Advancements in the preparation methods of artificial cell membranes with lipids
Wei
Yuan
ab,
Jiafang
Piao
ab and
Yuanchen
Dong
 *ab
*ab
aCAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: dongyc@iccas.ac.cn
bBeijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 10th May 2021
Abstract
In order to better understand the structure and function of biological cell membranes, various artificial systems have been developed. Among them, unilamellar lipid vesicle (liposome)-based artificial cell membranes have attracted great interest due to their inherently similar structure and compositions to cellular membranes. Diverse methodologies, including film hydration methods, emulsion templated technologies and FGA strategies, have been well established for simulating the cell membrane structure. In this review, advancements in the preparation methods of unilamellar liposomes will be summarized, and the applications, advantages and challenges of each method will also be discussed.
1. Introduction
A cell membrane is primarily composed of a phospholipid bilayer membrane, which is an important component of cells to separate the intracellular and extracellular environments. While it is necessary to protect the metabolic activities inside the cell, the cell membrane provides support for various functional membrane proteins, which facilitate the cellular activities, such as the transport of molecules,1 energy exchange,2 and signal transduction.3 Therefore, it is of great significance to investigate the functions of the cellular membrane and its molecular mechanism in order to understand the fundamental biological phenomena. However,the living cells are too complex to be studied directly, so artificial cell systems have been widely developed and utilized to simplify the relevant investigation due to their similar physicochemical properties to natural cell membranes.4–6Artificial cells are usually defined as ordered structures enclosed by a membrane, which could integrate complex biological reactions in living cells.7 Nowadays, several typically artificial membrane systems, including polymersomes,8 proteinosomes,9 colloidosomes,10 as well as liposomes,11 have been developed. Polymersomes are vesicles assembled from block copolymers, which could expand the properties of membranes. Proteinosomes are usually formed by chemically cross-linked protein–polymer nanoconjugates, providing an alternative method for membrane activation. Colloidosomes are microcapsules whose shells are composed of colloidal particles, and are widely applied in microencapsulation, such as the controlled release of cargoes. Liposomes are vesicles composed of lipids, which are most frequently used as a frame for artificial cells. Owing to their compositions and structures similar to native cell membranes, liposomes have attracted increasing attention and been widely investigated in biology-related fields.12
To prepare liposomes with similar properties to natural membrane systems, constructing liposomes with customized size and composition and a unilamellar structure is the essential target. During the last few decades, many methods have been developed, e.g., film-based hydration methodologies and emulsion templated methods. Taking advantage of different technologies, unilamellar liposomes could be prepared with sizes ranging from nanometers to hundreds of micrometers, allowing the construction of artificial systems to mimic the organelle or cell structures. Here, in this review, advancements in the preparation methods of liposomes will be summarized.
2. Cell membrane structure
Cell is the fundamental structural unit of biological systems, which perform the basic functions including autopoiesis, metabolism, genetics, and interactions with the environment. Cell membrane is the boundary of the cell, which plays important roles in protecting metabolic activities inside the cell.13 Since the cell structures were discovered, the composition and structure of the natural membrane system have been widely investigated and revealed during the last few decades.14,15 The phospholipid bilayer is the basic structural support of the cell membrane, in which the phospholipids arrange themselves with their polar heads to the outside, and their hydrophobic tails inside.16 Many proteins are integrated into the lipid bilayer or simply associated with the membrane. In particular, the membranes of cells are distributed in an asymmetric manner with different phospholipids, proteins, or carbohydrates on each leaflet.17 This asymmetry serves certain functions, such as the formation of a procoagulant interface upon cell disruption to trigger recognition events, the regulation of membrane budding, or the structural stability.18 The importance of the cell membrane has been widely demonstrated and it is of great significance to study the formation and functions of cell membranes. However, it is immensely complex to directly study biological membranes with a lot of unexpected or unknown influences. Therefore, there is an urgent need for simplified and controllable models to understand these fundamental biological functions.3. Methods for the preparation of unilamellar liposomes
Lipid vesicles (liposomes) have been developed for studying and emulating biological membranes.4,19 While the composition of artificial liposomes is similar to or the same as those of natural membranes, the size and structure of vesicles are also comparable to those of native cell membranes. Furthermore, asymmetric lipid membranes can also be constructed using special technologies, which further facilitate the investigation of the structure and function of natural cell membranes.Since the gentle hydration strategy was proposed by Bangham et al.,20 many strategies have been developed to construct liposome-based bio-simulated systems with similar properties to natural membrane systems. Here, we will summarize the recent progress of some strategies to prepare customized liposomes with controlled features (e.g., desired size, low polydispersity, tailored shapes, high yield production). Since the investigation of liposomes is such a broad field, it is impossible to cover all the technologies and here we will focus on the development of film hydration methods, emulsion templated strategies and frame-guided assembly.
3.1 Film hydration methodologies
Film hydration-based strategies are the earliest and most classical technologies for liposome fabrication.20,21 It should be noted that film hydration-based strategies can not only be employed to construct liposome structures, but can also be widely applied for other amphiphiles or block polymers.12,22 In the film hydration-based strategy, lipids or amphiphiles need to be dissolved in a volatile organic solvent (e.g., chloroform), which will be then removed by evaporation under a flow of argon or nitrogen, as shown in Fig. 1. It has been demonstrated that, during the evaporation step, phospholipid molecules will self-assemble into a stack of bilayers on a solid substrate.23 With the addition of an aqueous solution, the multi-lamellar membranes could be ruptured and separated from each other, which can be accelerated by extra forces, freezing, osmotic pressure, electric field, etc.24 Therefore, subsequent swelling would result in the re-fusion of the separated lipid lamellas, finally forming unilamellar vesicle structures. According to the swelling process, the film hydration-based strategy can be divided into (1) gentle hydration, (2) electroformation and (3) gel-assisted swelling. In this section, we will introduce these strategies and their recent progress below.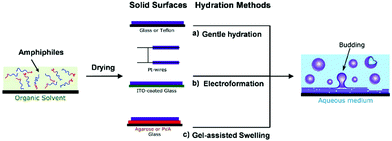 | ||
| Fig. 1 Schematic diagrams of the film hydration methods including (a) gentle hydration, (b) electroformation, and (c) gel-assisted hydration.12 © 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
It has been demonstrated that, during the swelling process, the electrostatic repulsion and steric effects of lipid head groups play an important role in the separation of lamellae, so it is difficult to prepare liposomes from electrostatically neutral lipids with a simply gentle hydration strategy.12 Furthermore, electrostatic screening under high ionic strength could also hinder repulsion, which causes strong influences of salt concentration on the preparation of liposomes.26 In order to address these problems, Yoshimura et al.27 presented a method to enhance the formation of oligolamellar liposomes by doping the lipids with sugar (glucose, mannose, and fructose) (Fig. 2A). Upon hydration, water would permeate through lipid lamellae to dissolve the sugar and the difference in osmotic pressure will gradually increase from the film to the glass surface. Consequently, the osmotic pressure could increase the interlamellar distance and improve the formation of unilamellar liposomes. Besides doping with sugar, other kinds of molecules, such as polymers,28 proteins29 and DNA,30 could also be employed to tune the osmotic pressure for enhanced liposome formation. Later, a similar technology was further developed to construct PEGylated liposomes, where the large molecular volume of PEG could increase the steric effects of the headgroup (Fig. 2B).31 It is also found that the simultaneous usage of PEGylated lipids and sugar could facilitate the formation of unilamellar liposomes synergistically even at low temperatures or physiological concentrations of metals. Therefore, unstable bio-molecules, e.g., transcription of RNA, could also be successfully accomplished within the GUVs by carrying out the gentle swelling process at low temperatures.
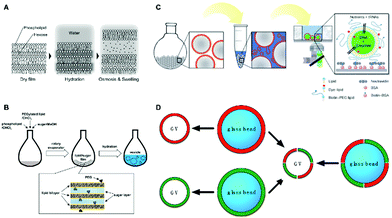 | ||
| Fig. 2 Gentle hydration-based methods for generating unilamellar liposomes. (A) Swelling of lipid bilayer membranes assisted by sugar.27 Copyright © 2009, American Chemical Society. (B) PEGylated lipid-and-sugar-doped gentle hydration.31 Copyright © 2015 The Authors. Published by Elsevier B.V. (C) Swelling of the lipid film on the glass beads.33 Copyright © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (D) Formation of hybrid lipid compositions with giant vesicles (GVs).34 Copyright © 2018, MDPI. | ||
In addition to the typically used planar solid supports, sphere glass bead substrates possessing a larger surface area can also be utilized to improve the formation of liposomes. It is found that the increased surface area of the substrates exhibits a higher yield of unilamellar liposomes, and, taking this into account, glass beads have become attractive to be used as substrates for liposome preparation.32 In 2012, a liposome encapsulating a minimal gene expression system was successfully created by using the porous bead matrix-assisted gentle hydration method, as shown in Fig. 2C.33 Assisted by a freeze–thaw procedure, a higher population of GUVs and more efficient encapsulation of biomolecules were achieved. While liposomes with hybrid lipid compositions could be produced from the beads when a hybrid lipid film is deposited, it could also be prepared by combining two sets of beads coated with different lipids with shaking under hydration (Fig. 2D).34
Overall, gentle hydration is a common and straightforward way for unilamellar liposome preparation. Owing to its facile procedure, a large amount of unilamellar lipid vesicles can be produced without specific equipment. However, there are also some shortcomings to be solved: the preparation is always time-consuming and the yield under physiological ion conditions is usually low; it is inevitable to produce both single- and multi-layer liposomes at the same time with high polydispersity. Furthermore, the encapsulation efficiency of the biomolecules (e.g., proteins and nucleic acids) is relatively poor.26
The electroformation technology was invented by Angelova and Dimitrov, and, in the first study, a direct current (DC) electric field was applied to generate lipid vesicles.37 As the DC electric field could result in bubbling due to water electrolysis, later, an alternating current (AC) electric field was introduced in the swelling process, which has become a common strategy.38 Compared with the gentle hydration methods, it is observed that the electroformation method could produce a higher population of unilamellar vesicles without significant defects. This could be explained by the fact that the electric field can generate mechanical stress (visualized as gentle agitation of the lipid layers by the alternate low-frequency field) to rupture the lipid bilayers and the formation of pieces, which could further bend and form unilamellar liposomes.
In the early electroformation method, the unilamellar liposomes are usually prepared under low ionic strength conditions because the swelling process will be hindered by the screening of the electric field induced by high ionic strength.39 In order to construct liposomes under high ionic strength or physiological conditions, different strategies have been developed. Han et al.36 applied a high-frequency AC field on the plasma-cleaned ITO electrode to generate giant liposomes (Fig. 3A). Under physiological conditions, it is possible for the high-concentration counterions to form an electric double layer near the electrode surface, which can hinder the formation of liposomes. However, the high-frequency AC could disrupt the electric double layer and induce abundant vesicles formed on the ITO electrode. Besides, the hydrophilic surface after plasma cleaning may also facilitate the hydration of the solid lipid film and the formation of lipid bilayers that subsequently bend and form vesicles. Therefore, the giant vesicles from various compositions of charged lipids or lipid mixtures could be electroformed under physiological conditions and even higher concentrations of NaCl. More interestingly, it was found that both the NaCl concentration and the AC field were associated with the diameter and formation of liposomes, respectively. Moreover, unilamellar liposomes with a high yield could also be generated under biorelevant conditions (e.g., PBS buffer and cell culture medium), which provides a promising way to prepare GUVs on a large scale under physiologically relevant conditions. Unilamellar liposomes could also be generated by using a specific sequence of increasing AC field in phosphate-buffered saline.40 Using a similar strategy, Bassereau and co-workers successfully reconstituted functional voltage-gated ion channels into cell-sized giant unilamellar vesicles (GUVs) by using buffers containing low (5 mM) or near-physiological salt concentrations (100 mM KCl, 4 mM HEPES (pH 7.2), 200 mM glucose).41 With lots of effort, to date, liposomes can be generated using various buffers with the development of the electroformation method.12,36,42
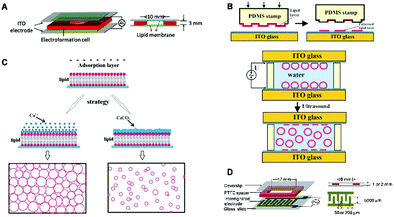 | ||
| Fig. 3 Electroformation-based methods for unilamellar liposome preparation. (A) Electroformation on ITO electrodes.36 © 2016 Elsevier B.V. All Rights Reserved. (B) Micro-contact printing technology involved an electroformation system for fabricating monodisperse liposomes.44 Reproduced from ref. 44 with permission from The Royal Society of Chemistry. (C) Ca-mediated electroformation platforms for liposome generation.46 Copyright © 2015, The Author(s). (D) The interdigitated ITO electrode-based electroformation platform for producing liposomes.47 Reproduced from ref. 47 with permission from The Royal Society of Chemistry. | ||
Although it is believed that the applied AC field can cause fluctuations in the bilayers facilitating the detachment of lamellae, the fusion process includes quick growth of vesicles, and thus the size of the resultant GUV population is often heterogeneous.43 To address this problem, in 2003, the Paunov group44 developed a technique for the generation of monodisperse giant liposomes based on a combination of micro-patterning of ITO glass slides with lipid solution and electroformation, as shown in Fig. 3B. Since the micro-patterned lipid films provide a sufficient distance between the lipid patches, the coalescence of neighbouring liposomes is prevented, which is an essential process affecting the final size and distribution of the liposomes. Hence, in this way, liposomes with narrow distribution could be generated. More importantly, the average size of the produced liposomes could be tuned by designing the features of the micro-pattern. Similarly, Misawa and colleagues45 utilized patterned supported lipid bilayers (SLBs) to prepare solvent-free giant vesicles (GVs). The SLBs were first formed on a patterned SiO2 surface through vesicle fusion, then the electroformation was carried out to obtain the liposomes. Through this method, the produced GVs possessed a mean size of 8.53 μm. For the same purpose, the Yang group46 demonstrated a new strategy by applying calcium ions (Ca2+) or a calcium carbonate (CaCO3) mineral layer on the dry film of lipids during the electroformation process. Under an AC field, the homogeneous Ca2+ adsorption layer was transformed into a patterned Ca2+ or CaCO3 “barrier”, and therefore liposomes could be generated only from the interspaces among the formed “barrier” when the electroosmotic flow passes over the lipid film surface. The “barrier” subsequently confined the growth and fusion of the GUVs, leading to the formation of high-quality and narrow-dispersed GUVs (Fig. 3C). From these results, it could be found that the film pattern would be useful for generating more uniform liposomes under electroformation.
In addition to conventional electrodes (e.g., ITO and Pt plates), new types of electrodes have also been developed to obtain unilamellar lipid vesicles. In 2013, the Han group47 developed a coplanar interdigitated electrode as a novel platform for the preparation of unilamellar liposomes (Fig. 3D). The interdigitated electrode itself provided a patterned substrate for the uniform deposition of lipid films, which broke the limitations of the opposite electrode layout. It was found that larger unilamellar liposomes could be generated when interdigitated electrodes with smaller widths were used under the same conditions. The size of the GUVs decreased with an elevated frequency at a constant amplitude. At a constant frequency of 10 Hz, the size of the GUVs increased with an increase in amplitude from 1 to 5 V and then decreased from 5 to 10 V. Recently, another novel type of electrode has also been utilized to readily generate giant unilamellar liposomes. In this study, readily available syringe needles were selected as stainless-steel electrodes, which could produce relatively monodisperse vesicles without observed lipid oxidation or hydrolysis.48 Besides syringe needles, the electroformation on copper electrodes has also been employed for stable giant liposome preparation, which proved to be easy, inexpensive, and convenient.49
In general, the electroformation method is a simple and relatively fast way to obtain unilamellar and pure liposomes with a relatively high yield, which is summarized in Table 1. Furthermore, different parameters, such as voltage, frequency, and time, can be easily and precisely adapted to match the types of lipids or the ionic strength. However, the size of the unilamellar liposomes prepared through electroformation is always heterogeneous and the oxidation of the encapsulated biomolecules also hinders its application. Additionally, special equipment is required to obtain liposomes.
| Composition | Electrode | Frequency | Voltage | Time | Ionic strength | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| EggL, DMPC | Pt | — | <3 V | — | Distilled water | Low | 37 |
| EPC, Chol/PC, PE/PC | ITO | 10 Hz | 1 V | 2 h | Milli-Q water | Low | 39 |
| DOPC, POPC, DMPC, DPPC, DOPS or DOTAP | ITO | 1 kHz | 2.5 V | 2 h | 100 mM NaCl | High | 36 |
| egg-PC | ITO | 10 Hz | 0.1 V | 25 min | 1 mM CaCl2 | High | 46 |
| EggPC, CHS | ITO Pt | 500 Hz | 260–1670 V m−1 | 5 min-overnight | PBS (10 mM, pH 7.4) | High | 40 |
| (DPhPC, EPG, EPA) SUVs | Pt | 10 Hz | 0.7 V | About 2 h | 100 mM KCl, 4 mM HEPES (pH 7.2), 200 mM glucose | High | 41 |
| DMPC | ITO | 10 Hz | 0.5 V | 1–1.5 h | 0.1865 M glucose solution | High | 44 |
| DOPC/BODIPY-HPC | Au | 10 Hz | 10 V | 1 h | 100 mM KCl, 25 mM HEPES/NaOH pH 7.4 | High | 45 |
| Egg PC, NBD-PE, TR-DHPE, DOPC | ITO | 1 Hz to 104 Hz | 1–10 V | 1–60 min | 200 mM of sucrose solution | High | 47 |
| DOPC/Chol | Stainless steel | 10 Hz, then 5 Hz | 5 V | 2 h + 30 min | 200 mM sucrose in deionized (DI) water | High | 48 |
The gel-assisted swelling method was first described by the Mayer group50 and agarose gel was used for enhanced swelling to form giant liposomes at physiological ionic strength (Fig. 4A). Due to the efficient swelling, the separation of the hybrid film were promoted, rendering the facile formation of unilamellar liposomes. More importantly, this method was much faster compared with the gentle hydration and electroformation methods which did not require any specialized equipment or lipids. Furthermore, this gel-assisted swelling was gentle enough to encapsulate bio-molecules and proteins including the active cytoskeletal actin-myosin networks, which have been demonstrated to be encapsulated with high efficiency.50 Later, based on a similar concept, Marques et al.51 developed a poly(vinyl alcohol) (PVA)-based swelling method for preparing liposomes. With the assistance of the PVA network, a variety of lipids or lipid hybrids were assembled into GUVs, which could also encapsulate biomolecules (proteins) efficiently.
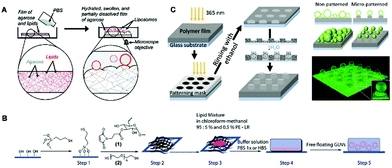 | ||
| Fig. 4 Various hydrogel-assisted swelling methods for the formation of unilamellar liposomes. (A) Liposome formation from hybrid films of agarose and lipids.50 Copyright © 2009, American Chemical Society. (B) Vesicle generation from dextran(ethylene glycol) hydrogels.52 Reproduced from Ref. 52 with permission from The Royal Society of Chemistry. (C) Fabrication of liposomes from the patterned PNIPAAm hydrogel.55 Copyright © 2019, American Chemical Society. | ||
Since the agarose and PVA hydrogel are cross-linked physically, these macro-molecules may be incorporated into the vesicles during hydration, which could change the physical properties of the membranes. To solve this problem, Kros et al.52 reported a chemically cross-linked dextran-poly(ethylene glycol) (Dex-PEG) hydrogel substrate to prepare vesicles with various compositions (Fig. 4B). In this study, it was observed that the size distribution of the liposomes could be controlled by tuning the degree of cross-linking within the gel network. Since then, the various types of chemically hydrogel substrates, including polyacrylamide (PAA),53 polydimethylsiloxane (PDMS),54 as well as poly(N-isopropylacrylamide) (PNIPAAm),55 have also been developed for preparing giant unilamellar vesicles.
It is found that the characteristics of the hydrogel substrate, including crosslinking density, thickness, surface chemistry, as well as morphology, affect the formation of unilamellar liposomes. In order to achieve a hydrogel substrate with controllable characteristics, the Malmstadt group56 reported an initiated chemical vapor deposition (iCVD) technique to generate porous charged polymer membranes with distinct morphologies and thicknesses, which could be further controlled to fabricate liposome low-polydispersity vesicles with higher yields. Later, an accessible method based on a patterned PNIPAAm hydrogel substrate for generating liposomes with controllable size was developed (Fig. 4C).55 For obtaining the patterned hydrogel membrane, a photolithography technology was used, which resulted in shallow circular holes with controllable diameters ordered in a precise square pattern. By performing the swelling process in these defined holes, unilamellar liposomes with narrow distribution were prepared within a few minutes.
To sum up, the gel-assisted method provides a fast, robust, and simple way to prepare liposomes with a high yield as well as controllable size. Importantly, it is suitable for the preparation of liposomes under physiological buffer conditions as well as with the incorporation of different types of charged lipids. However, the incorporation of hydrogels (e.g., PVA or agarose) into the membrane may affect the properties of the bilayer of the liposomes (e.g., interfacial tension and permeability), and there were also difficulties in detaching the vesicles from the surface of the hydrogel substrates. As a novel method, the gel-assisted method has shown great promising potential for the preparation of liposomes that can be further utilized to serve as an artificial cell model.
3.2 Emulsion templated methods for liposome construction
Although film hydration methods provide a simple way to prepare unilamellar liposomes, there are some limitations including the poor size control, low encapsulation efficiency of the biomolecules, as well as time consumption.Besides the simple film hydration methods, the emulsion templated technologies are also powerful tools to generate liposomes with the desired and controllable properties. In contrast to the hydration methods for generating liposomes through a process free of organic solvents, emulsion templated methods involve the use of organic solvents in the formation of liposomes. Because the emulsion involves both oil (organic) and aqueous phases, the methods can be generally classified into water-in-oil (W/O) emulsion templated and water-in-oil-in-water (W/O/W) double emulsion templated methods according to the number of involved phases. By controlling the properties of droplet emulsion, such as size, encapsulation, composition and symmetry, the desired liposomes possessing customized features can be achieved.
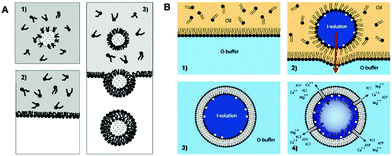 | ||
| Fig. 5 Water-in-oil (W/O) emulsion templated method for generating unilamellar symmetric liposomes. (A) Schematic representation of the synthesis of liposomes from the emulsion.57 Copyright © 2003, American Chemical Society. (B) Actin meshwork-containing symmetric liposome prepared through emulsion templated method.58 Copyright © 2009 Biophysical Society. Published by Elsevier Inc. All rights reserved. | ||
Pontani and co-workers58 successfully prepared symmetric unilamellar liposomes combined with actin, where the actin meshwork grew specifically from the inner monolayer of the liposome membrane (Fig. 5B). For this purpose, the first monolayer-stabilized droplets of I-solution containing actin were fabricated, followed by picking the second monolayer to form unilamellar vesicles surrounded by an O-buffer. The actin polymerization was accomplished by replacing the inner solution (I-solution) with a higher salt concentration solution (P-buffer). In a similar way, Elani et al.59 successfully prepared ternary and thermally-responsive GUVs (DOPC/DPPC/cholesterol) for the first time by using a mixed solution of lipids. This kind of liposome could be further used to study the phase behaviors, and is also beneficial for incorporating content release function into individual vesicles, which may find applications in smart synthetic biosystems. For improving the yield or efficiency of unilamellar liposome production, liposomes possessing high polydispersity were further purified by second centrifugation.60 So far, using the emulsion templated method, uniform liposomes with various compositions and encapsulation have been prepared.61–63
Since the inner and outer monolayers of liposomes are formed independently, Weitz et al.64 developed a symmetric method for constructing vesicles with different compositions in each leaflet, which is realized by creating chemically different lipid monolayers at the droplet and oil/water interface. Based on this method, Oiki et al.65 prepared asymmetric liposomes for protein insertion with controlled orientation. In this work, the KcsA potassium channels were added in the initial aqueous phase or the second aqueous solution, and they were successfully reconstituted with either an outside-out or inside-out orientation in the liposomes, which was due to the lipid-dependence of KcsA insertion (Fig. 6A). Similarly, the Lecommandoux group66 reported a hybrid polymer-lipid vesicle possessing an asymmetric membrane containing an inner polymer (PBut-b-PEO) monolayer and outer lipid (POPC) monolayer (Fig. 6B), which provided a way to prepare a cell-like model system. Furthermore, asymmetrical lipid vesicles have been recently developed as useful nanocarriers for biomolecular molecules, e.g., nucleic acids.67 Taking advantage of the emulsion templated method, liposomes with asymmetric membrane composition could be readily prepared.
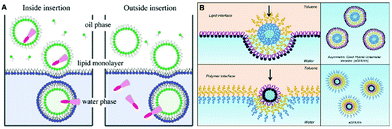 | ||
| Fig. 6 (A) Schematic illustration of the experimental protocol used to prepare liposomes containing KcsA in either orientation.65 Copyright © 2011, American Chemical Society. (B) Schematic representation of the generation of asymmetric giant hybrid unilamellar vesicles (aGHUV).66 © 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In order to obtain unilamellar liposomes with more controlled features, microfluidic devices have been combined with water-in-oil (W/O) droplet emulsions. In 2006, Lee et al.68 demonstrated a shear-focusing-based emulsion generation system for preparing unilamellar liposomes, as shown in Fig. 7A. In this work, the droplet emulsions containing the target encapsulated species were first produced in the microfluidic channel, followed by injection into an aqueous mixture of ethanol and water. As the oil (oleic acid) rapidly dissolved in ethanol, the organic solvent could be removed immediately, which facilitated the separation of the phospholipids from the liquid lipids, thus forcing the phospholipids to rearrange around the emulsion to obtain the vesicles. By using this method, various biological substances (e.g., cancer cells, yeast cells, nanosized proteins) could be efficiently encapsulated in the liposomes. Later, Paegel et al.69 developed a microfluidic assembly line for the stepwise synthesis of unilamellar liposomes (Fig. 7B). Droplet emulsions generated in a flow-focusing geometry flowed to a junction where a stable and reproducible flow-patterned interface was established from the oil and extracellular aqueous media. Continuous production of unilamellar liposomes was generated by a triangular post-mediated emulsion phase transfer process. In this way, unilamellar phospholipid vesicles with uniform and tunable size were realized, and the encapsulation efficiency of small-molecule cargoes was high (83%) compared to the bulk strategies such as gentle hydration and electroformation.
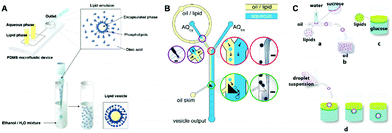 | ||
| Fig. 7 Unilamellar liposomes prepared from the emulsion templated method with the assistance of microfluidic devices. (A) A shear-focusing-based emulsion generation system for preparing unilamellar liposomes.68 Copyright © 2006, American Chemical Society. (B) Microfluidic assembly line for the stepwise synthesis of unilamellar liposomes.69 Copyright © 2011, American Chemical Society. (C) Microfluidic channel for preparing asymmetric giant unilamellar vesicles.70 Copyright © 2011, American Chemical Society. | ||
Microfluidic methods could also be applied to prepare asymmetric liposomes. In 2011, Malmstadt et al.70 reported a microfluidic channel for preparing compositionally asymmetric giant unilamellar vesicles (Fig. 7C). The droplet emulsions of one aqueous flow were generated in a channel containing an immiscible oil stream, and the first lipid monolayer of the emulsions was formed. When these monolayer-coated emulsions pass through the second oil–water interface, the second monolayer comprising another lipid was formed. Finally, vesicles with desired encapsulated contents and controlled composition of lipids were formed with centrifugation, which may find potential applications in the investigation of membrane biophysics. Later, a novel microfluidic platform with a channel meander was also developed to generate compositionally controlled unilamellar liposomes.71 The bending rigidity of these vesicles showed that asymmetry has a significant effect on the mechanical properties.
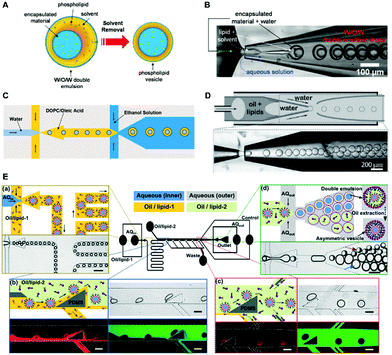 | ||
| Fig. 8 (A) Formation of liposomes through the double emulsion templated method.73 Copyright © 2008, American Chemical Society. (B–D) Double emulsion generated from various microfluidic devices. (B) Double emulsion generated in a microfluidic device that combined a co-flow and a flow-focusing geometry.73 Copyright © 2008, American Chemical Society. (C) The double emulsion prepared by consecutive flow-focusing channel geometries.74 Rights managed by AIP Publishing. (D) Monodisperse ultrathin shell double emulsion.78 © 2013 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (E) Asymmetric unilamellar vesicles from an integrated microfluidic device.81 Reproduced from ref. 81 with permission from The Royal Society of Chemistry. | ||
In 2008, Weitz et al.73 reported a microfluidic device that combined a co-flow and a flow-focusing geometry to prepare liposomes (Fig. 8B). The high uniformity in both the size and shape of the double emulsion could be tuned by adjusting the flow rate of each fluid phase and the diameter of each capillary in the device. A little later, a stable and biocompatible double emulsion was also prepared by using consecutive flow-focusing channel geometries (Fig. 8C), and lipid vesicles were then formed through a solvent extraction process.74 Since no toxic organic solvent was used, the double emulsion templated method enabled safe encapsulation and manipulation of the assortment of biological entities, such as cells, proteins, as well as nucleic acids. Recently, β-carotene-loaded double emulsion droplets have also been readily prepared for the formation of GUVs, which have potential applications in the protection and delivery of active compounds.75
In the double emulsion templated method, it is necessary to reduce the thickness of the oil shell to remove the oil, especially for the quality of GUVs, because the oil trapped in the formed liposomes may cause the exposure of sensitive biorelevant materials (e.g., nucleic acids, protein) to the organic solvent, which might either affect the activity of the biological macromolecules or make the giant liposomes extremely unstable.76,77 To address the issue of residual organic solvent, Weitz et al.78 developed a different microfluidic device to continuously produce monodisperse ultrathin shell double emulsion as a template to generate GUVs. As shown in Fig. 8D, by specially treating the surface of the tapered capillaries of the device, the double emulsion was forced to generate. The ultrathin oil shell could minimize the residual solvent that may be trapped within the bilayer membrane. Using the ultrathin shell double emulsion as the template, HeLa-based cell-free expression has been achieved within the engineered artificial cells. In order to deal with the problem of the time-consuming removal process of the organic solvent in the middle oil phase, rapid oil removal methods have been developed by using octanol or a copolymer surfactant (F-68).79,80 When using octanol or F-68, the interfacial tension between the two lipid monolayers decreased sharply. As a result, the oil was expelled from the shell, and the two lipid monolayers stuck together, forming a lipid bilayer membrane.
The advantages of the double emulsion templated method make it a powerful tool to prepare asymmetrical structures. By using the double emulsion templated method, Chiarot et al.81 successfully prepared asymmetric unilamellar vesicles with customized membrane composition, size, and luminal content. During fabrication, one special step was the replacement of the inner-leaflet-lipid solution with an outer-leaflet-lipid solution inside the channel (Fig. 8E), which enabled the produced vesicles to possess asymmetric membrane compositions. Most vesicles remained stable for several weeks, the membrane asymmetry was maintained for over 30 hours, and the transmembrane protein (alpha-hemolysin, αHL) can be reconstituted into the bilayer, which hold the potential to be employed as model systems in membrane biology or as vehicles for drug delivery. For a similar purpose, this method has been further utilized to construct vesicles possessing lipid composition of Gram-negative bacterial outer membrane (OM).82 It was observed that this technique generated monodisperse water-in-oil-in-water double emulsions at high-throughput. It was also found that the bending and expansion moduli of synthetic asymmetric membranes is physiologically relevant to the OM in Pseudomonas aeruginosa bacteria.
By the droplet emulsion templated methods, the vesicles with low polydispersity and high yield can be readily fabricated, which has also been summarized in Table 2, and the composition of the amphiphiles in the inner and outer layers of the vesicles is controllable. Moreover, the encapsulation of the desired hydrophilic cargoes can be achieved by doping with the inner water droplets. However, the oil is possibly trapped in the layers of the vesicles, which is likely to have undesired effects on the function of the vesicles when the biomolecules are embedded. Besides, the formation of droplet emulsions requires special microfluidic devices. In addition, the stability of droplet emulsions is also an annoying issue. For future preparation of biomimetic-stabilized liposomes, these concerns should be taken into consideration and addressed by integrating the methods.
| Methods | Size range, distribution | Encapsulation efficiency | Lamellarity | Asymmetric membrane | Yield | Residue of the organic solvent |
|---|---|---|---|---|---|---|
| W/O emulsion templated method | SUVs–GUVs, monodisperse | High | Unilamellar | Easy | High | Large |
| W/O/W double emulsion templated method | GUVs, monodisperse | High | Unilamellar | Relatively difficult | High | Small |
3.3 SUVs for mimicking the cell membrane system
To obtain sterilized liposomes, a heating method was proposed by Kikuchi et al.86 Interestingly, liposomes with a complete structure and high encapsulation efficiency were achieved after heating. Using a similar method, Mozafari et al.87 introduced nontoxic glycerol to the system and conducted the preparation process at 120 °C, which successfully prepared SUVs rapidly without harmful chemicals. Later, sterol was also demonstrated to be a useful liposome ingredient for generating SUVs at high temperatures.88 Besides the heating method, sonication is also one of the most widely employed technologies for preparing SUVs.24 There are two main sonication methods: bath sonication and probe sonication. Owing to the high energy of the sonicator probe tip, the former method involves immersion in liposome dispersion in a water/ice bath.62 Meanwhile, titanium may drop off the probe tip and pollute the solution. Therefore, the second sonication method is frequently used, which makes it easier to control the temperature of liposome dispersion, compared to probe sonication. Extrusion is also an important method to prepare nanosized SUVs, in which the liposome suspension is passed through a film filter with controlled pore size.89 An extruder carrying a pump that pushes suspensions through the films is always needed to accomplish the extrusion process. Extrusion is widely utilized in sizing the liposomes.90
 | ||
| Fig. 9 Various lipid-involved membrane mimicking systems from different scaffolds through the FGA method. (A) Shape-controlled vesicles constructed from the scaffold of AuNPs.93 © 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Virus-inspired enveloped DNA nanostructure from the scaffold of DNA nano-octahedrons.98 Copyright © 2014, American Chemical Society. (C) Liposome mimicking cell membrane structure obtained from the scaffold of the AuNPs.99 © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (D) Uniform liposomes prepared from the scaffold of the DNA origami ring.100 Copyright © 2017, Nature Publishing Group. (E) Liposomes in various shapes generated using DNA nanocages as outer templates.101 Copyright © 2017, Springer Nature. | ||
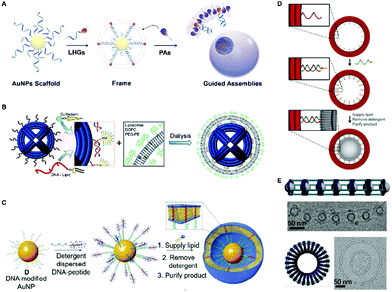
In principle, liposomes could also be prepared through frame-guided assembly, which is powerful to construct membranes with similar physicochemical properties to the natural membranes. In 2014, Shih et al.98 developed a virus-inspired enveloped DNA nanostructure using a similar method, which is illustrated in Fig. 9B. In their design, the lipid molecules were introduced to the DNA nano-octahedron (DNO) to preassemble onto outer handles. The initial solution was mixed with the lipids and surfactant (N-octyl-β-D-glucopyranoside), and after the surfactant was removed through a dialysis procedure, the lipid was guided around the DNO by the handles to form the bilayer structures. It has also been observed that the envelopment of the DNA nanostructures within the lipid bilayers conferred protection against nuclease digestion and the immune activation was decreased 2 orders of magnitude below control. Recently, using a similar dialysis procedure, a liposome mimicking cell membrane structure has been successfully constructed under physiological conditions (Fig. 9C).99 In this work, transmembrane α-helices peptides, which play dominant roles in the interactions between membrane proteins and the cell membrane, were selected as LHGs to form the initial frame. Owing to their well-matched hydrophobic interactions between the transmembrane peptides and lipids, cell structure-like vesicles were readily prepared. Both the α-helix conformation and transmembrane lengths of the peptides were demonstrated to benefit the formation as well as the stability of hetero-liposomes, and the FGA liposomes also exhibited enhanced stability compared to the liposomes prepared by conventional ways. Taking advantage of the biocompatibility and stability, the FGA liposomes were also employed to prepare novel drug delivery vehicles, which are promising in diagnostic imaging and cancer therapy applications.
Liposomes with controlled size can also be realized using DNA-origami rings as outer nano-templates. As illustrated in Fig. 9D, the lipid molecules were grafted onto the inner surface of the DNA rings to confine the liposome self-assembly during detergent removal.100 Both the lipid grafting density and the template size were important factors for generating the size-defined vesicles. By designing the template sizes and using various anchored lipid densities, uniform liposomes with predefined sizes were successfully prepared. Besides the predictable size, the method can also be utilized with diverse lipid compositions, which represents a general way to guide the formation of liposomes. For fully mimicking the complexity of biological membranes, the assembly, arrangement and remodeling of liposomes with designed geometries were exquisitely controlled by a set of reconfigurable DNA nanocages (Fig. 9E).101 Liposomes with high fidelity in tubular, toroid and other shapes were successfully prepared in vitro. Moreover, driven by the conformational changes of DNA nanocages, predictable membrane fusion and bending were also realized, which provides ideas for the systematic study of membrane mechanics.
In general, FGA provides a new, facile, and universal method for amphiphilic assembly, and exhibits promising application in the construction of liposomes with predictable shapes and sizes. The potential scaffold variability and assembly of amphiphiles make it possible in developing diverse amphiphilic assemblies. Combined with DNA nanotechnology, liposomes with higher complexity and curvature can also be generated. Nevertheless, for the preparation of liposomes, frames are needed to anchor the leading hydrophobic groups and a proper LHG density is also required because it cannot sustain the assembly of the amphiphiles if too low. Moreover, the diameter of liposomes constructed from this method is always of nanoscale dimensions, and the development of this technology to prepare large-sized liposomes remains to be explored.
4. Summary and perspective
Owing to their similar composition and bilayer structure to biological membranes, liposomes, such as giant unilamellar liposomes, have become ideal artificial cellular membrane models for studying and exploring the intrinsic formation and functions of cell membranes. Diverse methods, including film hydration methodologies, emulsion templated strategies, as well as frame-guided assembly (FGA) technologies, have been developed to construct desired liposomes. Generally, film hydration methods provide common and straightforward techniques for preparing high-quality unilamellar liposomes. The emulsion templated technologies offer powerful tools to fabricate vesicles with low polydispersity, a high yield, and controllable asymmetry. FGA provides a reliable means to construct structures with predictable shapes and sizes.While unilamellar liposomes with desired features, including size, uniformity, yield, symmetry, and composition, can be generated through these powerful methods, it should be noted that efficient encapsulation of biomolecules (e.g., nucleic acids, proteins) is important to render artificial cell models with certain functions. However, the current film hydration strategies still suffer from poor encapsulation, which was described previously, as the difficult trans-bilayer movements of large molecules restrict efficient incorporation.26 The nanosized liposomes generated from FGA limited the encapsulation of large-sized biomolecules. The emulsion templated methods may suffer from oil residues, which may affect the properties of the liposomes when the biomolecules are embedded. Therefore, developing novel or integrated strategies is necessary to increase encapsulation efficiency.
Meanwhile, although the construction of asymmetric liposomes possessing certain proteins has been demonstrated, the components of natural cellular membranes are far more complex than those of artificial membranes prepared using the present methods. In nature, cell membranes involved with many special lipids, membrane proteins or carbohydrates are still seldom incorporated in artificial systems. Furthermore, the orientation of membrane proteins is also important for the function of the cell membrane, which is still difficult to control by the currently reported strategies since the knowledge of interactions between the lipids and proteins is still scarce. Moreover, though the present artificial membrane systems with definite functions have been achieved, how to prepare more diverse and intricate systems is still in urgent need, which still requires the development of novel preparation methods.
In conclusion, based on the diverse well-established methods, controlled features of well-defined giant liposomes, including the encapsulation of biological molecules and asymmetric lipid membranes, have been achieved. It can be anticipated that, in the future, unilamellar liposomes prepared using these methods will keep providing powerful platforms to better understand complex natural biological phenomena.
Author contributions
DYC conceived the study and participated in its design and coordination. YW participated in the sequence alignment and drafted the manuscript. PJF helped in drafting the manuscript. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Natural Science Foundation of Beijing Municipality (Z180016), the National Science Foundation of China (21971248), and Beijing National Laboratory for Molecular Sciences.Notes and references
- J. Kulbacka, A. Choromańska, J. Rossowska, J. Weżgowiec, J. Saczko and M.-P. Rols, Cell membrane transport mechanisms: Ion channels and electrical properties of cell membranes, Transport across natural and modified biological membranes and its implications in physiology and therapy, 2017, 39 Search PubMed.
- R. B. Hamanaka and N. S. Chandel, Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes, Trends Biochem. Sci., 2010, 35, 505 Search PubMed.
- P. Liu, H. Cheng, T. M. Roberts and J. J. Zhao, Targeting the phosphoinositide 3-kinase pathway in cancer, Nat. Rev. Drug Discovery, 2009, 8, 627 Search PubMed.
- X. Wang, H. Du, Z. Wang, W. Mu and X. Han, Versatile Phospholipid Assemblies for Functional Synthetic Cells and Artificial Tissues, Adv. Mater., 2021, 33, 2002635 Search PubMed.
- Y. Zhu, X. Guo, J. Liu, F. Li and D. Yang, Emerging Advances of Cell-Free Systems toward Artificial Cells, Small Methods, 2020, 4, 2000406 Search PubMed.
- M. Komiya, M. Kato, D. Tadaki, T. Ma, H. Yamamoto, R. Tero, Y. Tozawa, M. Niwano and A. Hirano-Iwata, Advances in Artificial Cell Membrane Systems as a Platform for Reconstituting Ion Channels, Chem. Rec., 2020, 20, 730 Search PubMed.
- Y. Ding, F. Wu and C. Tan, Synthetic biology: A bridge between artificial and natural cells, Life, 2014, 4, 1092 Search PubMed.
- M. Marguet, C. Bonduelle and S. Lecommandoux, Multicompartmentalized polymeric systems: towards biomimetic cellular structure and function, Chem. Soc. Rev., 2013, 42, 512 Search PubMed.
- X. Huang, A. J. Patil, M. Li and S. Mann, Design and construction of higher-order structure and function in proteinosome-based protocells, J. Am. Chem. Soc., 2014, 136, 9225 Search PubMed.
- M. Li, R. L. Harbron, J. V. Weaver, B. P. Binks and S. Mann, Electrostatically gated membrane permeability in inorganic protocells, Nat. Chem., 2013, 5, 529 Search PubMed.
- R. Chandrawati and F. Caruso, Biomimetic liposome- and polymersome-based multicompartmentalized assemblies, Langmuir, 2012, 28, 13798 Search PubMed.
- E. Rideau, F. R. Wurm and K. Landfester, Self-Assembly of Giant Unilamellar Vesicles by Film Hydration Methodologies, Adv. Biosyst., 2019, 3, 1800324 Search PubMed.
- X. Cheng and J. C. Smith, Biological membrane organization and cellular signaling, Chem. Rev., 2019, 119, 5849 Search PubMed.
- P. L. Yeagle, Lipid regulation of cell membrane structure and function, FASEB J., 1989, 3, 1833 Search PubMed.
- H. T. McMahon and J. L. Gallop, Membrane curvature and mechanisms of dynamic cell membrane remodelling, Nature, 2005, 438, 590 Search PubMed.
- R. Mezzenga, J. M. Seddon, C. J. Drummond, B. J. Boyd, G. E. Schröder-Turk and L. Sagalowicz, Nature-Inspired design and application of lipidic lyotropic liquid crystals, Adv. Mater., 2019, 31, 1900818 Search PubMed.
- J. E. Rothman and J. Lenard, Membrane asymmetry, Science, 1977, 195, 743 Search PubMed.
- G. Di Paolo and P. De Camilli, Phosphoinositides in cell regulation and membrane dynamics, Nature, 2006, 443, 651 Search PubMed.
- X. Zhang, X. Shao, Z. Cai, X. Yan and W. Zong, The fabrication of phospholipid vesicle-based artificial cells and their functions, New J. Chem., 2021, 45, 3364 Search PubMed.
- A. D. Bangham and R. Horne, Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope, J. Mol. Biol., 1964, 8, 660 Search PubMed.
- J. P. Reeves and R. M. Dowben, Formation and Properties of Thin-walled Phospholipid Vesicles, J. Cell. Physiol., 1969, 73, 49 Search PubMed.
- E. Rideau, R. Dimova, P. Schwille, F. R. Wurm and K. Landfester, Liposomes and polymersomes: a comparative review towards cell mimicking, Chem. Soc. Rev., 2018, 47, 8572 Search PubMed.
- Y. P. Patil and S. Jadhav, Novel methods for liposome preparation, Chem. Phys. Lipids, 2014, 177, 8 Search PubMed.
- A. Jesorka and O. Orwar, Liposomes: technologies and analytical applications, Annu. Rev. Anal. Chem., 2008, 1, 801 Search PubMed.
- J. P. Colletier, B. Chaize, M. Winterhalter and D. Fournier, Protein encapsulation in liposomes: efficiency depends on interactions between protein and phospholipid bilayer, BMC Biotechnol., 2002, 2, 1 Search PubMed.
- P. Walde, K. Cosentino, H. Engel and P. Stano, Giant vesicles: preparations and applications, ChemBioChem, 2010, 11, 848 Search PubMed.
- K. Tsumoto, H. Matsuo, M. Tomita and T. Yoshimura, Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar, Colloids Surf., B, 2009, 68, 98 Search PubMed.
- L. M. Dominak and C. D. Keating, Polymer encapsulation within giant lipid vesicles, Langmuir, 2007, 23, 7148 Search PubMed.
- F. C. Tsai, B. Stuhrmann and G. H. Koenderink, Encapsulation of active cytoskeletal protein networks in cell-sized liposomes, Langmuir, 2011, 27, 10061 Search PubMed.
- 30 S. F. Shimobayashi and M. Ichikawa, Emergence of DNA-encapsulating liposomes from a DNA-lipid blend film, J. Phys. Chem. B, 2014, 118, 10688 Search PubMed.
- K. Shohda, K. Takahashi and A. Suyama, A method of gentle hydration to prepare oil-free giant unilamellar vesicles that can confine enzymatic reactions, Biochem. Biophys. Rep., 2015, 3, 76 Search PubMed.
- D. S. Dimitrov, J. Li, M. Angelova and R. K. Jain, Surface Effects in Preparation of Cell-Size Liposomes, FEBS Lett., 1984, 176, 398 Search PubMed.
- Z. Nourian, W. Roelofsen and C. Danelon, Triggered gene expression in fed-vesicle microreactors with a multifunctional membrane, Angew. Chem., Int. Ed., 2012, 51, 3114 Search PubMed.
- R. Tanasescu, U. Mettal, A. Colom, A. Roux and A. Zumbuehl, Facile and Rapid Formation of Giant Vesicles from Glass Beads, Polymers, 2018, 10, 54 Search PubMed.
- P. Méléard, L. A. Bagatolli and T. Pott, Giant Unilamellar Vesicle Electroformation, Methods Enzymol., 2009, 465, 161 Search PubMed.
- Q. Li, X. Wang, S. Ma, Y. Zhang and X. Han, Electroformation of giant unilamellar vesicles in saline solution, Colloids Surf., B, 2016, 147, 368 Search PubMed.
- M. I. Angelova and D. S. Dimitrov, Liposome Electroformation, Faraday Discuss. Chem. Soc., 1986, 81, 303 Search PubMed.
- D. S. Dimitrov and M. I. Angelova, Lipid swelling and liposome formation mediated by electric fields, J. Electroanal. Chem. Interfacial Electrochem., 1988, 253, 323 Search PubMed.
- M. I. Angelova, S. Soléau, P. Méléard, F. Faucon and P. Bothorel, Preparation-of-giant-vesicles-by exexternal AC electric fields. Kinetics and applications, Trends in colloid and interface science, 1992, 89, 127 Search PubMed.
- P. Lefrancois, B. Goudeau and S. Arbault, Electroformation of phospholipid giant unilamellar vesicles in physiological phosphate buffer, Integr. Biol., 2018, 10, 429 Search PubMed.
- S. Aimon, J. Manzi, D. Schmidt, J. A. Poveda Larrosa, P. Bassereau and G. E. Toombes, Functional reconstitution of a voltage-gated potassium channel in giant unilamellar vesicles, PLoS One, 2011, 6, 25529 Search PubMed.
- L. R. Montes, A. Alonso, F. M. Goni and L. A. Bagatolli, Giant unilamellar vesicles electroformed from native membranes and organic lipid mixtures under physiological conditions, Biophys. J., 2007, 93, 3548 Search PubMed.
- J. R. Howse, R. A. Jones, G. Battaglia, R. E. Ducker, G. J. Leggett and A. J. Ryan, Templated formation of giant polymer vesicles with controlled size distributions, Nat. Mater., 2009, 8, 507 Search PubMed.
- P. Taylor, C. Xu, P. D. Fletcher and V. N. Paunov, A novel technique for preparation of monodisperse giant liposomes, Chem. Commun., 2003, 1732 Search PubMed.
- N. Misawa, T. Motegi and R. Tero, Electroformation from patterned single-layered supported lipid bilayers for formation of giant vesicles with narrow size distribution, Appl. Phys. Express, 2014, 7, 117001 Search PubMed.
- F. Tao and P. Yang, Ca-mediated electroformation of cell-sized lipid vesicles, Sci. Rep., 2015, 5, 9839 Search PubMed.
- H. Bi, B. Yang, L. Wang, W. Cao and X. Han, Electroformation of giant unilamellar vesicles using interdigitated ITO electrodes, J. Mater. Chem. A, 2013, 1, 7125 Search PubMed.
- V. Pereno, D. Carugo, L. Bau, E. Sezgin, J. Bernardino de la Serna, C. Eggeling and E. Stride, Electroformation of Giant Unilamellar Vesicles on Stainless Steel Electrodes, ACS Omega, 2017, 2, 994 Search PubMed.
- H. G. Behuria, B. K. Biswal and S. K. Sahu, Electroformation of liposomes and phytosomes using copper electrode, J. Liposome Res., 2020, 1 Search PubMed.
- K. S. Horger, D. J. Estes, R. Capone and M. Mayer, Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength, J. Am. Chem. Soc., 2009, 131, 1810 Search PubMed.
- A. Weinberger, F. C. Tsai, G. H. Koenderink, T. F. Schmidt, R. Itri, W. Meier, T. Schmatko, A. Schroder and C. Marques, Gel-assisted formation of giant unilamellar vesicles, Biophys. J., 2013, 105, 154 Search PubMed.
- N. Lopez Mora, J. S. Hansen, Y. Gao, A. A. Ronald, R. Kieltyka, N. Malmstadt and A. Kros, Preparation of size tunable giant vesicles from cross-linked dextran(ethylene glycol) hydrogels, Chem. Commun., 2014, 50, 1953 Search PubMed.
- E. Parigoris, D. L. Dunkelmann, A. Murphy, N. Wili, A. Kaech, C. Dumrese, N. Jimenez-Rojo and U. Silvan, Facile generation of giant unilamellar vesicles using polyacrylamide gels, Sci. Rep., 2020, 10, 4824 Search PubMed.
- T. Fan, Q. Wang, N. Hu, Y. Liao, X. Chen, Z. Wang, Z. Yang, J. Yang and S. Qian, Preparation of giant lipid vesicles with controllable sizes by a modified hydrophilic polydimethylsiloxane microarray chip, J. Colloid Interface Sci., 2019, 536, 53 Search PubMed.
- J. Schultze, A. Vagias, L. Ye, E. Prantl, V. Breising, A. Best, K. Koynov, C. M. Marques and H. J. Butt, Preparation of Monodisperse Giant Unilamellar Anchored Vesicles Using Micropatterned Hydrogel Substrates, ACS Omega, 2019, 4, 9393 Search PubMed.
- N. Movsesian, M. Tittensor, G. Dianat, M. Gupta and N. Malmstadt, Giant Lipid Vesicle Formation Using Vapor-Deposited Charged Porous Polymers, Langmuir, 2018, 34, 9025 Search PubMed.
- S. Pautot, B. J. Frisken and D. A. Weitz, Production of Unilamellar Vesicles Using an InvertedEmulsion, Langmuir, 2003, 19, 2870 Search PubMed.
- L. L. Pontani, J. van der Gucht, G. Salbreux, J. Heuvingh, J. F. Joanny and C. Sykes, Reconstitution of an actin cortex inside a liposome, Biophys. J., 2009, 96, 192 Search PubMed.
- K. Karamdad, J. W. Hindley, G. Bolognesi, M. S. Friddin, R. V. Law, N. J. Brooks, O. Ces and Y. Elani, Engineering thermoresponsive phase separated vesicles formed via emulsion phase transfer as a content-release platform, Chem. Sci., 2018, 9, 4851 Search PubMed.
- K. Tsumoto, Y. Hayashi, J. Tabata and M. Tomita, A reverse-phase method revisited: Rapid high-yield preparation of giant unilamellar vesicles (GUVs) using emulsification followed by centrifugation, Colloids Surf., A, 2018, 546, 74 Search PubMed.
- Y. Ai, R. Xie, J. Xiong and Q. Liang, Microfluidics for Biosynthesizing: from Droplets and Vesicles to Artificial Cells, Small, 2020, 16, 1903940 Search PubMed.
- C. Has and P. Sunthar, A comprehensive review on recent preparation techniques of liposomes, J. Liposome Res., 2020, 30, 336 Search PubMed.
- A. Moga, N. Yandrapalli, R. Dimova and T. Robinson, Optimization of the Inverted Emulsion Method for HighYield Production of Biomimetic Giant Unilamellar Vesicles, ChemBioChem, 2019, 20, 2674 Search PubMed.
- S. Pautot, B. J. Frisken and D. A. Weitz, Engineering asymmetric vesicles, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10718 Search PubMed.
- M. Yanagisawa, M. Iwamoto, A. Kato, K. Yoshikawa and S. Oiki, Oriented reconstitution of a membrane protein in a giant unilamellar vesicle: experimental verification with the potassium channel KcsA, J. Am. Chem. Soc., 2011, 133, 11774 Search PubMed.
- A. Peyret, E. Ibarboure, J. F. Le Meins and S. Lecommandoux, Asymmetric Hybrid Polymer-Lipid Giant Vesicles as Cell Membrane Mimics, Adv. Sci., 2018, 5, 1700453 Search PubMed.
- M. B. C. de Matos, B. S. Miranda, Y. Rizky Nuari, G. Storm, G. Leneweit, R. M. Schiffelers and R. J. Kok, Liposomes with asymmetric bilayers produced from inverse emulsions for nucleic acid delivery, J. Drug Targeting, 2019, 27, 681 Search PubMed.
- Y. C. Tan, K. Hettiarachchi, M. Siu, Y. R. Pan and A. P. Lee, Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles, J. Am. Chem. Soc., 2006, 128, 5656 Search PubMed.
- S. Matosevic and B. M. Paegel, Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line, J. Am. Chem. Soc., 2011, 133, 2798 Search PubMed.
- P. C. Hu, S. Li and N. Malmstadt, Microfluidic fabrication of asymmetric giant lipid vesicles, ACS Appl. Mater. Interfaces, 2011, 3, 1434 Search PubMed.
- K. Karamdad, R. V. Law, J. M. Seddon, N. J. Brooks and O. Ces, Preparation and mechanical characterisation of giant unilamellar vesicles by a microfluidic method, Lab Chip, 2015, 15, 557 Search PubMed.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Monodisperse double emulsions generated from a microcapillary device, Science, 2005, 308, 537 Search PubMed.
- H. C. Shum, D. Lee, I. Yoon, T. Kodger and D. A. Weitz, Double emulsion templated monodisperse phospholipid vesicles, Langmuir, 2008, 24, 7651 Search PubMed.
- S. Deshpande, A. Birnie and C. Dekker, Stable, biocompatible lipid vesicle generation by solvent extraction-based droplet microfluidics, Biomicrofluidics, 2011, 5, 044113 Search PubMed.
- M. Michelon, Y. Huang, L. G. de la Torre, D. A. Weitz and R. L. Cunha, Single-step microfluidic production of W/O/W double emulsions as templates for β-carotene-loaded giant liposomes formation, Chem. Eng. J., 2019, 366, 27 Search PubMed.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi and K. Nejati-Koshki, Liposome: classification, preparation, and applications, Nanoscale Res. Lett., 2013, 8, 102 Search PubMed.
- K. Funakoshi, H. Suzuki and S. Takeuchi, Formation of giant lipid vesiclelike compartments from a planar lipid membrane by a pulsed jet flow, J. Am. Chem. Soc., 2007, 129, 12608 Search PubMed.
- L. R. Arriaga, S. S. Datta, S. H. Kim, E. Amstad, T. E. Kodger, F. Monroy and D. A. Weitz, Ultrathin shell double emulsion templated giant unilamellar lipid vesicles with controlled microdomain formation, Small, 2014, 10, 950 Search PubMed.
- S. Deshpande, Y. Caspi, A. E. Meijering and C. Dekker, Octanol-assisted liposome assembly on chip, Nat. Commun., 2016, 7, 10447 Search PubMed.
- N. N. Deng, M. Yelleswarapu and W. T. Huck, Monodisperse Uni- and Multicompartment Liposomes, J. Am. Chem. Soc., 2016, 138, 7584 Search PubMed.
- L. Lu, J. W. Schertzer and P. R. Chiarot, Continuous microfluidic fabrication of synthetic asymmetric vesicles, Lab Chip, 2015, 15, 3591 Search PubMed.
- S. Maktabi, J. W. Schertzer and P. R. Chiarot, Dewetting-induced formation and mechanical properties of synthetic bacterial outer membrane models (GUVs) with controlled inner-leaflet lipid composition, Soft Matter, 2019, 15, 3938 Search PubMed.
- S. G. Antimisiaris, S. Mourtas and A. Marazioti, Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery, Pharmaceutics, 2018, 10, 218 Search PubMed.
- L. Schoonen and J. C. van Hest, Compartmentalization Approaches in Soft Matter Science: From Nanoreactor Development to Organelle Mimics, Adv. Mater., 2016, 28, 1109 Search PubMed.
- L. Xu, P. Frederik, K. F. Pirollo, W. H. Tang, A. Rait, L. M. Xiang, W. Q. Huang, I. Cruz, Y. Z. Yin and E. H. Chang, Self-Assembly of a Virus-Mimicking Nanostructure System for Efficient Tumor-Targeted Gene Delivery, Hum. Gene Ther., 2002, 13, 469 Search PubMed.
- H. Kikuchi, A. CarlssoN, K. Yachi and S. Hirota, Possibility of heat sterilization of liposomes, Chem. Pharm. Bull., 1991, 39, 1018 Search PubMed.
- M. R. Mozafari, C. J. Reed, C. Rostron, C. Kocum and E. Piskin, Construction of stable anionic liposome-plasmid particles using the heating method: a preliminary investigation, Cell. Mol. Biol. Lett., 2002, 7, 923 Search PubMed.
- M. R. Mozafari, Liposomes: an overview of manufacturing techniques, Cell. Mol. Biol. Lett., 2005, 10, 711 Search PubMed.
- N. Berger, A. Sachse, J. Bender, R. Schubert and M. Brandl, Filter extrusion of liposomes using different devices: comparison of liposome size, encapsulation efficiency, and process characteristics, Int. J. Pharm., 2001, 223, 55 Search PubMed.
- G. Liu, S. Hou, P. Tong and J. Li, Liposomes: Preparation, Characteristics, and Application Strategies in Analytical Chemistry, Crit. Rev. Anal. Chem., 2020, 1 Search PubMed.
- M. P. Sheetz, Cell control by membrane-cytoskeleton adhesion, Nat. Rev. Mol. Cell Biol., 2001, 2, 392 Search PubMed.
- E. Luna and A. Hitt, Cytoskeleton--plasma membrane interactions, Science, 1992, 258, 955 Search PubMed.
- Y. Dong, Y. Sun, L. Wang, D. Wang, T. Zhou, Z. Yang, Z. Chen, Q. Wang, Q. Fan and D. Liu, Frame-guided assembly of vesicles with programmed geometry and dimensions, Angew. Chem., Int. Ed., 2014, 53, 2607 Search PubMed.
- Y. Dong and D. Liu, Frame-Guided Assembly of Amphiphiles, Chem. – Eur. J., 2015, 21, 18018 Search PubMed.
- Y. Dong, Z. Yang and D. Liu, Using Small Molecules to Prepare Vesicles with Designable Shapes and Sizes via Frame-Guided Assembly Strategy, Small, 2015, 11, 3768 Search PubMed.
- C. Zhou, Y. Zhang, Y. Dong, F. Wu, D. Wang, L. Xin and D. Liu, Precisely Controlled 2D Free-Floating Nanosheets of Amphiphilic Molecules through Frame-Guided Assembly, Adv. Mater., 2016, 28, 9819 Search PubMed.
- Y. Dong, Y. R. Yang, Y. Zhang, D. Wang, X. Wei, S. Banerjee, Y. Liu, Z. Yang, H. Yan and D. Liu, Cuboid Vesicles Formed by Frame-Guided Assembly on DNA Origami Scaffolds, Angew. Chem., Int. Ed., 2017, 56, 1586 Search PubMed.
- S. D. Perrault and W. M. Shih, Virus-inspired membrane encapsulation of DNA nanostructures to achieve in vivo stability, ACS Nano, 2014, 8, 5132 Search PubMed.
- C. Wang, J. Piao, Y. Li, X. Tian, Y. Dong and D. Liu, Construction of Liposomes Mimicking Cell Membrane Structure through Frame-Guided Assembly, Angew. Chem., Int. Ed., 2020, 59, 15176 Search PubMed.
- Y. Yang, J. Wang, H. Shigematsu, W. Xu, W. M. Shih, J. E. Rothman and C. Lin, Self-assembly of size-controlled liposomes on DNA nanotemplates, Nat. Chem., 2016, 8, 476 Search PubMed.
- Z. Zhang, Y. Yang, F. Pincet, M. C. Llaguno and C. Lin, Placing and shaping liposomes with reconfigurable DNA nanocages, Nat. Chem., 2017, 9, 653 Search PubMed.
| This journal is © the Partner Organisations 2021 |



