Dong Chen, Zhen Wu, Yingming Yao and Chen Zhu
Org. Chem. Front., 2018,5, 2370-2374
DOI:
10.1039/C8QO00558C,
Research Article
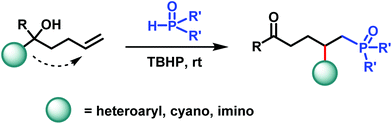
Disclosed herein is a novel and practical protocol for the radical-mediated phosphinoyl-functionalization of unactivated alkenes. The P-centered radicals are generated in the presence of TBHP under mild reaction conditions, which trigger the subsequent distal functional group migration. Both phosphine oxides and phosphonates are suitable precursors for the delivery of phosphinoyl radicals. As a result, phosphinoyl radicals along with another functional group such as heteroaryl, cyano, or imino are incorporated to alkenes concurrently. A wide range of synthetically valuable alkylphosphorus compounds are furnished in good yields.