A method for separating PEGylated Au nanoparticle ensembles as a function of grafting density and core size†
Feng
Lu
ab,
Tennyson L.
Doane
a,
Jun-Jie
Zhu
*b and
Clemens
Burda
*a
aDepartment of Chemistry, Case Western Reserve University, 10900 Euclid Ave., Cleveland, Ohio, 44106 USA. E-mail: burda@case.edu; Fax: +1-216-368-3006; Tel: +1-216-368-5313
bState Key Lab. of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, P. R. China. E-mail: jjzhu@nju.edu.cn; Fax: +86-25-8359-7204; Tel: +86-25-8359-7204
First published on 28th October 2013
Abstract
After ligand exchange with PEG, Au NPs with differently mixed surface functionalities co-exist in the as-synthesized sample. It is found that the poorly grafted nanoparticles can be simply removed using a chromatographic method, and the well grafted nanoparticles can be eluted as a function of the core size.
Nano-sized gold has become extremely attractive for scientists requiring a versatile platform for a variety of life science and biomedical applications, including biosensors, bio-imaging, diagnosis and drug delivery.1 In addition to their size and shape,2 surface ligands on gold nanoparticles (Au NPs) have been found to be a crucial parameter for bio-applications and heavily explored over the last few years.3 Among them, polyethylene glycol (PEG) grafting is one of the ubiquitously used techniques,4 which can significantly reduce nonspecific binding towards cells and serum proteins, and greatly extend the circulation half-life of Au NPs in vivo.5 Recently, ligand density was found to be another important parameter for altering the physical properties (e.g. hydrodynamic size, zeta potential and mobility)6 and the interaction of Au NPs with bio-environments.7 Importantly, a densely coated PEG shell was found to enable the nanoparticles to penetrate the blood–brain barrier which has long been considered as a large impediment for treatment of the central nervous system.8
Commonly, the maximum ligand density was expected to be achieved by adding excess ligands during a given ligand exchange reaction (the most common approach to obtain PEGylated Au NPs).9 However, our results show that a remarkable amount of poorly grafted crude products can still exist in the as-synthesized samples. Since the ligand exchange reaction is widely applied to gold nanoparticles, the research based on these non-uniform particle ensembles, often deriving from the ligand exchange, may lead to misleading and inaccurate results, which is of paramount importance in targeting, biodistribution and pharmacokinetics, but it has been largely neglected over the past years. Clearly, research needs to be focused on the non-uniformity of as-prepared NP ensembles.10 This increased awareness of the importance of NP surface properties has recently led to improved efforts, and some work has indeed aimed to obtain more uniform NP ensembles from the original NP samples.10a,11 Herein we show that poorly PEG grafted NPs can be removed by a simple chromatographic process, while well grafted particles can be eluted as a function of the gold core size (Scheme 1). Thus, this facile separation method provides a simple route to obtain well grafted and uniformly size-distributed Au NPs which will completely change the perspective and outlook for both fundamental research and practical applications involving Au NPs.
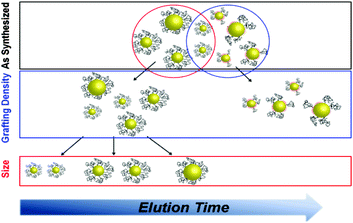 | ||
| Scheme 1 Schematic illustration of the separation process. First the NPs are separated by their grafting density, then by size. | ||
Initial PEGylated Au NPs were prepared via a well-established ligand exchange process between mPEG-thiols with different molecular weight (MW) and dodecylamine (DDA) capped Au NPs as described earlier.12 After the usual ligand exchange, the crude products with “surface ligand defects” (low grafting density and high residual DDA) should have a much longer retention time on the column, based on our initial thin layer chromatography (TLC) experiments (Fig. S1, ESI†), where we observed that PEGylated Au NPs migrated partially and DDA-capped Au NPs were retained at the starting point. Upon loading onto a silica gel column, Au NPs were found to be separated into both mobile and stationary fractions (Fig. 1A). The trapped fraction is significant (30–55% of the total amount), implying that there are at least two different populations of NPs in the original sample. It is notable that the ratio between these two fractions varies with the age of the sample. For 5 kDa PEG grafted NPs, after 7 months of storage, 73% of the Au NPs could migrate through the column, and after 12 months of storage the number increased up to 85%, which was almost double that of the fresh sample (45%). Earlier studies suggested that after initial coverage, further grafting should be very slow (7 days may be required for small molecules).13 In the case of PEG, further ligand exchange could be even more difficult due to the steric requirements of the long PEG chains already present on the surface. We found that separating nanoparticles using column chromatography is an excellent method to obtain samples that are densely grafted with PEG.
The two fractions from the 5 kDa-Au NP sample revealed significant differences in their assembly on the TEM grid (Fig. 2, Fig. S8, ESI†). In the case of the mobile fraction (F1), a uniform spacing of 9 nm was observed between each particle, while the gap between the trapped particles (F2) was much smaller (some even directly touching each other) with poor uniformity. These differences reveal that F1 has a dense and uniform coverage of PEG ligands compared with the trapped fraction. TEM images of F1 and F2 when redispersed in DI water revealed that a cluster structure was favored for F2 (Fig. S5, ESI†). We found that this is due to the residual hydrophobic DDA present on these trapped Au NPs. Besides, dynamic light scattering data show that the mobile fractions were all very narrow in size distribution with a polydispersity index (PDI) of <0.04 (Fig. S5, Table S1, ESI†). It can be concluded that the mobile fraction corresponds to the well-grafted Au NPs, while the trapped fraction corresponds to poorly-grafted ones.
 | ||
| Fig. 2 TEM images of Au NP fractions eluted (A) and retained (B) on the column with 14.3% aqueous methanol solution. Scale bar is 50 nm. | ||
Interestingly, Au NPs prepared using different PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au NP ratios (different average grafting densities) show different surface plasmon resonance (SPR) absorption peaks after being dried into a film (Fig. S4B, ESI†), thus providing a qualitative assessment of the average PEG shell thickness for each sample. These SPR peak shift and broadening are due to the strong interaction and coupling between neighboring Au NPs.14 For our 6 nm diameter Au NPs, the surface saturating PEG ratio is reached at 300
Au NP ratios (different average grafting densities) show different surface plasmon resonance (SPR) absorption peaks after being dried into a film (Fig. S4B, ESI†), thus providing a qualitative assessment of the average PEG shell thickness for each sample. These SPR peak shift and broadening are due to the strong interaction and coupling between neighboring Au NPs.14 For our 6 nm diameter Au NPs, the surface saturating PEG ratio is reached at 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which is in agreement with TLC data (Fig. S2, ESI†). Au NPs prepared in a ratio of 50
1 which is in agreement with TLC data (Fig. S2, ESI†). Au NPs prepared in a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5 kDa PEG
1 (5 kDa PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au NP), well below the minimum saturation coverage, were selected to further test the separation performance. After column separation, these particles showed an absorption peak at around 532 nm (Fig. S4A, ESI†) which was 18 nm blue shifted compared to the original sample. In comparison, the immobile fraction (F2) absorbed at 578 nm. This phenomenon serves as additional evidence of how important post-synthesis separation is, and that column chromatography can separate the populations of differently-grafted and agglomerated Au NPs.
Au NP), well below the minimum saturation coverage, were selected to further test the separation performance. After column separation, these particles showed an absorption peak at around 532 nm (Fig. S4A, ESI†) which was 18 nm blue shifted compared to the original sample. In comparison, the immobile fraction (F2) absorbed at 578 nm. This phenomenon serves as additional evidence of how important post-synthesis separation is, and that column chromatography can separate the populations of differently-grafted and agglomerated Au NPs.
The size separation was achieved simply by collecting the mobile fraction at different elution volumes. It was found that the absorption peak generally moved to longer wavelengths during the elution time (Fig. 1B), which often indicated the increase of gold core sizes.15 TEM of the original 1 kDa PEGylated Au NPs together with three selected fractions confirmed the separation of differently sized gold core populations (Fig. 3). All chromatographed fractions showed a narrow size distribution compared to the original batch. This size separation between 3.3 nm and 7.5 nm is very important, as the critical size for renal clearance is 5.5 nm.16 An increase of the average diameter (Fig. S7 and S8, ESI†) was also achieved for 5 kDa PEGylated Au NPs, but the size distribution was broader. This less effective separation was predictable from the small SPR peak shift (Fig. 1B). Our hypothesis is that the vdW forces of the Au NP cores toward the stationary phase lead to this size separation process.17 Although the polar interactions between the analyte and the stationary phase are commonly the major force to control the elution process, it should be relatively weak here, with a near unity Rf value on TLC. Au NPs with bigger cores have a stronger vdW interaction to silica beads (Fig. S9, see the ESI† for detailed calculations) and a longer retention time on the column is expected. The UvdW becomes much weaker when the PEG MW becomes larger than 2 kDa (PEG shell thickness > 9.6 nm). Thus, increasing PEG MW from 1 kDa to 5 kDa will weaken the vdW forces between silica and Au NP cores, making column chromatography less effective for core size separation (smaller absorption peak separation in Fig. 1B). With a shell thickness of 9.9 nm, 5 kDa (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) PEGylated Au NPs showed a boarder band and larger total elution volume compared to the corresponding NPs with a 500
1) PEGylated Au NPs showed a boarder band and larger total elution volume compared to the corresponding NPs with a 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. S3, ESI†). The vdW data combined with the profiles shown in Fig. 2 suggest that the ligand MW for an efficient size separation behavior is between 2 kDa and 5 kDa.
1 ratio (Fig. S3, ESI†). The vdW data combined with the profiles shown in Fig. 2 suggest that the ligand MW for an efficient size separation behavior is between 2 kDa and 5 kDa.
In summary, we show that the ligand exchange reaction time is much longer than generally expected; thus direct synthesis leads to a large amount of defective, even toxic nanoparticles. Remarkably, the chromatographic method revealed its abilities to eliminate the crude products and to separate the well-coated PEG Au NPs from the original sample. From the column, the well-grafted PEG-Au NPs are eluted as a function of the gold core size. Due to the versatility of this chromatography technique, we believe that the described method can be extended to other nanoparticle systems, thus providing a new avenue to more precisely control size and surface functionalities on nanoparticles.
This work is supported by National Natural Science Foundation of China (NSFC 21020102038).
Notes and references
- (a) X. Y. Wang and Z. J. Guo, Chem. Soc. Rev., 2013, 42, 202 RSC; (b) T. L. Doane and C. Burda, Chem. Soc. Rev., 2012, 41, 2885 RSC.
- C. Burda, X. B. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed.
- (a) T. A. Larson, P. R. Joshi and K. Sokolov, ACS Nano, 2012, 6, 9182 CrossRef CAS PubMed; (b) M. Colombo, S. Mazzucchelli, V. Collico, S. Avvakumova, L. Pandolfi, F. Corsi, F. Porta and D. Prosperi, Angew. Chem., Int. Ed., 2012, 51, 9272 CrossRef CAS PubMed.
- (a) C. D. Walkey, J. B. Olsen, H. B. Guo, A. Emili and W. C. W. Chan, J. Am. Chem. Soc., 2012, 134, 2139 CrossRef CAS PubMed; (b) S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer and W. C. W. Chan, Nano Lett., 2009, 9, 1909 CrossRef CAS PubMed.
- (a) H. S. Han, J. D. Martin, J. Lee, D. K. Harris, D. Fukumura, R. K. Jain and M. Bawendi, Angew. Chem., Int. Ed., 2013, 52, 1414 CrossRef CAS PubMed; (b) J. A. Hubbell and A. Chilkoti, Science, 2012, 337, 303 CrossRef PubMed.
- (a) K. M. Krueger, A. M. Al-Somali, M. Mejia and V. L. Colvin, Nanotechnology, 2007, 18, 475709 CrossRef; (b) J. Y. Kim, H. B. Kim and D. J. Jang, Electrophoresis, 2013, 34, 911 CrossRef CAS PubMed.
- (a) H. Hinterwirth, S. Kappel, T. Waitz, T. Prohaska, W. Lindner and M. Lammerhofer, ACS Nano, 2013, 7, 1129 CrossRef CAS PubMed; (b) B. Thierry and H. J. Griesser, J. Mater. Chem., 2012, 22, 8810 RSC.
- E. A. Nance, G. F. Woodworth, K. A. Sailor, T. Y. Shih, Q. G. Xu, G. Swaminathan, D. Xiang, C. Eberhart and J. Hanes, Sci. Transl. Med., 2012, 4, 149ra119 CrossRef PubMed.
- X. H. Xia, M. X. Yang, Y. C. Wang, Y. Q. Zheng, Q. G. Li, J. Y. Chen and Y. N. Xia, ACS Nano, 2012, 6, 512 CrossRef CAS PubMed.
- (a) J. K. Kim, M. D. Howard, T. D. Dziubla, J. J. Rinehart, M. Jay and X. L. Lu, ACS Nano, 2011, 5, 209 CrossRef CAS PubMed; (b) H. Portalès, N. Goubet, S. Sirotkin, E. Duval, A. Mermet, P.-A. Albouy and M.-P. Pileni, Nano Lett., 2012, 12, 5292 CrossRef PubMed.
- J. C. Olivier, R. Huertas, H. J. Lee, F. Calon and W. M. Pardridge, Pharm. Res., 2002, 19, 1137 CrossRef CAS.
- (a) T. L. Doane, Y. Cheng, A. Babar, R. J. Hill and C. Burda, J. Am. Chem. Soc., 2010, 132, 15624 CrossRef CAS PubMed; (b) Y. Cheng, J. D. Meyers, A. M. Broome, M. E. Kenney, J. P. Basilion and C. Burda, J. Am. Chem. Soc., 2011, 133, 2583 CrossRef CAS PubMed.
- (a) R. Guo, Y. Song, G. L. Wang and R. W. Murray, J. Am. Chem. Soc., 2005, 127, 2752 CrossRef CAS PubMed; (b) M. J. Hostetler, A. C. Templeton and R. W. Murray, Langmuir, 1999, 15, 3782 CrossRef CAS.
- Z. H. Xu, C. X. Li, X. J. Kang, D. M. Yang, P. P. Yang, Z. Y. Hou and J. Lin, J. Phys. Chem. C, 2010, 114, 16343 CAS.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042 CrossRef CAS PubMed.
- S. Zhang, Z. Chu, C. Yin, C. Zhang, G. Lin and Q. Li, J. Am. Chem. Soc., 2013, 135, 5709 CrossRef CAS PubMed.
- T. T. Morgan, H. S. Muddana, E. I. Altinoglu, S. M. Rouse, A. Tabakovic, T. Tabouillot, T. J. Russin, S. S. Shanmugavelandy, P. J. Butler, P. C. Eklund, J. K. Yun, M. Kester and J. H. Adair, Nano Lett., 2008, 8, 4108 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, detailed characterization of gold nanoparticles and vdW calculations. See DOI: 10.1039/c3cc47124a |
| This journal is © The Royal Society of Chemistry 2014 |


