A bioinspired antibacterial and photothermal membrane for stable and durable clean water remediation†
Yiyan
Yang‡
a,
Lei
Yang‡
a,
Fengying
Yang
a,
Wanjie
Bai
a,
Xueqian
Zhang
 a,
Haotian
Li
a,
Gaigai
Duan
a,
Haotian
Li
a,
Gaigai
Duan
 b,
Yuanting
Xu
b,
Yuanting
Xu
 *a and
Yiwen
Li
*a and
Yiwen
Li
 *a
*a
aCollege of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, 610065, China. E-mail: xuyt@scu.edu.cn; ywli@scu.edu.cn
bJiangsu Co-Innovation Centre of Efficient Processing and Utilization of Forest Resources International Innovation Centre for Forest Chemicals and Materials, College of Materials Science and Engineering, Nanjing Forest University, Nanjing 210037, China
First published on 8th November 2022
Abstract
Solar-driven steam generation has been considered as a prevalent and sustainable approach to obtain clean fresh water. However, the presence of microorganisms in seawater may cause the biofouling and degradation of polymeric photothermal materials and clog the channels for water transportation, leading to a decrease in solar evaporation efficiency during long-term usage. Herein, we have reported a facile strategy to construct a robust cellulose membrane device coated by tobramycin-doped polydopamine nanoparticles (PDA/TOB@CA). The PDA/TOB@CA membrane not only exhibited synergistic antibacterial behaviors with long-term and sustained antibiotic release profiles, but also achieved a high water evaporation rate of 1.61 kg m−2 h−1 as well as an evaporation efficiency of >90%. More importantly, the high antibacterial activity endowed the PDA/TOB@CA membrane with superb durability for stable reuse over 20 cycles, even in microbe-rich environments. Therefore, we envision that this study could pave a new pathway towards the design and fabrication of robust antibacterial and photothermal materials for long-term and stable clean water production.
New conceptsSolar-driven steam generation is emerging as a promising approach to mitigate the worldwide water crisis, and many achievements focusing on the increase of the evaporation rate have been made. However, the presence of microorganisms in seawater may cause the biofouling and degradation of photothermal materials, and clog the channels for water transportation, leading to a decrease in the solar evaporation efficiency during long-term usage. Herein, our study develops a bioinspired antibacterial and photothermal membrane coated by tobramycin-doped polydopamine nanoparticles. Benefiting from the synergistic antibacterial effect of sustained antibiotic release and photothermal ability of polydopamine, the obtained membrane not only exhibited a high water evaporation rate and efficient antibacterial activity, but also achieved superb durability for stable reuse over 20 cycles, even in microbe-rich environments. Our strategy for the design and development of this antibacterial and photothermal membrane can be generalized to abundant photothermal materials and polydopamine functional nanoparticles and further benefit a wide exploration across diverse applications. |
1. Introduction
With the fast development of modern industry and population expansion, environmental pollution has become a growing global problem,1–3 in which water pollution has raised more concerns due to its essential importance for human health. In order to alleviate the severe water scarcity problems,4,5 a variety of methods have been developed to obtain fresh water from sewage and seawater.6–8 Among them, solar-driven steam generation via interfacial heating has received increasing attention owing to the abundance of solar energy and negligible environmental impact.9–11 During the past years, various interfacial evaporators with rationally designed photothermal materials have been developed. Despite the inspiring achievements, most of them mainly focused on increasing evaporation rates.12–15 Unfortunately, interfacial evaporators come into contact with wastewater or seawater during use, which is often populated by numerous microorganisms (e.g., bacteria) for practical applications. These microorganisms may accumulate on the device surfaces, induce the formation of biofilms on evaporators and clog the channels of water transportation, finally leading to a decrease in the evaporation efficiency, unstable performance, and even degradation of photothermal materials.16–19 Therefore, it is highly desirable to develop suitable photothermal materials with anti-fouling properties to ensure the stable and efficient solar distillation efficacy of the evaporators. Traditional methods have often used chemicals for timely fouling removal, which not only increased energy consumption but also decreased the service life of the membrane. Compared with external chemicals for the membrane fouling removal, directly equipping photothermal materials with antimicrobial capability provides another better alternative for water purification.20,21 Whilst several types of preparation strategies have been well explored and applied to the antimicrobial material design, the high cost and redundant fabrication procedure severely limited their large-scale applications as a photothermal layer.22 Hence, more efforts should be devoted to the development of antibacterial and photothermal materials with cost-effective and facile-fabrication benefits for solar-driven steam generation.Nature usually offers plentiful resources and inspirations for researchers to design and develop different kinds of robust materials with unique structures and fascinating properties.23–25 Recently, bioinspired polydopamine (PDA) has attracted particular interest for surface modification,26–28 free-radical scavenging,29–31 and photothermal sterilization,32–34 based on mussel-inspired adhesion chemistry and excellent performance of natural melanin mimetics. In particular, the strong adhesive and outstanding light-harvesting properties of PDA make it a promising candidate as the building block for photothermal composite materials for solar distillation. Tobramycin (TOB) represents a class of naturally occurring broad-spectrum antibiotics with amino-functionalized glycosidic structures.35–37 The presence of abundant amino groups within TOB allows the copolymerization of TOB and the dopamine (DA) monomer through mussel-inspired polymerization and Schiff base/Michael addition reactions, which could generate the TOB-doped PDA nanoparticles with long-term and on-demand release of antibiotics.38–40 Notably, the integration of PDA and TOB into one system could enable the successful fabrication of robust materials with high photothermal efficiency and antibacterial performances, low cost, and durable stability for solar-driven steam generation. In this work, we described a strategy to prepare PDA/TOB NPs to coat the cellulose acetate membrane to obtain photothermal materials with efficient evaporation and antibacterial properties under sunlight irradiation. Additionally, benefiting from the synergistic effect of the robust nanomaterials, the resultant PDA/TOB@CA membranes exhibited excellent antibacterial activity, thus leading to efficient as well as durable solar evaporation performance even in a bacteria-rich environment, which demonstrated great potential in water remediation for practical applications.
2. Results and discussion
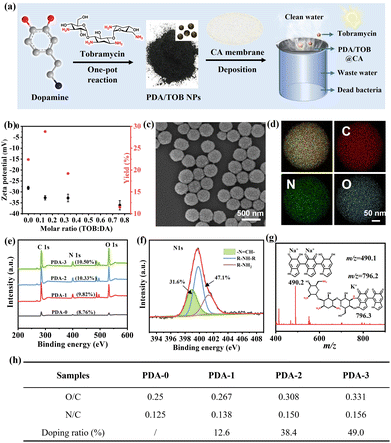
As illustrated in Fig. 1a, those new kinds of PDA/TOB NPs could be facilely acquired via a one-pot reaction of DA and TOB in an aqueous solution under magnetic stirring. Notably, the self-oxidation polymerization of DA was triggered by adding ammonia aqueous solution to adjust the solution to weakly alkaline conditions; meanwhile, TOB molecules also participated in the polymerization process through Schiff base and/or Michael addition reactions. According to the techniques in Fig. S1a (ESI†), a series of TOB-doped PDA NPs with different TOB doping contents were successfully synthesized and denoted as PDA-i (i = 0–3). Scanning electron microscopy (SEM) and dynamic light scattering (DLS) results clearly demonstrated that PDA-i (i = 0–3) NPs had sphere-like shapes with unimodal size distributions (Fig. S1b and S1d, ESI†). Additionally, the zeta potential values of PDA-i (i = 0–3) NPs were found to be less than −27 mV, suggesting their excellent dispersibility and stability in water (Fig. 1b). The magnifying SEM images, transmission electron microscopy (TEM) images and electron energy-loss spectroscopy (EELS) mapping images of PDA-1 NPs in Fig. 1c, Fig. S1d (ESI†), and Fig. 1d further confirmed the existence of C, N, and O elements, as well as their uniform distribution within NPs. The results were also supported by the results of X-ray photoelectron spectroscopy (XPS) in Fig. 1e and Fig. S2 (ESI†). Additionally, as shown in Fig. 1f, the high-resolution spectra of N 1s for PDA-1 NPs could be deconvoluted into three peaks:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C (398.6 eV), R–NH–R (399.6 eV), and R–NH2 (401.1 eV), respectively.41–43 This result indicated that the TOB unit was successfully doped into the PDA network. Moreover, the
N–C (398.6 eV), R–NH–R (399.6 eV), and R–NH2 (401.1 eV), respectively.41–43 This result indicated that the TOB unit was successfully doped into the PDA network. Moreover, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C (398.6 eV) species also demonstrated that DA and TOB had undergone a Schiff base reaction under weak base conditions. Another striking evidence was provided by electrospray ionization mass spectrometry (ESI-MS), which was conducted to identify the oligomeric intermediates of the polymerization reaction at the early stage. As shown in Fig. 1g and Fig. S3 (ESI†), a conjugate between PDA and TOB was observed, confirming that the both molecules are involved in the occurrence of Schiff base/Michael addition reactions, which was also consistent with the high-resolution XPS spectra of N 1s regions of PDA-1 NPs. Other m/z peaks and possible chemical structures of resultant intermediates were also listed here.
N–C (398.6 eV) species also demonstrated that DA and TOB had undergone a Schiff base reaction under weak base conditions. Another striking evidence was provided by electrospray ionization mass spectrometry (ESI-MS), which was conducted to identify the oligomeric intermediates of the polymerization reaction at the early stage. As shown in Fig. 1g and Fig. S3 (ESI†), a conjugate between PDA and TOB was observed, confirming that the both molecules are involved in the occurrence of Schiff base/Michael addition reactions, which was also consistent with the high-resolution XPS spectra of N 1s regions of PDA-1 NPs. Other m/z peaks and possible chemical structures of resultant intermediates were also listed here.
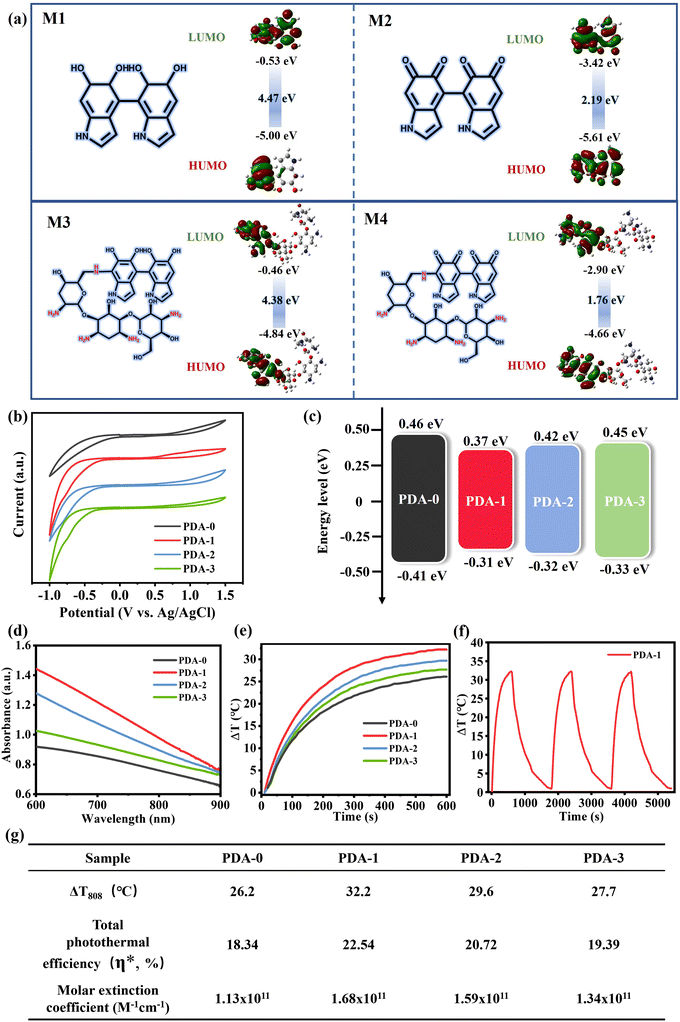
We then systematically investigated the photothermal conversion performance of PDA/TOB NPs and extensively analysed the mechanism underlying the improved light absorption capacity and photothermal effect of PDA/TOB NPs. Note that the formation of the donor–acceptor (D–A) domain within the samples by various oligomer conjugates could decrease the energy bandgaps of the PDA-based NPs and promote the electron delocalization, resulting in the enhancement of light absorption of the NPs for more energy intake.44–46 In order to verify these points in this system, a theoretical simulation of potential molecular bound structures within PDA/TOB NPs was first carried out by using the density functional theory (DFT) technique at the B3LYP/6-31G(d, p) level. As shown in Fig. 2a, based on the optimized structure, the lowest unoccupied molecular orbital (LUMO)/highest occupied molecular orbital (HOMO) levels of the typical molecular intermediates of PDA-i (i = 0–3) were calculated as M1 (−0.53/−5.00 eV), M2 (−3.42/−5.61 eV), M3 (−0.46/−4.48 eV) and M4 (−2.90/−4.66 eV), thus their energy bandgaps were calculated as 4.47, 2.19, 4.38 and 1.76 eV, respectively. Subsequently, we further investigated the electronic energy bandgaps (Eg) for all the PDA-i (i = 0–3) samples by electrochemical cyclic voltammetry (CV) (Fig. 2b and c), and the measurement techniques were based on our previously reported work.47 Notably, all PDA/TOB NPs indeed showed decreased energy bandgaps compared with pristine PDA-0, which was consistent with the above simulated calculation results of structural analysis. As can be seen in Fig. 2d, the Vis-NIR absorption spectra suggested that the doping of TOB remarkably facilitated the stronger light absorption performance across the visible and NIR regions compared with PDA-0. To investigate the photothermal performance of PDA/TOB NPs, the aqueous solutions of all samples (100 μg mL−1) were irradiated with an 808 nm laser at 1.5 W cm−2 for 10 min. As depicted in Fig. 2e, the average changes in the temperature (ΔT) of PDA-1, PDA-2, and PDA-3 solutions were found to be 32.2, 29.6, and 27.7 °C, respectively, all outperforming that of PDA-0 solution (26.2 °C). By taking PDA-1 as an example, the good stability of photothermal conversion performance was further verified by the temperature-increasing behaviours during three on/off cycles (Fig. 2f). Besides, the total photothermal efficiency (η*) values of PDA-i (i = 0–3) NPs were calculated as 18.34%, 22.54%, 20.72%, and 19.39% through the heating and cooling curves, respectively. Furthermore, the PDA/TOB NPs exhibited higher molar extinction coefficient compared with PDA-0 NPs which was in accordance with the photothermal efficiency results above. Based on the above results, it could be concluded that the doping of TOB enhanced the photothermal efficiency of PDA NPs, thus suggesting their tremendous potential for solar-driven evaporation applications (Fig. 2g).
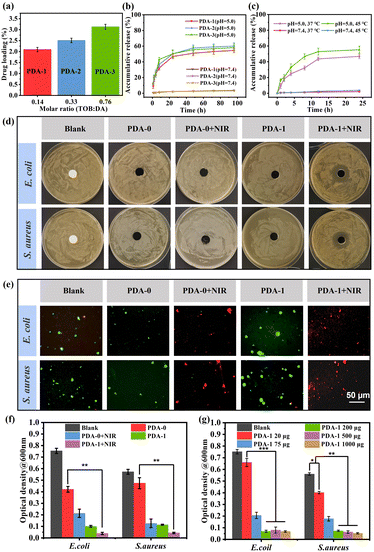
In order to ensure the stable photothermal performance of those bioinspired materials in microorganism-contaminated water, the antibacterial activity of PDA/TOB NPs was further studied. The drug loading contents and release profiles of PDA-i (i = 1–3) NPs could be regulated facilely by the tuning of TOB/DA ratios during the reaction process. As shown in Fig. 3a, the antibiotics loading content of PDA-i (i = 1–3) NPs increased gradually with the increase of the TOB/DA ratio, which was in accordance with the result of TOB doping ratio (Fig. 1h). We further investigated the TOB release profiles of PDA-i (i = 1–3), and the results are displayed in Fig. 3b. Compared with the TOB release behaviour of samples at pH = 7.4 (the normal physiological environment), all samples exhibited faster release profiles at pH = 5.0 (the normal bacterial colonization microenvironment of some typical bacteria, such as E. coli and S. aureus). It revealed that PDA-i (i = 1–3) NPs could release antibiotics in response to pH, which was explained by the dynamic imine bonds formed by a Schiff base reaction between PDA and TOB within the systems. The imine bond is unstable under acidic conditions. In the case of pH = 5.0, the imine bonds of PDA/TOB NPs were broken to release TOB.23 In consideration of their increasing temperature during the photothermal process, we also investigated the TOB release performance under different temperatures. After 24 h of incubation, less than 5% of TOB was released at 37 °C, while ∼60% of TOB was released at 45 °C under pH = 5.0 (Fig. 3c). It revealed the temperature-responsive release behaviour of PDA-i NPs, which might produce better antibacterial performance during the solar-driven evaporation process.
After verifying the photothermal efficiency and TOB release performance, two representative bacteria (E. coli and S. aureus) were selected to perform the zone of inhibition (ZOI) experiment to explore the antibacterial properties of the blank sample, PDA-0 and PDA-1 NPs. As shown in Fig. 3d, an obvious ZOI appeared in the medium after treating with PDA-1 under NIR irradiation, suggesting that the bacterial reproduction was inhibited significantly. This phenomenon could be attributed to the higher TOB amount released from PDA-1 NPs at higher temperature triggered by NIR light irradiation. Then, to identify the living bacteria in the culture environment, the samples were double stained for the live/dead count of E. coli and S. aureus. In Fig. 3e, no living bacteria could be observed after treatment with PDA-1 NPs under NIR irradiation, also indicating the excellent antibacterial ability of PDA-1 NPs. Additionally, it should be pointed out that there were also no live bacteria after treatment with PDA-0 under NIR irradiation, which meant that PDA-0 NPs exhibited a NIR-responsive antibacterial ability based on photothermal effect. Similar conclusions were obtained from the SEM images in Fig. S5 (ESI†). Therefore, in order to explore the synergistic antibacterial effect of photothermal property and antibiotics, we further conducted a quantitative study. As depicted in Fig. 3f, the antibacterial ability of PDA-0 NPs was restricted under normal circumstances and could be enhanced remarkably under NIR irradiation, which derived from the photothermal antibacterial effect of PDA. As for the PDA-1 NPs, they exhibited superb antibacterial ability even without NIR irradiation. In particular, this antibacterial efficiency was further improved when triggering the NIR irradiation. It could be attributed to the synergistic effect of the photothermal antibacterial effect of PDA, as well as the increased release of TOB at high temperature. To further explore the optimal usage amount of TOB-doped PDA NPs, we compared the antibacterial effect of PDA-1 NPs with different contents, and the result revealed that the 200 μg PDA-1 NPs was good enough for the antibacterial performance under NIR irradiation (Fig. 3g and Fig. S6, ESI†).
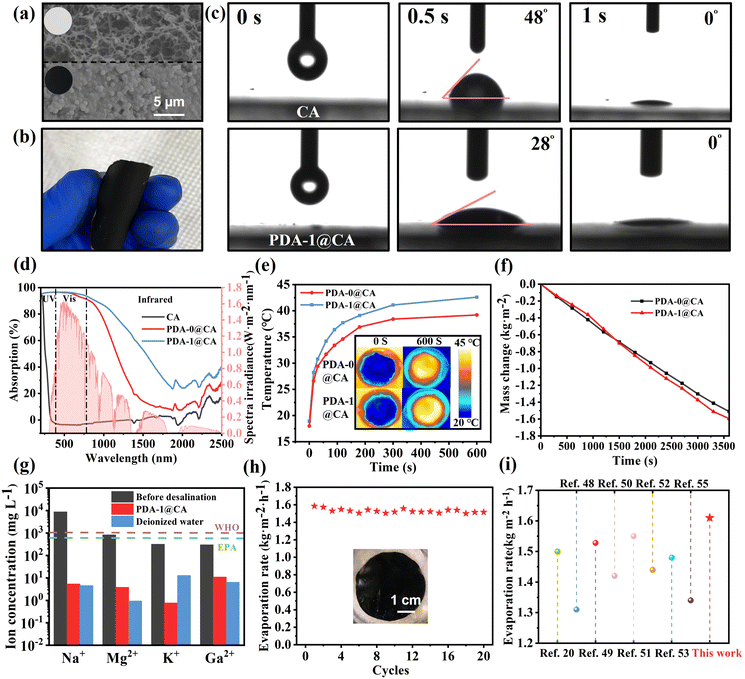
The excellent light absorption, photothermal properties, and antibacterial effect of PDA/TOB NPs provided a unique opportunity for the durable desalination. Among the samples, PDA-1 NPs were further selected to construct evaporation devices because of their excellent photothermal and antibacterial effects. Fig. 4a demonstrated the SEM images of the pristine CA membrane and PDA-1@CA membrane. A large number of NPs could be found on the surface of the modified membrane. Similar results could be visually observed from the inset in Fig. 4a. The colour of PDA-1@CA membrane turned to black, which confirmed the successful modification by PDA-1 NPs. More interestingly, the resulting PDA-1@CA membrane was quite flexible and could be easily curled and folded, revealing its favourable workability in practical applications (Fig. 4b). The hydrophilicity of the membrane has a great influence on water delivery in solar steam generation. As shown in Fig. 4c, a significant lower water contact angle of PDA-1@CA membrane indicated its superior hydrophilicity and water transport ability, which resulted from the existence of numerous hydrophilic functional groups (i.e. –OH and –NH2). Before the photothermal experiments, we investigated the UV-vis-NIR light absorption behaviours of membranes in the range of 200–2500 nm (Fig. 4d). The blank CA membrane exhibited extremely poor light absorption ability. After modification, both PDA-0@CA and PDA-1@CA membranes showed drastically preferable light absorption abilities. In particular, in the visible light range which covered the major wavelength range of sunlight, both PDA-0@CA and PDA-1@CA membranes covered over 90% light absorption. Note that the PDA-1@CA exhibited better light absorption ability than PDA-0@CA, especially in the NIR region, which resulted from the superior light absorption performance of PDA-1 NPs. Therefore, the PDA-1@CA membrane could be applied as light-absorbing layers better for solar-driven interfacial evaporation.
To intuitively analyse the photothermal behaviours of PDA-0@CA and PDA-1@CA membranes, the temperature changes of the evaporation surface were collected by an IR camera under one sun illumination. As displayed in Fig. 4e, after 600 s of irradiation, the average surface temperature of PDA-0@CA and PDA-1@CA membranes rose from room temperature (∼20 °C) to reach stable temperatures of 39.2 and 42.6 °C, respectively, which revealed the superior photothermal effect of the PDA-1@CA membrane. For quantitative assessment of water evaporation performance, an electrical balance was used to record the mass changes of water during the solar irradiation (Fig. 4f). The evaporation rate of the PDA-1@CA membrane (1.61 kg m−2 h−1) was also higher than that of the PDA-0@CA membrane (1.51 kg m−2 h−1) under one sun irradiation. Subsequently, according to a general formula η = mhLV/I, where m is the mass flux under solar illumination, hLV is the total enthalpy of water, and I is the intensity of solar illumination (1 kW m−2), the evaporation efficiency (η) of PDA-0@CA-, and PDA-1@CA-based evaporators were calculated to 90.1% and 92.4%, respectively. In order to evaluate the effect of desalination, the simulated saline water was treated by the PDA-1@CA membrane under solar irradiation, and inductively coupled plasma spectroscopy (ICP-OES) was used to evaluate the content of different metal ions. After desalination, there was a significant decrease in the concentration of Na+, which was well below the salinity levels stipulated by the World Health Organization (WHO) and the US Environmental Protection Agency (EPA). Correspondingly, other species of metal ions in the saline water, including Mg2+, K+, and Ca2+ were also decreased to a lower concentration after desalination (Fig. 4g). In addition, the durability and stability for a long term of steam generation of the PDA-1@ CA membranes were further investigated by cycling experiments. It was observed that the rugged device could be reused for more than 20 cycles under one sun illumination without material breakdown and the loss of desalination efficiency, thus confirming the good stability of the PDA-1@CA membrane (Fig. 4h). More importantly, we compared the evaporation performances of the PDA-1@CA composite in this study with many previous evaporators with antibacterial properties during recent years. As illustrated in Fig. 4i, the evaporation rate of the PDA-1@CA membranes was higher than that of many different types of evaporators previously reported,48–50 including MXene-based,51–54 Ag-based,55–57 PPy-based evaporators.20,58 Although some other devices exhibited high evaporation rates, their fabrication processes were usually complicated, or they exhibited an inability to suppress the colonization of bacteria for durable applications in microorganism-contaminated water.59
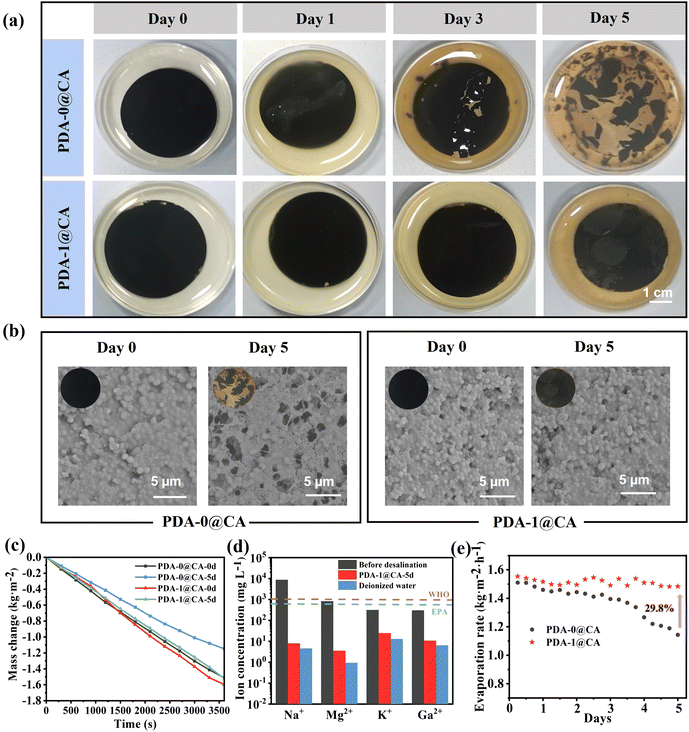
As discussed above, the PDA-0 NPs and PDA-1 NPs endowed the CA membranes with excellent photothermal effects and solar steam generation capacity. Nevertheless, there were numerous microorganisms in actual wastewater or seawater. The microorganisms might decompose the photothermal materials, clog the water transport channels and lead to a decrease in evaporation efficiency. As a result, we further explored the stability and long-term durability of the PDA-0@CA and PDA-1@CA membranes in the bacterial suspension. As shown in Fig. 5a, the PDA-0@CA membrane began to crack after being soaked in a suspension of E. coli bacteria for 3 days and decomposed severely after 5 days. In contrast, the PDA-1@CA membrane maintained intact morphology even after 5 days, owing to its favourable antibacterial properties. Similar conclusions could be obtained from the SEM images in Fig. 5b. Afterwards, we further investigated the water evaporation performances of these membranes after being soaked in bacterial suspension for different days. As demonstrated in Fig. 5c and Fig. S7 (ESI†), the evaporation rates of the PDA-0@CA membranes after being soaked for 0 days, 1 days, 3 days and 5 days were calculated to be 1.51, 1.46, 1.25, and 1.14 kg m−2 h−1 under one sun illumination, respectively. It suggested the decreased evaporation rates affected by bacteria. Interestingly, the PDA-1@CA membranes even after being soaked for 5 days not only kept a high evaporation rate of 1.52 kg m−2 h−1, but also exhibited an efficient desalination performance to achieve extremely low ion concentrations which are much lower than the salinity levels defined by WHO and EPA (Fig. 5d). More importantly, the treatment of soaking in bacterial suspension led to the decrease of the evaporation rates for the PDA-0@CA membrane during the multiple reuse process, while the antibacterial PDA-1@CA membrane still remained stable and durable evaporation performance even after soaking in bacterial suspension for 5 days (Fig. 5e). It indicated its promising application prospects and high economic benefits in the field of solar-driven steam generation. Finally, we further investigated the possible antibiotic release in the collected water after evaporation via the ninhydrin derivatization method. The result in Fig. S8 (ESI†) revealed that there was no TOB antibiotic in the purified water, thus avoiding the risk of antibiotic residue in this study.
3. Conclusions
In conclusion, we reported an antibacterial and photothermal membrane coated with TOB-doped PDA NPs for highly efficient and durable solar-driven steam generation. Owing to the enhanced light absorption, the obtained PDA-1@CA membrane displayed a water evaporation rate of 1.61 kg m−2 h−1 with an evaporation efficiency of 92.4% under 1 sun irradiation. In particular, benefiting from the synergistic antibacterial properties of photothermal effects and antibiotics, the PDA-1@CA membrane after being soaked in bacterial suspension for 5 days still exhibited outstanding stable and durable evaporation efficiency over 20 cycles. We believed that this work could supply novel ideas for reducing the biological pollution of the evaporator to ensure its long-term stability in solar-driven steam generation for freshwater production in an actual environment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21975167, 52225311 and 52003179), the Research Fund from Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Soochow University (KJS2116), and the Fundamental Research Funds for Central Universities.Notes and references
- Z. Li, X. Xu, X. Sheng, P. Lin, J. Tang, L. Pan, Y. V. Kaneti, T. Yang and Y. Yamauchi, ACS Nano, 2021, 15, 12535–12566 CrossRef CAS PubMed.
- T. Ding, Y. Zhou, W. L. Ong and G. W. Ho, Mater. Today, 2021, 42, 178–191 CrossRef CAS.
- X. Xu, S. Ozden, N. Bizmark, C. B. Arnold, S. S. Datta and R. D. Priestley, Adv. Mater., 2021, 33, 2007833 CrossRef CAS PubMed.
- M. Gao, L. Zhu, C. K. Peh and G. W. Ho, Energy Environ. Sci., 2019, 12, 841–864 RSC.
- X. Han, W. Wang, K. Zuo, L. Chen, L. Yuan, J. Liang, Q. Li, P. M. Ajayan, Y. Zhao and J. Lou, Nano Energy, 2019, 60, 567–575 CrossRef CAS.
- D. Qi, Y. Liu, Y. Liu, Z. Liu, Y. Luo, H. Xu, X. Zhou, J. Zhang, H. Yang, W. Wang and X. Chen, Adv. Mater., 2020, 32, 2004401 CrossRef CAS.
- Y. Zou, J. Zhao, J. Zhu, X. Guo, P. Chen, G. Duan, X. Liu and Y. Li, ACS Appl. Mater. Interfaces, 2021, 13, 7617–7624 CrossRef CAS.
- Y. Xu, J. Xu, X. Zhang, D. Yuan, G. Duan and Y. Li, Mater. Horiz., 2022, 9, 2496–2517 RSC.
- P. Tao, G. Ni, C. Song, W. Shang, J. Wu, J. Zhu, G. Chen and T. Deng, Nat. Energy, 2018, 3, 1031–1041 CrossRef.
- Z. Wang, X. Wu, F. He, S. Peng and Y. Li, Adv. Funct. Mater., 2021, 31, 201114 Search PubMed.
- X. Zhou, Y. Guo, F. Zhao, W. Shi and G. Yu, Adv. Mater., 2020, 32, 2007012 CrossRef.
- L. Li and J. Zhang, Nano Energy, 2021, 81, 105682 CrossRef CAS.
- N. Li, L. Qiao, J. He, S. Wang, L. Yu, P. Murto, X. Li and X. Xu, Adv. Funct. Mater., 2021, 31, 2008681 CrossRef CAS.
- H. Lu, W. Shi, F. Zhao, W. Zhang, P. Zhang, C. Zhao and G. Yu, Adv. Funct. Mater., 2021, 31, 2101036 CrossRef CAS.
- L. Hao, N. Liu, R. Niu, J. Gong and T. Tang, Sci. China Mater., 2022, 65, 201–212 CrossRef CAS.
- D. J. Miller, D. R. Dreyer, C. W. Bielawski, D. R. Paul and B. D. Freeman, Angew. Chem., Int. Ed., 2017, 56, 4662–4711 CrossRef CAS PubMed.
- J. Ge, D. Zong, Q. Jin, J. Yu and B. Ding, Adv. Funct. Mater., 2018, 28, 1705051 CrossRef.
- D. M. Warsinger, S. Chakraborty, E. W. Tow, M. H. Plumlee, C. Bellona, S. Loutatidou, L. Karimi, A. M. Mikelonis, A. Achilli, A. Ghassemi, L. P. Padhye, S. A. Snyder, S. Curcio, C. D. Vecitis, H. A. Arafat and J. H. Lienhard, Prog. Polym. Sci., 2018, 81, 209–237 CrossRef CAS.
- Y. Zhu, J. Wang, F. Zhang, S. Gao, A. Wang, W. Fang and J. Jin, Adv. Funct. Mater., 2018, 28, 1804121 CrossRef.
- Y. Xu, J. Ma, Y. Han, H. Xu, Y. Wang, D. Qi and W. Wang, Chem. Eng. J., 2020, 384, 123379 CrossRef CAS.
- B. Peng, Y. Gao, Q. Lyu, Z. Xie, M. Li, L. Zhang and J. Zhu, ACS Appl. Mater. Interfaces, 2021, 13, 37724–37733 CrossRef CAS.
- W. Qu, H. Zhao, Q. Zhang, D. Xia, Z. Tang, Q. Chen, C. He and D. Shu, ACS Sustainable Chem. Eng., 2021, 9, 11372–11387 CrossRef CAS.
- L. Yang, C. Wang, L. Li, F. Zhu, X. Ren, Q. Huang, Y. Cheng and Y. Li, Adv. Funct. Mater., 2022, 32, 2108749 CrossRef CAS.
- Z. Jia, M. Wen, Y. Cheng and Y. Zheng, Adv. Funct. Mater., 2021, 31, 2008821 CrossRef CAS.
- L. Yang, Y. Zou, W. Xia, H. Li, X. He, Y. Zhou, X. Liu, C. Zhang and Y. Li, Nano Res., 2021, 14, 969–975 CrossRef CAS.
- F. Xiong, S. Wei, H. Sheng, X. Han, W. Jiang, Z. Zhang, B. Li, H. Xuan, Y. Xue and H. Yuan, Int. J. Biol. Macromol., 2022, 201, 338–350 CrossRef CAS PubMed.
- P. Yang, F. Zhu, Z. Zhang, Y. Cheng, Z. Wang and Y. Li, Chem. Soc. Rev., 2021, 50, 8319–8343 RSC.
- C. Huang, X. Wang, P. Yang, S. Shi, G. Duan, X. Liu and Y. Li, Macromol. Rapid Commun., 2022, 2100916 CrossRef.
- T. Wang, Q. Fan, J. Hong, Z. Chen, X. Zhou, J. Zhang, Y. Dai, H. Jiang, Z. Gu, Y. Cheng and Y. Li, Small, 2021, 17, 2102485 CrossRef CAS.
- J. Hu, L. Yang, P. Yang, S. Jiang, X. Liu and Y. Li, Biomater. Sci., 2020, 8, 4940–4950 RSC.
- H. Zhang, C. Huang, J. Zhang, C. Wang, T. Wang, S. Shi, Z. Gu and Y. Li, Giant, 2022, 12, 100120 CrossRef CAS.
- Z. Wang, Y. Zou, Y. Li and Y. Cheng, Small, 2020, 16, 1907042 CrossRef CAS.
- P. Yang, S. Zhang, N. Zhang, Y. Wang, J. Zhong, X. Sun, Y. Qi, X. Chen, Z. Li and Y. Li, ACS Appl. Mater. Interfaces, 2019, 11, 42671–42679 CrossRef CAS.
- H. Cao, L. Yang, R. Tian, H. Wu, Z. Gu and Y. Li, Chem. Soc. Rev., 2022, 51, 4175–4198 RSC.
- J. Hu, L. Yang, X. Cheng, Y. Li and Y. Cheng, Adv. Funct. Mater., 2021, 31, 2103718 CrossRef CAS.
- X. Cheng, L. Li, L. Yang, Q. Huang, Y. Li and Y. Cheng, Adv. Funct. Mater., 2022, 32, 2206201 CrossRef CAS.
- M. Li, H. Wang, J. Hu, J. Hu, S. Zhang, Z. Yang, Y. Li and Y. Cheng, Chem. Mater., 2019, 31, 7678–7685 CrossRef CAS.
- Y. Fu, L. Yang, J. Zhang, J. Hu, G. Duan, X. Liu, Y. Li and Z. Gu, Mater. Horiz., 2021, 8, 1618–1633 RSC.
- S. Yu, G. Li, R. Liu, D. Ma and W. Xue, Adv. Funct. Mater., 2018, 28, 1707440 CrossRef.
- L. Yang, L. Li, H. Li, T. Wang, X. Ren, Y. Cheng, Y. Li and Q. Huang, Adv. Healthcare Mater., 2022, 11, 2200112 CrossRef CAS.
- R. A. Zangmeister, T. A. Morris and M. J. Tarlov, Langmuir, 2013, 29, 8619–8628 CrossRef CAS.
- F. Li, Z. Yu, H. Shi, Q. Yang, Q. Chen, Y. Pan, G. Zeng and L. Yan, Chem. Eng. J., 2017, 322, 33–45 CrossRef CAS.
- S. Rella, E. Mazzotta, A. Caroli, M. De Luca, C. Bucci and C. Malitesta, Appl. Surf. Sci., 2018, 447, 31–39 CrossRef CAS.
- Y. Zou, X. Chen, P. Yang, G. Liang, Y. Yang, Z. Gu and Y. Li, Sci. Adv., 2020, 6, eabb4696 CrossRef CAS.
- W. Bai, P. Yang, H. Liu, Y. Zou, X. Wang, Y. Yang, Z. Gu and Y. Li, Macromolecules, 2022, 55, 3493–3501 CrossRef CAS.
- W. Bai, P. Xiang, H. Liu, H. Guo, Z. Tang, P. Yang, Y. Zou, Y. Yang, Z. Gu and Y. Li, Macromolecules, 2022, 55, 6426–6434 CrossRef CAS.
- L. Yang, X. Guo, Z. Jin, W. Guo, G. Duan, X. Liu and Y. Li, Nano Today, 2021, 37, 101075 CrossRef CAS.
- Y. Yang, W. Que, J. Zhao, Y. Han, M. Ju and X. Yin, Chem. Eng. J., 2019, 373, 955–962 CrossRef CAS.
- Y. Xu, J. Ma, Y. Han, J. Zhang, F. Cui, Y. Zhao, X. Li and W. Wang, ACS Sustainable Chem. Eng., 2019, 7, 5476–5485 CrossRef CAS.
- H. Huang, L. Zhao, Q. Yu, P. Lin, J. Xu, X. Yin, S. Chen, H. Wang and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 11204–11213 CrossRef CAS PubMed.
- X. J. Zha, X. Zhao, J. H. Pu, L. S. Tang, K. Ke, R. Y. Bao, L. Bai, Z. Y. Liu, M. B. Yang and W. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 36589–36597 CrossRef CAS PubMed.
- H.-S. Guan, T.-T. Fan, H.-Y. Bai, Y. Su, Z. Liu, X. Ning, M. Yu, S. Ramakrishna and Y.-Z. Long, Carbon, 2022, 188, 265–275 CrossRef CAS.
- Z. Xie, Y. P. Peng, L. Yu, C. Xing, M. Qiu, J. Hu and H. Zhang, Sol. RRL, 2020, 4, 1900400 CrossRef.
- Y. Li, T. Wu, H. Shen, S. Yang, Y. Qin, Z. Zhu, L. Zheng, X. Wen, M. Xia and X. Yin, J. Cleaner Prod., 2022, 347, 131324 CrossRef CAS.
- J. Yang, Y. Chen, X. Jia, Y. Li, S. Wang and H. Song, ACS Appl. Mater. Interfaces, 2020, 12, 47029–47037 CrossRef CAS.
- X. Fan, H. Mu, Y. Xu, C. Song and Y. Liu, Int. J. Energy Res., 2022, 46, 8949–8961 CrossRef CAS.
- J. Xue, H. Gao, X. Wang, K. Qian, Y. Yang, T. He, C. He, Y. Lu and S. Yu, Angew. Chem., Int. Ed., 2019, 131, 14290–14294 CrossRef.
- M. Zhang, F. Xu, W. Liu, Y. Hou, L. Su, X. Zhang, R. Zhang, L. Zhou, X. Yan, M. Wang, X. Hou and Y. Cao, Nano Res., 2022 DOI:10.1007/s12274-021-4041-4.
- P. He, H. Bai, Z. Fan, L. Hao, N. Liu, B. Chen, R. Niu and J. Gong, J. Mater. Chem. A, 2022, 13378–13392 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2mh01151d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |