Open Access Article
Open Access ArticleAdvancements in removing common antibiotics from wastewater using nano zero valent iron
Shuxian Weia,
Chuan He b,
Lanyue Zhanga,
Canhua Li
b,
Lanyue Zhanga,
Canhua Li *ac,
Jiamao Li*d and
Gang DU
*ac,
Jiamao Li*d and
Gang DU a
a
aSchool of Metallurgical Engineering, Anhui University of Technology, Maanshan, Anhui 243002, China. E-mail: licanhua1979@163.com
bJiuquan Vocationl and Technical College, Jiuquan, GanSu 735000, China
cKey Laboratory of Metallurgical Emission Reduction & Resources Recycling (Ministry of Education), Anhui University of Technology, Maanshan, Anhui 243002, China
dSchool of Materials Science and Engineering, Anhui University of Technology, Maanshan, Anhui 243002, China. E-mail: lijiamao@ahut.edu.cn
First published on 20th August 2024
Abstract
The pollutants such as heavy metals, organic matter, and nitrates in soil and water pose challenges to environmental remediation technology. Nano zero valent iron has shown enormous potential in the field of environmental remediation due to its excellent adsorption performance. By using carbon based materials, rock minerals, biomolecules, etc., as supporting materials for nZVI, and through structural and performance modifications, its performance has been successfully optimized, reducing defects such as aggregation and easy oxidation of the material. This article compares and summarizes the modification effects of different loadings on nZVI, and comprehensively reviews the latest progress, preparation methods, and application of nZVI particles in soil and water remediation. Specifically, this article explores in detail the impact and mechanism of nZVI particles in commonly used antibiotics contaminated environments. Firstly, the combination methods of different types of materials with zero valent iron, as well as the synthesis methods and application scenarios of nZVI, were integrated. Secondly, the interaction mechanism between pollutants and nZVI was introduced in detail, including adsorption, redox reactions, and co-precipitation. Subsequently, environmental factors that affect repair efficiency were emphasized, such as pH value, coexisting components, oxygen, contact time, and temperature. Finally, the challenges faced by the application of nZVI in actual polluted soil and water bodies, as well as the prospects for its long-term efficacy and safety evaluation, are proposed to promote further development in the future.
1 Introduction
Antibiotics are widely used in medicine for disease prevention and control due to their antibacterial, and growth promoting properties. They are also used to promote animal growth in the fields of animal husbandry and aquaculture. The annual use of antibiotics worldwide is approximately 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 200
000 to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.1,2 In many countries, antibiotics added to animal feed and veterinary medicine account for over 50% of the total usage.3 The main types of antibiotics being used include sulfonamides, quinolones, tetracyclines, macrolides, and aminoglycosides, etc4,5. About 70% of antibiotic drugs worldwide are used in animal husbandry. Antibiotics are discharged into the environment through industrial wastewater from pharmaceutical companies, livestock and aquaculture wastewater, medical wastewater, and wastewater from sewage treatment plants. It has been found that a variety of antibiotics are retained in farmed fish, with hygromycin accounting for approximately 82.5%, enrofloxacin for approximately 30.0% and florfenicol for approximately 46.6% of the residues.6 Taking quinolone drugs as an example, over 80% of antibiotics are discharged into pipelines in the form of untreated excreta, which in turn enters groundwater, causing groundwater pollution and posing a threat to the ecological environment and human health.7 Antibiotics can also accumulate and remain in organs or tissues within organisms, posing a threat to water bodies, soil, and ecosystems. One major impact is the induction of resistance genes, leading to the spread of microbial resistance, which in turn affects the ecological environment and human health. In addition, metabolites of tetracycline antibiotics (TCs) and sulfonamide antibiotics (SAs) typically exhibit higher levels of mutagenicity and carcinogenicity.8 An in-depth study of the presence of antibiotics in a wide range of environmental media is necessary for a comprehensive assessment of the environmental hazards of antibiotics. Antibiotic contamination poses a threat to global human health, as it has adverse effects on human health through the food chain or accumulated media in the human body. Therefore, further exploration of targeted remedial measures is needed.
000 tons.1,2 In many countries, antibiotics added to animal feed and veterinary medicine account for over 50% of the total usage.3 The main types of antibiotics being used include sulfonamides, quinolones, tetracyclines, macrolides, and aminoglycosides, etc4,5. About 70% of antibiotic drugs worldwide are used in animal husbandry. Antibiotics are discharged into the environment through industrial wastewater from pharmaceutical companies, livestock and aquaculture wastewater, medical wastewater, and wastewater from sewage treatment plants. It has been found that a variety of antibiotics are retained in farmed fish, with hygromycin accounting for approximately 82.5%, enrofloxacin for approximately 30.0% and florfenicol for approximately 46.6% of the residues.6 Taking quinolone drugs as an example, over 80% of antibiotics are discharged into pipelines in the form of untreated excreta, which in turn enters groundwater, causing groundwater pollution and posing a threat to the ecological environment and human health.7 Antibiotics can also accumulate and remain in organs or tissues within organisms, posing a threat to water bodies, soil, and ecosystems. One major impact is the induction of resistance genes, leading to the spread of microbial resistance, which in turn affects the ecological environment and human health. In addition, metabolites of tetracycline antibiotics (TCs) and sulfonamide antibiotics (SAs) typically exhibit higher levels of mutagenicity and carcinogenicity.8 An in-depth study of the presence of antibiotics in a wide range of environmental media is necessary for a comprehensive assessment of the environmental hazards of antibiotics. Antibiotic contamination poses a threat to global human health, as it has adverse effects on human health through the food chain or accumulated media in the human body. Therefore, further exploration of targeted remedial measures is needed.
According to reports, the removal rate of antibiotics in sewage treatment plants ranges from 36% to 79% due to process limitations.9 Common treatment methods include traditional approaches like coagulation, filtration, or adsorption, as well as air flotation, membrane filtration, and biological methods. These techniques employ chemical and biological treatments to break down, convert, or precipitate pollutants through oxidation, reduction, neutralization, etc., thereby rendering harmful substances harmless or less harmful.10 However, these methods entail significant energy and equipment investments, leading to high costs and the risk of secondary pollution, thus increasing the environmental burden. Additionally, there are challenges related to low processing efficiency.
In recent years, nanoscale zero valent iron (nZVI) has been widely used for the removal of pollutants from water and soil due to its high specific surface area, excellent adsorption and reactivity.11 nZVI has Fe0 core and Fe oxide shell, where the core has reducing ability and the shell acts as a reaction site for chemical adsorption and electrostatic interactions. The mechanisms of reduction, absorption, precipitation, and mineralization have played a role in the removal of heavy metals from the aqueous phase using nZVI.12 However, due to the magnetism and high surface energy of the newly synthesized nZVI, it is prone to spontaneous agglomeration and oxidation under actual reaction conditions, leading to its rapid deactivation. The pollutant removal pathways determined by nZVI include adsorption, complexation, co precipitation, and surface mediated chemical reduction.13 However, the preparation of nZVI requires the use of strong reducing agents such as sodium borohydride/potassium, which increases the preparation cost, generates toxic gases, and poses a threat to the environment. Load based modification provides more active sites for nZVI by dispersing it onto a support carrier with a porous structure. This method can enhance the activity of nZVI, accelerate the reaction between pollutants and nZVI, and promote the degradation of pollutants. Common load materials include carbon based materials, clay minerals, polymer compounds, etc. In the treatment of polluted water, an increasing amount of theoretical and experimental evidence indicates that nZVI is an effective and widely applicable nano-material. The reaction mechanism of nZVI for the removal of heavy metals mainly includes adsorption, complexation, reduction, and co precipitation. This provides new solutions for dealing with other mixed system pollutants such as heavy metals, nitrates, organic matter, etc.
In summary, removing pollutants from wastewater has become an important issue that urgently needs to be addressed. However, the application of nZVI materials in the field of in situ remediation of environmental pollutants is currently limited. The defects of nZVI during use can be compensated for through load and other methods. Therefore, this article summarizes the materials loaded with nZVI, including carbon based materials, geological materials, and polymer compounds, whose mechanisms and application value in the remediation of antibiotic contaminated soil and water have not been systematically explored. The aim is to review the application, immobilization mechanism, and environmental impact of nZVI in the remediation of antibiotic contaminated soil and water. First, the synthesis methods of nZVI were summarized, and different types of loading materials and their combination with nZVI were introduced. Secondly, the environmental factors for the repair of commonly used antibiotics by nZVI were explored and the interaction mechanism between commonly used antibiotics and nZVI was summarized. In addition, this article summarizes the current situation of antibiotic pollution in water environment, soil, and sediment, aiming to draw attention to the issue of antibiotic pollution.
2 Preparation method of loaded nano zero valent iron
2.1 Preparation method of nano zero valent iron
Research on nZVI has progressed tremendously over the past two decades, with cleaner and more efficient preparation methods emerging. Some of these methods have been able to achieve pilot scale applications, even in industrial production, laying the foundation for the broader applications of nZVI. According to the morphological changes of nZVI, the preparation methods of nano zero valent iron can be divided into decomposition method and synthesis method.14 The decomposition method is to convert large iron particles into nanoscale particles. On the contrary, the synthesis method converts even smaller amounts of iron at the molecular level into nanoscale amounts. This chapter will focus on introducing commonly used preparation methods. Table 1 summarizes the characteristics of nZVI prepared by different methods. In addition to the methods described in Table 1, there are many other methods for preparing nZVI, such as electric explosion method,20 plasma reduction method,15 etc. Among them, the preparation of nZVI through liquid-phase reduction method is the most common and widely used method, ball milling method is a typical physical method,21 and nZVI prepared by green synthesis method has more reliable ecological safety, renewable materials, and good economy.| Methods | Process | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Liquid phase reduction method | Add highly reducing KBH4 or NaBH4 solution to Fe2+or Fe3+solution. After the reaction is complete, wash, separate, dry the solution, and reduce it to nZVI. Auxiliary methods: ultrasonic, microgravity, micro lotion, ball milling etc. | Easy to operate, mild conditions, and high product activity | Reducing agents are expensive and toxic; hydrogen and B(OH)3, a hazardous byproduct, are produced during the preparation process | 14 |
| Electrodeposition method | Preparation of nano iron particles using external current electrolysis of ferrous or trivalent iron salt solutions | High purity, small particle size | The tendency for particle aggregation is relatively high | 15 |
| Ball milling method | Under the action of rotating mechanical energy, zero valent iron powder is repeatedly compressed and broken by the grinding ball, continuously refining to the nanoscale, forming nZVI | Non toxic, high yield, low cost, and no excess by-products | The product has poor form and is prone to mixing impurities, resulting in high energy consumption | 16 |
| Green synthesis method | Extracts are prepared by using solvents and specific plants or organic compounds as reducing agents, and mixed with Fe2+or Fe3+ solutions to reduce them to nZVI | Green and environmentally friendly, extracts can reduce clumping without the need for additional dispersants | Long process time and low yield | 4 |
| Carbon thermal reduction method | Immerse the carbon source in an iron source solution and stir to disperse. After being filtered and dried, the iron source is thermally reduced by carbon in a high-temperature environment filled with protective gas. Auxiliary methods: aerosol, ultrasonic spray | Cheap raw materials | The production conditions are harsh, and producing hydrogen (flammable) and carbon monoxide (toxic) requires high energy and energy consumption | 17 |
| Hydrogen thermal reduction method | Reduce iron oxide to nZVI using hydrogen gas at around 500 °C | Less by-products | Long production time, dangerous processes, and high energy consumption | 18 |
| Evaporation–condensation method | The iron target is heated above the boiling point with a laser, and the iron atoms evaporate and condense rapidly in an inert gas atmosphere to form iron nanopowders. Auxiliary methods include laser ablation, electric arc and cathodic sput-tering | Low cost and environmentally friendly raw materials, good thermal conductivity of iron metal, high melting point, resulting in high purity, chemical activity, and surface cleanliness of iron nanoparticles, making it possible to prepare multi-component nanoparticles | Expensive equipment and high energy consumption | 19 |
This section will introduce different methods and their characteristics one by one. Cheng et al.22 prepared a series of sodium alginate/chitosan composite (SA/CTS) carbon aerogels with or without nano zero valent iron without supercritical process, and studied the relationship between the adsorption performance of carbon aerogels for Cr(VI). Including the relationship between pH value, adsorbent dosage and adsorption temperature. nZVI@CTS The composite material utilizes the excellent support structure of alginate, the high affinity between chitosan and Cr(VI), and the strong reducibility of nZVI, providing a relatively high specific surface area of 300 m2 g−1, excellent adsorption capacity of 204.35 mg g−1 at 318 K, and sufficient strength to maintain structural integrity for reuse. The preparation process is shown in Fig. 1a.
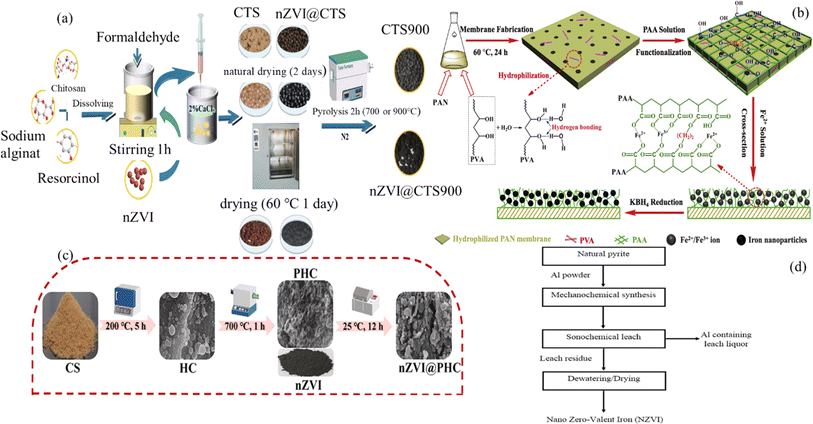 | ||
| Fig. 1 Process flow of four different nZVI preparation methods (a–d).22–25 | ||
Wang et al.23 initially developed hydrophilic and functionalized polyacrylonitrile (PAN) membranes through a two-step process involving the addition of polyvinyl alcohol and the in situ polymerization of acrylic acid. Furthermore, they incorporated nZVI into the modified membranes.The prepared PAA/PANnZVI (PPN) composite material exhibits good reactivity towards metronidazole (MNZ), with a conversion rate and reaction rate 2.03 and 4.77 times higher than nZVI. Meanwhile, PPN composite materials maintain enhanced stability and recyclability during repeated cycles. The preparation process diagram is shown in Fig. 1b.
To obtain porous hydrochar loaded nZVI (nZVI@PHC) composite materials, Cao et al.24 mixed 60 mg of nZVI, 100 mg of PHC, and 100 mL of anaerobic water in a conical flask and oscillated them for 12 h in a water bath oscillator at 25 °C and 200 rpm. Finally, the composite material was obtained through magnetic separation, centrifuged three times with anaerobic ethanol and distilled water, and then dried in a freeze-drying machine to avoid oxidation. nZVI@PHC was obtained. The preparation process diagram is shown in Fig. 1c.
B. N. Akhgar et al.25 prepared nZVI from natural pyrite concentrate through mechanochemical reactions and subsequent separation steps. Nano zero valent iron (nZVI) was prepared by mechanochemical treatment of natural pyrite using metallic aluminum powder and a sonochemical leaching method. Fe–Al2S3 compound was prepared by co-grinding natural pyrite and alumina using a planetary mill. Next, the sonochemical leaching method was used to prepare nZVI by leaching with 1.5 mol L−1 NaOH solution at 80 °C for 45 min. The prepared nZVI had a size of less than 100 nm and a specific surface area of 116 m2 g−1. The preparation process diagram is shown in Fig. 1d.
In summary, physical, chemical, and biosynthetic methods all prove effective in synthesizing nZVI. While the physical synthesis method is straightforward and environmentally friendly, it is constrained by the need for specialized synthesis equipment and relatively stringent synthesis conditions. In contrast, chemical and biosynthetic methods are more convenient and flexible to operate, with controllable reaction conditions. The synthesized nZVI has a more uniform size, providing more choices for the synthesis of nZVI. Regardless of the method used to synthesize nZVI, its surface structure may be affected by agglomeration or passivation, and further modification is needed to maintain its chemical activity. After decades of development, the preparation and modification technology of nano zero valent iron has become increasingly mature. Nonetheless, challenges remain regarding its stability, reaction efficiency, and the safety of nanoparticles, which pose obstacles to its engineering applications.
Among them, bimetallic modification is the modification of the structure of nZVI. Bimetallic particles accelerate the corrosion of Fe0 by electrochemical effects in combination with metals with high redox potentials, such as Pd, Pt, Co, Ni, Ag, and Cu. The incorporation of metal particles proves advantageous in hastening iron corrosion, augmenting hydrogen production, and expediting electron transfer. Nonetheless, the high cost of raw materials for the preparation of bimetallic particles and the potential for secondary contamination have limited their widespread use.25–28 From an application perspective, developing low-cost metal particles and loading them onto the surface of nZVI to form bimetallic particles is one of the future research directions. Table 2 outlines the strengths and weaknesses of bimetallic-modified nZVI.
| Modification methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Biochar bimetallic particles | Good dispersibility, high activity, and good stability | High preparation cost | 23 |
| Fe–Ag bimetallic nanoparticles | High efficiency, good performance | Expensive raw materials | 29 |
| Fe–Pd bimetallic nanoparticles | Acting as a reducing agent, efficient | Secondary pollution | 30 |
| Fe–Ni bimetallic nanoparticles | Fast, complete response | Requires other techniques | 31 |
| Fe Cu bimetallic nanoparticles | High activity, fast hydrogen production | Excessive inhibition | 32 |
Xie et al.33 uniformly loaded nZVI onto the surface and inner walls of nano-activated alumina (γ-Al2O3) using a liquid-phase reduction method, preparing magnetic nano zero-valent iron/activated alumina composite material (nZVI/γ-Al2O3). The material was characterized, and the adsorption isotherms and kinetics of five typical heavy metal ions Zn2+, Cu2+, Cd2+, Cr3+, and Mn2+ on nZVI/γ-Al2O3 were simulated. Additionally, the competitive adsorption and synergistic adsorption behavior in a multi-heavy metal ion system were investigated. The results indicated that magnetic nano zero-valent iron loaded on nano activated alumina not only overcame the agglomeration of nZVI particles caused by volume and surface interface effects but also kept nZVI in a stable high surface energy state. The modification of nZVI with bimetallic particles accelerated the corrosion process of iron, increased hydrogen production, and facilitated electron transfer. However, bimetallic particles were prone to secondary pollution and required modification using other expensive technologies, necessitating further research on them. At present, researchers are committed to exploring cleaner and more economical methods for modifying bimetallic particles.
The research suggests that incorporating magnetic nZVI onto nano-activated alumina effectively mitigated the agglomeration of nZVI particles due to volume and surface interface effects, while preserving nZVI in a stable high surface energy state.9–15,20,21 However, the use of bimetallic particles posed risks of secondary pollution and necessitated modification through other costly technologies. Thus, further investigation is warranted in this field.33
2.2 Preparation of loaded nano zero valent iron
In order to optimize nZVI and expand its application range, it is imperative to modify nZVI to a certain extent. Modification involves modifying the surface or structure of particles through physical or chemical means. Nanoparticles are loaded onto a carrier with a porous structure to increase the specific surface area of the nanoparticles. The modified nZVI can weaken the agglomeration effect of nanoparticles, improve the stability of nanoparticles in the environment, and to some extent, reduce the toxicity of nZVI to microorganisms.24 Carrier materials with adsorption properties can also accelerate the reaction between pollutants and nZVI, thereby promoting the degradation of pollutants. Load materials generally include clay minerals,29 metal oxide materials,33 carbon based materials,17 etc.| Materials | Structural form | Factors affecting removal | Disadvantages | Advantages | Ref. |
|---|---|---|---|---|---|
| Activated carbon (AC) | Highly porous with a wide range of pore size distribution from micro to macro pores. Particle size and shape can be adjusted according to application needs, such as powder, granule, column etc. | Pore structure development, large specific surface area, nZVI loaded on the pores of AC, functional groups such as –OH and –COOH on the surface are favorable for the binding of nZVI | The production cost is high, the adsorption capacity is limited, the selectivity is poor, and the regeneration process is complex | Low production cost and abundant precursor resources | 14 |
| Biochar (BC) | Porous structure, particle size can range from a few millimeters to tens of micrometers, with irregular morphology | Functional groups on the surface of biochar (e.g. hydroxyl, carboxyl, etc.) can form chemical bonds with pollutants | Low selectivity, uneven pore size distribution, difficult regeneration, unstable adsorption performance | High porosity, rich in oxygen-containing functional groups, simple production process, waste as raw material | 15 |
| Graphene nanotubes (GNs) | Hollow, long tubular structures with diameters in the nanometer range | nZVI can be loaded on the outside of carbon nanotubes or within the pores and cracks of a reticulated structure | Difficulty in dispersion, toxicity, and processing and handling | Easy to identify adsorption sites and mechanisms, with a very high specific surface area | 16 |
| Canotubes (CNT) | Two-dimensional planar structure with single or few layers, thickness of only one or a few carbon atoms, honeycomb hexagonal lattice structure | Graphene nanosheets with folded edges can support nZVI nanoparticles | High production costs, complex processing, unstable properties, and potential environmental and health risks | Large specific surface area, multiple active centers and functional groups, porous structure | 4 |
At present, the use of nano zero valent iron and carbon based nanocomposites for environmental remediation of water, soil, and other environments has become one of the hot topics of widespread concern for scholars around the world.29–32,34,35 Carbon based materials as carriers of nZVI can not only increase its specific surface area, reduce its aggregation, but also accelerate electron transfer efficiency. In addition, composite materials based on carbon based materials are difficult to completely separate from environmental media, increasing the risk of secondary pollution. The focus of future research lies in developing methodologies for the recovery and reuse of contaminants from environmental media.
Chen et al.36 employed agricultural waste straw biochar as a carrier for synthesizing zero-valent iron nanoparticles on straw biochar via the co-precipitation method. The removal of p-nitrophenol (PNP) was achieved through the activation of sodium persulfate (PS), and they investigated various factors influencing the degradation of PNP. The results indicated that at pH = 7, BC@nZVI exhibited the best activation effect when the dosage was 2 g L−1 and the PS concentration was 1 mM. During this condition, the removal of 40 mg L−1 PNP reached 91.7% within 10 min. By enhancing the dispersibility of nano zero-valent iron (nZVI), reducing its aggregation, and mitigating passivation, the activation performance of PS has been significantly improved. This provides a promising method for treating challenging pollutants such as PNP. BC@nZVI exhibits high stability, allowing for up to threefold repetition of catalytic experiments, thereby expanding the material's applicability and ensuring its reusability in water treatment applications.37
Mortazavia et al.38 developed a two-step synthesis method to immobilize nanoscale zero-valent iron (nZVI) particles onto activated carbon (AC/nZVI), enabling simultaneous adsorption and reduction of hexavalent chromium (Cr(VI)) from aqueous solutions. The two-step synthesis procedure was employed to fix nZVI particles onto activated carbon (AC/nZVI), exhibiting 33 times higher adsorption capacity and greater affinity towards Cr(VI) compared to AC alone. Meanwhile, Liu et al.39 utilized Fe2O3 and activated carbon (AC) as raw materials, subjected them to planetary ball milling followed by gas-phase reduction at 850 °C under a hydrogen atmosphere to prepare AC/nZVI composite materials. Experimental results demonstrated that the ball milling and gas-phase reduction method could partially achieve nanoscale Fe0 and load it onto AC, successfully synthesizing the nZVI-AC material. Zhang et al.40 synthesized efficient oxidative desulfurization (ODS) catalysts by encapsulating nZVI in self catalytic carbon nanotubes (CNTs). The nZVI@CNT catalyst demonstrates excellent catalytic performance and enhanced stability, with its initial performance remaining at 80% even after 6 cycles. This highlights effective optimization and regulation between carbon nanotubes and iron particles. It was verified that the unique coating structure and porous configuration contributed to enhancing desulfurization efficiency. The research results can provide reference for the effective synthesis strategies of nZVI high-efficiency oxidation desulfurization catalysts in practical industrial applications.
Chemical reduction technology utilizing nano zero-valent iron (nZVI) stands out as a highly effective approach for on-site remediation of contaminated water. Nevertheless, overcoming the associated surface passivation during the reaction process proved challenging, leading to a loss of reducing reactivity. Li et al.34 studied a novel system based on nZVI/graphene nanosheets (nZVI/GNS) for the effective removal of Cr(VI) from water. The experiment fixed nZVI on graphene to improve the stability of nZVI, while graphene coupling promoted electron transfer in nZVI and delayed the passivation of nZVI surface, thereby enhancing the performance of nZVI in removing Cr(VI). The two-dimensional structure of graphene may provide skeleton support for nZVI, thereby solving the bottleneck of aggregation and passivation. Mon et al.41 established the synthesis of nZVI/graphene from azalea leaves and FeCl3. During this process, it was confirmed that magnetic Fe3O4/amorphous carbon undergoes a phase transition to nZVI/graphene composite materials through a carbon thermal reduction process. The nZVI particles are sandwiched between graphene sheets with a diameter of approximately 16–40 nm, and the two-dimensional structure of graphene may provide skeletal support for nZVI, thereby solving the bottleneck of aggregation and passivation. Graphene provides good conductivity, skeleton support, and long-term electron release characteristics, which can accelerate the reduction of Cr(VI) to Cr(III) and exhibit fast and high Cr(VI) removal ability from aquifers.
2.2.2.1 Red mud. Red mud is a waste product from the aluminum oxide industry. Its high mechanical and chemical stability, along with its structural characteristics, make it an effective and sustainable stabilizing support medium.44,45
Sahu et al.46 successfully synthesized red mud-supported nZVI (RM-nZVI) composite materials using the sodium borohydride reduction method, where red mud served as a stable support medium for the development of red mud-modified nZVI. In the presence of red mud, sodium borohydride (NaBH4) was used to reduce iron and synthesize RM-nZVI. The morphological characteristics of RM-nZVI have been confirmed, indicating that it is in a diffusion state with small aggregation. The use of red mud as a carrier material enhanced the reactivity of RM-nZVI and reduced the aggregation of nZVI. Extensive characterization of RM-nZVI has confirmed that the removal of Hg2+ initially occurs through rapid physical adsorption, followed by the reduction of Hg2+ to Hg0. Adsorption mainly occurs on the surface of RM-nZVI, followed by reduction to Fe0. Red mud, as a supporting agent, has the advantages of strong adsorption capacity, low cost, renewability, and easy treatment in wastewater treatment. It can effectively adsorb organic matter and heavy metal ions, purify water quality, reduce treatment costs, and comply with sustainable development principles.
2.2.2.2 Zeolite. Zeolite, as an excellent supporting material, has attracted widespread attention due to its economic efficiency and environmental friendliness.47 It has abundant channels and pores, which can provide a large number of attachment sites for nZVI and pollutants. Zhao et al.48 synthesized nanoscale zero valent iron (nZVI) by liquid-phase reduction method, and modified the surface active agent polyethylene glycol (PEG-4000) onto molecular sieve to prepare zeolite (PZ)-nZVI composite material for adsorbing fluoroquinolone drugs (FQs). Compared with nZVI and molecular sieves, PZ-nZVI has higher removal efficiency, and the adsorption process of fluoroquinolone drugs (FQs) on PZ-nZVI is attributed to surface complexes (forming diacetate complexes), hydrophobic interactions, pore filling, and electrostatic interactions. In addition, it was found that using an external magnetic field can effortlessly separate PZ-nZVI from the mixed solution. Zeolite, as a supporting agent for wastewater treatment, has advantages such as strong adsorption capacity, selective adsorption, strong corrosion resistance, renewability, and wide application, which can effectively improve the efficiency of wastewater treatment and water quality purification.49
2.2.2.3 Bentonite. The nZVI based nanocomposites supported by clay mineral matrix are a promising technology for in situ remediation of groundwater and (sub) soil contaminated with chlorinated hydrocarbons such as trichloroethylene (TCE). However, little is known about the physical and chemical processes and interaction mechanisms between nZVI particles, clay minerals, and TCE. Baldermann50 fixed nZVI particles on a commercial bentonite matrix to prepare a novel nZVI nanocomposite material, and tested its performance in removing TCE from solution in an intermittent reactor. Compared with nZVI, nZVI- Bentonite exhibits higher TCE removal activity and efficiency (94%). Bentonite is a traditional low-cost and efficient adsorbent. Due to its richness, chemical and mechanical stability, high adsorption capacity, and unique structural characteristics, composite materials with nZVI have the potential to remove pollutants from wastewater.
2.2.2.4 Other geological materials. The use of minerals as loaders for adsorption in wastewater treatment has many advantages, including abundant resources, high surface area, renewability, environmental friendliness, and multifunctionality, which together promote the efficiency and sustainability of wastewater treatment.
Zhao et al.51 enhanced the denitrification effect of vertical flow constructed wetlands (VFCWs) using montmorillonite loaded nanoscale zero valent iron immobilized sodium alginate (SA/Mt nZVI). This study showed that SA/Mt nZVI, as a cost-effective material, is feasible and feasible for purifying VFCWs wastewater. The maximum NO3−–N removal efficiency reached 75.81 ± 1.6%. In addition, after adding SA/Mt nZVI, the biodiversity and richness of CW-nZVI are higher. The micro beads of SA/Mt nZVI improved water quality, reduced the burden of separation, and reduced the potential risk of nanomaterials to VFCW.
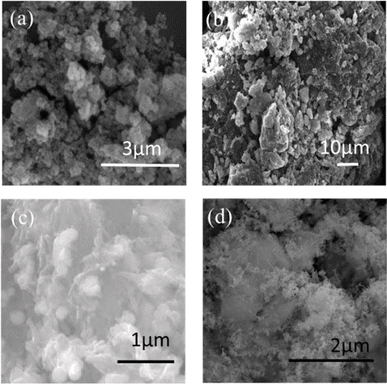
Ma et al.52 rapidly synthesized nZVI composite materials (PDA/ATP-nZVI) loaded with polydopamine (PDA) modified attapulgite (ATP) under acidic conditions. PDA/ATP-nZVI composite materials exhibit excellent Cr(VI) removal efficiency even in the presence of interfering ions under acidic conditions. The effect of low concentration coexisting ions on the removal of Cr(VI) can be ignored, while high concentration interfering ions can promote the removal of Cr(VI). The experiments showed that Fe2+ plays a key role in the reduction of Cr(VI) by PDA/ATP-nZVI. PDA enhances the elimination of Cr(VI) by supplying electrons to Cr(VI) and accelerating the conversion of Fe3+ to Fe2+. Fan et al.53 prepared nZVI by liquid-phase chemical reduction method and loaded it on expanded perlite to prepare nFe@EP materials used to remove phosphates from water bodies. Examined the initial pH, temperature, and nFe@EP The influence of added mass concentration and initial phosphate mass concentration. The results indicate that, nFe@EP good adsorption of phosphate in water, with a removal rate of 95.7% and an adsorption capacity of 31.9 mg g−1. Fig. 3 shows the scanning electron microscope images of nZVI loaded on different minerals, demonstrating the morphology of the different minerals. The loaded nZVI particles are mostly aggregated, and the nanoparticles have a large specific surface and porosity. Mineral-supported nZVI enhances its stability, dispersibility, and adsorption capacity, thereby improving efficiency and safety in environmental remediation and pollution control. Additionally, it adapts to various environmental conditions, offering a promising solution for environmental management.54
Jin et al.55 prepared nZVI by hydrothermal method and mixed it with polyvinyl alcohol (PVA) in solution to prepare a polyvinyl alcohol/nano-sized zero valent iron composite film with better structure and oxygen resistance. The results showed that with the increase of nZVI content, the glass transition temperature of the composite film gradually increased, the tensile strength and elongation after fracture first increased and then decreased, the oxygen permeability coefficient first decreased and then increased, and the thermal stability decreased.
Cheng et al.56 designed a series of sodium alginate/chitosan composite (SA/CTS) carbon aerogels and elucidated that the mechanism of adsorption of Cr(VI) mainly includes electrostatic interactions, hydrogen bonding attraction and redox reactions. Therefore, CTS and nZVI@CTS have a promising application in water and wastewater containing Cr(VI).
The intensification of industrial processes and human activities has caused heavy metal pollution in water, which has attracted global attention. We need to find an environmentally friendly and effective remedy. Zhao et al.57 prepared sodium alginate nZVI biochar composite material (CANRC) using calcium alginate encapsulation and liquid-phase reduction method. This composite material was used for the first time to remove Pb2+, Zn2+, and Cd2+ from water. CANRC is a heavy metal adsorbent with good regeneration performance, providing an effective method for removing heavy metals from wastewater. Gao et al.58 studied the effects of carboxymethyl cellulose sodium (CMC) as a stabilizer on the physicochemical properties, reactivity, and reusability of S-nZVI for the degradation of nitrobenzene (NB). Characterization shows that CMC improves the degree of vulcanization, significantly inhibits surface oxidation of S-nZVI, enhances hydrophobicity, and reduces electron transfer resistance. Meanwhile, CMC-S-nZVI exhibits superior reusability in NB degradation compared to S-nZVI.59 Table 4 summarizes the properties, structures, advantages, and disadvantages of common geological and biomacromolecule materials and loaded nanomaterials.
| Materials | Structural form | Factors affecting removal | Disadvantages | Advantages | Ref. |
|---|---|---|---|---|---|
| Red mud (RM) | The particle size affects its surface activity and reaction efficiency | Porous structure and high specific surface area, functional groups on the surface react with pollutants to improve adsorption efficiency | Poor selective adsorption performance, difficult to regenerate after use, may cause pollution, and needs to be treated before using | Low cost, easy to obtain, capable of achieving various functions such as photocatalysis and antibacterial | 46 |
| Zeolite | Regular shape and porous structure | Zeolite loaded nanoparticles can remove ionic pollutants from water through ion exchange reaction | Poor selective adsorption makes it difficult to regenerate after use, which may lead to secondary pollution | It has a high specific surface area, is easy to obtain, and has good ion exchange capacity | 47 |
| Bentonite | Similar to red mud | High specific surface area and good pore structure, capable of removing ionic contaminants from solution by ion exchange | Poor selective adsorption performance makes it difficult to regenerate after use, resulting in secondary pollution | A mineral with high specific surface area, good pore structure, excellent ion exchange capacity, and low cost and easy availability | 50 |
| Chitosan (CS) | Tens of microns to hundreds of microns, can be made into films or fibers | Amino and hydroxyl groups can adsorb pollutants through van der Waals forces and hydrogen bonds | Poor solubility in neutral or alkaline environments, and difficult to regenerate | Excellent biodegradability, good environmental protection, high adsorption capacity | 56 |
| Calcium alginate (CA) | When gel is formed, its thickness can vary from several microns to several millimeters | The gel with loaded nanoparticles is formed, and its surface and pores can absorb pollutants | Not very stable in acidic environments, and the regeneration process after use is complex | Able to form stable gels with good biocompatibility and biodegradability. Relatively cheap and easy to obtain | 57 |
| Polyvinyl alcohol (PVA) | Between tens and hundreds of micrometers | The loaded nanoparticles may react with contaminants to form colloidal precipitates that are removed from solution | Inherent low mechanical strength, poor solvent resistance in water | Good solubility in water for ease of preparation and application. Highly adjustable and biocompatible | 55 |
| Carboxymethyl cellulose sodium (CMC) | Tens of micrometers to hundreds of micrometers, forming a viscous solution or gel in water | Carboxymethyl groups can react chemically with loaded nanoparticles or contaminants | Poor stability: stable at extreme pH values | Good solubility in water, easy preparation, good adsorption capacity, and strong adjustability | 58 |
2.3 Application of loaded nano zero valent iron
Huang et al.61 used nZVI particles to quickly remove Cr6+from the solution. In the experiment, different nZVI particles of electric spray (E-nZVI) and non electric spray (NE-nZVI) were used to treat Cr6+solution with a concentration level of 100 mg mL−1. When the nZVI concentration reaches 0.93 mg mL−1, the removal rate approaches 100%. Based on the XPS results, no Cr6+was detected on the particle surface, and it can be concluded that the removal of Cr6+ is mainly achieved through precipitation and reduction.62 Pullin et al.49 discussed the application and mechanism of zero valent iron nanoparticles (nZVI) in the removal of Zn2+. This indicates that nZVI has a significant effect on the removal of Zn2+. Among them, pH and dissolved oxygen (DO) are important factors for the removal of Zn2+ by nZVI. DO improves the removal efficiency of Zn2+, and under oxygen-containing conditions, it can exhibit outstanding adsorption affinity. The FeOOH shell layer can improve the adsorption efficiency of nZVI. The removal efficiency of Zn2+ increases with the increase of pH. The acidic conditions reduce the removal efficiency of Zn2+ by nZVI, as the presence of H+ in the solution inhibits the formation of iron hydroxide (oxygen). In contrast, due to the multifunctional properties of nZVI and its inherent pH stability, which has a good tolerance to influent fluctuations, it is easy for nZVI to obtain stable and lower levels of Zn2+. In the case of nZVI, aqueous corrosion continuously releases Fe2+and OH−, and the reaction mechanism is shown in formula (1).
| Fe0 + 2H2O → Fe2+ + 2OH− + H2 | (1) |
The adsorption effect of nZVI on Zn2+: the surface of nZVI is composed of a layer of FeOOH, which has a prominent affinity for metal cations.49,50 The gradual corrosion of Fe0 by water leads to the formation of iron oxide in the form of discrete particles or deposits on the surface of nZVI, resulting in a significant increase in surface area.63
Liu et al.56 found through research that compared with other types of adsorbents, nZVI has higher adsorption capacity and is the most effective adsorbent for removing cadmium from aqueous solutions. The author points out that the extremely small particle size, large surface area, and high-density reaction and adsorption sites make nZVI a potential candidate for environmental applications, especially for Cd2+ removal. Compared with the newly produced biochar nZVI composite adsorbent, nZVI formed on biochar through adsorption and complexation processes.55–58,64,65
In summary, increasing the dosage of nZVI is beneficial for improving the removal efficiency of heavy metal ions in the removal process. Due to the competition between H+ and Pb2+for surface active sites, weak acidic conditions are more conducive to the removal of most heavy metal ions. The mechanism of using nZVI to remove heavy metal ions is mainly due to the combined effects of adsorption, reduction, and complexation adsorption, which precipitate iron minerals and form co precipitation. In summary, the characteristics of extremely small particle size, large surface area, and high-density reaction and adsorption sites enable nZVI to have better adsorption capacity. The mechanism of nZVI removal of heavy metals is shown in Fig. 4.
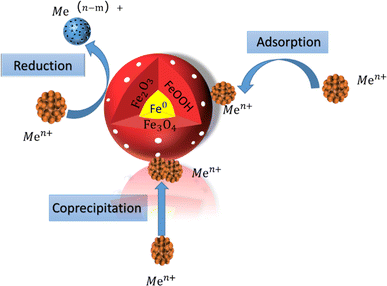 | ||
| Fig. 4 Reaction mechanism diagram of nzVI removal of heavy metals.66 | ||
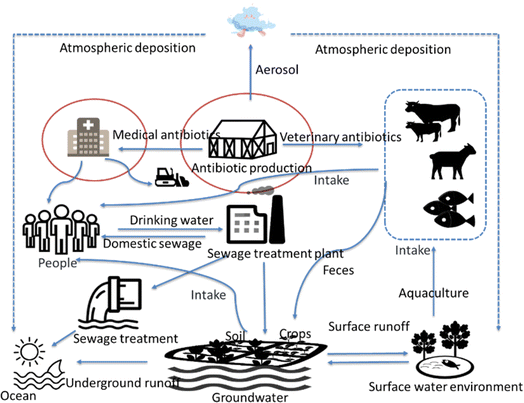 | ||
| Fig. 5 Source and fate of antibiotics in the environment.60,67–74 | ||
2.3.2.1 Tetracycline (TC). Tetracycline (TC) is a common antibiotic, however, long-term overuse can lead to an increase in bacterial resistance, making it more difficult to treat infections.72 In addition, tetracycline residues may enter the environment through wastewater and farmland, causing negative impacts on ecosystems and potentially posing a threat to health by entering the human body through the food chain. Therefore, measures must be taken to limit its use in order to protect the environment and human health.
In order to reduce the harm of tetracycline, it is necessary to use and manage tetracycline antibiotics reasonably, including controlling the rationality of agricultural use, strengthening environmental monitoring and governance, promoting rational use of drugs, and preventing the development of drug resistance. Wang et al.73 successfully prepared a modified membrane supported iron composite material PDA/PAN/BC-Fe0 (PPBN) by functionalizing hydrophilic porous polyacrylonitrile (PAN) membranes with sisal fiber biochar (SF-BC) and polydopamine (PDA) as carriers. Studied the effects of different reaction conditions and interference factors on the degradation performance of TC. In addition, the cycling and stability of PPBN were also evaluated. Effectively inhibiting the aggregation and release of nZVI particles, enhancing the reactivity and stability of PPBN.
The results show that the removal rate of TC by PPBN can reach 90.16% within 120 min, which is 36.16% higher than that of nZVI. After 6 regeneration cycles, the removal rate of TC by PPBN only decreased by 10.22%. Three degradation pathways for removing TC were proposed. The removal mechanism includes the synergistic effect of physical adsorption, chemical oxidation, and reduction, and the redox reaction induced by hydroxyl radicals (˙OH) and superoxide radicals (˙O2−) plays a key role. The research results provide a simple, efficient, and stable material for the treatment of antibiotic wastewater. Experiments have shown that the removal efficiency of TC is better under low pollutant concentration, high temperature, and neutral conditions.
2.3.2.2 Ciprofloxacin (CIP). Ciprofloxacin (CIP) is a commonly used fluoroquinolone antibiotic, mainly used in the medical and veterinary fields to treat bacterial infections. However, its residues may enter the human body and ecosystem through the food chain and environment, leading to increased drug resistance, environmental pollution, and the spread of bacterial resistance. To reduce its harm, it is necessary to limit its use, strengthen monitoring and management, promote rational drug use and aquaculture management, and strengthen drug resistance monitoring and research.
Chen et al.74 designed a combined system of polyvinylpyrrolidone (PVP) and nanoscale zero valent iron (nZVI) and copper (Cu) bimetallic particles (PVP nZVI/Cu) for the reduction of ciprofloxacin (CIP) under weak magnetic field (WMF) conditions. Further characterization of the surface morphology and physicochemical properties of the new catalytic material. In addition, the catalytic reactivity of PVP nZVI/Cu was measured in the presence or absence of WMF. The experimental results show that the introduction of WMF enhances the catalytic performance of the PVP nZVI/Cu system, with a maximum CIP removal rate of 95.6% (120 min). The study provides new insights into the removal of CIP by NZVI, and has prospects for its practical application.
The natural passivation phenomenon generated by the formation of surface oxide layers in nZVI is a bottleneck in exploring the complete potential of this material. B. Nandana et al.75 solved by in situ synthesis of nZVI/Cu bimetallic heterostructures. The passivation bypass of nZVI is achieved by synthesizing nZVI copper bimetallic nanoparticles (nZVI/Cu). Research has shown that nZVI/Cu has higher performance than bare nZVI. When using nZVI and nZVI/Cu as catalysts, the degradation efficiency of nZVI/Cu for TC and CIP was 99.99% and 98.59%, respectively, with rate constants of 0.0631 min−1 and 0.032 min−1. In addition, the removal rates of TET and CIP in the antibiotic mixture by nZVI/Cu were 99.34% and 99.9%, respectively. By utilizing the inherent magnetism of nZVI/Cu samples, catalysts can be easily recovered.
2.3.2.3 Oxytetracycline. Oxytetracycline, as a widely used antibiotic, mainly comes from the agricultural and medical fields. However, it may lead to hazards such as drug residues, environmental pollution, and the spread of bacterial resistance. To reduce these hazards, it is necessary to limit usage, strengthen monitoring and management, promote rational drug use and prevention measures, and strengthen drug resistance monitoring and research.
Yang et al.76 prepared a series of biochar supported nano zero valent iron catalysts (nZVI-BC) by co pyrolysis of soybean straw and Fe2O3 at different pyrolysis temperatures. It can effectively degrade tetracycline in water and reduce the total organic carbon content. In the future, it has potential application prospects. On the basis of preparing nZVI-BC, it should be possible to design and introduce more active functional groups and active sites to further improve the catalytic performance of the catalyst. However, in real water environments, the system is relatively complex and has limitations due to other pollutants such as heavy metals, pesticides, dyes, etc. Therefore, in future research, the problem of simultaneous removal and interaction of various pollutants should be addressed. Contribute to the development of advanced oxidation technology catalysts.
Nguyen et al.77 synthesized bimetallic palladium zero valent iron (Pd/nZVI/rGO) composite materials loaded with reduced graphene oxide using a one-step liquid-phase reduction method. Analysis shows that the presence of rGO flakes prevents the aggregation of Pd/nZVI nanoparticles and delays the transformation of iron corrosion products from magnetite/hematite to scale bluestone, resulting in more uniform dispersion of these nanoparticles. In addition, loading Pd/nZVI nanoparticles can effectively avoid the stacking of rGO sheets. The synthesized Pd/nZVI/rGO composite material is used to remove the antibiotic oxytetracycline (OTC) from aqueous solution. Research has found that introducing the optimal amount of rGO into Pd/nZVI nanoparticles significantly enhances the removal of OTC. The removal of OTC is the result of a combination of adsorption process, Fenton like reaction, and reduction reaction. The Pd/nZVI/rGO composite material exhibits better reusability than the original nZVI particles. A pathway for OTC degradation on Pd/nZVI/rGO nanocomposites was also proposed.
Li et al.79 prepared sulfurized nZVI (S-nZVI) using ball milling, vacuum chemical vapor deposition (CVD), and liquid-phase reduction techniques. Experiments have shown that the removal of 2,4,6-trichlorophenol (TCP) by nZVI and S-nZVI is based on surface adsorption and subsequent direct reduction of Fe0, in situ oxidation of ROS, and polymerization on the surface of these materials. During the reaction process, the corrosion products of these materials are transformed into crystalline Fe3O4 and α/β- FeOOH enhances the stability of nZVI and S-nZVI materials, facilitating electron transfer from Fe0 to TCP, as well as TCP's strong affinity for Fe or FeSx phases. All of these contribute to the high removal and mineralization performance of nZVI and sulfurized nZVI towards TCP in continuous cycling experiments. Zheng et al.89 prepared porous CaCO3 using microbial induced calcium carbonate precipitation (MICP) technology, which was then used as a carrier material for CaCO3@nZVI Composite materials. CaCO3@nZVI The main products of TCE degradation in composite materials are acetylene, ethylene, and ethane, and the synergistic effect of adsorption degradation is the main mechanism for removing TCE.
Jing et al.80 successfully synthesized nano zero valent iron reduced graphene oxide (NZVI rGO) in their research and applied it to the removal of 2,4-DCP. The characterization results indicate that nZVI particles were successfully loaded onto rGO nanosheets, improving the dispersion of the particles. The removal experiment showed that compared with bare nano zero valent iron, the presence of rGO significantly improved the removal efficiency of 2,4-DCP. The removal mechanism of 2,4-DCP during simultaneous removal is adsorption, which provides theoretical support for the treatment of combined pollution of chlorinated organic compounds and heavy metals.
Zarime et al.81 studied the removal of methylene blue (MB) using nano zero valent iron (Gr-nZVI) loaded on residual granite soil to investigate its potential use as an efficient adsorbent. According to adsorption analysis, compared with Gr-nZVI and nZVI, Gr exhibits higher MB removal adsorption capacity. Overall, it provides experimental support for the selection of nZVI carrier materials, which helps to promote the development and progress of green restoration materials.
However, using nZVI for organic pollutant remediation still faces some challenges. The application of nZVI-based materials for the removal of organic pollutants is still limited by the high cost of raw materials and the complexity of the synthesis method, which makes it difficult to develop industrialized practical applications in large quantities. Therefore, reducing the cost of synthesis and exploring new methods of preparation that increase the activity of nZVI, produce large scale outputs, and are adapted to real-world production are essential for realizing the widespread application of nZVI and its basic materials.82
Pei et al.84 successfully prepared nZVI/LDH nanocomposites by loading nano zero valent iron (nZVI) onto the surface of layered double hydroxides (LDH) using a liquid-phase reduction method. To adsorb coupled reduction of nitrate (NO3−–N), nZVI/LDH composite materials were prepared. The results showed that the removal rates of NO3−–N and total nitrogen by nZVI/LDH composite material within 180 min were 88.64% and 77.63%, respectively. The selectivity for N2 was 55.21%, and the selectivity for ammonia nitrogen was only 1.86%. The mechanism of NO3−–N synergistic adsorption reduction degradation, including rapid adsorption of initial NO3−–N, was proposed by measuring the content of NO3−–N, nitrite NO3−–N, and NH4+–N in the aqueous and adsorbed phases during the reaction process. LDH, as a loading material for nZVI, significantly reduces the agglomeration of nZVI and provides more Fe0 active sites. This is mainly due to the synergistic effect of nZVI and LDH in nZVI/LDH composite materials. Therefore, nZVI/LDH composite materials can be applied as an efficient and stable material in real water. Zhou et al.85 studied and prepared a novel composite filler consisting of tea polyphenols, nZVI, and modified polyethylene carrier (TP nZVI/PE). The removal rate of nitrate by TP-nZVI/PE composite microorganisms is 79.88 ± 0.17%, which is three times that of TP-NZVI/PE. The oxidized nZVI is converted into Fe2+/Fe3+, which is easy to adsorb nitrate and then co precipitate, which is conducive to further removal of nitrate.
The rapid, environmentally friendly preparation and efficient application of nZVI exhibit excellent performance in the reduction and removal of nitrate. The above study not only enriches the application of nZVI and biological denitrification treatment technology. The possible mechanisms in nitrate removal are shown in Fig. 6.
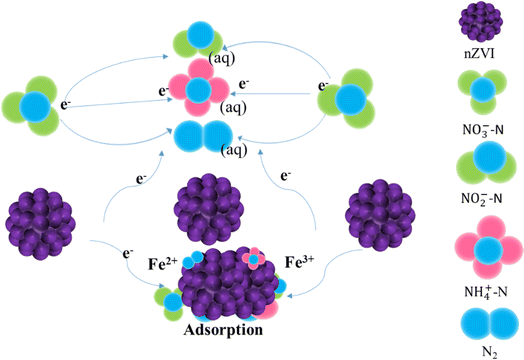 | ||
| Fig. 6 Possible mechanism of nitrate removal by nano zero valent iron.83–85 | ||
3 Factors affecting the removal of typical antibiotics from wastewater by nano zero valent iron
3.1 pH
In actual water environments, the pH value of wastewater varies greatly, so the pH value of the solution has a significant impact on the removal performance of pollutants in wastewater. In order to investigate the effect of pH on the removal performance of TC, Wang et al.67 explored and compared the removal of TC under five different pH gradients. At pH 2.5, 5.5, 7.0, 9.0, and 11.0, the removal rates of TC were 78.69%, 81.42%, 89.61%, 80.33%, and 71.58%, respectively. Overall, the modified membrane loaded iron composite material PDA/PAN/BC-Fe0 (PPBN) prepared in the experiment showed better removal efficiency of TC in neutral and weakly acidic environments, but poorer in strongly acidic or alkaline environments. This is because the excess H generated under strong acid conditions has a scavenging effect on –OH active substances, leading to a decrease in reaction activity; In alkaline environments, the generated iron hydroxide tends to form a passivation layer on the material surface,86 which hinders the contact between pollutants and active sites.pH value is a key factor in the nZVI reaction process. Chen et al.74 designed a composite material consisting of polyethylene pyrrolidone (PVP) and nanoscale zero valent iron (nZVI) and copper (Cu) bimetallic particles (PVP nZVI/Cu). In the presence or absence of an external weak magnetic field (WMF), the pH (2–10) increased from 2 to 6 with the initial pH, and the removal rate of CIP increased from 48.8% to 85.7%. However, as the initial pH further increased to 10, the removal efficiency significantly decreased to 19.7%. The mechanism proposed for the decrease in catalytic ability at pH < 6.0 is the extremely rapid corrosion of PVP-nZVI/Cu at low pH, resulting in H2 (eqn (2)) covering the surface of the composite material, which will limit the interaction between CIP and PVP-NZVI/Cu. In addition, the partial dissolution of PVP-nZVI/Cu at pH 2.0 also weakened the adsorption of CIP on PVP-nZVI/Cu particles, leading to a decrease in CIP removal efficiency. However, as the pH value continues to increase, the passivation layer formed by the precipitation of iron hydroxide on the surface of PVP-NZVI/Cu in high pH solutions hinders the corrosion of iron (eqn (3)). As the initial solution pH increases, the CIP removal rate gradually decreases. Acidic and neutral conditions are more conducive to the removal of CIP than alkaline conditions. This is because the Fe2+ formed by the corrosion of nZVI under acidic conditions can activate PS. As the pH decreases, the concentration of Fe2+ in the solution increases, and the removal rate of CIP also increases.
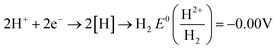 | (2) |
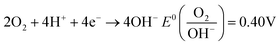 | (3) |
As the initial solution pH rises, the removal rate of CIP gradually diminishes. Acidic and neutral conditions prove more favorable for CIP removal compared to alkaline environments. This phenomenon arises from the activation of PS by Fe2+ generated through nZVI corrosion under acidic conditions. With decreasing pH, the concentration of Fe2+ in the solution rises, correspondingly enhancing the removal efficiency of CIP.
3.2 Temperature
The temperature effect is an important physicochemical parameter for evaluating the removal effect of adsorbents on harmful substances in water. The removal of TC is also affected by the reaction system temperature. Wang et al.73 achieved a removal rate of 90.16%, 91.67%, and 93.12% within 120 min at reaction temperatures of 298 K, 308 K, and 318 K, respectively. This indicates that the removal efficiency of TC increases with the increase of reaction temperature, but the improvement is not significant, attributed to the limited reaction sites of the material. Raising the reaction temperature can increase the reaction rate and shorten the reaction time. It can be seen that as the pyrolysis temperature increases, the removal rate of TC increases. As the pyrolysis temperature increases, Fe transforms from Fe3O4 to nZVI, and nZVI has a better activation effect on TC than Fe3O4, resulting in an increase in TC removal rate. It can be seen that the environmental temperature within a certain range during adsorption has a positive impact on the removal rate and speed of pollutants, which means an increase in equilibrium adsorption capacity.Chen et al.74 investigated the relationship between the removal efficiency of CIP by PVP nZVI/Cu particles and reaction temperature (288.15–308.15 K). It can be seen that the removal efficiency increases with the increase of temperature. Under weak magnetic field conditions, the removal rate of CIP increased from 86.2% to 98.4%. Higher temperatures may increase the migration rate of CIP from solution to nano-particles. In addition, higher temperatures can also accelerate the corrosion of PVP-NZVI/Cu.
3.3 The influence of anions and cations
In addition, the actual polluted water environment contains complex components, and there may be many different types and concentrations of anions and cations. Therefore, experiments were conducted to investigate the effects of different concentrations of anions and cations commonly found in water environments on TC removal. Cations (such as Na+) and anions (such as Cl−) also have an impact on the removal ability.Wang et al.87 prepared a new aluminum hydroxide gel coated with nanometer zero valent iron (AHG@nZVI), studied AHG@nZVI Studied the presence of NaCl in aqueous solutions of different concentrations. As the salt concentration increased from 0.1 mol L−1 to 0.5 mol L−1, the TC removal capacity (mg g−1) increased by 12.8% and 18%, respectively. The removal effect of NaCl on TC is significant. However, high ion concentration (1 mol L−1 NaCl) often reduces the removal ability of TC. At low chloride ion concentrations (0.1–0.5 mol L−1 NaCl), the coating on the surface of nZVI inhibits the formation of chloride complexes with iron oxide, thereby playing a positive role in the removal of TC. In contrast, at 1 mol L−1 NaCl, the concentration of competitive anions reached the competitive saturation point, leading to AHG@NZVI The adsorption capacity decreases.85 Research has found that cations K+, Ca2+, and Mg2+ all have inhibitory effects on the removal of TC,73 with the inhibition intensity in the order of Ca2+ > Mg2+ > K+. The reason may be that Ca2+and Mg2+ in aqueous solutions are more likely to hydrolyze to form precipitates in the form of hydroxides than Na+ and K+, leading to masking of reaction sites. When the concentration of Ca2+and Mg2+ increases to 10 mM, the inhibitory effect becomes more pronounced and the removal rate decreases significantly, which may be attributed to the easy reaction of Ca2+ and Mg2+ with iron hydroxide (oxygen). The removal effect of anions on TC varies, with SO42− having a weaker inhibitory effect and HCO3− having a more pronounced inhibitory effect. This may be because bicarbonate can act as a scavenger and inhibitor of hydroxyl radicals,88 producing OH− and inhibiting the production of ˙OH in the reaction system, thereby reducing reactivity.89
3.4 Other influencing factors
In the wastewater treatment process, nZVI acts as an electron donor, while antibiotics and dissolved oxygen act as electron acceptors to form a competitive relationship. Dissolved oxygen reacts with nZVI to generate Fe2O3 and Fe3O4, which attach to the surface of iron particles, reducing the surface active sites of nZVI and leading to a decrease in the reduction rate of nZVI.90 The dosage of nZVI is proportional to the rate of antibiotic removal within a certain range, but excessive dosage may bring other side effects, such as potential harm to the environment. The particle size and morphology of nZVI can affect its specific surface area and activity. The smaller the particle size, the larger the specific surface area, and the faster the reaction rate, resulting in better removal efficiency. When using nZVI to remove antibiotics, these factors should be optimized according to the actual situation to improve removal efficiency.914 The interaction mechanism between typical antibiotics and nano zero valent iron removal
4.1 Adsorption
Adsorption is a low-cost, proficient, and widely used method for removing antibiotics from the environment. Due to the increase in specific surface area and stability, the adsorption performance of C-nZVI particles has been significantly improved compared to using nZVI and carbon based materials alone. Carbon based materials have porous surfaces and abundant oxygen-containing functional groups, providing adsorption sites for heavy metal ions. On the other hand, nZVI with a core–shell structure contains surface iron hydroxide and oxides, which also enhance the adsorption capacity of heavy metal ions. Liang et al.92 analyzed the degradation process of tetracycline using biochar loaded sulfurized nano zero valent iron, which was mainly divided into two stages. In the first stage, tetracycline molecules can be quickly adsorbed onto the surface of S-nZVI/MB; This is due to the specific structure of modified biochar and nano zero valent iron during the degradation and removal process of tetracycline; The presence of graphite structure and aromatic rings in modified biochar can also form π–π interactions with tetracycline molecules, further enhancing adsorption; The FeS or FeS2 generated on the surface of sulfurized nano zero valent iron has hydrophobicity, which can increase the probability of contact between tetracycline molecules and S-nZVI/MB. The fitting results of adsorption kinetics and adsorption isotherm models indicate that the adsorption of tetracycline by S-nZVI/MB is a combination of chemical adsorption and physical adsorption.93 The possible reaction mechanism for the degradation and removal of tetracycline can be further inferred, as shown in Fig. 7.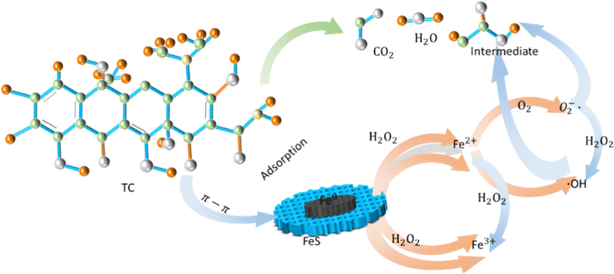 | ||
| Fig. 7 Possible mechanism diagram of nZVI removal of tetracycline.92 | ||
4.2 Oxidation-reduction reaction
nZVI mainly involves adsorption and reduction pathways in the process of removing antibiotics. Ardelfatah. et al.94 studied the adsorption and removal of tetracycline (TC) by green plant synthesized castor oil (Ricinus communis Linn.)-nZVI. In this study, a removal mechanism was discussed, where iron nanoparticles can still effectively remove TC. Secondly, Fe0 reacts with H2O and releases electrons, utilizing H+ to produce highly reducing active hydrogen. Finally, certain cationic molecules receive electrons from active hydrogen, causing –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –C
N and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– to be attributed to the cleavage of the benzene ring.95,96 The reaction mechanism is shown in the Fig. 8. In summary, RCL-nZVI can remove TC antibiotics through adsorption and reduction processes, where antibiotics are adsorbed on iron oxide and masking agents, and then reduced by zero valent iron. Therefore, TC will mainly exist in the form of cations, zwitterions, and anions. The formation and properties of iron oxide coated on the surface of Fe0 nanoparticles affect their interactions with different pollutants. On the other hand, in the presence of oxygen, iron nanoparticles can still effectively remove TC. Secondly, Fe0 reacts with H2O and releases electrons, utilizing H+ to produce highly reducing active hydrogen. Finally, certain cationic molecules receive electrons from active hydrogen, causing –C
C– to be attributed to the cleavage of the benzene ring.95,96 The reaction mechanism is shown in the Fig. 8. In summary, RCL-nZVI can remove TC antibiotics through adsorption and reduction processes, where antibiotics are adsorbed on iron oxide and masking agents, and then reduced by zero valent iron. Therefore, TC will mainly exist in the form of cations, zwitterions, and anions. The formation and properties of iron oxide coated on the surface of Fe0 nanoparticles affect their interactions with different pollutants. On the other hand, in the presence of oxygen, iron nanoparticles can still effectively remove TC. Secondly, Fe0 reacts with H2O and releases electrons, utilizing H+ to produce highly reducing active hydrogen. Finally, certain cationic molecules receive electrons from active hydrogen, causing –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –C
N and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C – to be attributed to the cleavage of the benzene ring.16,96
C – to be attributed to the cleavage of the benzene ring.16,96
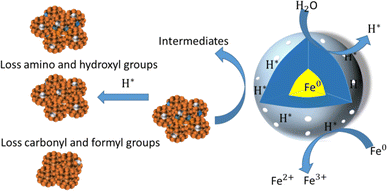 | ||
| Fig. 8 Schematic diagram of the possible reaction mechanism of nZVI for removing pollutants in redox reactions.94 | ||
According to the experiment 97,98 in the particle electrode loaded with nZVI mainly removes CIP from the system through adsorption, reduction, and ˙OH oxidation in a three-dimensional electrode reactor. Due to the attack of ˙OH, the C–F bond in CIP breaks, and the F on quinolones is replaced by ˙OH through a defluorination process to generate new compounds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C next to the carboxyl group on quinolones is destroyed due to hydroxylation, achieving the goal of degradation. Product analysis shows that the cleavage of ammonia, carbonyl, hydroxyl, and dimethylamino groups is the main pathway for TC conversion. Chemical reactions are induced by electron or atomic hydrogen (H*) species by oxidizing nZVI with H2O/H*.
C next to the carboxyl group on quinolones is destroyed due to hydroxylation, achieving the goal of degradation. Product analysis shows that the cleavage of ammonia, carbonyl, hydroxyl, and dimethylamino groups is the main pathway for TC conversion. Chemical reactions are induced by electron or atomic hydrogen (H*) species by oxidizing nZVI with H2O/H*.
Wang et al.99 found that Fenton oxidation (Fenton oxidation refers to the addition of hydrogen peroxide and iron ions), usually Fe2+, to the treated water to produce hydroxyl radicals (˙OH), which have strong oxidizing properties when oxidizing organic pollutants. Fenton oxidation can efficiently degrade organic compounds in organic wastewater and is one of the effective water treatment methods. The contribution of three reaction mechanisms, namely specific coordination and material adsorption, to the removal of OTC from materials. Fenton oxidation is the main mechanism for OTC removal. For the removal of OTC by specific coordination, it may be that after the addition of water absorbing gel coated nano zero valent iron (PPAA-nZVI), a certain amount of Fe3+ is dissolved in the material, and specific coordination occurs with OTC; Subsequently, the nZVI inside the material is oxidized, producing ˙OH, at which point Fenton oxidation begins to dominate; As the reaction time increases, the oxidation of Fenton begins to weaken, and at the same time, there is more Fe3+in the solution, enhancing the specific coordination effect.
| Fe0 + O2 + 2H+ → 2[H] → Fe2+ + H2O | (4) |
| Fe2+ + H2O2 → Fe3+ + OH− + ˙OH | (5) |
4.3 Complexation
Complexation plays an important role in adsorbing organic compounds, especially in the fields of environmental science and engineering. Complex refers to a stable complex formed between two or more compounds. In the environment, organic matter usually exists in a dissolved state, and chelation can affect their adsorption behavior on solid surfaces, thereby affecting their migration and removal in water, soil, or wastewater treatment processes.Due to flocculation, a certain amount of TC can be adsorbed. Wang et al.99 used PPAA-nZVI to remove OTC from water. The experimental analysis showed that there are three ways to remove OTC: firstly, the ˙OH generated by the Fenton system oxidizes and degrades OTC; the second is that Fe3+ in the solution chelates with OTC, resulting in specific coordination; The third is the adsorption of OTC by the surface active sites of the material. Sun et al.100 prepared a sulfurized nano zero valent iron loaded on blast furnace slag using liquid-phase reduction method (S-nZVI@BFS) Materials were used to remove oxytetracycline (OTC) from wastewater. The degradation mechanism shifts from Fendon oxidation and complexation precipitation to electrostatic adsorption.101 Discovered under acidic conditions S-nZVI@BFS The dissolved iron ions can undergo complexation precipitation reaction with oxytetracycline, generating insoluble products in water.
5 Conclusion
At present, sewage treatment is an urgent environmental problem that needs to be solved. nZVI nanocomposites have high specific surface area and high reactivity, and have been proven to be excellent remediation materials that can reduce heavy metal pollution in soil and water. This article reviews the latest progress in the classification and synthesis methods of nZVI. A summary was made on the types of load materials and their effects for load type nZVI. Interactions with commonly used antibiotics through adsorption, electrostatic interactions, co precipitation, ion exchange, complexation, and redox reactions. Meanwhile, environmental conditions such as pH, coexisting components, oxygen, contact time, and temperature regulate the efficiency of antibiotic remediation. The remediation of soil and water pollution based on nZVI is a promising technology that should be further explored and implemented in the near future. In the future, research needs to be conducted in the following areas:(1) Further research is needed to explore the effectiveness and mechanisms of joint pollutant remediation, as well as simultaneous and selective remediation of target pollutants. Currently, nZVI primarily focuses on single pollutant systems in antibiotic contamination remediation, with insufficient understanding of composite heavy metal or nitrate organic compound pollution systems.
(2) Clean preparation methods and novel modification techniques for nZVI are currently hot topics in experimental research. Improving its physical and chemical properties can address the issue of performance degradation due to spontaneous aggregation.
(3) Green preparation methods involving biological and microbial extraction of reducing agents will reduce residual toxicity after removing heavy metals from water, thereby minimizing secondary pollution. However, addressing the high cost associated with new preparation and modification methods remains a significant challenge for industrial applications, although it would facilitate broader adoption.
Data availability
The study did not generate any new or original data.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We are grateful to the sponsors of this work. Anhui University of Technology, School of Metallurgical Engineering and School of Materials Science and Engineering, Jiuquan Vocational and Technical College, Key Laboratory of Metallurgical Emission Reduction & Resources Recycling (Ministry of Education), Anhui Provincial Central Leading Local Science and Technology Development Special Project (No. 202107d06050012); Anhui University Graduate Scientific Research Project (No. YJS202110333); Gansu Province University Teacher Innovation Fund Project, (No. 2024B-342), Natural Science Foundation of Gansu Province, (No. 24JRRF006).References
- J. Dutta and A. Malaa, Removal of antibiotic from the water environment by the adsorption technologies: a review, Water Sci. Technol., 2020, 82(3), 401–426 CAS.
- A. Oluwatosin, S. Salsabil and H. Patricia, et al., Accumulation of antibiotics in the environment: Have appropriate measures been taken to protect Canadian human and ecological health?, Ecotoxicol. Environ. Saf., 2024, 280(116513), 0147–6513 Search PubMed.
- Q. Zhang and X. Qi, et al., Developmental Toxicity of Cu and Tetracycline on Zebrafish Embryos (Danio rerio), Asian J. Ecotoxicol., 2015, 10(05), 35–46 CAS.
- M. T. Liu, Y. Y. Duan and H. J. Ding, Research progress on carbon-based adsorbents for sulfonamide antibiotics removal in water, Water Purif. Technol, 2024, 43(02), 51 Search PubMed.
- W. Q. Yang, J. Li and Z. L. Yao, et al., A review on the alternatives to antibiotics and the treatment of antibiotic pollution: Current development and future prospects, Sci. Total Environ., 2024, 926(171757), 0048–9697 Search PubMed.
- B. B. Tang, Z. H. Zhang and X. Lu, et al., Advances in Phytoremediation of antibiotics in breeding wastewater, Jiangsu Agric. Sci., 2017, 33(01), 224–232 Search PubMed.
- P. F. Zhang, X. W. Liu and L. Jie, et al., Current research in processes for antibiotic Removal from Livestock wastewater, Water Purif. Technol, 2018, 37(04), 60 Search PubMed.
- D. He, J. Li and W. Yu, et al., Deciphering the removal of antibiotics and the antibiotic resistome from typical hospital wastewater treatment systems, Sci. Total Environ., 2024, 926, 171806 CrossRef CAS.
- H. Gao, B. Li and Z. W. Yao, Advances in research on the presence and environmental behavior of antibiotics in the marine environment, Environ. Chem., 2023, 42(3), 779–791 CAS.
- S. Sharma and M. Mansoor Ahammed, Application of modified water treatment residuals in water and wastewater treatment: A review, Heliyon, 2023, 9(5), 15796 CrossRef PubMed.
- A. Liu, J. Liu and J. Han, et al., Evolution of nanoscale zero-valent iron (nZVI) in water: Microscopic and spectroscopic evidence on the formation of nano- and micro-structured iron oxides, J. Hazard. Mater., 2017, 322, 129–135 CrossRef CAS PubMed.
- J. Liu, T. H. Gu and W. Wang, et al., Surface Chemistry and Phase Transformation of Nanoscale Zero-Valent Iron (nZVI) in Aquatic Media, Acta Chim. Sin., 2019, 77(02), 121–129 CrossRef CAS.
- J. Sheng, H. Y. FU and W. Wang, et al., Research progress on the characterization and modification of nanoscale zero-valent iron for applications, Environ. Chem., 2020, 39(11), 2959–2978 CAS.
- H. Dong, C. Zhang and K. Hou, et al., Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution, Sep. Purif. Technol., 2017, 188, 188–196 CrossRef CAS.
- J. Li, C. Chen and K. Zhu, et al., Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd(II) removal, J. Taiwan Inst. Chem. Eng., 2016, 59, 389–394 CrossRef CAS.
- L. Huang, X. Weng and Z. Chen, et al., Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity, Spectrochim. Acta, Part A, 2014, 130, 295–301 CrossRef CAS.
- J. He, L. Ai, Y. Wang, et al., Carbothermal Synthesis of Aerosol-Based Iron-Carbon Nanocomposites for Adsorption and Reduction of Cr (VI), Nanoscale Zerovalent Iron Particles for Environmental Restoration, 2019, pp. 495–510 Search PubMed.
- C. L. Chun, D. R. Bare and D. W. Matson, et al., Characterization and reactivity of iron nanoparticles prepared with added Cu, Pd, and Ni, Environ. Sci. Technol., 2010, 44(13), 5079–5085 CrossRef CAS PubMed.
- B. A. De, T. Lovaglio and A. Galasso, et al., Iron and iron oxide nanoparticles obtained by ultra-short laser ablation in liquid, Appl. Surf. Sci., 2015, 353, 433–438 CrossRef.
- C. C. Peng, J. X. Wang and Z. B. Tong, et al., Progress in the Fabrication of Powder materials Using Electric Explosion Method, J. Mater. Sci. Eng., 2013, 31(04), 608–613 CAS.
- S. L. Li, W. Yan and W. X. Zhang, et al., Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling, Green Chem., 2009, 11(10), 1618–1626 RSC.
- R. Cheng, J. Li and S. Li, et al., An improved preparation method of carbon aerogels derived from alginate/chitosan with a superiority of promoting nZVI for chromium (VI) ions removal from aqueous solution, Microporous Mesoporous Mater., 2023, 352, 112512 CrossRef CAS.
- X. Wang, P. Liu and J. Ma, et al., Preparation of novel composites based on hydrophilized and functionalized polyacrylonitrile membrane-immobilized NZVI for reductive transformation of metronidazole, Appl. Surf. Sci., 2017, 396, 841–850 CrossRef CAS.
- B. Cao, J. Qu and W. Bian, et al., Porous hydrochar loaded nZVI as an efficient catalyst to activate persulfate for phenol degradation: Performance and mechanism, J. Clean. Prod., 2024, 141221 CrossRef CAS.
- B. Akugar and P. J. I. J. O. M. P. Pourghahramani, Implementation of sonochemical leaching for preparation of nano zero-valent iron (NZVI) from natural pyrite mechanochemically reacted with Al, Int. J. Miner. Process., 2017, 164, 1–5 CrossRef.
- B. Kakavandi, R. R. Kalantlry and M. Farzadkia, et al., Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles, J. Environ. Sci. Health Environ. Sci. Eng., 2014, 12, 1–10 CrossRef PubMed.
- L. He, S. Wang and F. Luo, et al., Unravelling the bifunctional role of biochar in promoting nZVI/Ni towards complete dechlorination of trichloroethylene: Not only a carbonouces support, Chem. Eng. J., 2024, 481, 148634 CrossRef CAS.
- T. Wang, J. Su and X. Jin, et al., Functional clay supported bimetallic nZVI/Pd nanoparticles used for removal of methyl orange from aqueous solution, J. Hazard. Mater., 2013, 262, 819–825 CrossRef CAS PubMed.
- L. N. Shi, Y. M. Lin and X. Zhang, Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr (VI) from aqueous solution, Chem. Eng. J., 2011, 171(2), 612–617 CrossRef CAS.
- W. Liang, G. Wang and C. Peng, et al., Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review, J. Hazard. Mater., 2022, 426, 127993 CrossRef CAS PubMed.
- M. Sun, L. Zou and P. Wang, et al., Nano valent zero iron (NZVI) immobilized CNTs hollow fiber membrane for flow-through heterogeneous Fenton process, J. Environ. Chem. Eng., 2022, 10(3), 107806 CrossRef CAS.
- G. Gopal, H. Sankar and C. Natarajan, et al., Tetracycline removal using green synthesized bimetallic nZVI-Cu and bentonite supported green nZVI-Cu nanocomposite: A comparative study, J. Environ. Manage., 2020, 254, 109812 CrossRef CAS PubMed.
- W. M. Xie, X. L. Bi and Z. J. Huang, et al., Adsorption mechanism of different heavy metals on the magnetic nano-zerovalent iron supported by nano-active alumina, Acta Sci. Circumstantiae, 2020, 40(08), 2732–2740 CAS.
- X. Li, L. Ai and J. Jiang, Nanoscale zerovalent iron decorated on graphene nanosheets for Cr(VI) removal from aqueous solution: Surface corrosion retard induced the enhanced performance, Chem. Eng. J., 2016, 288, 789–797 CrossRef CAS.
- Y. Zhang, B. Q. Su and C. Zhang, et al., Research on the removal of Cr(VI)from wastewater by pectin-stabilized nanoscale zero-valent iron, Ind. Water Treat., 2016, 36(09), 47–50 CrossRef.
- L. Chen, J. Zhang and Q. Li, et al., Removal of p-nitrophenols by BC@nZVI activated persulfate: A study of key factors and mechanisms, J. Environ. Chem. Eng., 2023, 11(6), 111483 CrossRef CAS.
- J. Y. Guo, J. Y. Jiang and Y. H. Chen, et al., Synthesis of nZVI-BC composite for persulfate activation to degrade pyrene: Performance, correlative mechanisms and degradation pathways, Process Saf. Environ. Prot., 2022, 162, 733–745 CrossRef CAS.
- S. Mortazavian, H. An and D. Chun, et al., Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies, Chem. Eng. J., 2018, 353, 781–795 CrossRef CAS.
- S. Liu, X. Xia and B. Xin, et al., Study on mechanism of adsorption of Cr(VI)by modified activated carbon loaded with nano-zero-valent iron, J. Univ. Sci. Technol. Liaoning, 2023, 46(04), 287–295 Search PubMed.
- G. Zhang, F. Yang and W. Yang, et al., N-doped carbon nanotube encapsulated nZVI as a high-performance bifunctional catalyst for oxidative desulfurization, Particuology, 2023, 81, 109–118 CrossRef CAS.
- M. P. Phyu, C. P. Phyu and L. Chanadana, et al., Bio-waste assisted phase transformation of Fe3O4/carbon to nZVI/graphene composites and its application in reductive elimination of Cr(VI) removal from aquifer, Sep. Purif. Technol., 2023, 306, 122632 CrossRef.
- Y. Vieira, M. S. Netto and É. C. Lima, et al., An overview of geological originated materials as a trend for adsorption in wastewater treatment, Geosci. Front., 2022, 13(1), 101150 CrossRef CAS.
- L. Yu, Y. Li and M. Yi, et al., Research Progress in Removal of Contaminants by Clay-supported Nanoscale Zero-valent Iron Composites, J. Zhejiang Univ., 2018, 38(07), 34–45 Search PubMed.
- Y. Li, B. Xu and H. Yang, et al., Promotion of Cu/Ce supported red mud for NO removal from low and medium temperature flue gas, J. Fuel Chem. Technol., 2024, 52(3), 362–371 CrossRef CAS.
- X. R. Yang, C. H. Li and W. Q. Ma, et al., Study on the adsorption performance of Cu2+ in wastewater by red mud, Desalination Water Treat., 2024, 317, 2100254 Search PubMed.
- M. K. Sahu, R. K. Patel and S. Kurwadkar, Mechanistic insight into the adsorption of mercury (II) on the surface of red mud supported nanoscale zero-valent iron composite, J. Contam. Hydrol., 2022, 246, 103959 CrossRef CAS PubMed.
- I. Liaquat, R. Munir and N. A. Abbasi, et al., Exploring zeolite-based composites in adsorption and photo-catalysis for toxic wastewater treatment: Preparation, mechanisms, and future perspectives, Environ. Pollut., 2024, 123922 CrossRef CAS PubMed.
- Q. J. Zeng, X. Zhou and C. Huang, et al., Enhanced removal of Cd(II) from aqueous solution by nanoscale zero-valent iron coupled with white rot fungus, China Environ. Sci., 2022, 42(7), 3174–3183 CAS.
- A. Pullin, R. A. Crane and D. J. Morgan, et al., The effect of common groundwater anions on the aqueous corrosion of zero-valent iron nanoparticles and associated removal of aqueous copper and zinc, J. Environ. Chem. Eng., 2017, 5(1), 1166–1173 CrossRef.
- A. Baldermann, S. Kaufhold and R. Dohrmann, et al., A novel nZVI–bentonite nanocomposite to remove trichloroethene (TCE) from solution, Chemosphere, 2021, 282, 131018 CrossRef CAS.
- Y. Zhao, X. Cao and X. Song, et al., Montmorillonite supported nanoscale zero-valent iron immobilized in sodium alginate (SA/Mt-NZVI) enhanced the nitrogen removal in vertical flow constructed wetlands (VFCWs), Bioresour. Technol., 2018, 267, 608–617 CrossRef CAS PubMed.
- B. Ma, J. Yao and Z. Chen, et al., Superior elimination of Cr(VI) using polydopamine functionalized attapulgite supported nZVI composite: Behavior and mechanism, Chemosphere, 2022, 287, 131970 CrossRef CAS.
- C. Fan, J. Q. Meng and B. Guo, Expanded Pearlite-Loaded Nano Fe0 for efficiently removaling phosphates in wastewater, Mod. Chem. Ind., 2023, 43(02), 117 Search PubMed.
- Y. H. Wang, Q. Q. Gao and L. Zhang, et al., The removal of p-nitrophenol by sulfurized nano zero valent iron supported by bentonite, Environmental Pollution & Control, 2019, 41(03), 329–333 Search PubMed.
- X. T. Jin, J. J. Zhuo and Z. H. Zhang, et al., Preparation and Properties of Polyvinyl Alcohol/Nano-Scale Zero-Valent Iron Composite Films, Packag. J., 2023, 15(03), 25–30 Search PubMed.
- R. Cheng, J. Li and S. Li, et al., An improved preparation method of carbon aerogels derived from alginate/chitosan with a superiority of promoting nZVI for chromium(VI) ions removal from aqueous solution, Microporous Mesoporous Mater., 2023, 352, 112512 CrossRef CAS.
- R. Zhao, B. Wang and P. Wu, et al., Calcium alginate-nZVI-biochar for removal of Pb/Zn/Cd in water: Insights into governing mechanisms and performance, Sci. Total Environ., 2023, 894, 164810 CrossRef CAS PubMed.
- F. Gao, M. Zhang and W. Zhang, et al., Synthesis of carboxymethyl cellulose stabilized sulfidated nanoscale zero-valent iron (CMC-S-nZVI) for enhanced reduction of nitrobenzene, Sep. Purif. Technol., 2023, 315, 123704 CrossRef CAS.
- W. Wang, Y. Hua and S. Li, et al., Removal of Pb(II) and Zn(II) using lime and nanoscale zero-valent iron (nZVI): A comparative study, Chem. Eng. J., 2016, 304, 79–88 CrossRef CAS.
- R. Radhakrishnan l, M. Datchanamoorthy and D. Narayanasamy, et al., Unveiling global public interest and seasonal patterns of antibiotics and antibiotic resistance: An infodemiology study with implications for public health awareness and intervention strategies, Int. J. Med. Inf., 2023, 179(105231), 386–5056 Search PubMed.
- P. Huang, Z. Ye and W. Xie, et al., Rapid magnetic removal of aqueous heavy metals and their relevant mechanisms using nanoscale zero valent iron (nZVI) particles, Water Res., 2013, 47(12), 4050–4058 CrossRef CAS PubMed.
- L. Liu, P. W. Hu and R. Q. Gao, et al., Removal of Chromate(VI)from Water Using Kaolinite Enhanced by Nano-Zero-Valent Iron and Its Mechanism, Bull. Chin. Ceram. Soc., 2021, 40(05), 1529 Search PubMed.
- C. S. Kuo, D. T. F. Kuo and A. Chang, et al., Rapid debromination of tetrabromobisphenol A by Cu/Fe bimetallic nanoparticles in water, its mechanisms, and genotoxicity after treatments, J. Hazard. Mater., 2022, 432, 128630 CrossRef CAS PubMed.
- J. Werner, A. Zgoła-grześkowiak and T. Grześkowiak, et al., Biopolymers-based sorbents as a future green direction for solid phase (micro)extraction techniques, Trac. Trends Anal. Chem., 2024, 173, 117659 CrossRef CAS.
- M. T. F. De, A. S. Andate, J. Scapinello, et al., Biopolymers as Support Materials for Photocatalysts During Wastewater Treatment [M], Reference Module in Materials Science and Materials Engineering, Elsevier, 2024 Search PubMed.
- S. X. Wei, C. He and C. H. Li, et al., Study on the removal of heavy metals from wastewater by nano-scale zero-valent iron and its preparation method, Nonferrous Met. Sci. Eng., 2023, 1–13 Search PubMed.
- H. Ji, Y. L. Yang and R. G. Xing, et al., Pollution characteristics and ecological risk assessment of sulfonamide antibiotics in the Xi’an section of the Weihe River, Environmental Pollution & Control, 2023, 45(04), 521–527 Search PubMed.
- Y. T. Zhang, M. Wang and W. Cheng, et al., Effects of water environmental factors and antibiotics on bacterial community in urban landscape lakes, Aquat. Toxicol., 2023, 265, 106740 CrossRef CAS PubMed.
- J. Cui, L. Fu and B. Tang, et al., Occurrence, ecotoxicological risks of sulfonamides and their acetylated metabolites in the typical wastewater treatment plants and receiving rivers at the Pearl River Delta, Sci. Total Environ., 2020, 709, 136192 CrossRef CAS PubMed.
- M. Khmaissa, H. Zouari-mechichi and G. Sciara, et al., Pollution from livestock farming antibiotics an emerging environmental and human health concern: A review, J. Hazard. Mater. Adv., 2024, 13, 100410 CrossRef CAS.
- B. Madhogaria, S. Banerjee and A. Kundu, et al., Efficacy of new generation biosorbents for the sustainable treatment of antibiotic residues and antibiotic resistance genes from polluted waste effluent, Infect. Med., 2024, 3(1), 100092 CrossRef PubMed.
- X. Zhang, T. Cai and S. Zhang, et al., Contamination distribution and non-biological removal pathways of typical tetracycline antibiotics in the environment: A review, J. Hazard. Mater., 2024, 463, 132862 CrossRef CAS.
- X. Wang, J. Tong and J. Ma, Design of sisal fibre biochar/poly(dopamine)/NZVI@PAN membrane for efficient degradation of tetracycline, React. Funct. Polym., 2023, 192, 105704 CrossRef CAS.
- L. Chen, T. Yuan and R. Ni, et al., Multivariate optimization of ciprofloxacin removal by polyvinylpyrrolidone stabilized NZVI/Cu bimetallic particles, Chem. Eng. J., 2019, 365, 183–192 CrossRef CAS.
- B. Nandana, S. John and K. E. Velmaiel, et al., Passivation bypassed Zero valent Iron based heterostructures for effective removal of persistent antibiotics by solar driven photo-Fenton process, J. Environ. Chem. Eng., 2024, 12(1), 111567 CrossRef CAS.
- Y. Yang, J. Zhu and Q. Zeng, et al., Enhanced activation of peroxydisulfate by regulating pyrolysis temperature of biochar supported nZVI for the degradation of oxytetracycline, J. Taiwan Inst. Chem. Eng., 2023, 145, 104775 CrossRef CAS.
- C. H. Nguyan, M. L. Tran and T. T. T. Van, et al., Efficient removal of antibiotic oxytetracycline from water by Fenton-like reactions using reduced graphene oxide-supported bimetallic Pd/nZVI nanocomposites, J. Taiwan Inst. Chem. Eng., 2021, 119, 80–89 CrossRef.
- Q. Li, Z. Chen and H. Wang, et al., Removal of organic compounds by nanoscale zero-valent iron and its composites, Sci. Total Environ., 2021, 792, 148546 CrossRef CAS PubMed.
- L. Li, H. Jin and N. Luo, et al., Sulfurized nano zero-valent iron prepared via different methods: Effect of stability and types of surface corrosion products on removal of 2,4,6-trichlorophenol, Ecotoxicol. Environ. Saf., 2023, 256, 114864 CrossRef CAS PubMed.
- Q. Jing, W. You and S. Qiao, et al., Comprehensive understanding of adsorption and reduction on 2,4-DCP and Cr(VI) removal process by NZVI-rGO: Performance and mechanism, J. Water Proc. Eng., 2023, 51, 103413 CrossRef.
- N. A. Zarime, B. Soleman and W. Z. W. Yaacob, et al., Removal of Methylene Blue by Granitic Residual Soil Supported Nano Zero Valent Iron (Gr-nZVI): Kinetic and Equilibrium Studies, Desalination Water Treat., 2024, 317, 100099 CrossRef.
- D. S. Ken and A. Sinha, Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts, Environ. Nanotechnol. Monit. Manag., 2020, 14, 100344 Search PubMed.
- M. Apte, N. Nadavade and S. S. Sheikh, A review on nitrates' health benefits and disease prevention, Nitric Oxide, 2024, 142, 1–15 CrossRef CAS.
- Y. Pei, W. Cheng and R. Liu, et al., Synergistic effect and mechanism of nZVI/LDH composites adsorption coupled reduction of nitrate in micro-polluted water, J. Hazard. Mater., 2024, 464, 133023 CrossRef CAS PubMed.
- Y. Zhou and X. Li, Green synthesis of modified polyethylene packing supported tea polyphenols-NZVI for nitrate removal from wastewater: Characterization and mechanisms, Sci. Total Environ., 2022, 806, 150596 CrossRef CAS PubMed.
- H. Dong, Z. Jiang and C. Zhang, et al., Removal of tetracycline by Fe/Ni bimetallic nanoparticles in aqueous solution, J. Colloid Interface Sci., 2018, 513, 117–125 CrossRef CAS.
- X. Wang, B. Zhang and J. Ma, et al., Novel synthesis of aluminum hydroxide gel-coated nano zero-valent iron and studies of its activity in flocculation-enhanced removal of tetracycline, J. Environ. Sci., 2020, 89, 194–205 CrossRef CAS.
- J. Yang, M. Zhu and X. Wang, et al., Poly(vinylidene fluoride) membrane supported nano zero-valent iron for metronidazole removal: Influences of calcium and bicarbonate ions, J. Taiwan Inst. Chem. Eng., 2015, 49, 113–118 CrossRef CAS.
- T. Zheng, D. Hou and N. Wu, et al., Synergistic effect and removal mechanism of trichloroethylene (TCE) by nano scale zero-valent iron (nZVI) supported on biological calcium carbonate (CaCO3), J. Environ. Chem. Eng., 2023, 11(6), 111573 CrossRef CAS.
- W. Qing, D. D. Sun and Y. X. Cao, et al., Research and Progress on Removing Nitrate from Water by Nano-zero-valent Iron, China Water & Wastewater, 2023, 39(16), 21–28 Search PubMed.
- L. Chen, J. K. Zhang and Q. X. Li, et al., Removal of p-nitrophenols by BC@nZVI activated persulfate: A study of key factors and mechanisms, J. Environ. Chem. Eng., 2023, 11(6), 111483 CrossRef CAS.
- S. Liang, DEGRADATION OF TETRACYCLINE BY SULFIDE NANO ZERO VALENT IRON SUPPORTED ON BIOCHAR [D], MPhil thesis, Donghua university, Shanghai, 2021.
- Y. X. Yang, J. F. Zhu and Q. Z. Zeng, et al., Enhanced activation of peroxydisulfate by regulating pyrolysis temperature of biochar supported nZVI for the degradation of oxytetracycline, J. Taiwan Inst. Chem. Eng., 2023, 145, 104775 CrossRef CAS.
- A. M. Abdelfatah, M. Fawzy and M. E. El-khouly, et al., Efficient adsorptive removal of tetracycline from aqueous solution using photosynthesized nano-zero valent iron, J. Saudi Chem. Soc., 2021, 25(12), 101365 CrossRef CAS.
- K. Sravanthi, D. Ayodhya and P. Y. Swamy, Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles, Mater. Sci. Energy Technol., 2019, 2(2), 298–307 Search PubMed.
- L. Zhang, Z. X. Song and T. J. Dong, et al., Mitigating mechanism of nZVI-C on the inhibition of anammox consortia under long-term tetracycline hydrochloride stress: Extracellular polymeric substance properties and microbial community evolution, J. Hazard. Mater., 2023, 52, 131035 CrossRef PubMed.
- C. Xiao and H. Li Y. Zhao, et al., Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes, J. Environ. Manage., 2020, 275, 111262 CrossRef CAS PubMed.
- X. W. Ji, Preparation of Iron Nanocomposite Particle Electrodes and Their Use in a Three-Dimensional Electro-Chemical System for the Removal of Ciprofloxacin from Water, MPhil thesis, 2023.
- Y. Y. Wang, H. L. Shi and M. Yuan, et al., Removal of OTC from water using hydrogel coated nZVI, J. Chin. Inst. Environ. Eng., 2023, 17(6), 1828–1840 Search PubMed.
- Q. N. Sun, R. B. Zhang and M. J. Deng, et al., Removal of Oxytetracycline from Water Using Blast Furnace Slag Loaded Sulfide Nanoscale Zero-valent Iron, Environ. Sci., 2021, 42(02), 867–873 Search PubMed.
- S. X. Wei, H. Chuan and C. H. Li, et al., Study on the removal of heavy metals from wastewater by nano-scale-zero-valent iron and its preparation method, Nonferrous Met. Sci. Eng., 2024, 36(1311), 1674–9669 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |