Recent advances in organocatalytic asymmetric multicomponent reactions
Peng-Ying Jiang
ab,
San Wu
a,
Jun (Joelle) Wang
 *b,
Shao-Hua Xiang
*b,
Shao-Hua Xiang
 a and
Bin Tan
a and
Bin Tan
 *a
*a
aShenzhen Grubbs Institute and Department of Chemistry, Southern University of Science and Technology, Shenzhen, 518055, China. E-mail: tanb@sustech.edu.cn
bDepartment of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China. E-mail: junwang@hkbu.edu.hk
First published on 2nd April 2025
Abstract
Multicomponent reactions enable the simultaneous formation of multiple chemical bonds in a single synthetic operation, efficiently combining three or more reactants to access structurally complex molecules. The integration of chiral catalysts into such processes establishes a well-defined asymmetric environment, facilitating precise stereochemical control and delivering enantioenriched products containing stereogenic elements. In recent years, environmentally benign organocatalytic strategies have emerged as a powerful platform for asymmetric multicomponent reactions, demonstrating remarkable versatility in constructing diverse molecular architectures with high enantioselectivity. This review systematically categorizes recent advances in this field based on organocatalyst types, with a focus on their roles in distinct reaction mechanisms, key intermediates, and substrate activation modes. Representative transformations are discussed to illustrate design principles and challenges in achieving stereocontrol within multicomponent systems.
10th anniversary statementAs an organic chemistry researcher, I feel deeply honored to contribute a review on advances in organocatalytic asymmetric multicomponent reactions to the 10th anniversary collection of Organic Chemistry Frontiers (OCF). In early 2016, my collaborators and I published our first paper in OCF, and five years later, it was instrumental as one of the five representative works we presented in our successful application for the Guangdong Provincial Natural Science Award. Undoubtedly, this emerging journal has played an important role in the growth of many young domestic researchers like me. Over the past decade, OCF has become an indispensable force in disseminating the development of organic chemistry in China. On this momentous occasion, I wish that this journal will continue to provide a timely window for many researchers to showcase original research results and also provide an excellent platform for young people to learn and exchange ideas. |
1. Introduction
Multicomponent reactions (MCRs) offer a unique opportunity to combine simple, readily available raw materials into intricate, structurally complex molecules through a single, streamlined process.1 These transformations exhibit remarkable potential across a broad spectrum of fields, including drug discovery and natural product synthesis,2–8 due to their high convergence, atom economy, and skeletal diversity.9–12 In fact, with the prevalence of chiral molecules in the current drug market, the development of enantioselective multicomponent reactions has garnered significant attention in recent years. However, the complexity of these reactions, which often involve three or more components simultaneously, makes the system extremely intricate. The challenge lies in the inherent side reactions and background reactions that make chemo-, regio-, and stereoselectivity control in asymmetric multicomponent reactions (AMCRs) a substantial obstacle to overcome.Asymmetric catalysis stands as the most effective method for obtaining a single stereoisomer. By introducing a chiral catalytic system, it not only creates an asymmetric environment for enantioinduction but also enables the amplification of the desired reactivity, ultimately providing an efficient means to control stereoselective MCRs (Fig. 1).
The past decades have witnessed asymmetric catalysis at the forefront of the synthetic chemistry area, driven by the exploitation of diverse catalytic systems and activation modes,13–18 especially enantioselective organocatalysis. In comparison with transition metal catalysis, this emerging catalytic platform features relatively high catalyst stability, excellent functional group tolerance, and environmental sustainability, along with the crucial advantage of avoiding metal residues in the products, making it highly appealing in chiral drug research and development.19–21 Asymmetric organocatalysis has exhibited great potential in AMCRs, successfully revitalizing a wide range of time-honoured yet highly valuable MCRs, including the Biginelli reaction, the Passerini reaction, the Ugi reaction, etc. In the early years, several elegant reviews had been published, which summarized the research status in this field in great detail.22–25 This review mainly focuses on summarizing and analysing the significant achievements within the last ten years, specifically examining the utilization of different chiral organocatalysts such as amines, thioureas and Brønsted acids. The representative substrate coverage under different chiral catalytic systems will be pointed out in detail, and discussions will be carried out around the mechanisms, activation and stereocontrol modes and proposed transition states.
2. Chiral Brønsted acid catalysis
2.1 Asymmetric three-component reaction
The Biginelli reaction is one of the earliest discovered multicomponent reactions, dating back to 1893. It typically involves the reaction of an aldehyde (1), a (thio)urea (2), and a β-ketoester (3) in the presence of an acidic catalyst to form a dihydropyrimidinone (DHPM, 4) product. In recent decades, chiral Brønsted acids26–28 have emerged as powerful organocatalysts for asymmetric imine activation.29–31 Mechanistic studies of the Biginelli reaction have suggested that chiral phosphoric acids (CPAs)32–34 hold great promise as catalysts for controlling enantioselectivity. This hypothesis was validated in 2006 when the Gong group reported the first asymmetric Biginelli reaction, using an H8-BINOL-derived (R)-C1 catalyst. This pioneering work enabled the synthesis of a broad range of DHPMs with high enantiopurity (88–97% ee).35 In 2009, they expanded the range of substrates through the regulation of (R)-C2, enabling the accommodation of cyclic and linear alkyl ketones (5) with inferior reactivity (Biginelli-like reaction). However, both the yield and enantiocontrol were visibly decreased when aromatic ketones were employed. The effect of the 3,3′-substituents of CPAs on the stereochemical control was also theoretically rationalized.36 In 2017 and 2020, Hu and co-workers disclosed the applicability of another class of CPAs (C3, C4) in the asymmetric Biginelli reaction. These chiral catalysts with tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL) as the core skeleton had previously been reported to facilitate the asymmetric Mannich reaction.37 Control experiments verified that the hydrogen atoms of both hydroxyl groups on the catalyst are of crucial importance.38,39 However, centrally chiral catalysts exhibited lower enantiocontrol and narrower substrate generality compared to those with axially chiral skeletons (Fig. 2). After that, Zou,40 Xiao41 and Silvani42 independently made certain contributions to this transformation, paving a convenient avenue towards several pharmaceutical intermediates.Besides the canonical Biginelli reaction, CPA catalysts also displayed remarkable enantiocontrol ability in the trapping of imines with other substrates via hydrogen-bonding activation. In 2008, an asymmetric three-component reaction of α,β-unsaturated aromatic aldehydes (6), amines (7) and β-ketoesters with (R)-C5 was developed by Gong's group, producing dihydropyridines (8) with high enantioselectivities. In this reaction, α,β-unsaturated iminium is generated from α,β-unsaturated aldehyde and primary amine under CPA catalysis. Subsequent asymmetric conjugate addition and cyclization lead to the target product (Fig. 3a).43 Similarly, Sun reported an asymmetric aza-Diels–Alder reaction between in situ-formed imines and indoles (10) in the presence of 1,1-spirobiindane-7,7′-diol-based (R)-C6. The subsequent intramolecular ring-opening of oxetanes oriented at the ortho-position of aromatic aldehydes (9) from the cyclized amines afforded structurally complex molecules (11) containing indoline, tetrahydroquinoline, and tetrahydroisoquinoline moieties. These products exhibited excellent diastereomeric ratios (dr) and ee values (Fig. 3b).44 Additionally, nucleophilic TMSCN was amenable and the CPA-promoted asymmetric three-component Strecker reaction provided α-aminonitriles with excellent efficiency but low enantiocontrol.45 In 2013, the enantioselective interruption of imines with (2-ethynylphenyl)methanol and its analogs (12) was realized by Gong. The relay three-component reaction enabled by the synergistic Au(I)/(R)-C2 catalysis offered an expeditious route towards the synthesis of highly enantioenriched spiroacetals (Fig. 3c).46 In the same year, Lin and co-workers disclosed an asymmetric three-component Povarov reaction to generate benzo[e]indolizidines with excellent yield and stereocontrol.47
The Passerini reaction is a classic isocyanide-based three-component reaction. It offers a rapid route for the assembly of α-acyloxyamides (17) via a one-pot reaction of a carboxylic acid (15), an aldehyde (1) and an isocyanide (16). Since one stereogenic center is generated, great efforts have been devoted to control the stereochemistry to expand the synthetic utility of this reaction. Attempts have primarily focused on chiral Lewis acid systems until Tan's work in 2015. In this reaction, the organocatalyst (R)-C7 and carboxylic acid 15 form a heterodimer, which serves as a bifunctional catalyst to activate both the aldehyde (1) and isocyanide (16). The CPA dictates the stereochemical information to the nitrilium intermediate. The formation of the heterodimer may simultaneously enhance the acidity of the CPA and the nucleophilicity of the carboxylic acid, rendering the addition of the carboxylic acid to the nitrilium intermediate easier. This strategy not only delivered α-acyloxyamides with excellent enantiocontrol, but also demonstrated remarkable substrate generality, particularly for the aldehyde component. Both aryl and alkyl aldehydes, even sterically hindered pivalaldehyde, could be efficiently converted to the corresponding α-acyloxyamides (Fig. 4).48
In 1959, Ugi and co-workers subtly included another component, amine, in the Passerini reaction, and the resulting Ugi four-component reaction gave α-acylamino amides as the product with water as the only by-product. The introduction of an additional component notably enhances the flexibility of this reaction and greatly expands the structural diversity of the products. As a result, it has been widely employed in the construction of a vast array of heterocyclic and macrocyclic structures.49 However, the increased complexity of the reaction system also presents a significant challenge in achieving effective stereoinduction.50 Considering that the introduction of carboxylic acid not only adds complexity to the reaction but also leads to undesired background reactions, Zhu and co-workers conducted pioneering research on the catalytic asymmetric three-component reactions of aldehydes, anilines, and α-isocyanoacetamides (18). Through the facilitation of the CPA (R)-C8, this reaction successfully afforded 5-(1-aminoalkyl)-5-aminooxazoles 19 in excellent yields with moderate to good enantioselectivities (Fig. 5a).51 This work laid the foundation for their development of a streamlined variant of the catalytic asymmetric Ugi four-component reaction, in which the carboxylic acid and aldehyde components were ingeniously integrated into a single compound 20. Nonetheless, this Ugi four-center three-component reaction only accommodated six substrates and necessitated 30–40 mol% loading of (R)-C9 to give satisfactory ee values for the 3-oxo-2-arylisoindoline-1-carboxamide products 21 (Fig. 5b).52 Mechanistic investigations indicate that the enantiocontrol is contributed to the dynamic kinetic resolution of achiral Int-III generated from imine–enamine isomerization of chiral Int-I rather than the C–C bond-forming process. It should be mentioned that the utilization of secondary amides instead of primary amines in a one-pot reductive Ugi-type three-component reaction was established by the Huang group via direct activation and transformation of amides. This reaction provided rapid access to a series of α-acetamidoamides with carboxylic acid and isocyanide as the other two components.53
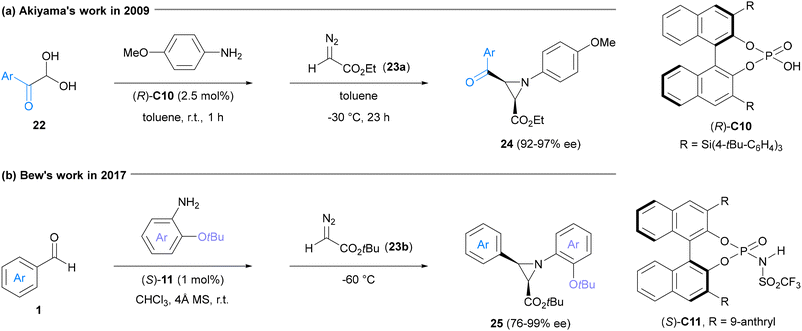
Optically active aziridines hold significant potential across diverse research fields, particularly as versatile synthons in synthetic chemistry. The pioneering achievement of asymmetric multicomponent reactions in the synthesis of such compounds (24) was realized in 2009 by the Akiyama group through CPA-catalyzed asymmetric cycloaddition of imines and diazoacetate 23a. However, this enantioselective aza-Darzens reaction was confined to benzoyl-substituted aldehydes (22), limiting its further application (Fig. 6a).54
In 2017, Bew and co-workers established a relatively broad asymmetric three-component aza-Darzens reaction. The key to this transformation was the judicious choice of a CPA-derived Brønsted acid catalyst (S)-11, which possesses stronger acidity. This enabled the reaction to accommodate a wide range of commonly used (hetero)aryl aldehydes. Additionally, the presence of a tert-butoxy group at the ortho-position of the aromatic amines played a crucial role in achieving high stereochemical induction of aziridines (25). This effect was facilitated through both hydrogen bonding interactions and steric influences (Fig. 6b).55
In 2019, Studer and co-workers reported an enantioselective three-component Minisci reaction, providing a valuable synthetic route to pharmaceutically relevant γ-amino acid derivatives (29). This cascade reaction, initiated from α-bromocarbonyl compounds (26), enamides (27), and quinolines or pyridines (28), proceeded with high levels of chemoselectivity, regioselectivity, and enantioselectivity under mild conditions. The dual catalysis by photoredox and CPA was crucial to achieving these selective outcomes. The mechanism involves the initial single-electron transfer in α-bromocarbonyls (26), which generates an alkyl radical. This radical subsequently reacts with the electron-rich enamine, resulting in the formation of a nucleophilic radical. Driven by the bifunctional activation of the catalyst (R)-12, which involves both quinoline/pyridine and the resulting radical species, the enantioselective nucleophilic addition of iminium takes place, ultimately yielding the desired γ-amino acid derivatives (29). Chiral 1,2-diamine (30) derivatives could also be achieved with the same strategy (Fig. 7).56
Aside from central chirality, AMCRs have also been successfully applied in the construction of axially and helically chiral architectures. In 2015, Doyle reported a three-component cascade reaction of in situ-formed enamines with 2,3-diketoesters (31) under Brønsted acid catalysis to afford 5-vinyl-pyrrole and 4-hydroxy-indole derivatives in generally moderate to good yields via successive aldol/cyclization/aromatization.57 Inspired by this pioneering work, an asymmetric variant was realized by Lin and co-workers using aryl amines bearing sterically hindered groups at the ortho-position. This reaction furnished various atropisomeric N-arylindole compounds (33) in good yields with excellent enantioselectivities. Experimental results revealed that the catalyst (R)-13 derived from the 3,3,3′,3′-tetramethyl-1,1′-spirobiindane-7,7′-diol (TM-SPINOL) skeleton exhibited better stereoinduction ability than those derived from commonly used cores, including BINOL and SPINOL (Fig. 8a).58
In 2023, AMCR was effectively harnessed by Yang and co-workers in the synthesis of chiral azahelicenes (36) via a one-pot reaction involving polycyclic aryl amines (34), aldehydes (1), and amides (35a). This transformation comprises two sequential processes. The first is the asymmetric Povarov reaction, where imines are intercepted by electron-rich olefins in the presence of (R)-14. Subsequently, the resulting chiral tetrahydroquinolines are oxidized to yield the final azahelicene products (36a) through an aromatization-enabled central-to-helical chirality conversion. When diene substrates 35b were utilized, the asymmetric cyclization of the terminal alkene with the imine takes place to give 36b as products. These compounds were found to possess unique acid/base-triggered switching of photophysical properties (Fig. 8b).59 More recently, the same group realized the enantioselective Groebke–Blackburn–Bienaymé reaction by means of organocatalysis. Using the catalyst (R)-15, various 6-aryl-2-aminopyridines (37), aldehydes (1), and isocyanides (16) were efficiently converted to structurally diverse imidazo[1,2-a]pyridine atropisomers (38) in high to excellent yields with excellent enantiopurities. Control experiments demonstrated that the remote hydrogen bonding donor on the substrate plays a crucial role in achieving high stereoselectivity in the reaction (Fig. 8c).60
Besides CPA, other chiral Brønsted acid catalysts have also been used in AMCRs. In 2012, Maruoka reported an asymmetric Ugi-type reaction using hydrazides (39) instead of traditional amines. In this reaction, the BINOL-embedded axially chiral dicarboxylic acid catalyst (R)-16 was utilized to facilitate the asymmetric addition of isocyanides (16) to the in situ generated acyclic azomethine imines. The intramolecular trapping of the resulting nitrilium ion intermediates with the oxygen atoms of hydrazides generates a series of chiral heterocyclic compounds (40). Notably, the 2-benzoyl group of the isocyanides can be efficiently removed to afford benzoxazole derivatives (41) with excellent efficiency and enantiocontrol. This transformation is achieved through a one-pot treatment of the Ugi-type reaction mixture with K2CO3 in CF3CH2OH (Fig. 9a).61 In 2016, axially chiral disulfonimide (R)-17 was elegantly adopted by Rodríguez and co-workers to promote the enantioselective three-component reaction of aldehydes (1), aromatic amines (7) and enol ethers (42). Compared with the traditional CPAs, this type of Brønsted acid catalyst harbors one more basic site. It was found that the installation of substituents at the 3,3′-positions of (R)-17 gave rise to the configurational inversion of pyrrolo[1,2-a]indole derivatives (43) and computational studies were performed to explain this unusual enantioinversion phenomenon (Fig. 9b).62
In addition, a chiral task-specific ionic liquid, merging a chiral phosphate anion and a nonchiral Brønsted acid-appended imidazolium cation, was effectively employed to catalyze the asymmetric three-component Biginelli reaction by the Neto group in 2018.63 On the other hand, Peng and co-workers combined CPA and silver carbonate to catalyze a cyclization/Mannich tandem reaction of 2-alkynylbenzaldehydes, aromatic amines, and dimethylphosphonate in 2020. Good stereoinduction was observed in the desired cyclic α-aminophosphonate products.64
2.2 Asymmetric four-component reaction
As previously stated, the typical Ugi reaction involves four distinct components: a carbonyl compound, an amine, an acid, and an isocyanide. Due to the complexity of its reaction mechanism, research on asymmetric variations of this well-established reaction has primarily focused on three-component reactions with simplified systems. Despite numerous attempts, the four-component Ugi reaction remained elusive until Tan's groundbreaking work in 2018. In this study, CPAs were utilized as the most effective catalysts. It was hypothesized that the greater acidity of CPA compared to carboxylic acid facilitates the acceleration of the target reaction's kinetics and surpasses the background reactions. Furthermore, the self-assembled heterodimerization between CPA and carboxylic acid results in an enhancement of acidity of the catalyst and an increase in the nucleophilicity of the carboxylic acid, effectively realizing catalytic asymmetric control. Simultaneously, the rapid formation of imines and the preferential activation of carbonyl groups under CPA catalysis can effectively suppress the Passerini reaction and other undesired side reactions. The developed reaction exhibited broad substrate generality. Both aliphatic and aromatic aldehydes were accommodated through slight modification of the CPA catalyst, offering a library of more than 80 α-acylamino amides (44) in generally good yields with excellent enantioselectivities. Further elaboration of the highly enantioenriched products via post-condensation enabled a rapid approach for synthesizing pharmaceutically relevant molecules. DFT calculations were performed by Houk and co-workers to demonstrate the reaction mechanism and origins of stereoselectivity (Fig. 10a).65 In 2020, the Tan group applied this catalytic system to the asymmetric Ugi reaction with H2O in situ generated along with the formation of imines instead of carboxylic acids to provide the α-amino amides with high enantiocontrol.66Three years later, access to β-amino acids and their derivatives (46) was achieved through the re-design of the catalytic asymmetric four-component Ugi reaction. In this work, the C1-synthon isocyanide component was replaced with the ambiphilic C2-synthon ynamide (45). The employment of CPA-derived N-triflylphosphoramide derivatives with stronger acidity as catalysts is crucial to facilitate this transformation. Three classes of β-amino amides bearing one or two contiguous carbon stereocenters, totaling over one hundred, were accessible through altering ynamides or oxygen nucleophiles. Notably, this approach can be utilized in the late-stage modification of drugs (Fig. 10b),67 demonstrating its practicality and flexibility in synthetic chemistry.
Besides the classic examples shown above, chiral Brønsted acids have also been used in some other AMCRs in the synthesis of chiral heterocyclic compounds.68 In these reactions, the initial step is usually the formation of imines or their derivatives. Through hydrogen-bonding activation of chiral Brønsted acids, imines or their tautomers undergo asymmetric cycloaddition with the other component to afford the desired products. As for the construction of spiro structures, which was comprehensively summarized by Franz and co-workers69 in 2021, we will not delve into further details here.
3. Chiral amine catalysis
Amines react with carbonyl compounds to form imines or enamines as transient intermediates. These reactive species can undergo subsequent transformations with diverse coupling partners, while the amine catalyst is regenerated under mild work-up conditions. To harness this reactivity for asymmetric synthesis, chiral amine catalysts, particularly secondary amines, have been strategically designed to activate carbonyl substrates and orchestrate enantioselective bond-forming processes. The structural tunability of these chiral catalysts, achieved through modular substitution patterns on their frameworks, enables precise stereochemical induction across a broad spectrum of transformations.70 Notably, such amine-based catalytic systems have been successfully implemented in AMCRs, demonstrating their versatility in constructing complex molecular architectures with high stereocontrol.As early as 2000, List reported the first example of the asymmetric three-component Mannich reaction of aldehydes, acetone and p-anisidine employing proline C23 as the catalyst, providing a rapid access towards chiral β-amino ketones 47 through the nucleophilic addition of enamine which was generated by activating acetone to form imine intermediates. Generally, relatively high enantioselectivities and poor yields were obtained for aromatic aldehydes, while aliphatic aldehyde substrates resulted in compromised enantiocontrol but improved efficiency (Fig. 11a).71 Subsequently, in 2005, Jørgensen and co-workers reported the discovery of a chiral amine C24-catalyzed AMCR of α,β-unsaturated aldehydes. This reaction combines iminium and enamine activation to generate active iminium ion intermediates, which then lead to a highly enantioselective addition of thiols (48) at the β-carbon center. The resulting enamines are trapped by azodicarboxylates (49), acting as the electrophiles. Upon treatment with NaBH4, cyclization products 50 bearing two stereogenic centers were formed with generally good to high diastereocontrol and remarkable enantiocontrol (Fig. 11b).72 Apart from thiols, succinimide was also an amenable nucleophile for this protocol to give the corresponding products stereoselectively.73 Utilizing the same tactic, Macmillan and co-workers realized the asymmetric chlorofunctionalization of α,β-unsaturated aldehydes with chlorinated quinone 51 as the electrophilic chlorine source (Fig. 11c).74 Notably, structurally novel bridged benzoxazocines could be formed through a γ-selective Mannich-initiated one-pot cascade reaction when electron-rich aromatic amines and electron-deficient salicylaldehydes were incorporated into this type of transformation.75
In 2006, a chiral amine C26 catalytic enamine–iminium–enamine triple activation sequence was realized by the Enders group, employing aldehydes, α,β-unsaturated aldehydes and nitroolefins (53) as starting materials. This AMCR furnished tetra-substituted cyclohexene derivatives (54) possessing four contiguous stereogenic centers with complete enantiocontrol via successive Michael, Michael, and aldol reactions. Despite modest chemical yields and only moderate dr values in most cases, this three-component multistep reaction demonstrated exceptional versatility in constructing a broad array of multi-functionalized cyclohexene derivatives. These derivatives are highly valuable as versatile chiral building blocks in organic synthesis (Fig. 12a).76 Afterwards, the same group further expanded the range of the developed AMCR and a variety of complex chiral molecules 55–60 were readily accessed (Fig. 12b).77–81 Additionally, the Jørgensen group disclosed an enantioselective three-component reaction involving two different α,β-unsaturated aldehydes and malononitrile in 2007, utilizing a similar strategy. Through consecutive iminium–iminium–enamine activation, two stereogenic centers were formed in the cyclohexene products 61 under the catalysis of C24 (Fig. 12c).82
Aside from Michael-type addition, chiral amine catalysts were also engaged in asymmetric 1,3-dipolar cycloaddition reactions. In 2007, inspired by the two-component reaction between nitrones and α,β-unsaturated aldehydes for the synthesis of isoxazolidines reported by MacMillan and co-workers,83 and by virtue of chiral amine-enabled iminium activation, Córdova's group proposed an asymmetric three-component variant through the in situ generation of nitrones (63) from hydroxylamines (62) and aldehydes (1). Considering the slight epimerization of the resulting aldehyde products (65) upon silica-gel column chromatography, reduction with NaBH4 was performed to give the chiral alcohols (64) as the final products (Fig. 13a).84 In the same year, they performed a similar reaction by substituting hydroxylamine with 2-aminomalonate. Through the 1,3-dipolar cycloaddition of in situ-generated imines with chiral amine-activated iminium intermediates, highly functionalized pyrrolidine derivatives were readily obtained with enantioselectivities exceeding 90%.85 In 2020, Liu and co-workers reported a chiral amine-catalyzed asymmetric multicomponent reaction (AMCR) involving hydroxylamine, acrylaldehyde, and acetal-containing enones (66). The reaction began with the formation of cyclic hemiacetals (67) through an aza-Michael reaction, facilitated by iminium activation. Subsequent enamine activation of these cyclic hemiacetals enabled a Michael addition with the acetal-containing enones, yielding highly functionalized hemiacetals (68). To simplify the reaction system, (±)-camphorsulfonic acid (CSA) was added to give bisacetal-containing bicyclic isoxazolidines with four continuous stereocenters (69) as the final products for analysis (Fig. 13b).86
Chiral amines have also participated in the asymmetric Biginelli reaction as co-catalysts. In 2008, Feng and co-workers reported their success through the combination of a chiral amine (C27) and an achiral Brønsted acid. Two independent activation modes were involved in this reaction. Imines were in situ generated from aldehydes and urea under Brønsted acid catalysis, while the chiral amine catalyst promoted the formation of enamine intermediates from β-ketoesters. Aromatic, heteroaromatic, and fused-ring aldehydes were all found to be suitable substrates for this transformation and afforded dihydropyrimidines with good to excellent enantiocontrol but poor yields (Fig. 14a).87 Given the specific activation ability and unique activation mode of chiral secondary amine catalysts towards aldehydes or ketones, structurally novel molecules may be readily accessed through rational introduction of other components. In 2021, harnessing the high reactivity of aromatic isobenzopyrylium ions, Jørgensen and co-workers realized the asymmetric synthesis of chiral tetrahydronaphthols (72) containing four contiguous stereocenters from the reaction of isochromenes (71), α,β-unsaturated aldehydes and H2O via dienamine catalysis. In this transformation, a catalytic amount of Brønsted acid HCl was added to release the reactive isobenzopyrylium intermediates. Notably, the resulting product was efficiently utilized in the construction of octahydrobenzo[h]isoquinoline and [2.2.2]octane skeletons (Fig. 14b).88
In addition to secondary amines, chiral primary amines derived from enantiopure cinchona alkaloids have also demonstrated remarkable capabilities in catalyzing organic reactions. Unlike secondary amines, primary amine catalysis provides unique opportunities to address substrates with significant steric hindrance, such as ketones and α,α-disubstituted aldehydes. For its application in AMCR, a pioneering work was reported by the Melchiorre group in 2009. They demonstrated the activation of α,β-disubstituted enals by C29 through a well-defined iminium/enamine tandem sequence. This approach enabled the stereoselective synthesis of various valuable precursors of α-amino acids (73), which feature two adjacent stereogenic centers. Azodicarboxylates (49) were employed as electrophiles, while indoles and thiols served as nucleophiles (Fig. 15a).89 In 2018, Chen and co-workers developed an AMCR involving aldehydes, isoxazol-5(4H)-ones (74), and 3-substituted 2-cyclopentenones (75). This reaction, catalyzed by primary amine C30, enabled the rapid construction of highly enantioenriched spirocyclic frameworks (76) through the presented mechanistic pathway (Fig. 15b).90
4. Chiral thiourea-derived organocatalysts
Bifunctional chiral thiourea-amine organocatalysts have garnered significant attention in asymmetric synthesis due to their unique ability to simultaneously activate both electrophilic and nucleophilic reactants. This dual activation mode is highly advantageous for enhancing reaction rates and achieving superior enantiocontrol. The application of such catalysts in AMCRs has been extensively explored.91 In this context, we will highlight several representative research findings.The asymmetric Strecker reaction is a powerful strategy for the enantioselective synthesis of α-amino acid derivatives. However, one major limitation of the traditional Strecker reaction is the use of toxic hydrogen cyanide (HCN). To address this challenge, a modified three-component acyl-Strecker reaction has been developed, employing acyl cyanides as an alternative source of cyanide ions (CN−). In 2007, the first organocatalytic asymmetric variant of this AMCR was realized by the List group where various α-aminonitriles (78) were assembled with excellent yields and enantioselectivities from aldehydes, amines and acyl cyanides (77) in the presence of the chiral thiourea-based organocatalyst C31. Both aryl and aliphatic aldehydes were amenable for this reaction; however, compromised enantiocontrol was obtained when simple alkylamines were evaluated (Fig. 16).92
The Petasis reaction is also a very famous multicomponent reaction and has a lot of applications in organic synthesis. In 2007, the Takemoto group reported that a newly designed chiral thiourea catalyst C32 could activate organoboric acids and facilitate the enantioselective variant of the Petasis-type transformation of quinoline compounds even at low temperatures. A high degree of enantiocontrol was achieved in the reaction of various quinoline compounds (79) and alkenyl boronic acids (80). It was proposed that the 1,2-amino alcohol moiety on the catalyst activated the boronate nucleophile while thiourea activated the electrophilic N-acylated quinolinium salt via hydrogen bonds (Fig. 17a).93 In 2012, Yuan and co-workers realized a catalytic enantioselective three-component Petasis reaction among salicylaldehydes, amines, and organoboronic acids with a chiral thiourea catalyst C33 bearing both central and axial chirality elements. This reaction gave a wide range of alkylaminophenols (81) with generally good yields and high enantioselectivities. It should be mentioned that a cyclic binaphthyl-derived boronate ester fragment is proposed through the diol exchange of the BINOL moiety in the catalyst with the boronic acid reactant (Fig. 17b).94
The application of chiral thiourea-amine bifunctional catalysts in the asymmetric Biginelli reaction was introduced in 2011 by the Chen group. The identification of a sugar-glycosylated chiral phase-transfer catalyst C34·TfOH is essential for high enantiocontrol. It is suggested that the thiourea entity activates the condensed α,β-unsaturated imine through hydrogen bonding interaction, and the primary amine functionality enables the generation of an active enamine from β-ketoester for the subsequent nucleophilic addition event (Fig. 18a).95 Soon after, Bolm and co-workers attempted this reaction with chiral sulfoximine-derived bifunctional thiourea catalysts. However, none of the evaluated chiral catalysts provided satisfactory stereoinduction in the desired DHPM products.96 In 2016, Han and co-workers developed an organocatalyst C35 which was self-assembled from a cinchona alkaloid-based thiourea and an L-proline derivative for promoting the asymmetric Biginelli reaction with excellent efficiency and enantiocontrol. When the two catalysts were used separately, both the yield and enantioselectivity were significantly decreased (Fig. 18b).97 Moreover, diverse variants of the asymmetric Biginelli reaction with aldehydes and amines as substrates were achieved using this type of catalyst by means of hydrogen bonding interaction between thiourea and imine, which directed enantioselective Mannich-type reactions.98–103
Besides, chiral thiourea-based bifunctional catalysis has also been applied in some other types of transformations. For instance, Xu, Dixon and co-workers reported a highly enantioselective protocol for the preparation of cyclohexanes (83) with multiple stereogenic centers via synergistic catalysis of a thiourea-amine and a cyclic secondary amine using asymmetric Michael addition as one of the key steps. In the reaction pathway, the thiourea-amine promotes the Michael addition between malonate esters (82) and nitroalkenes via bifunctional activation. After that, secondary Michael addition occurs from the resulting chiral adducts to iminiums generated from the cyclic secondary amine and α,β-unsaturated aldehydes. Subsequent cyclization gives chiral cyclohexanes as the final products. By altering the stereochemical configurations of the proline catalysts used in this reaction, different diastereomers could be rapidly accessed with high enantiocontrol (Fig. 19a).104 In 2010, they expanded this chemistry to AMCRs of aldehydes, nitroalkenes and imines (84). In contrast, enamine activation of aldehydes by the chiral amine catalyst was involved in this reaction, and fully substituted cyclic hemiaminals (85) were synthesized with nearly complete enantiocontrol (Fig. 19b).105 Apart from synergistic catalysis, the introduction of a chiral amine into chiral thiourea catalysts also demonstrated good stereoinduction ability in the construction of cyclic structures involving enantioselective Michael addition.106–109
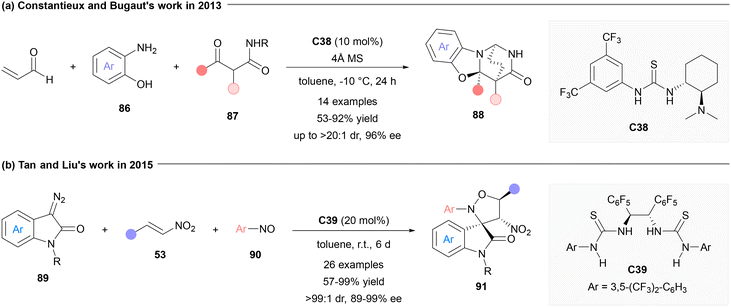
Furthermore, AMCRs under bifunctional chiral thiourea catalysis have also provided efficient synthetic routes towards more complex structures. For instance, in 2013, functionalized 2,6-diazabicyclo-[2.2.2]octanone structures (88) were assembled by Constantieux, Bugaut and co-workers from aminophenols (86), β-ketoamides (87), and acrolein using catalyst C38. Generally, moderate to good stereocontrol could be obtained for the probed substrates (Fig. 20a).110 On the other hand, Tan, Liu and co-workers realized the construction of highly enantioenriched spirocycles employing chiral thiourea catalytic AMCR in 2015. With diazooxindoles (89), nitroalkenes and nitrosoarenes (90) as starting materials, a series of spirooxindole derivatives were synthesized in a highly diastereoselective and enantioselective manner by using bis-thiourea catalyst C39. They proposed that the nitroalkene and nitroso components were successively activated by the catalyst for nucleophilic additions (Fig. 20b).111 This strategy was also effectively utilized in forging other novel spirocyclic frameworks with multiple stereogenic centers.112–118 Noteworthily, when the thiourea moiety was substituted by a squaramide bearing two hydrogen-bonding sites, the resulting squaramide-amine catalysts were also able to catalyze various AMCRs with similar catalytic modes.119–121
Additionally, an asymmetric three-component reductive coupling reaction facilitated by a chiral thiourea catalyst was developed by Johnson's group in 2016, starting from dimethyl phosphite, benzylidene pyruvates (92) and aldehydes. The triaryliminophosphorane functionality on C40 is essential for the high stereoinduction of the vicinal polyfunctionalized stereocenters in product 93. In the proposed mechanistic pathway, the reaction is initiated by a Pudovik addition, in which the deprotonated dimethyl phosphite reacts with compound 92. This step is followed by a phospha-Brook rearrangement, generating an enolate intermediate that can be stereoselectively trapped by aldehydes to yield the desired products (Fig. 21).122
5. Other chiral catalysts
CPA and its derivatives have emerged as a privileged class of Brønsted acids in catalytic asymmetric synthesis. Their chiral diol cores have consistently demonstrated remarkable performance in a variety of enantioselective transformations. In a previous study, it was shown that incorporating an alcohol moiety into the chiral catalyst can lead to interaction with boronic acids, thereby directing stereochemical induction during the addition to activated quinolines.93 Meanwhile, chiral diols can activate achiral boronates via transesterification, thereby generating chiral boronates that subsequently enable asymmetric alkynylation123 and allylation124 reactions.Motivated by these achievements, the Schaus group realized the enantioselective three-component Petasis reaction of alkenyl boronates (94), secondary amines, and ethyl glyoxylate to synthesize chiral α-amino acid esters (95) under the catalysis of chiral biphenol C41 (Fig. 22a).125 Subsequently, several variants of this reaction were reported, successively exploiting a similar strategy.126–128 In 2012, Thomson and co-workers proposed that by utilizing sulfonylhydrazide as the initiator, a Petasis-type coupling reaction could occur between α-hydroxyaldehydes and alkynyl trifluoroborate salts to afford allene structures in the presence of a Lewis acid. This reaction is also known as the traceless Petasis reaction.129 In 2017, a catalytic asymmetric version of the traceless Petasis reaction was reported by the groups of Schaus and Thomson with 1,1′-bi-2-naphthol (BINOL)-derived chiral diols as catalysts. Starting from the glycolaldehyde dimer, the hydrazone species is formed through the reaction with sulfonylhydrazide (96). This intermediate can react with chiral alkynyl boronates which are generated in situ from catalyst C42 with achiral alkynyl boronates (97) to afford propargyl hydrazides. After the loss of sulfinic acid and the extrusion of nitrogen, chiral allenes 98a were delivered. This protocol was also applicable to the synthesis of chiral allenes 98b utilizing alkynyl aldehydes and allyl boronates as reactants (Fig. 22b).130 Moreover, by utilizing the interaction between the hydroxyl group and boron, in 2014, Wulff and co-workers synthesized a group of chiral catalysts with boroxine as the core skeleton by subjecting various chiral biaryl ligands to an amine, water, BH3·SMe2 and a phenol. The in situ-formed catalysts possess compelling potential to enhance the asymmetric three-component Ugi reaction, involving an aldehyde, a secondary amine, and an isonitrile.131 To gain a comprehensive understanding of the recent advancements in the Petasis reaction, it is advisable to refer recent reviews132,133 for additional details.
As is well known, α,β-unsaturated aldehydes can act as nucleophiles bearing two nucleophilic centers and undergo enantioselective cyclization reactions through polarity inversion under the catalysis of chiral N-heterocyclic carbenes (NHCs). Exploiting this unique reactivity, Tan's group accomplished an NHC C43-catalyzed AMCR of an α,β-unsaturated aldehyde, an isoquinolinium (99) and an alcohol. Substituted benztropines (100) with four contiguous stereocenters were produced in a highly diastereo- and enantioselective manner through a double Mannich reaction of isoquinolines that were activated by benzyl-type bromide. Notably, a four-component reaction was also viable under the developed catalytic system (Fig. 23).134
More recently, Cramer et al. designed a class of chiral thiazole-based carbenes with three highly tunable sites. Through subtle elaboration of the NHC catalyst, an asymmetric three-component radical dicarbofunctionalization reaction was developed. Unlike the classic NHC catalysis, the Breslow intermediate formed by the reaction of C44 with an aldehyde undergoes a single-electron transfer with difluoroalkyl bromides (101), generating a reactive difluoroalkyl radical and a persistent ketyl radical intermediate via a Breslow enolate. After that, a radical addition of the difluoroalkyl radical to olefins (102) occurs to give a new radical species. Subsequent radical coupling of the adduct to the olefins (102) affords the highly enantioenriched β-difluoroalkylated α-chiral ketones (103), and the control of enantioselectivity is achieved during this process (Fig. 24).135–137
6. Conclusions
In this review, we present a concise summary of recent advances in organocatalytic AMCRs. Undoubtedly, the rapid evolution of asymmetric organocatalysis has led to the emergence of diverse catalytic systems and novel activation modes, enabling precise stereochemical control over challenging multicomponent transformations. These breakthroughs have created unprecedented opportunities for the efficient and divergent assembly of structurally complex frameworks containing multiple stereogenic centers, thereby establishing valuable platforms for developing chiral pharmaceutical candidates. However, current methodologies predominantly rely on stereoselective additions to imines or employ odorous isocyanide components, resulting in limited substrate generality and mechanistically constrained pathways. Furthermore, practical implementations remain underdeveloped. To address these challenges, future research could exploit photochemical or electrochemical strategies to activate inert substrates, design innovative organocatalysts with tailored activation modes, and develop customized AMCR protocols targeting privileged chiral scaffolds in medicinal chemistry. Particular emphasis should be placed on expanding reaction diversity while maintaining stereochemical fidelity, ultimately bridging the gap between methodological innovation and practical synthetic applications.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2022YFA1503703), the National Natural Science Foundation of China (22425011, 22231004, and 22271135), the Guangdong Innovative Program (2019BT02Y335), the Guangdong Basic and Applied Basic Research Foundation (2024B1515020055), the New Cornerstone Science Foundation through the Xplorer Prize, the Hong Kong Scholars Program (XJ2024-007), the Shenzhen Science and Technology Program (JCYJ20210324120205016 and KQTD20210811090112004) and high-level special funds (G03050K003). The authors appreciate the assistance of SUSTech Core Research Facilities.References
- A. Dömling, W. Wang and K. Wang, Chemistry and Biology of Multicomponent Reactions, Chem. Rev., 2012, 112, 3083–3135 CrossRef.
- B. B. Touré and D. G. Hall, Natural Product Synthesis Using Multicomponent Reaction Strategies, Chem. Rev., 2009, 109, 4439–4486 Search PubMed.
- D. Wang, F. Xiao, F. Zhang and G.-J. Deng, Three-Component Synthesis of 2-Heteroaryl-3-hydroxybenzo[b]-thiophenes under Transition-Metal-Free Conditions, Chin. J. Chem., 2021, 39, 2483–2488 CAS.
- P. Jiang, Z. Shan, S. Chen, Q. Wang, S. Jiang, H. Zheng and G.-J. Deng, Metal-Free Synthesis of Benzo[a]phenanthridines from Aromatic Aldehydes, Cyclohexanones, and Aromatic Amines, Chin. J. Chem., 2022, 40, 365–370 CrossRef CAS.
- W. Zuo, Y. Cheng, Z. Zhu, L. Zuo, X. Geng, Z. Li and L. Wang, Visible-Light-Induced Domino Cyclization to Access Pyrido[2,3-d]pyrimidine-2,4-diones via a Radical-Polar Crossover Reaction, Chin. J. Chem., 2024, 42, 2346–2350 CrossRef CAS.
- X.-B. Wu, J.-X. Shi, Y.-M. Ou, H.-J. Jiang, Y.-J. Fang, Q.-M. Wang, Q. Gao and J. Yu, Sequential Enantioselective Ugi-4CR/post-Ugi Transformation Strategy: A Precise Construction of Structurally Diverse Azaspiro Polycyclic Scaffolds, Sci. China: Chem., 2024, 67, 576–586 CAS.
- L. Tang, G. Lv, T. Wu, L. Zhang, X. Wang, F. Jia, Q. Zhou and G. Zou, Photoredox-Catalyzed Three-Component Carbotrifluoromethylation of Alkenes via Radical–Radical Cross-Coupling, Org. Chem. Front., 2024, 11, 4708–4715 CAS.
- H. Chen, X. Shang, N. Jiang, Q. Zhou, K.-W. Tang, L.-J. Zhong and Y. Liu, Photoinduced Copper-Catalyzed Three-Component Alkylarylation of Alkenes Involving C–S Bond Cleavage of Sulfonium Salts, Org. Chem. Front., 2025, 12, 1498–1505 CAS.
- Y. Zhou and B. Liu, Metal-Free Visible-Light-Induced Borylative/Silylative Pyridylation of Vinylarenes, Org. Chem. Front., 2025, 12, 142–147 CAS.
- L. Ding, G. Yang, L. Luo, Y. Ma, J. Shi, D. Liang and Y. Li, DABCO-Mediated Photoelectrochemical Three-Component Sulfonocyclization of 3-Aza-1,5-dienes, Chin. J. Chem., 2025, 43, 491–500 CAS.
- T. Li, W. Wang, M. Dong, Z. Zhang, S. Yu, Z. Chen, S. Wei and D. Yi, Synergistic Brønsted Base/Photoredox-Catalyzed Three-Component Coupling with Malonates to Synthesize δ-Hydroxy Esters and δ-Keto Esters, Chin. J. Chem., 2024, 42, 957–962 CrossRef CAS.
- E. Koranteng, Z.-C. Shu, Y.-Y. Liu, Q. Yang, B. Shi, Q.-X. Wu, F. Tan, L.-Q. Lu and W.-J. Xiao, Metallaphotoredox-Catalyzed Three-Component Couplings for Practical Synthesis of Ureas and Carbamates, Chin. J. Chem., 2024, 42, 264–270 CrossRef CAS.
- M. Belal, Z. Li, X. Lu and G. Yin, Recent Advances in the Synthesis of 1,1-Diarylalkanes by Transition-Metal Catalysis, Sci. China: Chem., 2021, 64, 513–533 CrossRef CAS.
- B. Xiao, T.-Y. Sun, J. Zhang and Y.-D. Wu, Theoretical Insight Into the Activity and Selectivity in Palladium/Ming-Phos-Catalyzed Three-Component Asymmetric Synthesis of Gem-Diarylmethine Silanes, Sci. China: Chem., 2023, 66, 2817–2827 CAS.
- J.-B. Lin, D.-S. Ji and P.-F. Xu, Catalytically Generated Noncovalent Ammonium Dienolate: A Versatile Platform for the Development of Organocatalytic Asymmetric Cascade Reactions, Sci. China: Chem., 2024, 67, 2524–2546 Search PubMed.
- J. Wang and X. Li, Rhodium-Catalyzed Enantio- and Diastereoselective Carboamidation of Bicyclic Olefins toward Construction of Remote Chiral Centers and Axis, Sci. China: Chem., 2023, 66, 2046–2052 Search PubMed.
- Y. Zhang, J. Wu, L. Ning, Q. Chen, X. Feng and X. Liu, Enantioselective Synthesis of Tetrasubstituted Allenes via Addition/Arylation Tandem Reaction of 2-Activated 1,3-Enynes, Sci. China: Chem., 2023, 66, 526–533 CAS.
- X. He, F. Zhou, Q. Wang, X. Wang, Z. Chang, X. Zhang and Z. Lian, Enantioselective Synthesis of α-Chiral Sulfonates through Palladium-Catalyzed Tsuji–Trost Sulfonylation with Sulfur Dioxide, Org. Chem. Front., 2025, 12, 1274–1283 CAS.
- J. Alemán and S. Cabrera, Applications of Asymmetric Organocatalysis in Medicinal Chemistry, Chem. Soc. Rev., 2013, 42, 774–793 Search PubMed.
- N. Brodt and J. Niemeyer, Chiral Organophosphates as Ligands in Asymmetric Metal Catalysis, Org. Chem. Front., 2023, 10, 3080–3109 CAS.
- X.-L. Liu, M.-L. Gong, X. Yang, P. Tian and Q.-H. Li, Recent Progress in Asymmetric Rearrangement Reactions Mediated by Chiral Brønsted Acids, Org. Chem. Front., 2024, 11, 4934–4953 CAS.
- D. J. Ramón and M. Yus, Asymmetric Multicomponent Reactions (AMCRs): The New Frontier, Angew. Chem., Int. Ed., 2005, 44, 1602–1634 CrossRef.
- C. de Graaff, E. Ruijter and R. V. A. Orru, Recent Developments in Asymmetric Multicomponent Reactions, Chem. Soc. Rev., 2012, 41, 3969–4009 RSC.
- C. M. R. Volla, I. Atodiresei and M. Rueping, Catalytic C–C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis, Chem. Rev., 2014, 114, 2390–2431 CrossRef CAS.
- P. S. G. Nunes, H. D. A. Vidal and A. G. Corrêa, Recent Advances in Catalytic Enantioselective Multicomponent Reactions, Org. Biomol. Chem., 2020, 18, 7751–7773 RSC.
- T. Akiyama, Strong Brønsted Acids, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed.
- D. Kampen, C. M. Reisinger and B. List, Chiral Brønsted Acids for Asymmetric Organocatalysis, Top. Curr. Chem., 2010, 291, 395–456 CrossRef CAS.
- X.-L. Liu, M.-L. Gong, X. Yang, P. Tian and Q.-H. Li, Recent Progress in Asymmetric Rearrangement Reactions Mediated by Chiral Brønsted Acids, Org. Chem. Front., 2024, 11, 4934–4953 RSC.
- T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid, Angew. Chem., Int. Ed., 2004, 43, 1566–1568 CrossRef CAS PubMed.
- D. Uraguchi and M. Terada, Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation, J. Am. Chem. Soc., 2004, 126, 5356–5357 CrossRef CAS PubMed.
- A. Ting and S. E. Schaus, Organocatalytic Asymmetric Mannich Reactions: New Methodology, Catalyst Design, and Synthetic Applications, Eur. J. Org. Chem., 2007, 5797–5815 CrossRef CAS.
- D. Parmar, E. Sugiono, S. Raja and M. Rueping, Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates, Chem. Rev., 2014, 114, 9047–9153 CAS.
- R. Maji, S. C. Mallojjala and S. E. Wheeler, Chiral Phosphoric Acid Catalysis: from Numbers to Insights, Chem. Soc. Rev., 2018, 47, 1142–1158 RSC.
- X. del Corte, E. M. de Marigrta, F. Palacios, J. Vicario and A. Maestro, An Overview of the Applications of Chiral Phosphoric Acid Organocatalysts in Enantioselective Additions to C=O and C=N bonds, Org. Chem. Front., 2022, 9, 6331–6399 RSC.
- X.-H. Chen, X.-Y. Xu, H. Liu, L.-F. Cun and L.-Z. Gong, Highly Enantioselective Organocatalytic Biginelli Reaction, J. Am. Chem. Soc., 2006, 128, 14802–14803 CrossRef CAS PubMed.
- N. Li, X.-H. Chen, J. Song, S.-W. Luo, W. Fan and L.-Z. Gong, Highly Enantioselective Organocatalytic Biginelli and Biginelli-Like Condensations: Reversal of the Stereochemistry by Tuning the 3,3′-Disubstituents of Phosphoric Acids, J. Am. Chem. Soc., 2009, 131, 15301–15310 CrossRef CAS PubMed.
- T. Akiyama, Y. Saitoh, H. Morita and K. Fuchibe, Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid Derived from TADDOL, Adv. Synth. Catal., 2005, 347, 1523–1526 CrossRef CAS.
- X. Hu, R. Zhang, J. Xie, Z. Zhou and Z. Shan, Synthesis of a Novel Sterically Hindered Chiral Cyclic Phosphoric Acid Derived from L-Tartaric Acid and Application to the Asymmetric Catalytic Biginelli Reaction, Tetrahedron: Asymmetry, 2017, 28, 69–74 CrossRef CAS.
- X. Hu, J. Guo, C. Wang, R. Zhang and V. Borovkov, Stereoselective Biginelli-Like Reaction Catalyzed by a Chiral Phosphoric Acid Bearing Two Hydroxy Groups, Beilstein J. Org. Chem., 2020, 16, 1875–1880 CrossRef CAS PubMed.
- Y. Guo, Z. Gao, X. Meng, G. Huang, H. Zhong, H. Yu, X. Ding, H. Tang and C. Zou, Highly Enantioselective Biginelli Reaction of Aliphatic Aldehydes Catalyzed by Chiral Phosphoric Acids, Synlett, 2017, 2041–2045 CrossRef CAS.
- Y. Guo, Z. Gao, K. Wang, J. Li, X. Bi, L. Guo, H. Liu, E. Shi and J. Xiao, Chiral Spirocyclic Phosphoric Acid-Catalyzed Synthesis of 4-Alkyl-3,4-dihydropyrimidin-2(1H)-one Derivatives by Asymmetric Biginelli Reactions, Asian J. Org. Chem., 2020, 9, 626–630 CrossRef CAS.
- M. Stucchi, G. Lesma, F. Meneghetti, G. Rainoldi, A. Sacchetti and A. Silvani, Organocatalytic Asymmetric Biginelli-Like Reaction Involving Isatin, J. Org. Chem., 2016, 81, 1877–1884 CrossRef CAS PubMed.
- J. Jiang, J. Yu, X.-X. Sun, Q.-Q. Rao and L.-Z. Gong, Organocatalytic Asymmetric Three-Component Cyclization of Cinnamaldehydes and Primary Amines with 1,3-Dicarbonyl Compounds: Straightforward Access to Enantiomerically Enriched Dihydropyridines, Angew. Chem., Int. Ed., 2008, 47, 2458–2462 CrossRef CAS PubMed.
- Z. Chen, B. Wang, Z. Wang, G. Zhu and J. Sun, Complex Bioactive Alkaloid-Type Polycycles through Efficient Catalytic Asymmetric Multicomponent Aza-Diels–Alder Reaction of Indoles with Oxetane as Directing Group, Angew. Chem., Int. Ed., 2013, 52, 2027–2031 CrossRef CAS PubMed.
- G.-W. Zhang, D.-H. Zheng, J. Nie, T. Wang and J.-A. Ma, Brønsted Acid-Catalyzed Efficient Strecker Reaction of Ketones, Amines and Trimethylsilyl Cyanide, Org. Biomol. Chem., 2010, 8, 1399–1405 RSC.
- H. Wu, Y.-P. He and L.-Z. Gong, Direct Access to Enantioenriched Spiroacetals through Asymmetric Relay Catalytic Three-Component Reaction, Org. Lett., 2013, 15, 460–463 CrossRef CAS PubMed.
- D. Huang, F. Xu, T. Chen, Y. Wang and X. Lin, Highly Enantioselective Three-Component Povarov Reaction Catalyzed by SPINOL-Phosphoric Acids, RSC Adv., 2013, 3, 573–578 CAS.
- J. Zhang, S.-X. Lin, D.-J. Cheng, X.-Y. Liu and B. Tan, Phosphoric Acid-Catalyzed Asymmetric Classic Passerini Reaction, J. Am. Chem. Soc., 2015, 137, 14039–14042 CrossRef CAS PubMed.
- L. A. Wessjohann, D. G. Rivera and O. E. Vercillo, Multiple Multicomponent Macrocyclizations (MiBs): A Strategic Development Toward Macrocycle Diversity, Chem. Rev., 2009, 109, 796–814 CrossRef CAS PubMed.
- J. C. Flores-Reyes, A. Islas-Jácome and E. González-Zamora, The Ugi Three-Component Reaction and Its Variants, Org. Chem. Front., 2021, 8, 5460–5515 RSC.
- T. Yue, M.-X. Wang, D.-X. Wang, G. Masson and J. Zhu, Brønsted Acid Catalyzed Enantioselective Three-Component Reaction Involving the α-Addition of Isocyanides to Imines, Angew. Chem., Int. Ed., 2009, 48, 6717–6721 CrossRef CAS PubMed.
- Y. Zhang, Y.-F. Ao, Z.-T. Huang, D.-X. Wang, M.-X. Wang and J. Zhu, Chiral Phosphoric Acid Catalyzed Asymmetric Ugi Reaction by Dynamic Kinetic Resolution of the Primary Multicomponent Adduct, Angew. Chem., Int. Ed., 2016, 55, 5282–5285 CrossRef CAS.
- J.-F. Zheng, X.-Y. Qian and P.-Q. Huang, Direct Transformation of Amides: A One-Pot Reductive Ugi-Type Three-Component Reaction of Secondary Amides, Org. Chem. Front., 2015, 2, 927–935 RSC.
- T. Akiyama, T. Suzuki and K. Mori, Enantioselective Aza-Darzens Reaction Catalyzed by A Chiral Phosphoric Acid, Org. Lett., 2009, 11, 2445–2447 CrossRef CAS PubMed.
- S. P. Bew, J. Liddle, D. L. Hughes, P. Pesce and S. M. Thurston, Chiral Brønsted Acid-Catalyzed Asymmetric Synthesis of N-Aryl-cis-Aziridine Carboxylate Esters, Angew. Chem., Int. Ed., 2017, 56, 5322–5326 CrossRef CAS PubMed.
- D. Zheng and A. Studer, Asymmetric Synthesis of Heterocyclic γ-Amino-Acid and Diamine Derivatives by Three-Component Radical Cascade Reactions, Angew. Chem., Int. Ed., 2019, 58, 15803–15807 CrossRef CAS PubMed.
- Q. Sha, H. Arman and M. P. Doyle, Three-Component Cascade Reactions with 2,3-Diketoesters: A Novel Metal-Free Synthesis of 5-Vinyl-Pyrrole and 4-Hydroxy-Indole Derivatives, Org. Lett., 2015, 17, 3876–3879 CrossRef CAS.
- L. Wang, J. Zhong and X. Lin, Atroposelective Phosphoric Acid Catalyzed Three-Component Cascade Reaction: Enantioselective Synthesis of Axially Chiral N-Arylindoles, Angew. Chem., Int. Ed., 2019, 58, 15824–15828 CrossRef CAS PubMed.
- W. Liu, T. Qin, W. Xie, J. Zhou, Z. Ye and X. Yang, Enantioselective Synthesis of Azahelicenes through Organocatalyzed Multicomponent Reactions, Angew. Chem., Int. Ed., 2023, 62, e202303430 CrossRef CAS.
- S. Hong, W. Liu, C. Zhang and X. Yang, Atroposelective Synthesis of Axially Chiral Imidazo[1,2-a]pyridines via Asymmetric Multicomponent Reaction, Sci. Adv., 2024, 10, eadr6135 CrossRef CAS.
- T. Hashimoto, H. Kimura, Y. Kawamata and K. Maruoka, A Catalytic Asymmetric Ugi-type Reaction With Acyclic Azomethine Imines, Angew. Chem., Int. Ed., 2012, 51, 7279–7281 CrossRef CAS.
- A. Galván, A. B. González-Pérez, R. Álvarez, A. R. de Lera, F. J. Fañanás and F. Rodríguez, Exploiting the Multidentate Nature of Chiral Disulfonimides in a Multicomponent Reaction for the Asymmetric Synthesis of Pyrrolo[1,2-a]indoles: A Remarkable Case of Enantioinversion, Angew. Chem., Int. Ed., 2016, 55, 3428–3432 CrossRef.
- H. G. O. Alvim, D. L. J. Pinheiro, V. H. Carvalho-Silva, M. Fioramonte, F. C. Gozzo, W. A. da Silva, G. W. Amarante and B. A. D. Neto, Combined Role of the Asymmetric Counteranion-Directed Catalysis (ACDC) and Ionic Liquid Effect for the Enantioselective Biginelli Multicomponent Reaction, J. Org. Chem., 2018, 83, 12143–12153 CAS.
- L. Zou, J. Huang, N. Liao, Y. Liu, Q. Guo and Y. Peng, Catalytic Asymmetric Three-Component Reaction of 2-Alkynylbenzaldehydes, Amines, and Dimethylphosphonate, Org. Lett., 2020, 22, 6932–6937 CAS.
- J. Zhang, P. Yu, S.-Y. Li, H. Sun, S.-H. Xiang, J. Wang, K. N. Houk and B. Tan, Asymmetric Phosphoric Acid–Catalyzed Four-Component Ugi Reaction, Science, 2018, 361, eaas8707 Search PubMed.
- J. Zhang, Y.-Y. Wang, H. Sun, S.-Y. Li, S.-H. Xiang and B. Tan, Enantioselective Three-Component Ugi Reaction Catalyzed by Chiral Phosphoric Acid, Sci. China: Chem., 2020, 63, 47–54 CrossRef CAS.
- J. Wei, J. Zhang, J. K. Cheng, S.-H. Xiang and B. Tan, Modular Enantioselective Access to β-Amino Amides by Brønsted Acid-Catalysed Multicomponent Reactions, Nat. Chem., 2023, 15, 647–657 CAS.
- J. Yu, F. Shi and L.-Z. Gong, Brønsted-Acid-Catalyzed Asymmetric Multicomponent Reactions for the Facile Synthesis of Highly Enantioenriched Structurally Diverse Nitrogenous Heterocycles, Acc. Chem. Res., 2011, 44, 1156–1171 CAS.
- Y. Wang, A. A. Cobo and A. K. Franz, Recent Advances in Organocatalytic Asymmetric Multicomponent Cascade Reactions for Enantioselective Synthesis of Spirooxindoles, Org. Chem. Front., 2021, 8, 4315–4348 CAS.
- S. Mukherjee, J.-W. Yang, S. Hoffmann and B. List, Asymmetric Enamine Catalysis, Chem. Rev., 2007, 107, 5471–5569 CAS.
- B. List, The Direct Catalytic Asymmetric Three-Component Mannich Reaction, J. Am. Chem. Soc., 2000, 122, 9336–9337 CAS.
- M. Marigo, T. Schulte, J. Franzén and K. A. Jørgensen, Asymmetric Multicomponent Domino Reactions and Highly Enantioselective Conjugated Addition of Thiols to α,β-Unsaturated Aldehydes, J. Am. Chem. Soc., 2005, 127, 15710–15711 CrossRef CAS PubMed.
- H. Jiang, J. B. Nielsen, M. Nielsen and K. A. Jørgensen, Organocatalysed Asymmetric β-Amination and Multicomponent syn-Selective Diamination of α,β-Unsaturated Aldehydes, Chem. – Eur. J., 2007, 13, 9068–9075 CrossRef CAS PubMed.
- Y. Huang, A. M. Walji, C. H. Larsen and D. W. C. Macmillan, Enantioselective Organo-Cascade Catalysis, J. Am. Chem. Soc., 2005, 127, 15051–15053 CrossRef CAS PubMed.
- L. K. Ransborg, M. Overgaard, J. Hejmanowska, S. Barfüsser, K. A. Jørgensen and Ł. Albrecht, Asymmetric Formation of Bridged Benzoxazocines through an Organocatalytic Multicomponent Dienamine-Mediated One-Pot Cascade, Org. Lett., 2014, 16, 4182–4185 CrossRef CAS.
- D. Enders, M. R. M. Hüttl, C. Grondal and G. Raabe, Control of four stereocentres in a triple cascade organocatalytic reaction, Nature, 2006, 441, 861–863 CrossRef CAS.
- D. Enders, M. R. M. Hüttl, J. Runsink, G. Raabe and B. Wendt, Organocatalytic One-Pot Asymmetric Synthesis of Functionalized Tricyclic Carbon Frameworks from a Triple-Cascade/Diels–Alder Sequence, Angew. Chem., Int. Ed., 2007, 46, 467–469 CrossRef CAS PubMed.
- D. Enders, C. Wang, M. Mukanova and A. Greb, Organocatalytic Asymmetric Synthesis of Polyfunctionalized 3-(Cyclohexenylmethyl)-Indoles via a Quadruple Domino Friedel–Crafts-Type/Michael/Michael/Aldol Condensation Reaction, Chem. Commun., 2010, 46, 2447–2449 RSC.
- N. Erdmann, A. R. Philipps, I. Atodiresei and D. Enders, An Asymmetric Organocatalytic Quadruple Cascade Initiated by a Friedel–Crafts-Type Reaction with Electron-Rich Arenes, Adv. Synth. Catal., 2013, 355, 847–852 CrossRef CAS.
- S. Dochain, F. Vetica, R. Puttreddy, K. Rissanen and D. Enders, Combining Organocatalysis and Lanthanide Catalysis: A Sequential One-Pot Quadruple Reaction Sequence/Hetero-Diels–Alder Asymmetric Synthesis of Functionalized Tricycles, Angew. Chem., Int. Ed., 2016, 55, 16153–16155 CrossRef CAS PubMed.
- M. Kumar, P. Chauhan, S. J. Bailey, E. Jafari, C. von Essen, K. Rissanen and D. Enders, Organocatalytic Oxa-Michael/Michael/Michael/Aldol Condensation Quadruple Domino Sequence: Asymmetric Synthesis of Tricyclic Chromanes, Org. Lett., 2018, 20, 1232–1235 CrossRef CAS PubMed.
- A. Carlone, S. Cabrera, M. Marigo and K. A. Jørgensen, A New Approach for an Organocatalytic Multicomponent Domino Asymmetric Reaction, Angew. Chem., Int. Ed., 2007, 46, 1101–1104 CrossRef CAS PubMed.
- W. S. Jen, J. J. M. Wiener and D. W. C. Macmillan, New Strategies for Organic Catalysis: The First Enantioselective Organocatalytic 1,3-Dipolar Cycloaddition, J. Am. Chem. Soc., 2000, 122, 9874–9875 CrossRef CAS.
- R. Rios, I. Ibrahem, J. Vesely, G.-L. Zhao and A. Córdova, A Simple One-Pot, Three-Component, Catalytic, Highly Enantioselective Isoxazolidine Synthesis, Tetrahedron Lett., 2007, 48, 5701–5705 CrossRef CAS.
- I. Ibrahem, R. Rios, J. Vesely and A. Córdova, Organocatalytic Asymmetric Multi-Component [C+NC+CC] Synthesis of Highly Functionalized Pyrrolidine Derivatives, Tetrahedron Lett., 2007, 48, 6252–6257 CrossRef CAS.
- J.-P. Pei, X.-J. Lv, C.-J. Peng and Y.-K. Liu, Asymmetric Organocatalytic Multicomponent Reactions for Efficient Construction of Bicyclic Compounds Bearing Bisacetal and Isoxazolidine Moieties, Chem. Commun., 2020, 56, 12765–12768 RSC.
- J. Xin, L. Chang, Z. Hou, D. Shang, X. Liu and X. Feng, An Enantioselective Biginelli Reaction Catalyzed by a Simple Chiral Secondary Amine and Achiral Brønsted Acid by a Dual-Activation Route, Chem. – Eur. J., 2008, 14, 3177–3181 CrossRef CAS PubMed.
- Y. Liu, J. A. Izzo, D. Mcleod, S. Ričko, E. B. Svenningsen, T. B. Poulsen and K. A. Jørgensen, Organocatalytic Asymmetric Multicomponent Cascade Reaction for the Synthesis of Contiguously Substituted Tetrahydronaphthols, J. Am. Chem. Soc., 2021, 143, 8208–8220 CrossRef CAS PubMed.
- P. Galzerano, F. Pesciaioli, A. Mazzanti, G. Bartoli and P. Melchiorre, Asymmetric Organocatalytic Cascade Reactions with α-Substituted α,β-Unsaturated Aldehydes, Angew. Chem., Int. Ed., 2009, 48, 7892–7894 CrossRef CAS PubMed.
- W. Xiao, Z. Zhou, Q.-Q. Yang, W. Du and Y.-C. Chen, Organocatalytic Asymmetric Four-Component [5 + 1 + 1 + 1] Cycloadditions via a Quintuple Cascade Process, Adv. Synth. Catal., 2018, 360, 3526–3533 CrossRef CAS.
- T. Parvin, R. Yadav and L. H. Choudhury, Recent Applications of Thiourea-Based Organocatalysts in Asymmetric Multicomponent Reactions (AMCRs), Org. Biomol. Chem., 2020, 18, 5513–5532 RSC.
- S. C. Pan and B. List, Catalytic Asymmetric Three-Component Acyl-Strecker Reaction, Org. Lett., 2007, 9, 1149–1151 CrossRef CAS PubMed.
- Y. Yamaoka, H. Miyabe and Y. Takemoto, Catalytic Enantioselective Petasis-Type Reaction of Quinolines Catalyzed by a Newly Designed Thiourea Catalyst, J. Am. Chem. Soc., 2007, 129, 6686–6687 CrossRef CAS PubMed.
- W.-Y. Han, Z.-J. Wu, X.-M. Zhang and W.-C. Yuan, Enantioselective Organocatalytic Three-Component Petasis Reaction among Salicylaldehydes, Amines, and Organoboronic Acids, Org. Lett., 2012, 14, 976–979 CAS.
- Y. Wang, J. Yu, Z. Miao and R. Chen, Bifunctional Primary Amine-Thiourea–TfOH (BPAT·TfOH) as a Chiral Phase-Transfer Catalyst: the Asymmetric Synthesis of Dihydropyrimidines, Org. Biomol. Chem., 2011, 9, 3050–3054 CAS.
- M. Frings, I. Thomé and C. Bolm, Synthesis of Chiral Sulfoximine-Based Thioureas and Their Application in Asymmetric Organocatalysis, Beilstein J. Org. Chem., 2012, 8, 1443–1451 CAS.
- Z. Hang, J. Zhu, X. Lian, P. Xu, H. Yu and S. Han, A Highly Enantioselective Biginelli Reaction Using Self-Assembled Methanoproline–Thiourea Organocatalysts: Asymmetric Synthesis of 6-Isopropyl-3,4-Dihydropyrimidines, Chem. Commun., 2016, 52, 80–83 RSC.
- X. Li, H. Deng, S. Luo and J.-P. Cheng, Organocatalytic Three-Component Reactions of Pyruvate, Aldehyde and Aniline by Hydrogen-Bonding Catalysts, Eur. J. Org. Chem., 2008, 4350–4356 CAS.
- S. Bai, X. Liang, B. Song, P. S. Bhadury, D. Hu and S. Yang, Asymmetric Mannich Reactions Catalyzed by Cinchona Alkaloid Thiourea: Enantioselective One-Pot Synthesis of Novel β-Amino Ester Derivatives, Tetrahedron: Asymmetry, 2011, 22, 518–523 CrossRef CAS.
- Q. Guo and J. C.-G. Zhao, Highly Enantioselective Three-Component Direct Mannich Reactions of Unfunctionalized Ketones Catalyzed by Bifunctional Organocatalysts, Org. Lett., 2013, 15, 508–511 CAS.
- A. S. Demir and S. Basceken, Study of Asymmetric Aldol and Mannich Reactions Catalyzed by Proline–Thiourea Host–Guest Complexes in Nonpolar Solvents, Tetrahedron: Asymmetry, 2013, 24, 515–525 CrossRef CAS.
- F. Cruz-Acosta, P. de Armas and F. García-Tellado, Water-Compatible Hydrogen-Bond Activation: A Scalable and Organocatalytic Model for the Stereoselective Multicomponent Aza-Henry Reaction, Chem. – Eur. J., 2013, 19, 16550–16554 CrossRef CAS PubMed.
- S. Bai, S. Liu, Y. Zhu, K. Zhao and Q. Wu, Antiviral Bioactivity of Chiral β-Amino Acid Ester Derivatives Synthesized through a One-Pot, Solvent-Free Asymmetric Mannich Reaction, Synlett, 2018, 1921–1925 CrossRef CAS.
- Y. Wang, R.-G. Han, Y.-L. Zhao, S. Yang, P.-F. Xu and D. J. Dixon, Asymmetric Organocatalytic Relay Cascades: Catalyst-Controlled Stereoisomer Selection in the Synthesis of Functionalized Cyclohexanes, Angew. Chem., Int. Ed., 2009, 48, 9834–9838 CrossRef CAS PubMed.
- Y. Wang, D.-F. Yu, Y.-Z. Liu, H. Wei, Y.-C. Luo, D. J. Dixon and P.-F. Xu, Multiple-Organocatalyst-Promoted Cascade Reaction: A Fast and Efficient Entry into Fully Substituted Piperidines, Chem. – Eur. J., 2010, 16, 3922–3925 CrossRef CAS PubMed.
- R. Imashiro, H. Uehara and C. F. Barbas III, One-Pot Enantioselective Syntheses of Iminosugar Derivatives Using Organocatalytic anti-Michael–anti-Aza-Henry Reactions, Org. Lett., 2010, 12, 5250–5253 CrossRef CAS PubMed.
- D. Enders, G. Urbanietz, E. Cassens-Sasse, S. Keeß and G. Raabe, Control of Six Contiguous Stereocenters in an Asymmetric Organocatalytic One-Pot Michael/Michael/Aldol Addition Sequence, Adv. Synth. Catal., 2012, 354, 1481–1488 CrossRef CAS.
- H. R. Tan, H. F. Ng, J. Chang and J. Wang, Highly Enantioselective Assembly of Functionalized Tetrahydroquinolines with Creation of an All-Carbon Quaternary Center, Chem. – Eur. J., 2012, 18, 3865–3870 CrossRef CAS PubMed.
- W. Hou, Q. Wei, G. Liu, J. Chen, J. Guo and Y. Peng, Asymmetric Multicomponent Sulfa-Michael/Mannich Cascade Reaction: Synthetic Access to 1,2-Diamino-3-Organosulfur Compounds and 2-Nitro Allylic Amines, Org. Lett., 2015, 17, 4870–4873 CrossRef CAS PubMed.
- M. M. Sanchez Duque, O. Baslé, Y. Génisson, J.-C. Plaquevent, X. Bugaut, T. Constantieux and J. Rodriguez, Enantioselective Organocatalytic Multicomponent Synthesis of 2,6-Diazabicyclo[2.2.2]octanones, Angew. Chem., Int. Ed., 2013, 52, 14143–14146 CrossRef CAS PubMed.
- M.-Y. Wu, W.-W. He, X.-Y. Liu and B. Tan, Asymmetric Construction of Spirooxindoles by Organocatalytic Multicomponent Reactions Using Diazooxindoles, Angew. Chem., Int. Ed., 2015, 54, 9409–9413 CrossRef CAS PubMed.
- W.-T. Wei, C.-X. Chen, R.-J. Lu, J.-J. Wang, X.-J. Zhang and M. Yan, Enantioselective Synthesis of 3,3′-Dihydropyrryl-Spirooxindoles via an Organocatalytic Three-Component Reaction, Org. Biomol. Chem., 2012, 10, 5245–5252 RSC.
- B. Zhou, Y. Yang, J. Shi, Z. Luo and Y. Li, Synthesis of Six-Membered Spirocyclic Oxindoles with Five Consecutive Stereocenters in an Asymmetric Organocatalytic One-Pot Michael/Michael/Aldol Addition Sequence, J. Org. Chem., 2013, 78, 2897–2907 CrossRef CAS PubMed.
- X. Huang, K. Pham, W. Yi, X. Zhang, C. Clamens, J. H. Hyatt, J. P. Jasinsk, U. Tayvah and W. Zhang, Recyclable Organocatalyst-Promoted One-Pot Asymmetric Synthesis of Spirooxindoles Bearing Multiple Stereogenic Centers, Adv. Synth. Catal., 2015, 357, 3820–3824 CrossRef CAS.
- X. Huang, M. Liu, K. Pham, X. Zhang, W.-B. Yi, J. P. Jasinski and W. Zhang, Organocatalytic One-Pot Asymmetric Synthesis of Thiolated Spiro-γ-Lactam Oxindoles Bearing Three Stereocenters, J. Org. Chem., 2016, 81, 5362–5369 CrossRef CAS PubMed.
- L.-L. Zhang, J.-W. Zhang, S.-H. Xiang, Z. Guo and B. Tan, Stereoselective Construction of Complex Spirooxindoles via Bisthiourea Catalyzed Three-Component Reactions, Chin. J. Chem., 2018, 36, 1182–1186 CrossRef CAS.
- L.-L. Zhang, J.-W. Zhang, S.-H. Xiang, Z. Guo and B. Tan, Remote Control of Axial Chirality: Synthesis of Spirooxindole–Urazoles via Desymmetrization of ATAD, Org. Lett., 2018, 20, 6022–6026 CAS.
- L. Zhu, X. Ren, Z. Liao, J. Pan, C. Jiang and T. Wang, Asymmetric Three-Component Cyclizations toward Structurally Spiro Pyrrolidines via Bifunctional Phosphonium Salt Catalysis, Org. Lett., 2019, 21, 8667–8672 CAS.
- L. Tian, X.-Q. Hu, Y.-H. Li and P.-F. Xu, Organocatalytic Asymmetric Multicomponent Cascade Reaction via 1,3-Proton Shift and [3+2] Cycloaddition: An Efficient Strategy for the Synthesis of Oxindole Derivatives, Chem. Commun., 2013, 49, 7213–7215 RSC.
- M. Blümel, P. Chauhan, R. Hahn, G. Raabe and D. Enders, Asymmetric Synthesis of Tetrahydropyridines via an Organocatalytic One-Pot Multicomponent Michael/Aza-Henry/Cyclization Triple Domino Reaction, Org. Lett., 2014, 16, 6012–6015 CrossRef PubMed.
- Y. Zhong, X. Zhao, X. Zhao, D. Zhang, W. Li, S. Wei, F. Liu, J. Yu, G. Li and D. Wang, Multi-Component Syntheses of 2-Pyrrolines and Organocatalytic Asymmetric Syntheses of Functionalized Chiral 2-Pyrrolines, Org. Chem. Front., 2021, 8, 664–669 RSC.
- M. A. Horwitz, B. P. Zavesky, J. I. Martinez-Alvarado and J. S. Johnson, Asymmetric Organocatalytic Reductive Coupling Reactions between Benzylidene Pyruvates and Aldehydes, Org. Lett., 2016, 18, 36–39 CrossRef CAS PubMed.
- T. R. Wu and J. M. Chong, Ligand-Catalyzed Asymmetric Alkynylboration of Enones: A New Paradigm for Asymmetric Synthesis Using Organoboranes, J. Am. Chem. Soc., 2005, 127, 3244–3245 CAS.
- S. Lou, P. N. Moquist and S. E. Schaus, Asymmetric Allylboration of Ketones Catalyzed by Chiral Diols, J. Am. Chem. Soc., 2006, 128, 12660–12661 CAS.
- S. Lou and S. E. Schaus, Asymmetric Petasis Reactions Catalyzed by Chiral Biphenols, J. Am. Chem. Soc., 2008, 130, 6922–6923 CAS.
- G. Muncipinto, P. N. Moquist, S. L. Schreiber and S. E. Schaus, Catalytic Diastereoselective Petasis Reactions, Angew. Chem., Int. Ed., 2011, 50, 8172–8175 CAS.
- X. Shi, W. F. Kiesman, A. Levina and Z. Xin, Catalytic Asymmetric Petasis Reactions of Vinylboronates, J. Org. Chem., 2013, 78, 9415–9423 CAS.
- C. S. Marques, P. McArdle, A. Erxleben and A. J. Burke, Accessing New 5-α-(3,3-Disubstituted Oxindole)-Benzylamine Derivatives from Isatin: Stereoselective Organocatalytic Three Component Petasis Reaction, Eur. J. Org. Chem., 2020, 3622–3634 CAS.
- D. A. Mundal, K. E. Lutz and R. J. Thomson, A Direct Synthesis of Allenes by a Traceless Petasis Reaction, J. Am. Chem. Soc., 2012, 134, 5782–5785 CAS.
- Y. Jiang, A. B. Diagne, R. J. Thomson and S. E. Schaus, Enantioselective Synthesis of Allenes by Catalytic Traceless Petasis Reactions, J. Am. Chem. Soc., 2017, 139, 1998–2005 CAS.
- W. Zhao, L. Huang, Y. Guan and W. D. Wulff, Three-Component Asymmetric Catalytic Ugi Reaction—Concinnity from Diversity by Substrate-Mediated Catalyst Assembly, Angew. Chem., Int. Ed., 2014, 53, 3436–3441 CAS.
- P. Wu, M. Givskov and T. E. Nielsen, Reactivity and Synthetic Applications of Multicomponent Petasis Reactions, Chem. Rev., 2019, 119, 11245–11290 CAS.
- K. J. Gonzalez, C. Cerione and B. M. Stoltz, Strategies for the Development of Asymmetric and Non-Directed Petasis Reactions, Chem. – Eur. J., 2024, 30, e202401936 CAS.
- J.-H. Xu, S.-C. Zheng, J.-W. Zhang, X.-Y. Liu and B. Tan, Construction of Tropane Derivatives by the Organocatalytic Asymmetric Dearomatization of Isoquinolines, Angew. Chem., Int. Ed., 2016, 55, 11834–11839 CAS.
- S. Jana and N. Cramer, Tunable Thiazolium Carbenes for Enantioselective Radical Three-Component Dicarbofunctionalizations, J. Am. Chem. Soc., 2024, 146, 35199–35207 CAS.
- Z. Lin, G. Lv, J. Liu, J. Tang, B. Mu, J. Chen, T. Huang, Z. Yang and Y. Wu, Cooperative NHC/Photoredox Catalysis: Three-Component Reaction of Aroyl Fluorides, Styrenes and Oxalates, Chin. J. Chem., 2024, 42, 511–515 CAS.
- A. Jialingbieke, X. Hu, Z. Liu, X. Lin, Y. Yin, J. Li, Y. Huang and D. Du, Organocatalytic Radical Aminoacylation of Alkenes for β-Aminoketone Synthesis, Sci. China: Chem., 2025, 68, 1002–1008 CAS.
| This journal is © the Partner Organisations 2025 |