Selective removal of Ca2+ from brackish water by electrodialysis desalination: process optimization and application
Abstract
Exploration and application of unconventional water sources, particularly brackish water, have emerged as key strategies for addressing freshwater scarcity. Electrodialysis (ED) is utilised in brackish water desalination to eliminate Ca2+ owing to its cost-effectiveness and eco-friendliness. This study integrated electrochemical impedance spectroscopy to examine the effects of voltage, flow rate, and initial concentration on the migration numbers of Na+ and Ca2+ ions as well as the selectivity coefficient for Ca2+ during ED. The experimental results revealed that a lower voltage, flow rate, and initial concentration enhanced the selective removal of Ca2+ compared to Na+, which was linked to variations in the boundary layer thickness near the membrane. The maximum 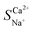 reached 2.48 at an initial concentration of 3.3 mmol L−1, with a voltage of 6 V and an influent flow rate of 36 L h−1. In addition, a 2 month pilot study was conducted using brackish groundwater from northwestern China. This indicates a stable effluent and high efficiency of Ca2+ removal during brackish water treatment via the ED process. The Ca2+ concentration in the effluent remained below 20 mg L−1 with a daily water production efficiency of 90%. This study offers valuable insights into ED technology applicable to the desalination and hardness reduction of brackish water.
reached 2.48 at an initial concentration of 3.3 mmol L−1, with a voltage of 6 V and an influent flow rate of 36 L h−1. In addition, a 2 month pilot study was conducted using brackish groundwater from northwestern China. This indicates a stable effluent and high efficiency of Ca2+ removal during brackish water treatment via the ED process. The Ca2+ concentration in the effluent remained below 20 mg L−1 with a daily water production efficiency of 90%. This study offers valuable insights into ED technology applicable to the desalination and hardness reduction of brackish water.

- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...