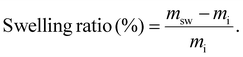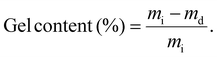Enhancing the robustness of thiol–thioester covalent adaptable networks through reversible thiol–Michael masking
Anna
Stasiuk
 ab,
Alexis
Millan
ab,
Alexis
Millan
 a,
Elena
Rigo
a,
Mathias
Destarac
a,
Elena
Rigo
a,
Mathias
Destarac
 a and
Marc
Guerre
a and
Marc
Guerre
 *a
*a
aLaboratoire SOFTMAT, Université de Toulouse, CNRS UMR, 5623, 118 route de Narbonne, 31062 Toulouse, France. E-mail: marc.guerre@cnrs.fr
bInstitute of Chemistry and Chemical Technologies, Lviv Polytechnic National University, 12 Bandera Str., Lviv, 79013, Ukraine
First published on 23rd December 2025
Abstract
Covalent adaptable networks (CANs) offer an appealing alternative to traditional thermosets by combining mechanical robustness with reprocessability through dynamic covalent chemistry. Thiol–thioester CANs are particularly promising, but their reliance on free thiols to enable network rearrangement also promotes creep and permanent deformation. Here, we present a simple reversible thiol-protection strategy to overcome this issue. Free thiols are temporarily “masked” via a reversible thiol–Michael reaction, limiting chain mobility and improving mechanical resistance while preserving network dynamics. To demonstrate this concept, we developed a thioester-based CAN in which thiols are masked as dithioacetal groups. Model studies confirmed that the masked thiols can be released on demand and participate in thiol–thioester exchanges, with both steps catalysed by TBD. A reference network with unprotected thiols (C-FT) was compared to the protected analogue (C-BT). Although thiol protection slowed down relaxation due to incomplete dissociation, increasing catalyst concentration compensated for this effect, enabling similar relaxation kinetics while preserving enhanced mechanical resistance. This reversible thiol-protection strategy provides a simple and effective approach to mitigate creep in thioester-based CANs without compromising reprocessability, and could be extended to other nucleophile-activated dynamic chemistries.
Introduction
Covalent adaptable networks (CANs) are a category of thermoset materials that undergo covalent bond rearrangements while preserving the characteristics of thermoset properties such as mechanical robustness and chemical stability.1–4 Owing to this chemical arrangement, CANs can be reprocessed like thermoplastics or glass, positioning them at the interface between thermoplastic and thermoset materials. These dynamic materials are usually composed of dynamic bonds that can be triggered via diverse stimuli such as heat or light. To date, two types of dynamic exchange mechanisms have been incorporated into CAN materials: dissociative and associative mechanisms. Associative networks undergo reversible exchanges with constant network connectivity during the reorganization process.5 Because of their enhanced network stability, associative networks, also coined vitrimers, attracted significant attention with various dynamic chemistries already implemented.6–12 On the other hand, dissociative networks follow a reversible addition mechanism, leading predominantly to the breaking and reforming of chemical bonds resulting in a significant modification of the network structure upon activation.13 Recently, thiol-thioester exchange (TTE) developed by Bowman and co-workers14–21 has shown interesting characteristics such as relatively low activation energy (approximately 30 kJ mol−1) and the possibility to tailor the exchange rates through the judicious selection of a basic or nucleophilic catalyst. While significant efforts have been devoted to assessing the potential of this chemistry concerning the reactivity of thiols in relation to thioesters18,22 and the influence of the nucleophilicity/basicity of the catalyst,14 there has been limited work focused on improving material properties, particularly in terms of creep. Indeed, this chemistry relies on a high concentration of pendant thiols within the network to facilitate thiol–thioester exchange, with most formulations typically containing a ratio of two thiols to one thioester.23 At lower thiol concentrations, the reaction is slow, and the material cannot be efficiently reshaped within a reasonable timeframe. However, a high concentration of unprotected thiols can introduce significant drawbacks, including side reactions caused by oxidation, increased deformation under external stress, and a high swelling ratio.18 Furthermore, the rapid base-catalysed thiol–thioester exchange observed at room temperature may result in pronounced creep, particularly in materials with low glass transition temperature.24 This corresponds to a common dilemma observed in CANs, where bond exchange needs to be highly activated and rapid during processing at elevated temperatures but should be sufficiently slow or deactivated during regular use to effectively minimize creep. In order to improve the creep resistance of CANs, several approaches were employed including, but not limited to, combination of exchange chemistries,25–27 functional groups traps,28 phase segregation,29–31 use of stimuli-activated catalysts,24,32–34 supramolecular additives,35,36 or the mixing of dynamic crosslinkers with static37–40 or supramolecular crosslinkers.41–48Herein, we propose to use a similar strategy as recently introduced by Du Prez and co-workers on vitrimers with reversibly trapped amines,28 but this time applied to thiol–thioester exchange. The “free” pendant thiols, which are detrimental for thiol/thioester exchanges, will be reversibly masked through a simple thiol-Michael addition to form dithioacetals (Scheme 1). Through catalytic activation of the thioacetals, the generation of nucleophilic thiols is expected to be gradually released for exchange. The reversible thiol–Michael platform was deliberately selected based on previous work on alkynone-based dynamic covalent systems.49 In contrast to classical Michael acceptors, alkynone derivatives display markedly slower retro-Michael kinetics due to their reduced electrophilicity, enabling a gradual and temperature-controlled release of free thiols. The released thiols can then reversibly react at lower temperature to reform the starting materials with protected thiols. The materials are expected to be as reversible as their thiol–thioester counterparts, with all thiols reversibly protected, reducing creep and protecting thiols from potential side reactions. One of the advantages of this strategy is that both exchange mechanisms (i.e., thiol–thioester exchange and thioacetal exchange) can be activated by the same 1,5,7-triazabicyclo[4.4.0]dec-5-en (TBD) basic catalyst.18,50
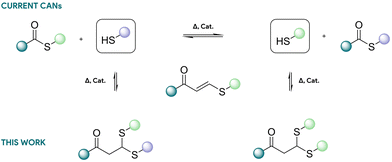 | ||
| Scheme 1 Thiol-thioester exchange mechanism (top) and reversible protection of free thiols trough Michael addition (bottom). | ||
Experimental
Materials
Dodecanethiol, triazabicyclodecene (TBD), sodium bicarbonate (NaHCO3), magnesium sulfate (MgSO4), sodium sulfate (Na2SO4), p-toluenesulfonic acid monohydrate (TsOH·H2O), pentaerythritol tetrakis(3-mercaptopropionate), 1,3,5-trioxane, 3,3,3-trifluoropropionic acid, azobisisobutyronitrile (AIBN), ethyl acetate, cyclohexane, chloroform, toluene, methanol, allyl alcohol, dichloromethane and tetrahydrofuran were all purchased from Sigma-Aldrich. 3-Butyn-2-one was purchased from Fluorochem.Synthesis
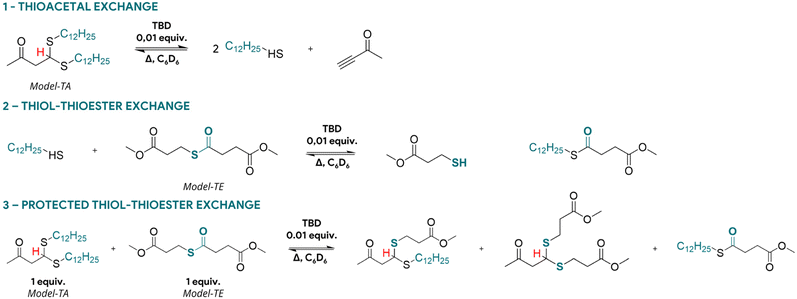 | ||
| Scheme 2 Distinct exchange reactions occurring during the protected thiol–thioester (3) model reaction: (1) thiol–thioester exchange and (2) thioacetal exchange. | ||
1H NMR (300 MHz, CDCl3), δ (ppm), Fig. S2: 4.25 (t, –CH2–CH(–S–)–S–), 2.89 (d, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2–CH–), 2.52–2.72 (m, –S–CH2–(CH2)10), 1.25 and 1.55 (s, –(CH2)10–), 0.88 (t, –(CH2)10–CH3).
C–CH2–CH–), 2.52–2.72 (m, –S–CH2–(CH2)10), 1.25 and 1.55 (s, –(CH2)10–), 0.88 (t, –(CH2)10–CH3).
1H NMR (300 MHz, CD3OD), δ (ppm), Fig. S3: 3.70 and 3.69 (s, –CH3), 3.14 (t, –CH2–CH2–S–), 2.91 (t, OC–CH2–CH2–S–), 2.66 (m, OC–(CH2)2–CO).
1H NMR (300 MHz, CDCl3), δ (ppm), Fig. S4: 5.76–5.89 (m, –CH2–CH2–S–), 5.12–5.27 (m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) ), 4.49–4.52 (m, –O–CH2–CH
), 4.49–4.52 (m, –O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.06 (t, OC–CH2–CH2–S–), 2.82 (t, OC–CH2–CH2–S–), 2.62–2.55 (m, –SCO–CH2–CH2–COO–).
CH2), 3.06 (t, OC–CH2–CH2–S–), 2.82 (t, OC–CH2–CH2–S–), 2.62–2.55 (m, –SCO–CH2–CH2–COO–).
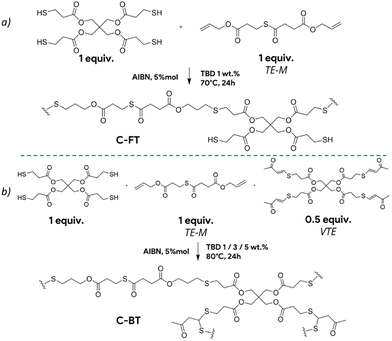 | ||
| Scheme 3 Network structures and curing conditions for C-FT with 100% thiol excess (top) and corresponding C-BT (bottom) CAN. | ||
1H NMR (300 MHz, CDCl3), δ (ppm), Fig. S5: 7.54–7.61 and 7.04–7.11 (m, –S–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CO), 6.35 and 6.15 (dd, –S–CH
CH–CO), 6.35 and 6.15 (dd, –S–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CO), 4.11–4.19 (m, –CH2–O–CO–), 3.12–2.96 (m, –S–CH2–CH2–CO–), 2.79–2.65 (m, –S–CH2–CH2–CO–), 2.23 (d, –CO–CH3).
CH–CO), 4.11–4.19 (m, –CH2–O–CO–), 3.12–2.96 (m, –S–CH2–CH2–CO–), 2.79–2.65 (m, –S–CH2–CH2–CO–), 2.23 (d, –CO–CH3).
Model reaction
Model-TE (0.07 g, 0.3 mmol, 1 equiv.), TBD (0.4 mg, 0.003 mmol, 0.01 equiv.) and 1,3,5-trioxane (0.03 g, 0.3 mmol, 1 equiv.) were mixed in 6 mL of toluene and stirred before adding Model-TA (0.14 g, 0.3 mmol, 1 equiv.). The mixture was then heated at 60 °C and for each NMR spectrum of Fig. 1, 1 mL of solution was taken and added to an NMR tube containing 1 mL of C6D6 and a drop of 3,3,3-trifluoropropionic acid required to quench the reaction. | ||
| Fig. 1 Evolution of the CH proton signal of the dithioacetal at 60 °C during the exchange reaction, alongside the corresponding structures of all dithioacetals formed (right). | ||
Materials synthesis and characterization
Swelling ratio was calculated using the following equation:
Gel content was calculated using the following equation:
Instrumentation
Results and discussion
Model reaction
To evidence the possibility of reversible releasing thiols from thioacetals with subsequent thiol-thioester exchange, a model reaction depicted in Scheme 2(3) was conducted. Thioacetals bearing two dodecyl groups were mixed with a thioester molecule in the presence of 0.01 equiv. of TBD as catalyst. No free thiols were available to induce the thiol-thioester exchange. The reaction was heated at 60 °C in deuterated benzene, the dithioacetal being expected to dissociate in alkynone and in two free thiols (Scheme 2(1)) which are then capable of reacting with the thioester moiety (Scheme 2(2)). Upon exchange of the dodecyl thiol with the thioesters, the corresponding thiol (methyl 3-mercaptopropionate) then reacts back to the alkynone reforming a new thioacetal. The formation of this thioacetal can be easily followed from the NMR signal attributed to the CH of the thioacetal. A typical triplet at 4.45 ppm (Fig. 1, initial) corresponding to the dithioacetal with alkane chains is slowly replaced by two new triplets corresponding to the mono and bi-substituted dithioacetal at 4.42 ppm and 4.40 ppm, respectively (Fig. 1). Since all the reactions are concurrent, it is not possible to effectively assess a reaction rate and evaluate kinetics for each exchange pathway. Moreover, after 3 hours an equilibrium is reached with all three dithioacetals present in the system. This exchange pathway was therefore found suitable for the design of thioester-based CAN. Full NMR spectra are available in Fig. S1.Influence of free thiols concentration
Before comparing the effect of thiol masking on materials, the influence of free thiol concentration on the overall exchange kinetics was investigated. Five different materials were synthesized following the synthetic scheme described in Scheme 3a. The molar ratio between the thioester and the tetra-thiol was adjusted to leave 100%, 77%, 50%, 30% and 5% of free thiols, enabling efficient thiol-thioester exchange. These materials are designated in the text as C-FT. For all these formulations, the quantity of TBD was fixed at 1 wt%. All materials were characterized by DSC, which revealed glass transition temperatures (Tg) ranging from −29 °C to −15 °C, with the lowest corresponding to the highest thiol concentrations (see Fig. S20 for the DSC curves and Table S1 for the thermomechanical properties). The 14 °C increase in Tg observed with decreasing thiol content is attributed to a higher crosslink density of the materials. All these materials were then subjected to stress relaxation experiments (Fig. S12–S17 for non-normalized, normalized stress relaxation and corresponding Arrhenius dependency). The fastest stress relaxation was observed for the material containing 100% free thiols, with relaxation times of 99 s at 150 °C. In contrast, lower content of free thiols resulted in significantly slower relaxation behaviour. Fig. 2 compares the relaxation curves of each material at 140 °C, clearly showing the strong influence of thiol excess on the relaxation kinetics. For the material with only 5% thiol excess, only partial relaxation was observed, making it impossible to evaluate the temperature dependency. Overall, the activation energy for viscous flow was found to depend on the thiol concentration. It decreased from 194 kJ mol−1 at 30% thiol (C-FT/30%) to values in the range of 86–111 kJ mol−1 for thiol concentrations between 50% and 100%, where it tended to plateau (Fig. S19). It is also important to note the significant decrease in the non-normalized modulus with increasing temperature, which is counterintuitive for an associative-type exchange mechanism such as the thiol–thioester exchange (see Fig. S12–S16).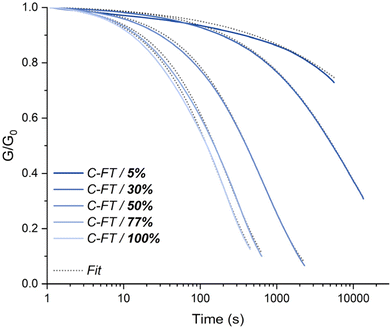 | ||
| Fig. 2 Stress relaxation profiles of C-FT materials containing 5, 30, 50, 77 and 100% of free thiols at 140 °C. | ||
We suspect that this decrease arises from an acyl-transfer exchange process, which can occur in TBD-catalysed reactions.51 The decrease in crosslink density with increasing thiol content and constant catalyst concentration can be explained by an acyl-transfer side reaction occurring in TBD-catalysed systems. In this mechanism, TBD can react with thioesters to form transient acyl–TBD intermediates, temporarily removing a fraction of thioester bonds from the network. At higher thiol concentrations, the generation of thiolate species is enhanced, which accelerates nucleophilic attack and increases the overall rate of acyl-transfer events. This competitive equilibrium could explain the observed decrease in modulus with temperature in TBD-catalysed systems. These results highlight the importance of carefully considering and evaluating the evolution of the modulus with temperature, as it may be influenced by various parameters, particularly in catalysed systems. For the rest of the article and subsequent thiol-masking study, the formulation containing 100% free thiols was selected. This formulation was chosen to more effectively assess the efficiency of our thiol-blocking strategy and its impact on the material's properties, while also maintaining consistency with the formulations reported by Bowman and co-workers.
Influence of thiol masking and catalyst
Based on this 100% excess formulation consisting of 1/1 ratio of thioester and the tetra-thiol, two systems were investigated and strictly compared: one containing free, unmasked thiols (Scheme 3a), and another containing masked thiols (Scheme 3b). The materials are designated as C-FT for the CAN containing free thiols as previously mentioned and C-BT for the CAN containing masked (protected) thiols. The free thiols were protected with vinyl thioacetal groups (see Fig. S5 for NMR characterization), ensuring that no thiol groups remained unreacted as illustrated in Scheme 3b. The notation C-BT-X% and C-FT-X% provides additional information on the amount of added TBD on the systems, which has been optimized later on. TBD catalyses both thioacetal exchange and thiol-thioester exchange reactions and was set at 1 wt% for both C-FT and C-BT materials. With the protection of thiols, an increase in crosslinking density was observed with a shift in Tg from −28.5 °C for C-FT-1% versus 3.9 °C for C-BT-1% (Fig. S18), both materials being elastomers at room temperature. To characterize the dynamic properties of these formulations, stress relaxation experiments were conducted at temperatures ranging from 150 °C to 90 °C for C-FT-1% (Fig. 3) and from 150 °C to 110 °C for C-BT-1% (Fig. S9). All relaxation times, fitting parameters and profiles are available in the SI (Tables S2–S5 and Fig. S10–S17).As a comparison, the relaxation times were 22 min at 150 °C for C-BT-1% compared to 37s for the same temperature for C-FT-1%. Regarding the flow activation energy, both materials remain within the same range with values of 111 (±1) kJ mol−1 and 137 (±6) kJ mol−1 for C-FT-1% and C-BT-1%, respectively. We suspect that the slower relaxation observed for C-BT-1% results from the competitive catalysis of TBD, which, at the same concentration, must catalyse both thiol-thioester and thioacetal exchange reactions. In addition, the dissociation of thioacetals appears to be limited, as seen in the non-normalized stress relaxation data (SI), where the contribution of dissociation is much less pronounced. This non-quantitative dissociation suggests that the concentration of free thiols remains lower than in the case of C-FT-1%, thereby slowing down the overall exchange kinetics.
In order to demonstrate the possibility of finely tuning the exchange dynamics and potentially achieving relaxation behaviour similar to that of the C-FT-1% reference materials, the amount of catalyst was adjusted and two additional C-BT formulations were synthesized. These formulations contained the same concentration of masked thiols but included 3 wt% and 5 wt% of TBD, respectively. As shown in Fig. S9–S11, the relaxation time can be easily tuned by varying the catalyst concentration, even when the thiols were protected as thioacetals. With 5 wt% TBD, a relaxation time comparable to those of the C-FT-1% materials were achieved (Fig. 3). This represents a significant added value, as similar exchange dynamics are obtained while maintaining mechanical integrity. Regarding the Arrhenius dependency (Fig. 4), activation energies of 111 (±1) kJ mol−1 and 142 (±3) kJ mol−1 were estimated for C-FT-1% and C-BT-5%. Values obtained at high temperatures were not used for linear fittings as thiol moieties may have undergone oxidation and elongate relaxation times. All properties of the C-FT-1% and C-BT-1%, C-BT-3% and C-BT-5% and detailed in Table 1.
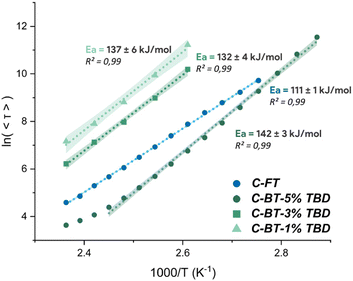 | ||
| Fig. 4 Arrhenius plot of C-FT and C-BT using stress relaxation times to determine activation energies (confidence bands shown as shaded regions). | ||
| Sample | Free thiols (%) | Masked thiols (%) | TBD (wt%) | T g (°C) | E a (kJ mol−1) | Swelling ratio (%) | Soluble fraction (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CHCl3 | THF | CHCl3 | THF | |||||||
| C-FT-1% | R0 | 100 | — | 1 | −29 | 111 ± 1 | 536 | 201 | 33 | 29 |
| R1 | 100 | — | 1 | −30 | 169 ± 8 | 340 | 279 | 38 | 42 | |
| C-BT-1% | — | 100 | 1 | 1 | 137 ± 6 | 104 | 89 | 2 | 2 | |
| C-BT-3% | — | 100 | 3 | 3 | 132 ± 4 | 194 | 64 | 2 | 0 | |
| C-BT-5% | R0 | — | 100 | 5 | 4 | 124 ± 3 | 206 | 104 | 12 | 4 |
| R1 | — | 100 | 5 | −18 | 124 ± 4 | 244 | 98 | 11 | 4 | |
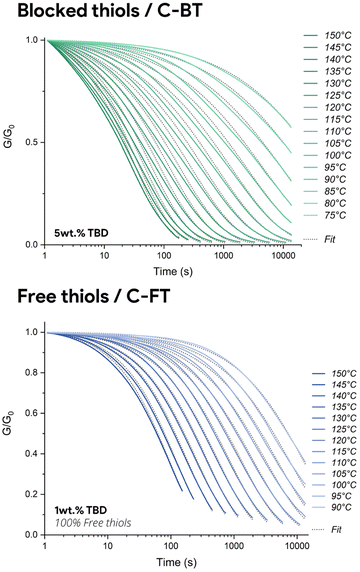
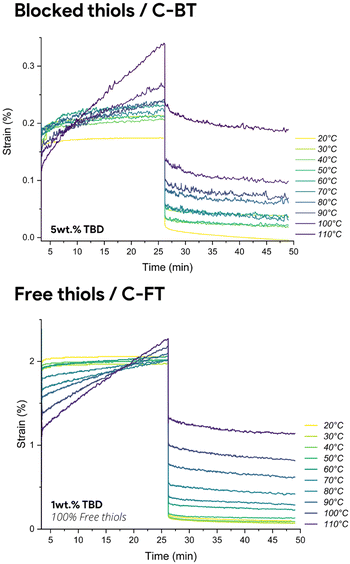
Furthermore, isothermal creep experiments were setup at multiple temperatures and under a constant stress of 1500 Pa for both materials (Fig. 5). Maximum and residual strains, as well as creep rates and recovery percentages are reported in Table S6 for both formulations. To enable a more rigorous comparison of creep resistance between C-FT-1% and C-BT-5%, it is essential to consider not only the qualitative creep profiles but also the quantitative deformation parameters under identical loading conditions. Although both materials were subjected to the same applied stress (1500 Pa), the different strain amplitudes are therefore intrinsic to the materials and directly reflect their mechanical resistance. The much lower maximum strain reached by C-BT-5% results from its higher effective crosslink density and reduced chain mobility induced by thiol masking. When analysed quantitatively, the masked network systematically exhibits lower creep rates, reduced maximum strain, and substantially lower residual strain across all temperatures. At 110 °C, for example, C-BT-5% displays a creep rate of only 1.43 × 10−4%·min−1, compared to 7.31 × 10−4%·min−1 for C-FT-1%, and its residual strain decreases from 1.14% to 0.19%, corresponding to an approximately six-fold reduction in permanent deformation. While the material becomes significantly more resistant to deformation at service temperature, the exchange kinetics at elevated temperature are fully preserved, and even accelerated by increasing the catalyst concentration (〈τ〉 = 2671 s for C-FT-1% vs 〈τ〉 = 864 s for C-BT-5% at 110 °C). This quantitative analysis confirms that reversible thiol protection effectively reduces creep while preserving the exchange kinetics required for efficient reprocessing.
The soluble fraction clearly reflects the distinct network architectures of the free-thiol (C-FT) and thiol-masked (C-BT) materials. As expected for thiol–thioester CANs formulated with a large excess of free thiols, the C-FT networks exhibit a high soluble fraction, consistent with their lower effective crosslink density and increased chain mobility. In contrast, introducing the reversible thiol-masking strategy drastically reduces solubility: all C-BT materials display soluble fractions below 15%, demonstrating that thiol protection effectively increases network integrity.
Reprocessing experiments were carried out on both the free-thiol (C-FT-1%) and masked-thiol (C-BT-5%) networks under identical conditions in order to directly compare their behaviour upon thermal reshaping (Fig. S6 and S7). As noted previously, both materials could be successfully remoulded without visible macroscopic defects, demonstrating that network rearrangement remains active in all cases. A darkening was observed for the masked system, and this effect seems to indicate that the catalyst, rather than the thiol-masking strategy itself, is mainly responsible for the colour evolution during repeated processing. Importantly, despite this aesthetic change, the essential thermomechanical and dynamic properties (Tg, relaxation times, activation energies) were largely maintained after reprocessing for both materials, with only a moderate slowing of the relaxation kinetics (Fig. S8, S9, S18 and S20). This slowdown is fully consistent with partial thiol oxidation occurring in both networks and does not compromise the ability of the materials to undergo bond exchange at elevated temperatures. Altogether, these results confirm that reversible thiol masking does not hinder the high-temperature reprocessability of the network.
Conclusions
In the past, thioester-based CANs relied on the presence of free thiols to initiate exchange reactions, which often led to significant creep and residual strain. In this work, we developed a new type of thioester-based CAN in which the free thiols are temporarily masked as dithioacetal groups, enhancing the mechanical properties while maintaining comparable dynamic behaviour. A model reaction confirmed that thiols can be reversibly released from the thioacetal groups and subsequently participate in thioester exchange through thiol-thioester exchange reactions, both processes being catalysed by TBD. Once the feasibility of the reaction was confirmed, a reference network containing free, unprotected thiols (C-FT) was synthesized for comparison with the novel material incorporating dithioacetal-masked thiols (C-BT). Although incomplete thiol dissociation was observed in materials containing masked thiols, resulting in overall slower relaxation dynamics, increasing the catalyst concentration effectively compensated for this effect. This implies that the released thiols, even when present at lower concentrations, exchange more rapidly through thiol–thioester reactions. Ultimately, this demonstrates that materials with comparable or even faster relaxation kinetics at high temperatures can be achieved while keeping all thiols reversibly trapped within thioacetal units, thereby improving mechanical resistance. Creep experiments further confirmed this improvement: under the same applied stress, C-FT-1% exhibited a higher residual strain than C-BT-5%, clearly highlighting the benefits of thiol protection in thioester-based CANs. The C-BT-5% network was also successfully reprocessed multiple times, and its dynamic properties were preserved, despite a colour change attributed to minor oxidation. Overall, this work introduces a promising strategy to mitigate creep in thioester-based CANs through reversible thiol protection, maintaining the dynamic adaptability of the network while improving its mechanical stability. Beyond this study, fine tuning of the Michael acceptor structure through different electron-withdrawing groups could offer a promising strategy to precisely regulate thiol-release kinetics and further tailor the balance between mechanical stability and network dynamics in future covalent adaptable networks. This reversible thiol protection strategy could also be extended to other dynamic chemistries relying on nucleophilic activation, offering a generic route to reduce creep without compromising recyclability.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: model reactions, NMR, DSC, Stress relaxations and fitting data. See DOI: https://doi.org/10.1039/d5py01081k.In addition, raw data are available are available from the corresponding author upon reasonable request.
References
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643–2653 CrossRef CAS.
- C. N. Bowman and C. J. Kloxin, Angew. Chem., Int. Ed., 2012, 51, 4272–4274 CrossRef CAS PubMed.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 Search PubMed.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 Search PubMed.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 Search PubMed.
- B. Hendriks, J. Waelkens, J. M. Winne and F. E. Du Prez, ACS Macro Lett., 2017, 6, 930–934 CrossRef CAS.
- W. A. Ogden and Z. Guan, J. Am. Chem. Soc., 2018, 140, 6217–6220 CrossRef CAS.
- P. Chakma, Z. A. Digby, M. P. Shulman, L. R. Kuhn, C. N. Morley, J. L. Sparks and D. Konkolewicz, ACS Macro Lett., 2019, 8, 95–100 CrossRef CAS PubMed.
- J. Huang, L. Zhang, Z. Tang, S. Wu and B. Guo, Compos. Sci. Technol., 2018, 168, 320–326 CrossRef CAS.
- G. Prasanna Kar, M. Osman Saed and E. Michael Terentjev, J. Mater. Chem. A, 2020, 8, 24137–24147 RSC.
- F. Asempour, E. Laurent, T. Bride and M. Maric, Eur. Polym. J., 2024, 219, 113402 Search PubMed.
- S. Guggari, F. Magliozzi, S. Malburet, A. Graillot, M. Destarac and M. Guerre, Polym. Chem., 2024, 15, 1347–1357 RSC.
- X. Cui, N. Jiang, J. Shao, H. Zhang, Y. Yang and P. Tang, Macromolecules, 2023, 56, 772–784 CrossRef CAS.
- B. T. Worrell, S. Mavila, C. Wang, T. M. Kontour, C.-H. Lim, M. K. McBride, C. B. Musgrave, R. Shoemaker and C. N. Bowman, Polym. Chem., 2018, 9, 4523–4534 RSC.
- C. Wang, S. Mavila, B. T. Worrell, W. Xi, T. M. Goldman and C. N. Bowman, ACS Macro Lett., 2018, 7, 1312–1316 CrossRef CAS.
- S. Mavila, B. T. Worrell, H. R. Culver, T. M. Goldman, C. Wang, C.-H. Lim, D. W. Domaille, S. Pattanayak, M. K. McBride, C. B. Musgrave and C. N. Bowman, J. Am. Chem. Soc., 2018, 140, 13594–13598 CrossRef CAS PubMed.
- N. Sowan, Y. Lu, K. J. Kolb, L. M. Cox, R. Long and C. N. Bowman, Polym. Chem., 2020, 11, 4760–4767 RSC.
- N. J. Bongiardina, K. F. Long, M. Podgórski and C. N. Bowman, Macromolecules, 2021, 54, 8341–8351 CrossRef CAS.
- B. J. Carberry, J. E. Hergert, F. M. Yavitt, J. J. Hernandez, K. F. Speckl, C. N. Bowman, R. R. McLeod and K. S. Anseth, Biofabrication, 2021, 13, 044104 CrossRef CAS.
- J. J. Hernandez, A. L. Dobson, B. J. Carberry, A. S. Kuenstler, P. K. Shah, K. S. Anseth, T. J. White and C. N. Bowman, Macromolecules, 2022, 55, 1376–1385 CrossRef CAS.
- J. J. Hernandez, S. P. Keyser, A. L. Dobson, A. S. Kuenstler and C. N. Bowman, Macromolecules, 2024, 57, 1426–1437 Search PubMed.
- M. Podgórski, N. Spurgin, S. Mavila and C. N. Bowman, Polym. Chem., 2020, 11, 5365–5376 RSC.
- B. T. Worrell, M. K. McBride, G. B. Lyon, L. M. Cox, C. Wang, S. Mavila, C.-H. Lim, H. M. Coley, C. B. Musgrave, Y. Ding and C. N. Bowman, Nat. Commun., 2018, 9, 2804 CrossRef PubMed.
- F. Van Lijsebetten, T. Debsharma, J. M. Winne and F. E. Du Prez, Angew. Chem., Int. Ed., 2022, 61, e202210405 CrossRef CAS PubMed.
- D. J. Fortman, R. L. Snyder, D. T. Sheppard and W. R. Dichtel, ACS Macro Lett., 2018, 7, 1226–1231 CrossRef CAS PubMed.
- M. Chen, L. Zhou, Y. Wu, X. Zhao and Y. Zhang, ACS Macro Lett., 2019, 8, 255–260 CrossRef CAS PubMed.
- M. Podgórski, S. Mavila, S. Huang, N. Spurgin, J. Sinha and C. N. Bowman, Angew. Chem., Int. Ed., 2020, 59, 9345–9349 CrossRef PubMed.
- F. Van Lijsebetten, K. De Bruycker, Y. Spiesschaert, J. M. Winne and F. E. Du Prez, Angew. Chem., Int. Ed., 2022, 61, e202113872 CrossRef CAS.
- G. J. M. Formon, S. Storch, A. Y.-G. Delplanque, B. Bresson, N. J. Van Zee and R. Nicolaÿ, Adv. Funct. Mater., 2023, 33, 2306065 Search PubMed.
- L. M. A. Joosten, P. Cassagnau, E. Drockenmuller and D. Montarnal, Adv. Funct. Mater., 2024, 34, 2306882 CrossRef CAS.
- H. Sun, I. Göde, M. Bonnard, V. Liautard, M. Pomes-Hadda, S. Aime, L. Chabaud, P. Edera, A. Guérinot, M. Pucheault and R. Nicolaÿ, ACS Mater. Lett., 2025, 7, 2534–2540 CrossRef CAS.
- W. Alabiso, B. Sölle, D. Reisinger, G. Guedes de la Cruz, M. Schmallegger, T. Griesser, E. Rossegger and S. Schlögl, Angew. Chem., Int. Ed., 2023, 62, e202311341 Search PubMed.
- G. Vozzolo, M. Ximenis, D. Mantione, M. Fernández and H. Sardon, ACS Macro Lett., 2023, 12, 1536–1542 CrossRef CAS.
- D. Reisinger, M. U. Kriehuber, M. Bender, D. Bautista-Anguís, B. Rieger and S. Schlögl, Adv. Mater., 2023, 35, 2300830 Search PubMed.
- A. Quinteros-Sedano, B. Bresson, N. J. Van Zee and R. Nicolaÿ, ACS Mater. Lett., 2024, 6, 877–884 CrossRef CAS.
- G. J. M. Formon, J. Jayaratnam, C. Guibert, N. J. Van Zee and R. Nicolaÿ, Macromolecules, 2024, 57, 8277–8286 CrossRef CAS.
- L. Li, X. Chen, K. Jin and J. M. Torkelson, Macromolecules, 2018, 51, 5537–5546 Search PubMed.
- J.-H. Chen, D.-D. Hu, Y.-D. Li, F. Meng, J. Zhu and J.-B. Zeng, Polymer, 2018, 143, 79–86 CrossRef CAS.
- J. J. Cash, T. Kubo, D. J. Dobbins and B. S. Sumerlin, Polym. Chem., 2018, 9, 2011–2020 RSC.
- A. Breuillac, A. Kassalias and R. Nicolaÿ, Macromolecules, 2019, 52, 7102–7113 CrossRef CAS.
- J. A. Neal, D. Mozhdehi and Z. Guan, J. Am. Chem. Soc., 2015, 137, 4846–4850 CrossRef CAS PubMed.
- Y. Liu, Z. Tang, D. Wang, S. Wu and B. Guo, J. Mater. Chem. A, 2019, 7, 26867–26876 RSC.
- Y. Liu, Z. Tang, S. Wu and B. Guo, ACS Macro Lett., 2019, 8, 193–199 CrossRef CAS.
- Y. Chen, Z. Tang, Y. Liu, S. Wu and B. Guo, Macromolecules, 2019, 52, 3805–3812 CrossRef CAS.
- S. Wang, S. Ma, Q. Li, X. Xu, B. Wang, K. Huang, Y. Liu and J. Zhu, Macromolecules, 2020, 53, 2919–2931 CrossRef CAS.
- F. I. Altuna, U. Casado, I. E. dell'Erba, L. Luna, C. E. Hoppe and R. J. J. Williams, Polym. Chem., 2020, 11, 1337–1347 RSC.
- H. Zhang, A. van Hertrooij, T. Schnitzer, Y. Chen, S. Majumdar, R. A. T. M. van Benthem, R. P. Sijbesma and J. P. A. Heuts, Macromolecules, 2023, 56, 6452–6460 CrossRef CAS.
- L. Wang, Y. Liu, N. Hao, Y. Qiao, W. Zeng, L. Wei and A. Du, Polymer, 2023, 265, 125595 CrossRef CAS.
- B. Ghalei, K. Wakimoto, C. Y. Wu, A. P. Isfahani, T. Yamamoto, K. Sakurai, M. Higuchi, B. K. Chang, S. Kitagawa and E. Sivaniah, Angew. Chem., Int. Ed., 2019, 58, 19034–19040 CrossRef CAS.
- N. Van Herck, D. Maes, K. Unal, M. Guerre, J. M. Winne and F. E. Du Prez, Angew. Chem., Int. Ed., 2020, 59, 3609–3617 CrossRef CAS PubMed.
- R. C. Pratt, B. G. G. Lohmeijer, D. A. Long, R. M. Waymouth and J. L. Hedrick, J. Am. Chem. Soc., 2006, 128, 4556–4557 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |