Radical-mediated remote migration of quinoxalinones†
Chenyang
Chang‡
a,
Qi
Zhang‡
a,
Xinxin
Wu
 a and
Chen
Zhu
a and
Chen
Zhu
 *ab
*ab
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu 215123, People's Republic of China. E-mail: chzhu@suda.edu.cn
bFrontiers Science Center for Transformative Molecules and Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, People's Republic of China. E-mail: chzhu@sjtu.edu.cn
First published on 20th March 2023
Abstract
Disclosed herein is the first example of radical-mediated remote migration of quinoxalinones. The quinoxalinonyl-functionalization of alkenes employs the quinoxalinone-substituted tertiary bishomoallylic alcohols as substrates, proceeds through intramolecular 1,4-quinoxalinone migration, and gives rise to complex γ-quinoxalinone-substituted aliphatic ketones. A set of external radicals is compatible with this method. The protocol features broad tolerance of functional groups, good adaptability to various external radicals and high product diversity, and opens a new door for the synthesis of quinoxalinone derivatives.
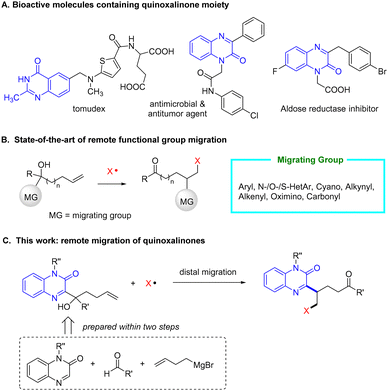
Nitrogen-containing heteroarenes are extensively present in natural products and drug molecules,1 among which quinoxalinone often serves as the pharmacophore of bioactive compounds. For instance, they are widely exploited in anti-tumour and anti-bacteria agents, HIV-1 reverse transcriptase inhibitors, anticoagulants, and hypoglycaemic agents (Scheme 1A).2 In view of broad prospects in application, the preparation of quinoxalinone derivatives has attracted great attention over the past few years.3 The most common approaches rely on the Minisci reaction by adding various alkyl radicals to the parent quinoxalinone.4 Alkenes are easily available and can act as the precursor of alkyl radicals that engage in the Minisci reaction.5 Nevertheless, this approach is generally limited to activated alkenes, in particular styrene derivatives which only generate benzyl radicals in the reaction.
 | ||
| Scheme 1 Importance of quinoxalinone derivatives and the preparation via remote migration of quinoxalinone. | ||
Remote functional group migration (FGM) provides an efficient strategy for the transformations of unactivated alkenes.6 We and others have comprehensively investigated the FGM reactions, where a portfolio of functional groups including aryls,7 heteroaryls,8 cyano,9 alkynyl,10 alkenyl,11 oximino,12 and carbonyl,12a,13 showcase excellent migratory aptitudes (Scheme 1B). We conceive to study the migration of quinoxalinones and apply it to the construction of complex quinoxalinone derivatives. While the migration of several azaarenes, such as (benzo)thiazole, (benz)oxazole, (benz)imidazole, pyridine, quinoline, and pyrimidine, has been well established, the migrating behavior of quinoxalinone remains unaddressed.
Herein, we provide proof-of-principle studies for radical-mediated remote migration of quinoxalinones (Scheme 1C). The transformation employs strategically designed quinoxalinone-substituted tertiary bishomoallylic alcohols as substrates, which are conveniently accessed in two steps, the Minisci-type reaction of adding a carbonyl radical to quinoxalinone followed by the nucleophilic addition of Grignard reagent to the resulting ketone.14 The reaction of the tertiary alcohols with various external radicals readily proceeds, leading to complex γ-quinoxalinone-substituted aliphatic ketones, otherwise difficult to synthesize, through radical difunctionalization of unactivated alkenes. Remarkably, this is the first example of radical-mediated quinoxalinone migration.
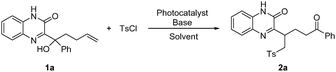
At the outset, a reaction parameters survey was implemented with the reaction of quinoxalinone-substituted tertiary alcohol 1a and tosyl chloride (Table 1). The reaction proceeded with the use of fac-Ir(ppy)3 as a photosensitizer, Na2CO3 as a base, and acetonitrile as a solvent under blue-light irradiation, leading to the desired product 2a. Then various solvents were investigated (entries 1–6). Among those commonly used solvents, acetonitrile delivered the best yield. A set of bases was subsequently examined, in which no one delivered a better yield than Na2CO3 (entries 7–11). Photosensitizers were also briefly screened, showing that others were not as effective as the original one (entries 12–16). Finally, light intensity was evaluated and the yield was further improved to 74% under the irradiation of 18 W blue light (entry 18). Control experiments indicated that the reaction could not occur in the absence of either light or photosensitizer (entries 19 and 20).
| Entry | Base | Photocatalyst | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), TsCl (0.3 mmol, 1.5 equiv.), base (0.2 mmol, 1.0 equiv.) and photocatalyst (3 mol %) in solvent (2.0 mL) were irradiated with 30 W blue LEDs at rt under N2. b Isolated yield. c 5 W blue LEDs. d 18 W blue LEDs (460 nm). e No photocatalyst. f In the dark. | ||||
| 1 | Na2CO3 | fac-Ir(ppy)3 | CH3CN | 70 |
| 2 | Na2CO3 | fac-Ir(ppy)3 | EtOAc | 30 |
| 3 | Na2CO3 | fac-Ir(ppy)3 | DCM | 18 |
| 4 | Na2CO3 | fac-Ir(ppy)3 | THF | 26 |
| 5 | Na2CO3 | fac-Ir(ppy)3 | DMF | 15 |
| 6 | Na2CO3 | fac-Ir(ppy)3 | Acetone | 22 |
| 7 | K2CO3 | fac-Ir(ppy)3 | CH3CN | 43 |
| 8 | K2HPO4 | fac-Ir(ppy)3 | CH3CN | 45 |
| 9 | NaHCO3 | fac-Ir(ppy)3 | CH3CN | 55 |
| 10 | KHCO3 | fac-Ir(ppy)3 | CH3CN | 64 |
| 11 | NaOAc | fac-Ir(ppy)3 | CH3CN | 51 |
| 12 | Na2CO3 | [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 | CH3CN | 31 |
| 13 | Na2CO3 | Ir(ppy)2(dtbbpy)PF6 | CH3CN | 38 |
| 14 | Na2CO3 | Ru(bpy)3Cl2·6H2O | CH3CN | 28 |
| 15 | Na2CO3 | 4CzIPN | CH3CN | 61 |
| 16 | Na2CO3 | Eosin Y | CH3CN | 24 |
| 17c | Na2CO3 | fac-Ir(ppy)3 | CH3CN | 65 |
| 18d | Na2CO3 | fac-Ir(ppy)3 | CH3CN | 74 |
| 19e | Na2CO3 | — | CH3CN | N.R. |
| 20f | Na2CO3 | fac-Ir(ppy)3 | CH3CN | N.D. |
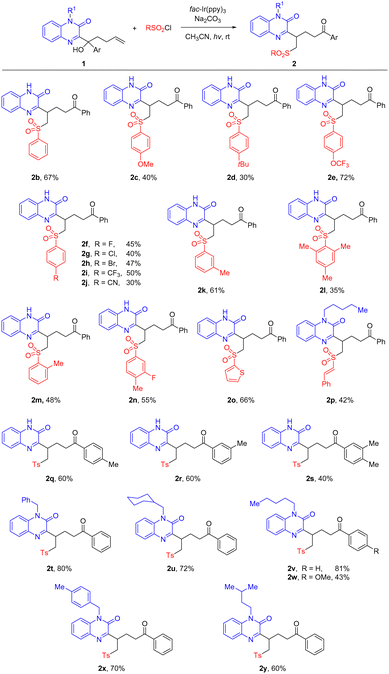
With the optimized reaction conditions in hand, we turned our attention to defining the scope of sulfonyl radicals and tertiary alcohol substrates (Scheme 2), in order to assess the compatibility of functional groups. Firstly, the substituent on the aryl sulfonyl chlorides was investigated. The substrates bearing either electron-rich or deficient groups were apt to afford the corresponding products (2b–2k). Many common groups such as halides and cyano were tolerated in the reaction. Steric hindrance caused a decline in yield, as shown in the examples bearing an ortho-substituent (2l and 2m). Heteroaryls such as thienyl sulfonyl radicals and vinyl sulfonyl radicals were amenable to the reaction, giving rise to the corresponding products 2o and 2p in useful yields. The substituent of tertiary alcohols 1 could also be varied. Adding extra functional groups on the aryl, such as methyl, did not interfere with the reaction (2q–2s). In addition to the parent quinoxalinone, the migration of functionalized quinoxalinones with protecting groups was investigated. The quinoxalinone bearing either a benzyl or alkyl protecting group readily migrated in the reaction, leading to the corresponding products within appreciably improved yields (2t–2y).
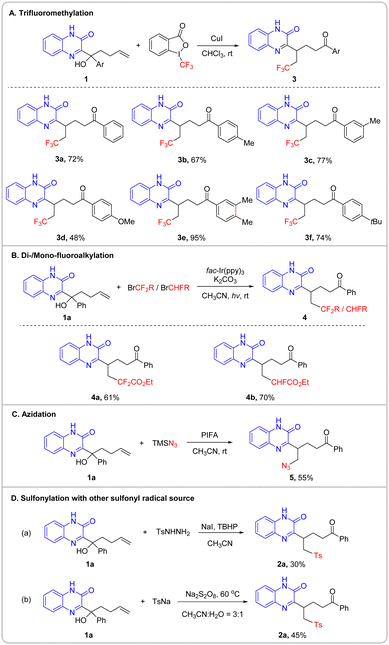
Other types of external radicals instead of sulfonyl radicals that triggered the migration reaction were explored, to assess the generality of the protocol (Scheme 3). Trifluoromethyl radicals derived from Togni's reagent in the presence of catalytic CuI readily added to the substrates, leading to the trifluoromethylation products in modest to high yields (Schemes 3A, 3a–3f). Likewise, changing the substitution of tertiary alcohols 1 did not impede the desired transformations. The reaction of 1a with difluoroalkyl and monofluoroalkyl radicals generated under photochemical conditions smoothly proceeded to give the corresponding products in good yields (Schemes 3B, 4a and 4b). Moreover, the reaction of 1a with azido radicals furnished the azidation product in a useful yield (Scheme 3C, 5). Other sulfonyl radical precursors such as TsNHNH2 and TsNa were also suitable for the radical sulfonylation, giving rise to product 2a, respectively (Scheme 3D).
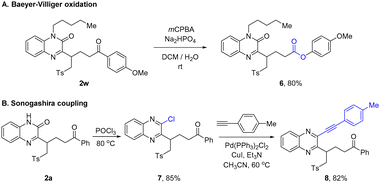
The utility of this method was further manifested by converting the product to other valuable molecules. For instance, ketone product 2w could be transformed into ester 6 by Baeyer–Villiger oxidation (Scheme 4A). The treatment of quinoxalinone with POCl3 resulted in 2-chloroquinoxaline 7, which was subject to Sonogashira coupling to afford the complex alkyne product 8 (Scheme 4B).
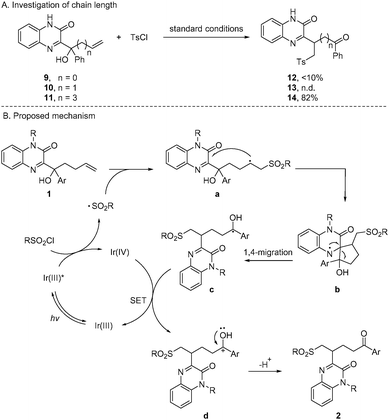
The chain length of tertiary alcohols was investigated to probe the preferable transition state of the migrating step (Scheme 5A). While the reaction of 9 (n = 0) and 10 (n = 1) did not provide satisfactory outcomes, the conversion of 11 (n = 3) to product 14 delivered a high yield similar to that of 1a. These results suggested that 1,4- or 1,5-migration of quinoxalinones readily proceeded via a kinetically favored five- or six-membered transition state.
On the basis of the experimental results, a plausible mechanism is depicted in Scheme 5B. Firstly, the transition of Ir(III) species to the excited state is enabled by visible-light irradiation, and generates a sulfonyl radical from sulfonyl chloride and Ir(IV) species via SET. Adding the sulfonyl radical to 1 gives rise to alkyl radical a, which undergoes intramolecular cyclization of quinoxalinone to generate a spiro intermediate b. The subsequent N-centered radical promoted ring opening accomplishes the 1,4-migration of quinoxalinone, and results in ketyl radical d. Single-electron oxidation of c to cation d by Ir(IV) species followed by deprotonation furnishes final product 2, and regenerates Ir(III) species to perpetuate the photoredox catalytic cycle.
In summary, we have disclosed the radical-mediated remote migration of quinoxalinones for the first time. It presents a new supplement to the precedent examples of functional group migration. The reaction proceeds through intramolecular 1,4-quinoxalinone migration, leading to a variety of complex γ-quinoxalinone-substituted aliphatic ketones in synthetically useful yields. The protocol features wide tolerance of functional groups, good adaptability to external radicals and high product diversity, and opens a new door for the synthesis of quinoxalinone derivatives.
We are grateful for the financial support from the National Natural Science Foundation of China (Grant no. 22171201, 22001185, 21971173), the Natural Science Foundation of Jiangsu Province (BK20200852), the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201905), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of interest
There are no conflicts to declare.Notes and references
- For selected reviews: (a) J. P. Michael, Nat. Prod. Rep., 1999, 16, 675 RSC; (b) A. A. Kalinin and V. A. Mamedov, Chem. Heterocycl. Compd., 2011, 46, 1423 CrossRef CAS; (c) L. Zhang, X. Peng, G. L. V. Damu, R. Geng and C.-H. Zhou, Med. Res. Rev., 2014, 34, 340 CrossRef CAS PubMed; (d) M. D. Matveeva, R. Purgatorio, L. G. Voskressensky and C. D. Altomare, Future Med. Chem., 2019, 11, 2735 CrossRef CAS PubMed.
- For selected reviews: (a) A. Carta, S. Piras, G. Loriga and G. Paglietti, Mini-Rev. Med. Chem., 2006, 6, 1179 CrossRef CAS PubMed; (b) X. Li, K.-H. Yang, W.-L. Li and W.-F. Xu, Drugs Future, 2006, 31, 979 CrossRef CAS; (c) M. Demeunynck and I. Baussanne, Curr. Med. Chem., 2013, 20, 794 CAS; (d) L. Shi, W. Hu, J. Wu, H. Zhou, H. Zhou and X. Li, Mini-Rev. Med. Chem., 2018, 18, 392 CrossRef CAS PubMed.
- For selected reviews: (a) X. Jiang, K. Wu, R. Bai, P. Zhang and Y. Zhang, Eur. J. Med. Chem., 2022, 229, 114085 CrossRef CAS PubMed; (b) V. Mamedov, RSC Adv., 2016, 6, 42132 RSC; (c) Q. Ke, G. Yan, J. Yu and X. Wu, Org. Biomol. Chem., 2019, 17, 5863 RSC; (d) J. Berenguer, G. Blay, J. Pedro and C. Vila, Eur. J. Org. Chem., 2020, 6148 CrossRef.
- For selected examples: (a) Y. Gao, Z. Wu, L. Yu, Y. Wang and Y. Pan, Angew. Chem., Int. Ed., 2020, 59, 10859 CrossRef CAS PubMed; (b) J. Yang, S. Zhu, F. Wang, F. Qing and L. Chu, Angew. Chem., Int. Ed., 2021, 60, 4300 CrossRef CAS PubMed; (c) P. Ghosh, N. Kown, S. Kim, S. Han, S. Lee, W. An, N. Mishra, S. Han and I. Kim, Angew. Chem., Int. Ed., 2021, 60, 191 CrossRef CAS PubMed; (d) J. Yu, X. Zhang, X. Wu, T. Liu, Z.-Q. Zhang, J. Wu and C. Zhu, Chem, 2023, 9, 472 CrossRef CAS.
- For selected examples: (a) X. Li, J. Grokopf, C. Jandl and T. Bach, Angew. Chem., Int. Ed., 2021, 60, 2684 CrossRef CAS PubMed; (b) M. Bhuyan, S. Sharma and G. Baishya, Org. Biomol. Chem., 2022, 20, 1462 RSC; (c) D. Zheng and A. Studer, Org. Lett., 2019, 21, 325 CrossRef CAS PubMed.
- For selected reviews on FGM, see: (a) X. Wu, S. Wu and C. Zhu, Tetrahedron Lett., 2018, 59, 1328 CrossRef CAS; (b) X. Wu and C. Zhu, Acc. Chem. Res., 2020, 53, 1620 CrossRef CAS PubMed; (c) X. Wu, Z. Ma, T. Feng and C. Zhu, Chem. Soc. Rev., 2021, 50, 11577 RSC; (d) Y. Wei, X. Wu and C. Zhu, Synlett, 2022, 1017 CAS.
- For selected examples: (a) D. Wu, M. Christian, C. Daniliuc and A. Studer, J. Am. Chem. Soc., 2021, 143, 9320 CrossRef PubMed; (b) T. Zhou, F. Luo, M. Yang and Z. Shi, J. Am. Chem. Soc., 2015, 137, 14586 CrossRef CAS PubMed; (c) W. Kong, M. Casimiro, E. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480 CrossRef CAS PubMed; (d) N. Fuentes, W. Kong, F.-S. Luis, E. Merino and C. Nevado, J. Am. Chem. Soc., 2015, 137, 964 CrossRef CAS PubMed; (e) X. Liu, F. Xiong, X. Huang, P. Li and X. Wu, Angew. Chem., Int. Ed., 2013, 52, 6962 CrossRef CAS PubMed; (f) P. Gao, Y. Shen, R. Fang, X. Hao, Z. Qiu, F. Yang, X. Yan, Q. Wang, X. Gong, X. Liu and Y. Liang, Angew. Chem., Int. Ed., 2014, 53, 7629 CrossRef CAS PubMed; (g) Z. Li, M. Wang and Z. Shi, Angew. Chem., Int. Ed., 2021, 60, 186 CrossRef CAS PubMed.
- For selected examples: (a) Z. Wu, D. Wang, Y. Liu, L. Huan and C. Zhu, J. Am. Chem. Soc., 2017, 139, 1388 CrossRef CAS PubMed; (b) H. Zhang, L. Kuo, D. Chen, M. Ji, X. Bao, X. Wu and C. Zhu, Org. Lett., 2020, 22, 5947 CrossRef CAS PubMed; (c) J. Yu, Z. Wu and C. Zhu, Angew. Chem., Int. Ed., 2018, 57, 17156 CrossRef CAS PubMed; (d) J. Liu, S. Wu, J. Yu, C. Lu, Z. Wu, X. Wu, X.-S. Xue and C. Zhu, Angew. Chem., Int. Ed., 2020, 59, 8195 CrossRef CAS PubMed; (e) J. Jeon, Y.-T. He, S. Shin and S. Hong, Angew. Chem., Int. Ed., 2020, 59, 281 CrossRef CAS PubMed; (f) M. Ji, X. Wang, J. Liu, X. Wu and C. Zhu, Sci. China: Chem., 2021, 64, 1703 CrossRef CAS; (g) M. Ji, C. Chang, X. Wu and C. Zhu, Chem. Commun., 2021, 57, 9240 RSC.
- For selected examples: (a) Z. Wu, R. Ren and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 10821 CrossRef CAS PubMed; (b) R. Ren, L. Huan and C. Zhu, Adv. Synth. Catal., 2017, 359, 3052 CrossRef CAS; (c) M. Ji, Z. Wu, J. Yu, X. Wan and C. Zhu, Adv. Synth. Catal., 2017, 359, 1959 CrossRef CAS; (d) M. Ji, Z. Wu and C. Zhu, Chem. Commun., 2019, 55, 2368 RSC; (e) C. Chang, H. Zhang, X. Wu and C. Zhu, Chem. Commun., 2022, 58, 1005 RSC.
- For selected examples: (a) Y. Xu, Z. Wu, J. Jiang, Z. Ke and C. Zhu, Angew. Chem., Int. Ed., 2017, 56, 4545 CrossRef CAS PubMed; (b) X. Tang and A. Studer, Chem. Sci., 2017, 8, 6888 RSC; (c) J. Liu, W. Li, J. Xie and C. Zhu, Org. Chem. Front., 2018, 5, 797 RSC; (d) M. Wang, H. Zhang, J. Liu, X. Wu and C. Zhu, Angew. Chem., Int. Ed., 2019, 58, 17646 CrossRef CAS PubMed.
- For selected examples: (a) X. Tang and A. Studer, Angew. Chem., Int. Ed., 2018, 57, 814 CrossRef CAS PubMed; (b) A. Hossay, M. Josep, M. Mostafa, J. Derek, R. Robin, F. Natalie and C. Jonathan, Angew. Chem., Int. Ed., 2019, 58, 2418 CrossRef PubMed; (c) J. Yu, H. Zhang, X. Wu and C. Zhu, CCS Chem., 2021, 3, 1426 Search PubMed; (d) Y. Wei, H. Zhang, X. Wu and C. Zhu, Angew. Chem., Int. Ed., 2021, 60, 20215 CrossRef CAS PubMed.
- For selected examples: (a) J. Yu, D. Wang, Y. Xu, Z. Wu and C. Zhu, Adv. Synth. Catal., 2018, 360, 744 CrossRef CAS; (b) N. Wang, J. Wang, Y. Guo, L. Li, Y. Sun, Z. Li, H. Zhang, Z. Guo, Z. Li and X. Liu, Chem. Commun., 2018, 54, 8885 RSC.
- For selected examples: (a) Z.-L. Li, X.-H. Li, N. Wang, N.-Y. Yang and X.-Y. Liu, Angew. Chem., Int. Ed., 2016, 55, 15100 CrossRef CAS PubMed; (b) C. Morrill, A. Peter, I. Amalina, E. Pye, G. Crisenza, N. Kaltsoyannis and D. Procter, J. Am. Chem. Soc., 2022, 144, 13946 CrossRef CAS PubMed; (c) W. Yao, G. Zhao, Y. Wu, L. Zhou, U. Mukherjee, P. Liu and M. Ngai, J. Am. Chem. Soc., 2022, 144, 3353 CrossRef CAS PubMed; (d) G. Zhao, W. Yao, I. Kevlishvili, J. Mauro, P. Liu and M. Ngai, J. Am. Chem. Soc., 2021, 143, 8590 CrossRef CAS PubMed.
- (a) X. Zeng, C. Liu, X. Wang, J. Zhang, X. Wang and Y. Hu, Org. Biomol. Chem., 2017, 15, 8929 RSC; (b) J.-W. Yuan, J.-H. Fu, S.-N. Liu, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Biomol. Chem., 2018, 16, 3203 RSC; (c) J. Lu, X.-K. He, X. Cheng, A.-J. Zhang, G.-Y. Xu and J. Xuan, Adv. Synth. Catal., 2020, 362, 2178 CrossRef; (d) L.-Y. Xie, Y.-S. Bai, X.-Q. Xu, X. Peng, H.-S. Tang, Y. Huang, Y.-W. Lin, Z. Cao and W.-M. He, Green Chem., 2020, 22, 1720 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06887g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |