesterol fraction on “bilayer fluidity”. Chapman had studied phase behavior of glycerides and soaps by infrared spectroscopy. He found that outside surfaces of cells had lipids with no net charge, but that the inner surface of cells was negative. His stated goal was to make biomedical devices that were bio-compatible.7 He was also a pioneer in the use of differential scanning calorimetry of model membranes.8
In living cells the most important case of an asymmetric bilayer, and indeed the only closely studied case in living cells, is the cell plasma membrane.10–19 Yet finding out the compositions of each leaflet of a PM has proven difficult, and includes efforts over more than 50 years. Complicating these efforts, researchers wish to elucidate the PM only of living cells, because their PM changes when the cell dies.20–22
A potential goal for these discussions: given an increasingly complex picture of the asymmetric bilayer, it would be useful to have starting points on which we can all agree. On the other hand, we are not assembled here in order to agree! But I will attempt to sketch some starting points.
Significant progress is happening now,4 50 years after researchers first recognized asymmetry of rbc plasma membrane bilayers.13 Much bilayer physical chemistry had been worked out by Chapman, by Thompson and many others, before plasma membrane asymmetry was established, including general characteristics of lipid order and motion and cholesterol behaviors.6,8 These types of order are characteristic of the class of order termed “liquid crystal”.9 Note that this class of order is not at all necessarily a liquid or a crystal, although many researchers even today continue to mistake the name of this category as a literal description of phase order and motion. And for asymmetric bilayers there must be new categories of order, such as lipids of the cytoplasmic leaflet that are organized by an apposing Lo domain in the exoplasmic leaflet into “induced order domains”, with probable but as yet unknown connections of such induced order domains to ordered proteins of, for example, an actin–myosin polymer.23,24
2. How asymmetry is established
I will refer now to two vexing issues with experimental asymmetry research of the plasma membranes found in nature: The first difficulty is the purity of the PM for study. For all living cells, with perhaps the single exception of the rbc, obtaining a pure preparation of the plasma membrane has not been possible, mainly because of contamination with the more numerous other membranes inside the cell, which are typically present in many-fold excess over the PM.25The second problem is to chemically analyze each leaflet of this asymmetric rbc membrane. Methods used to analyze each leaflet typically include either (1) chemically reactive small molecules, or (2) phospholipase enzymes. As researcher Alain Zachowsky concisely summarized regarding measurement of rbc asymmetry, “None of the methods utilized is ‘perfect’. Each one has its own drawbacks and advantages”.26 But so far, all are in at least broad agreement with the asymmetric leaflet compositions determined by the van Deenen researchers 50 years ago, as shown in Fig. 1.
 | ||
Fig. 1 From Verkleij et al. 1973 (ref. 13) originally BBA, 323![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 Fig. 13. Note that error bars, which are likely significant, are not available. 190 Fig. 13. Note that error bars, which are likely significant, are not available. | ||
Methods to quantitatively measure the rbc lipid asymmetry of PM leaflets have provided somewhat less quantitation than is sought for some proposed research that intrigues us, about how the two different leaflets interact.4,27 I will attempt to clarify this troubling puzzle of asymmetry quantitation that is affecting our future progress in understanding this new state of matter.
Many of us have done both chemical synthesis and chemical analysis, myself included. I can report that “organic chemistry quantitation” can be difficult to achieve at ±2%, with some exceptions, for example, thin layer chromatography, TLC. When steps in addition to TLC are required, even ±5% can be a challenge. For quantitating asymmetry of a plasma membrane, we have an additional and significant problem that I will address here: the difficulty to establish that the catalyzed hydrolysis reactions of the exoplasmic leaflet lipids have gone to completion. This confronts us with a different kind of error that must be addressed, a systematic error.
With living cells, precise lipid analysis involves knowing that the PM is intact, and has not become leaky nor rearranged its leaflet compositions. The rbc, with its readily detected content of hemoglobin, is perhaps the best possible living cell for affirming an intact plasma membrane (Fig. 2).
Another aspect is that even the most simple plasma membrane has many chemical components, and also presents the investigator with a material that is inherently difficult to work with, being inhomogeneous in having both an aqueous part, and a water insoluble part that is organized into the two monolayer leaflets. Researchers seek a lipid analysis of each monolayer, exoplasmic and cytoplasmic.
The starting point for lipid quantitation is what is most accurately measured, the total of each PM lipid, without regard to leaflet assignment. This measurement for each lipid does not require any enzyme or reactive chemical agent, merely the solubilization of all rbc lipids in organic solvent, typically in methanol and chloroform.17 One such 2-dimensional chromatogram of the extracted rbc lipids by the van Deenen researchers is shown in Fig. 3. Thus, for many decades, researchers have known with perhaps ±2% accuracy the total quantity of each phospholipid, as well as the total cholesterol, in the rbc plasma membrane.
 | ||
| Fig. 3 2D TLC of MeOH then CHCl3 extraction of red blood cell lipids showing each phospholipid. Originally Fig. 3 of Roelefsen & Zwaal 1976.18 By phosphate assay of each scraped spot,17 quantitation is achieved. | ||
Next, and more challenging by far, is to find for each lipid the fraction that is in the exoplasmic leaflet, and then by subtraction from the known total for that phospholipid, find its fraction in the cytoplasmic leaflet. Incomplete lipase-catalyzed reaction of phospholipids in the exoplasmic leaflet would lead to the unreacted fraction of those lipids being wrongly assigned to the cytoplasmic leaflet, thus potentially a significant systematic error.
In his study in 1972, Mark Bretscher found little PE (phosphatidylethanolamine) and no PS (phosphatidylserine) in the exoplasmic rbc leaflet, based on little reaction with a non-penetrating, strongly radioactive probe that he devised, FMMP shown in Fig. 4. Bretscher had synthesized FMMP originally for protein study.11 FMMP reacts most strongly with primary amines, thus is especially sensitive for PE and PS. Gordesky and Marinetti in 1973 (ref. 12) used the now more familiar non-penetrating probe TNBS, Fig. 5, to find no PS in the exoplasmic PM leaflet, but they did find about 30% of total PE to be in the exoplasmic leaflet.
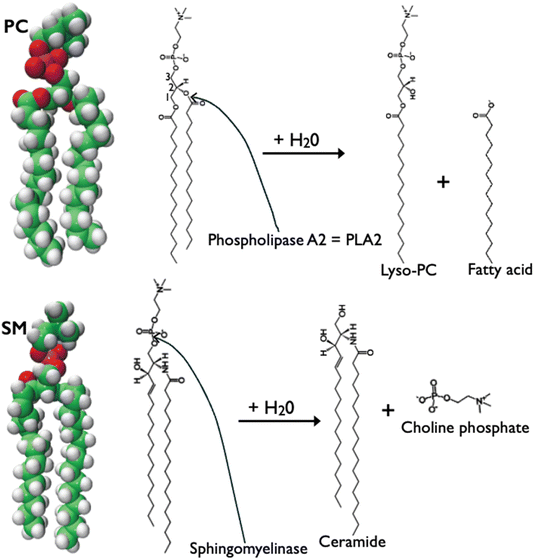
Thus, a key point for rbc quantitative lipid asymmetry analysis is the need for 100% hydrolysis of all PC and all SM (sphingomyelin) in the exoplasmic leaflet via the lipase enzymes shown in Fig. 6, added to the outside of the rbc. Fig. 7 shows where these lipase reactions take place on the rbc surface. If 100% hydrolysis is not achieved, then given the method for the quantitation of each lipid from TLC-determined totals for each, some PC (phosphatidylcholine) and SM actually in the exoplasmic leaflet would be wrongly assigned to the cytoplasmic leaflet. Yet a significant problem for those of us now reading the many papers from the van Deenen group is that key experimental details, for example, regarding manipulations of rbc's, lipid extraction and analysis, and especially monolayer studies of lipase enzymes, are scattered among many papers in ref. 13–19, 26 and 28–30 but also in other publications from the van Deenen groups.
Putting aside these inconveniences for us readers, these researchers made the important discovery that the lipase reaction catalyzed by PLA2 does not go to completion unless the exoplasmic leaflet monolayer pressure is low enough for the PLA2 to function. This enzyme must interact with the monolayer, and monolayer pressure is a variable. Fortuitous for these measurements, a pressure reduction occurs during the next step,31 SMase action, as measured by monolayer studies with a Wilhelmy plate and Langmuir trough.28–30 In brief, the sequence of first PLA2 action, followed by SMase action, followed by additional PLA2 catalysis, is important, as illustrated in Fig. 8. This behavior of exoplasmic monolayer leaflet pressure increasing from PLA2 reaction product formation, followed by exoplasmic leaflet pressure decreasing because of SMase reaction product formation, is thus a reasonable, but not directly proven proposal,33–37 even with the catalysis observations per se not in question. More recent PLA2 studies include molecular dynamics simulations to reveal monolayer pressure to be involved at several stages of PLA2 insertion into the bilayer and at several steps of enzymatic activity.33,35–37
 | ||
| Fig. 8 Illustrations of molecular models (from Avanti Polar Lipids website) showing that PLA2-catalyzed hydrolysis reaction of PC increases monolayer pressure with both products remaining in the bilayer, whereas SMase enzymatic reaction decreases monolayer pressure, with choline phosphate, soluble in water, leaving the monolayer, and product ceramide32 being a more condensed lipid than is SM. Double headed arrows give approximate relative indication of the lipid area. | ||
To state again the most important difficulty for lipid quantitation, enzyme-catalyzed lipase reactions with lipids on the PM surface must go to completion. Otherwise, unreacted lipids on the surface, especially PC and PE, would be mistakenly assigned to be inner leaflet lipids. However, separate use of PLA2 and SMase is not sufficient for these monolayer reactions to go to completion. The van Deenen groups showed that pressure builds up in the exoplasmic leaflet as reaction products fatty acid and lyso-PC accumulate: PLA2-catalyzed hydrolysis slows, then stops, interpreted initially to be caused by lack of PLA2 penetration into the monolayer. Recent studies confirm the decreased activity of PLA2 at high monolayer pressure, but offer additional explanations32–36 beyond simply PLA2 monolayer penetration. The monolayer pressure seems to be significantly relieved by SMase action13–19 as illustrated in the pictures in Fig. 8. Hence, the importance of the sequence of steps to be first PLA2 catalysis of PC hydrolysis, where van Deenen researchers discovered leaflet pressure unavoidably builds up, followed by SMase catalysis, where leaflet pressure drops. These studies showing a role for monolayer pressure in chemical reactions within a cell plasma membrane might be the first such chemical implications for monolayer pressure in cell biology. An excellent discussion of monolayer pressure can be found in ref. 38.
3. Phospholipid quantitation numbers
In this way, 76% of the total PC was found to be in the exoplasmic leaflet, including by use of the most efficient PLA2 for rbc lipids, from Naja naja, the Indian king cobra.13 82% of the total PM SM is degraded by SMase from Staph. aureus, and is thus assigned to the exoplasmic leaflet. But the source of the PLA2 also matters, with PLA2 from bee venom digesting only 58% of total rbc PC,4,17 leading to an incorrect conclusion that the unhydrolysed PC is entirely in the cytoplasmic leaflet. Further, by use of the lipase sequence of first, catalyzed PC hydrolysis by PLA2 from Naja naja, then catalyzed hydrolysis of SM by SMase which reduces monolayer pressure, followed by further PLA2-catalyzed hydrolysis of the remaining PC, 20% of all plasma membrane PE is then also hydrolyzed. Therefore another important asymmetry result is that approximately 20% of all plasma membrane PE seems to be in the exoplasmic leaflet,13,14 in agreement with the external TNBS reaction with PE.12 From these experiments, PLA2 catalyzed hydrolysis of substrate PE might be more efficient in the unusual new monolayer of lyso-PC, fatty acid, and ceramide.Without attention to these leaflet pressure effects on PC and PE hydrolysis catalyzed by PLA2, not only would about 8% of the total PC not be assigned to the exoplasmic leaflet, but all of that unreactive 8% would be assigned to the cytoplasmic leaflet. Use of PLA2 from bee venom,17 being a less efficient PLA2 when applied to the rbc, would further miss-assign 18% of the total PC to be in the cytoplasmic leaflet, an especially large error when a researcher seeks to establish numbers of phospholipids in each leaflet.4,39 Lack of attention to these issues of PLA2 source and monolayer pressure thus causes systematic errors, not random errors, in assignment of PC and PE to the two leaflets.
For counting all phospholipids in each leaflet, the van Deenen studies yield approximately equal numbers of phospholipids in each leaflet.13 For counting all phospholipids in each leaflet, original data from Lorent et al. (2020)4 together with added quantification of other minor lipids and GPI anchored proteins to estimate the total lipid abundance of both leaflets from Doktorova et al. (2025)39 found 2× more phospholipids in the cytoplasmic leaflet.
The van Deenen researchers concluded that PLA2 fails to penetrate a monolayer to reach its substrate when monolayer pressure is too high. Specifically, their monolayer studies showed that for N. naja PLA2, at film pressure 35 erg per sq cm = 35 mN m−1, PLA2 stops working.28–30 This behavior of PLA2 at higher monolayer pressures has been confirmed over the ensuing decades, even while the molecular-level explanation is more complex than simply PLA2 penetration of the monolayer (Fig. 9).33–37
 | ||
| Fig. 9 PLA2 and its target lipid, modified from Fig. 4 and 5 of Dennis et al..34 (A) Water-soluble PLA2 penetrates exoplasmic monolayer to bind its PC substrate; (B) 2-chain PC with sn-2 acyl linkage in active site of PLA2 enzyme.33–37 Surrounding bilayer not shown. | ||
4. Asymmetric bilayers beyond the rbc plasma membrane
Did the van Deenen researchers get it right?! Reading their papers 50 years ago, I did not notice the importance of the sequence: to first add PLA2 to the rbc, then wait about one hour for catalysis to proceed, then to add SMase to catalyze SM hydrolysis, then finally to allow PLA2 hydrolysis to proceed again. This attention to pressure in a monolayer leaflet calls attention to surface chemistry. This author needed immensely useful discussions with a former van Deenen group member, Gerrit van Meer, in order to clarify these important experimental details.In the many decades that followed studies by the van Deenen researchers, techniques have been described that yield more convenient forms for study of plasma membranes, such as latex beads or glass slides coated with adherent plasma membrane, or inducing cells to create closed vesicle “GPMVs” formed from plasma membrane.40–43 Yet, no improvement of the method for PLA2-catalyzed hydrolysis of PC in the rbc exoplasmic leaflet seems to have been published.
What is the interest in asymmetric bilayers now, in 2025? We will be hearing and discussing the enormous range of thinking, computation, and wet-lab experiments regarding asymmetric bilayers. For eukaryotic, and especially mammalian plasma membranes, the exoplasmic leaflet has a large fraction of high melting temperature phospholipids, most notably sphingomyelin, whereas such lipids are rare in other cell membranes. Studies of multicomponent mixtures having a high melting phospholipid have shown that rich phase behavior is typical.44 An interesting case of asymmetric bilayer behavior occurs when one leaflet is phase-separated, but not the other leaflet. One example is when liquid-ordered (Lo) and disordered (Ld) domains coexist in an exoplasmic leaflet.
5. The unexpected phenomenon of induced order
A specific result from asymmetric bilayer imaging studies, with clear implications for asymmetric cell plasma membrane behaviors, is precisely this case of Lo + Ld coexistence in the exoplasmic leaflet of a model mammalian plasma membrane. We have found that induced order domains are formed in a model cytoplasmic leaflet of the apposed monolayer. This is work done by my former colleague Dr Thais Enoki, currently a Young Investigator at the University of Sao Paulo Institute of Physics. This and other work from Dr Enoki reveal the immense value of image data to establish the properties of asymmetric bilayers, because with different dyes localized to each leaflet, individual properties of each leaflet are clearly marked, and moreover clearly show the spatial relationships of the Lo phase in one leaflet to the induced domain in the apposed leaflet.A plasma membrane case of special interest is when an exoplasmic leaflet has coexisting Lo + Ld phases (“raft + non-raft”), with the cytoplasmic leaflet being a one-phase disordered mixture of phospholipids, predominantly PE and PS, along with cholesterol. A behavior of such asymmetric bilayers is that the Lo phase in the exoplasmic leaflet can induce an ordered phase in the otherwise disordered phase of the cytoplasmic leaflet.23,45–50 This behavior of “induced order” across leaflets was first observed in model systems more than 15 years ago by researchers in Lukas Tamm's group, by separately imaging each leaflet of asymmetric bilayers.45,46 This induced order seems to be a normal and characteristic property of asymmetric bilayers: an ordered Lo phase induces “Lo-like” order in DOPC/chol, whereas an ordered Lβ (gel) phase induces “Lβ-like” order in DOPC in the other leaflet.50
By use of multicomponent lipid mixtures to model the cytoplasmic leaflet, we plan to find out which of these lipids become the induced ordered domains, and which remain disordered. By abundance, the predominant cytoplasmic leaflet lipids are PS, PE and cholesterol. We also want to find out the behavior of the quantitatively minor but multifunctional phospholipid PIP2 in these asymmetric bilayers.51 These questions involve, for example, whether or not PS in a cytoplasmic leaflet is concentrated into a more negatively charged and ordered domain than previously appreciated, and whether PIP2 co-localizes with such concentrated PS. Other researchers are now asking similar questions by use of data that do not require imaging.52–55
We have so far obtained data only with the simple system, DOPC/chol, that nonetheless is an informative model for a disordered cytoplasmic leaflet.48–50,56 Our long term goal is to understand the more complex mixture of PS and PE, both having PUFA-containing chains, together with PIP2 and cholesterol in a disordered cytoplasmic multicomponent model mixture. DOPC is a useful model for a phospholipid with PUFA-containing chains, in part because DOPC has been found to have phase behaviors in multi-component mixtures that are rather like the phase behaviors of PUFA-chain phospholipids,57 and in part because DOPC, unlike lipids having PUFA chains, is stable to oxygen and to intense confocal microscope illumination.58
To obtain good image data it is useful to prepare asymmetric bilayers that are sufficiently large, tens of microns. Generic hemifusion of a supported lipid bilayer with a GUV having the composition that becomes the outer leaflet of the aGUV, is shown in Fig. 10. Confocal fluorescence microscopy images below in Fig. 12 show induced order domains in a DOPC/chol leaflet being precisely superimposed across from the thermodynamically stable Lo domains of an aGUV leaflet of (DSPC or SM)/DOPC/chol. With this preparation, the symmetric GUV's stable Lo + Ld coexistence becomes the phase-separated inner leaflet of a large asymmetric vesicle (Fig. 11).
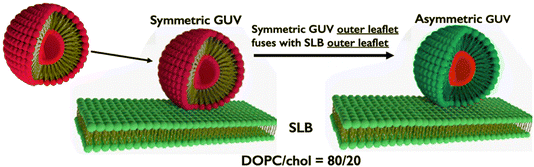 | ||
| Fig. 10 aGUV preparation by use of hemifusion. The symmetric GUV hemifuses with lipids of a supported lipid bilayer. The original GUV inner leaflet is retained, a new aGUV outer leaflet forms with lipids of the SLB composition. From ref. 49 (Fig. 1). | ||
 | ||
| Fig. 11 Left: schematic of an aGUV. The upper right image illustrates the two compositionally different leaflets, with cholesterol as yellow rectangles. This outer leaflet was formed from an SLB of DOPC/chol = 80/20 and includes the green fluorescent dye, TopFluor-PC. Lower right image: the phospholipids and cholesterols shown as more realistic molecular models. The thermodynamically stable Lo and Ld phases are indicated, and the induced order domains are enclosed by black rectangles.23 Modified from Fig. 2 of ref. 23. | ||
Confocal imaging data of the DOPC/chol leaflet, Fig. 12, show that the brightly green-colored Ld region is superimposed over the brightly red-colored Ld regions of the phase-separated (DSPC or SM)/DOPC/chol inner leaflet of the aGUV. The minimally-colored Lo domain of (DSPC or SM)/DOPC/chol and the induced order domain of DOPC/chol are also superimposed. Here, because of the implications for cell biology, we emphasize Lo domains causing the order, which lead to “Lo-like” induced order. However, when the cholesterol fraction of the phase-separated monolayer is low, below about 0.16, the coexisting phases are Lβ + Ld, and the induced order domain has Lβ-like order.57
 | ||
| Fig. 12 Fluorescence image data. One symmetric GUV in (A), and three examples of induced order (B–D). Confocal images show green domains of the outer aGUV leaflet to be precisely superimposed on the red domains of the inner leaflet. (A) Symmetric GUV of DSPC/DOPC/chol having phase-separated Lo + Ld. Image is the GUV equator. Red DiD indicates the Ld phase, the connecting Lo phase is dark. Lo order is intrinsic thermodynamic order, not induced; (B) Z-stack of an aGUV with one large Ld and one large Lo domain. DiD in the inner leaflet labels Ld red, leaving Lo dark. TFPC in the outer leaflet labels Ld green, leaving induced order domain dark. This induced order domain is entirely DOPC/chol; (C) aGUV equator showing 3 phase domains. The inner leaflet red DiD presents Ld, leaving Lo dark. The outer leaflet green TFPC prefers Ld, leaving the induced order domain dark; (D) Z-stack of aGUV showing many phase domains. DiD in the inner leaflet labels Ld red, leaving Lo dark. TFPC in the outer leaflet labels Ld green, leaving the induced order regions dark. Scale bars indicate 5 microns. Modified from Fig. 3 and 5 of ref. 59. | ||
The two monolayer aGUV leaflets meet only at the bilayer midplane. This midplane is almost entirely methyl and methylene groups. This “chemically simple” midplane area seems to be the site that determines the energy of the asymmetric bilayers.23 One way to illustrate clearly this midplane role is shown in Fig. 13.
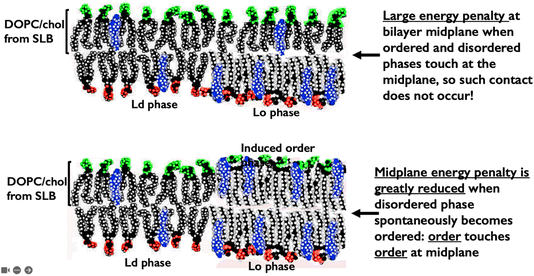 | ||
| Fig. 13 Importance of the asymmetric bilayer midplane area. The upper image shows contact at the bilayer midplane of disordered with ordered phases that would incur a large energy penalty. The lower image shows induced order domains to appear spontaneously to reduce this energy penalty. This would be the same midplane energy penalty that in symmetric GUVs keeps phase-separated domains in their familiar perfect superposition.44 | ||
5.1 Summary of induced order image data in asymmetric bilayers
(1) Hemifusion of a symmetric GUV with a supported lipid bilayer creates an aGUV model of a cell plasma membrane, which enables the obtaining of image information by confocal fluorescence microscopy.(2) An Lo phase domain induces order in an otherwise highly disordered apposing DOPC/chol monolayer. The mechanism could be that induced order reduces a large energy penalty at the bilayer midplane for contact of order with disorder.
(3) Implication: membrane rafts in a PM exoplasmic leaflet could induce order in a cytoplasmic leaflet that has mostly PUFA-containing lipids plus cholesterol.
(4) If the cytoplasmic leaflet has induced order domains coexisting together with disordered domains, then PE, PS, PIP2 and membrane proteins would partition between these two different phase environments. We do not yet know whether cytoplasmic PE or cytoplasmic PS becomes the induced order domain in actual cytoplasmic leaflets.
6. Organization of these discussions into four themes
Here I introduce the four session themes of this meeting, and add my own thoughts for each theme (shown in italics).6.1. Plasma membrane asymmetry and lipid homeostasis
The omics era has provided much data on phospholipids, sphingolipids, sterols and proteins located in different membranes, although information on their functional relevance remains limited. This session will address challenges such as measuring trans-leaflet lipid distribution, including the spatiotemporal relation to overall lipid composition, and dissecting secondary lipid messengers from lipid ‘players’ with respect to bulk membrane properties.(a) For wet lab studies, obtaining a purity of membrane without losing membrane integrity (for example, plasma membrane completely purified away from endoplasmic reticulum), is exceedingly difficult. 60
(b) To establish spatiotemporal relationships, image data are helpful. 49
6.2. Engineering plasma membrane mimics
Quantification of physicochemical properties of realistic plasma membrane mimics hinges on the controlled production of asymmetric bilayers in the presence and absence of integral or peripheral membrane proteins. In this theme, recently developed asymmetric membrane preparation procedures and how these might be improved will be discussed.(a) Lipid exchange via exchange proteins can be useful for both preparation and analysis of asymmetric bilayer models.61,62
(b) New methods involve new uncertainties. Almost any recently developed asymmetric membrane preparation could be tested for a close result to the known asymmetry established for red blood cells.
6.3. Structure and dynamics of asymmetric membranes
Principles from physics and physical chemistry have now been placed within a framework that enables calculation of both membrane tension and membrane curvature. In this session we will seek to obtain physicochemical insights from lipid-only plasma membrane mimics. The session will include minimum realistic mimics of mammalian or bacterial plasma membranes, as well as the role of cholesterol in tuning and maintaining membrane asymmetry.(a) For wet-lab studies, surface chemistry, especially monolayer methods with a Langmuir trough or similar device, can provide quantitation of monolayer pressure for individual monolayer compositions. 63
(b) Some preparation methods involve organic solvents or detergents that can remain in the bilayer. 64
6.4. Proteins in asymmetric membranes
This session will focus on integral proteins functionally reconstituted into asymmetric membranes. Topics will include folding and reconstitution of proteins in asymmetric lipid membranes, control of protein directionality, and stability of lipid asymmetry in the presence of integral proteins.(a) Membrane models include the parameter of bilayer thickness, and when biological matching to proteins or else leaflet coupling is paramount, DPPC chains might not be long enough. DPPC chains are shorter than those of Hi-Tm PM lipids found in mammalian cells. Instead, DSPC but especially SM are good Hi-Tm lipid choices that closely match natural Hi-Tm lipid length. 65
(b) When organic solvents are used for protein solubilization, the protein can be trapped for weeks in a non-physiological structure. 66
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts of interest to declare.References
- E. Gorter and F. Grendel, J. Exp. Med., 1925, 41(4), 439–443 CrossRef CAS PubMed.
- S. L. Foley, M. Varma, A. Hossein and M. Deserno, Emerging Top. Life Sci., 2023, 7(1), 95–110 CrossRef CAS PubMed.
- R. Lipowsky, R. Ghosh, V. Satarifard, A. Sreekumari, M. Zamaletdinov, B. Rózycki, M. Miettinen and A. Grafmüller, Biomolecules, 2023, 13, 926–987 CrossRef CAS PubMed.
- J. H. Lorent, K. R. Levental, L. Ganesan, G. Rivera-Longsworth, E. Sezgin, M. Doktorova, E. Lyman and I. Levental, Nat. Chem. Biol., 2020, 16, 644–652 CrossRef CAS PubMed.
- L. L. M. van Deenen, Prog. Chem. Fats Other Lipids, 1966, 8, 1–127 CrossRef.
- M. B. Sankaram and T. E. Thompson, Proc. Natl. Acad. Sci. USA, 1991, 88, 8686–8690 CrossRef CAS PubMed.
- D. Chapman, Langmuir, 1993, 9, 39–45 CrossRef CAS.
- D. Chapman, Q. Rev. Biophys., 1975, 8, 185–235 CrossRef CAS PubMed.
- P.-G. de Gennes and J. Prost, The Physics of Liquid Crystals, Oxford University Press, 1993, vol. 83 Search PubMed.
- M. S. Bretscher, Nature New Biol., 1972, 236, 11–12 CrossRef CAS PubMed.
- M. S. Bretscher, J. Mol. Biol., 1972, 71, 523–528 CrossRef CAS PubMed.
- S. E. Gordesky and G. V. Marinetti, Biochem. Biophys. Res. Commun., 1973, 50, 1027–1031 CrossRef CAS PubMed.
- A. J. Verkleij, R. F. A. Zwaal, B. Roelofsen, P. Comfurius, D. Kastelijn and L. L. M. van Deenen, Biochim. Biophys. Acta, 1973, 323, 178–193 CrossRef CAS PubMed.
- C. M. Colley, R. F. A. Zwaal, B. Roelofsen and L. L. M. van Deenen, Biochim. Biophys. Acta, 1973, 307, 74–82 CrossRef CAS PubMed.
- R. Verger, M. C. E. Mieras and G. H. de Haas, J. Biol. Chem., 1973, 248(11), 4023–4034 CrossRef CAS PubMed.
- R. F. A. Zwaal, B. Roelofsen and C. M. Colley, Biochim. Biophys. Acta, 1973, 300, 159–182 CrossRef CAS PubMed.
- R. F. A. Zwaal, B. Roelofsen, P. Comfurius and L. L. M. van Deenen, Biochim. Biophys. Acta, 1975, 406, 83–96 CrossRef CAS PubMed.
- B. Roelofsen and R. F. A. Zwaal, Methods in Membrane Biology, ed. E. D. Korn, Springer Science, 1976, ch. 2 Search PubMed.
- J. A. F. Op den Kamp, Annu. Rev. Biochem., 1979, 48, 47–71 CrossRef CAS PubMed.
- J. F. R. Kerr, A. H. Wyllie and A. R. Currie, Br. J. Cancer, 1972, 26, 239–257 CrossRef CAS PubMed.
- A. H. Wyllie, J. Clin. Pathol., 1974, 27, 35–4210 CrossRef.
- V. A. Fadok, D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton and P. M. Henson, J. Immunol., 1992, 148(7), 2207–2216 CrossRef CAS.
- G. W. Feigenson, J. Huang and T. A. Enoki, J. Am. Chem. Soc., 2023, 145, 21717–21722 CrossRef CAS PubMed.
- I. Meenakshi, S. M. Rao, S. Mayor and R. Sowdhamini, Front. Cell Dev. Biol., 2023, 11, 1168050 CrossRef PubMed.
- T. D. Pollard, W. C. Earnshaw, J. Lippincott-Schwartz and G. Johnson, Cell Biology E-Book, Elsevier Health Sciences, 2022 Search PubMed.
- A. Zachowski, Biochem. J., 1993, 295, 1–14 CrossRef PubMed.
- M. Doktorova, J. L. Symons, J. Schegel, E. Sezgin, H. Wang, E. R. Lyman, K. R. Levental and I. Levental, Biophys. J., 2024, 123(3), 512a CrossRef.
- R. Demel, W. S. M. Geurts van Kessel, R. F. A. Zwaal, B. Roelofsen and L. L. M. van Deenen, Biochim. Biophys. Acta, 1975, 406, 97–107 CrossRef CAS PubMed.
- R. A. Demel, Methods Enzymol., 1974, 32, 539–544 CAS.
- R. Verger and G. H. de Haas, Chem. Phys. Lipids, 1973, 10, 127–136 CrossRef CAS PubMed.
- F. Pattus, A. J. Slotboom and G. H. de Haas, Biochemistry, 1979, 18(13), 2691–2697 CrossRef CAS PubMed.
- A. Alonso and F. Goni, Annu. Rev. Biophys., 2018, 47, 633–654 CrossRef CAS PubMed.
- S. A. Khan and M. A. Ilies, Int. J. Mol. Sci., 2023, 24, 1353–1388 CrossRef CAS PubMed.
- E. A. Dennis, J. Cao, Y. H. Hsu, V. Magrioti and G. Kokotos, Chem. Rev., 2011, 111, 6130–6185 CrossRef CAS PubMed.
- E. A. Dennis, J. Biol. Chem., 2022, 298, 101873 CrossRef CAS PubMed.
- S. Suladze, S. Cinar, B. Sperlich and R. Winter, J. Am. Chem. Soc., 2015, 137, 12588–12596 CrossRef CAS PubMed.
- A. S. Alekseeva, P. E. Volynsky, N. A. Krylow, V. P. Chernikov, E. L. Vodovozova and I. A. Boldyrev, Biochim. Biophys. Acta, 2021, 1863, 183481 CrossRef CAS PubMed.
- B. Roelofsen and J. A. Op den Kamp, Membrane Biogenesis, Springer, Berlin Heidelberg, 1988, pp. 1–13 Search PubMed.
- M. Doktorova, J. L. Symons, X. Zhang, H.-Y. Wang, J. Schlegel, J. H. Lorent, F. A. Heberle, E. Sezgin, E. Lyman, K. R. Levental and I. Levental, Cell, 2025 DOI:10.1016/j.cell.2025.02.034.
- V. D. Vacquier, Dev. Biol., 1975, 43, 62–74 CrossRef CAS PubMed.
- M. G. Wetzel and E. D. Korn, J. Cell Biol., 1969, 43, 90–104 CrossRef CAS PubMed.
- K. R. Levental and I. Levental, Curr. Top. Membr., 2015, 75, 25–57 CAS.
- B. G. Monasterio, N. Jimenez-Rojo, A. B. Garcia-Arribas, H. Riezman, F. Goni and A. Alonso, Int. J. Mol. Sci., 2020, 21, 2643 CrossRef CAS PubMed.
- J. Korlach, P. Schwille, W. W. Webb and G. W. Feigenson, Proc. Natl. Acad. Sci. USA, 1999, 96, 8461–8466 CrossRef CAS PubMed.
- V. Kiessling, C. Wan and L. K. Tamm, Biochim. Biophys. Acta, 2009, 1788, 64–71 CrossRef CAS PubMed.
- C. Wan, V. Kiessling and L. K. Tamm, Biochemistry, 2008, 47(7), 2190–2198 CrossRef CAS PubMed.
- Q. Wang and E. London, Biophys. J., 2018, 115(4), 664–678 CrossRef CAS PubMed.
- E. London, Acc. Chem. Res., 2019, 52(8), 2382–2391 CrossRef CAS PubMed.
- T. A. Enoki and G. W. Feigenson, Biophys. J., 2019, 117(6), 1037–1050 CrossRef CAS PubMed.
- T. A. Enoki, J. Wu, F. A. Heberle and G. W. Feigenson, Biochim. Biophys. Acta, 2021, 1863(6), 183586 CrossRef CAS PubMed.
- Y. Wen, V. M. Vogt and G. W. Feigenson, Annu. Rev. Biochem., 2021, 90, 681–707 CrossRef CAS PubMed.
- F. S. Leomil, M. Stephen, S. Pramanik, K. A. Riske and R. Dimova, Langmuir, 2024, 40, 4719–4731 CrossRef CAS PubMed.
- E. London, Membranes, 2022, 12, 870 CrossRef CAS PubMed.
- M. Krompers and H. Heerklotz, Membranes, 2023, 13, 267 CrossRef CAS PubMed.
- G. Pabst and S. Keller, Trends Biochem. Sci., 2024, 49, 333–345 CrossRef CAS PubMed.
- G. W. Feigenson and T. A. Enoki, Biophys. J., 2023, 122(6), 925–930 CrossRef CAS PubMed.
- T. M. Konyakhina and G. W. Feigenson, Biochim. Biophys. Acta, 2016, 1858, 153–161 CrossRef CAS PubMed.
- N. F. Morales-Penningston, J. Wu, E. R. Farkas, S. L. Goh, T. M. Konyakhina, J. Y. Zheng, W. W. Webb and G. W. Feigenson, Biochim. Biophys. Acta, 2010, 1798, 1324–1332 CrossRef CAS PubMed.
- T. A. Enoki and G. W. Feigenson, Biochim. Biophys. Acta, 2022, 1864, 183995 CrossRef CAS PubMed.
- D. F. H. Wallach and P. S. Lin, Biochim. Biophys. Acta, 1973, 300, 211–254 CrossRef CAS PubMed.
- P. E. DiCorleto, J. B. Warach and D. B. Zilversmit, J. Biol. Chem., 1979, 254, 7795–7802 CrossRef CAS PubMed.
- K. W. A. Wirtz and L. L. M. van Deenen, Trends Biochem. Sci., 1977, 2, 49–51 CrossRef CAS.
- J. J. Giner-Casares, G. Brezesinski and H. Mohwald, Curr. Opin. Colloid Interface Sci., 2014, 19, 176–182 CrossRef CAS.
- D. Lombardo and M. A. Kiselev, Pharmaceutics, 2022, 14, 543 CrossRef CAS PubMed.
- R. G. Cutler and M. P. Mattson, Mech. Ageing Dev., 2001, 122, 895–908 CrossRef CAS PubMed.
- W. R. Veatch and E. R. Blout, Biochemistry, 1974, 13, 5257–5264 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |