Proton conductive Zr-based MOFs
Xin
Chen
and
Gang
Li
 *
*
College of Chemistry and Green Catalysis Center, Zhengzhou University, Zhengzhou 450001, Henan, P. R. China. E-mail: gangli@zzu.edu.cn
First published on 13th August 2020
Abstract
The applications of crystalline solid-state proton conductive materials in fuel cells, proton sieving, electrochemical sensing and biochemistry are in the foreground, among which proton conducting metal–organic frameworks are favored by researchers for their structural diversity, functional design and modification. As one class of promising candidates for proton conductors, Zr-based metal–organic frameworks (MOFs) have attracted considerable attention. Therefore, the proton conductivity of such complexes will be comprehensively summarized for the first time by us. Herein, the proton conductivity properties of these MOFs with ordered porous structures, outstanding thermal stability, remarkably high water stability and chemical stability will be reviewed. These MOFs are classified and summarized according to the types of constructed organic ligands, such as carboxylic, phosphoric, and nitrogenous ligands. Consequently, the preparation strategy, structural characteristics, proton conductivity, conduction mechanism and application value are discussed. Finally, based on our experimental experience and literature review, the future development direction and application of this type of proton conducting MOF are assessed and highlighted.
1. Introduction
In today's world, with the rapid development of global industry and the improvement of people's living standards, global energy consumption continues to reach an unprecedented level, which leads to the rapid consumption of fossil fuels such as oil and natural gas. The resulting energy crisis and environmental pollution have become two major problems that must be solved. To solve these two problems, people hope to use more clean energy sources or new alternative energy such as fuel cells (FCs).1 In this context, proton exchange membrane fuel cells (PEMFCs) are favoured by many researchers for their advantages of efficient energy conversion and nearly zero emission and so on.2 In PEMFCs, a proton exchange membrane (PEM) provides a channel for proton transport, making protons pass across the film from the anode to the cathode, and forming a circuit with the electron transfer in the external circuit to provide current to the outside world. Therefore, the performance of PEMs plays a very important role in the performance of PEMFCs. As one core component, the PEM directly determines the service life and performance of PEMFCs. Therefore, the design, preparation and development of proton exchange membranes with outstanding performance have attracted much attention.2–5To date, the most commonly used PEM is the Nafion series perfluorinated sulfonic acid membrane with high proton conductivity (σ) and good chemical stability. Nevertheless, such a membrane has the following disadvantages: (1) it is very difficult to synthesize and sulfonate perfluorinated substances, which makes the film formation difficult and leads to high costs; (2) requirements of high temperature and H2O content. The optimal operating temperature of Nafion series films is 70–90 °C; exceeding this temperature range will cause a sharp decrease in water content and a rapid decline in electrical conductivity; (3) some hydrocarbons, such as methanol (MeOH), have high permeability and are not suitable for use as PEMs in direct methanol fuel cells (DMFC). (4) The poor crystalline structural characteristics of such membranes make it impossible to deeply analyse and study the proton conductive mechanism, which limits the demand for improving the proton conductivity.6,7 Therefore, the development of new and inexpensive proton-conducting materials with excellent performance has been a subject of intensive research.
Recently, the research of an important family of crystalline solid materials, such as metal–organic frameworks (MOFs),8–20 covalent organic frameworks (COFs),21–24 and hydrogen-bonded organic frameworks (HOFs)25–30 acting as promising conductors has attracted great attention. In addition to the structural modifying ability and functional modulability of such crystalline materials, which are well known to us, they also have high crystallinity, which provides a good material basis for the in-depth study of the proton conduction mechanism.
Here we will focus on the proton conducting properties of MOFs with ordered structures, which are assembled by metal ions or clusters with bridging organic ligands. Since a 2D copper(II) MOF, [(HOC2H4)2-dtoa-Cu] (dtoa = dithiooxamide) with proton conductivity was reported for the first time in 1979,31 a great number of studies have been conducted on proton conductive MOFs, especially in recent years. Through a large number of explorations, it has been found that MOFs can be a good candidate for proton conductors for the following reasons: first, the porous structures of MOFs contain rich H-bonds and water clusters, which can carry out proton transmission quickly and efficiently; second, the single crystal products of MOFs are relatively easy to obtain, and their fine structural characteristics can be obtained through X-ray crystallography, which provides convenience for the exploration of the proton conduction mechanism; third, the proton conductivity of MOFs can be tuned by selecting different metal ions or modifying multi-functional organic ligands or the post-modification method. Although the research on the proton conductivity of such MOFs has been summarized and evaluated by several groups covering phosphate MOFs,32 carboxylate MOFs,33 and so on,34–39 most of these reviews are broad and do not include specific reviews of MOFs of a certain metal atom, especially zirconium-based MOFs. It has been found in past studies that a significant number of proton conducting MOFs are of low stability, which limits their further practical application.33 Therefore, it is one of the preconditions to search for MOFs with ultrahigh structural and chemical stability for proton conductivity research.
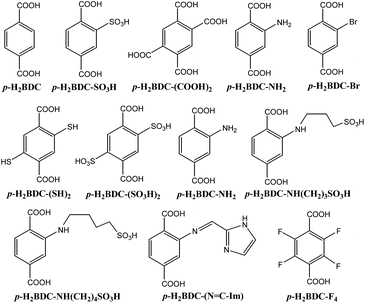
Since the Cavka group first reported a terephthalic zirconium MOF, UiO-66 (UiO = University of Oslo) in 2008,40 there is great interest in this class of MOFs with superior water stability and chemical stability. After twelve years of research and exploration, people have carried out in-depth studies on various properties of Zr-based MOFs including UiO series, PCN series, NU series and DUT series.41–43 Nevertheless, until now, there has not been a systematic and comprehensive review of the research of these MOFs in the field of proton conductivity, although there have been many recent exciting research developments. In this review, we will summarize the design strategy, structural characteristics, proton conductivity and proton conduction mechanism of such MOFs, and look forward to the future development trend. We hope that this review will enable us to design and prepare novel proton-conductive zirconium-based MOFs more efficiently and quickly for future applications. Scheme 1 gives the organic linkages appearing in the UiO series MOFs. Table 1 lists the proton conducting Zr-based MOFs described so far.
| Organic linkages | MOFs | Structures | Syntheses | σ/S cm−1 | E a/eV | Ref. |
|---|---|---|---|---|---|---|
| p-H2BDC | Zr6O4(OH)4.6(p-BDC)5.7 | 3D | Ligand defect control method | 1.30 × 10−5 (65 °C, 95% RH) | 0.25 | 44 |
| p-H2BDC | Zr6O4(OH)5.6(p-BDC)5.2 | 3D | Ligand defect control method | 6.61 × 10−5 (65 °C, 95% RH) | 0.29 | 44 |
| p-H2BDC | Zr6O4(OH)6.8(p-BDC)4.6 | 3D | Ligand defect control method | 1.01 × 10−3 (65 °C, 95% RH) | 0.36 | 44 |
| p-H2BDC | Zr6O4(OH)4(p-BDC)5.3(O2CCH3)1.4 | 3D | Ligand defect control method | 2.75 × 10−5 (65 °C, 95% RH) | 0.29 | 44 |
| p-H2BDC | Zr6O4(OH)4.8(p-BDC)5.6 | 3D | Ligand defect control method | 2.63 × 10−4 (65 °C, 95% RH) | 0.32 | 44 |
| p-H2BDC | Zr6O4(OH)6(p-BDC)5 | 3D | Ligand defect control method | 6.93 × 10−3 (65 °C, 95% RH) | 0.22 | 44 |
| p-H2BDC-SO3H | Zr6O4(OH)8(p-BDC-SO3H)4.2·67H2O | 3D | Solution reaction | 1.93 × 10−3 (65 °C, 95% RH) | 0.25 | 45 |
| p-H2BDC-SO3H | Zr6O4(OH)8(p-BDC-SO3H)4·65H2O | 3D | Solution reaction | 2.40 × 10−3 (65 °C, 95% RH) | 0.25 | 45 |
| p-H2BDC-SO3H | Zr6O4(OH)8(p-BDC-SO3H)4·80H2O | 3D | Solution reaction | 5.62 × 10−3 (65 °C, 95% RH) | 0.24 | 45 |
| p-H2BDC-SO3H | Zr6O4(OH)8(p-BDC-SO3H)3.8·153H2O | 3D | Post-modified method | 3.46 × 10−3 (65 °C, 95% RH) | 0.25 | 45 |
| p-H2BDC-SO3H | UiO-66-SO3H | 3D | Solvothermal synthesis | 3.4 × 10−3 (30 °C, ∼97% RH) | 0.27 | 50 |
| p-H2BDC-(COOH)2 | UiO-66–2COOH | 3D | Solution reaction | 1.0 × 10−3 (30 °C, ∼97% RH) | 0.18 | 50 |
| p-H2BDC-NH2 | UiO-66-NH2 | 3D | Solvothermal synthesis | 1.40 × 10−5 (30 °C, ∼97% RH) | 0.40 | 50 |
| p-H2BDC | UiO-66 | 3D | Solvothermal synthesis | 7.54 × 10−6 (30 °C, ∼97% RH) | 0.44 | 50 |
| p-H2BDC-Br | UiO-66-Br | 3D | Solvothermal synthesis | 2.23 × 10−7 (30 °C, ∼97% RH) | 0.78 | 50 |
| p-H2BDC | UiO-66 | 3D | Solvothermal synthesis | 2.5 × 10−5 (80 °C, 90% RH) | - | 51 |
| p-H2BDC-(SH)2 | UiO-66(SH)2 | 3D | Micarowave reaction | 4.3 × 10−6 (80 °C, 90% RH) | 0.23 | 51 |
| p-H2BDC-(SO3H)2 | UiO-66(SO3H)2 | 3D | Post-synthetic oxidation | 8.4 × 10−2 (80 °C, 90% RH) | 0.32 | 51 |
| p-H2BDC-F4 | UiO-66-F4 | 3D | Solution reaction | — | — | 52 |
| p-H2BDC-NH(CH2)3SO3H | PSM 1 | 3D | Post-synthetic modification | 1.64 × 10−1 (80 °C, 90% RH) | 0.107 | 53 |
| p-H2BDC-NH(CH2)4SO3H | PSM 2 | 3D | Post-synthetic modification | 4.66 × 10−3 (80 °C, 90% RH) | 0.292 | 53 |
| p-H2BDC-NH2 | UiO-66-NH2 | 3D | Solvothermal synthesis | 3 × 10−6 (80 °C, 98% RH) | 54 | |
| p-H2BDC-NH2 + p-H2BDC-SO3H | UiO-66-AS | 3D | Post-synthetic modification | 1.7 × 10−4 (80 °C, 98% RH) | 54 | |
p-H2BDC-SO3H + p-H2BDC-(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-Im) C-Im) |
IM-UiO-66-AS | 3D | Post-synthetic modification | 1.54 × 10−1 (80 °C, 98% RH) | 0.2 | 54 |
| IM-UiO-66-AS@PP (60 wt%) | Film | Solution-casting method | 1.19 × 10−2 (80 °C, 98% RH) | 0.32 | 54 | |
| p-H2BDC-(COOH)2 | UiO-66-2COOH | 3D | Solution reaction | 2.3 × 10−3 (90 °C, 95% RH) | 0.17 | 56 |
| p-H2BDC-NH2 | UiO-66-NH2 + UiO-66-SO3H/Nafion-0.6 | Film | Solution-casting method | 0.256 (90 °C, 95% RH) | — | 57 |
| p-H2BDC-SO3H | ||||||
| p-H2BDC-SO3H | CS/UiO-66-SO3H-6 | Film | Solution-casting method | 1.52 × 10−3 (90 °C, anhydrous) | 0.105 | 58 |
| p-H2BDC-NH2 | UiO-66-NH2-15 | Film | Solution-casting method | 0.0564 (100 °C, 98% RH) | 0.12 | 58 |
| p-H2BDC-NH2 | CS/UiO-66-SO3H-6 + UiO-66-NH2-15 | Film | Solution-casting method | 5.2 × 10−2 (100 °C, 98% RH) | 0.131 | 58 |
| p-H2BDC-SO3H | 3.78 × 10−3 (120 °C, anhydrous) | 0.189 | ||||
| p-H2BDC-NH2 | GO@UiO-66-NH2/Nafion-0.6 | Film | Solution-casting method | 0.303 (90 °C, 95% RH) | — | 59 |
| 3.403 × 10−3 (90 °C, anhydrous) | — | |||||
| p-H2BDC-NH2 | SPEN/UiO-66-NH2-5 | Film | Solution-casting method | 1.351 × 10−1 (80 °C, hydrated in H2O) | — | 60 |
| p-H2BDC-NH2 | UiO-66-NH2@NFs/Nafion | Film | Impregnating method | 0.27 (80 °C, 100% RH) | — | 61 |
| p-H2BDC-SO3H | SPEEK/UiO-66-SO3H@GO-10 | Film | Solution-casting method | 0.268 (70 °C and 95% RH) | — | 62 |
| p-H2BDC | UiO-66(200)/Nafion-2 | Film | Solution-casting method | 0.207 (110 °C and 95% RH) | — | 63 |
| p-H2BDC-SO3H | UiO-66-SO3H/Nafion-2 | Film | Solution-casting method | 0.189(110 °C and 95% RH) | — | 63 |
| p-H2BDC-SO3H | BSP/Zr-Cr-SO3H-0.5% | Film | Solvothermal synthesis | 0.154 (80 °C and 100% RH) | — | 64 |
| H2bpdc | Him@UiO-67 | 3D | Evaporation method | 1.52 × 10−3 (130 °C, anhydrous) | 0.36 | 65 |
| H2bpdc-4SO2Me4F | Zr-bpdc-4SO2Me4F | 3D | Oxidation method | 1.75 × 10−4 (100 °C, 98% RH) | — | 66 |
| H3BTC | MOF-808 | 3D | Solvothermal synthesis | 7.58 × 10−3 (42 °C, 99% RH) | 0.37 | 68 |
| MOF-808@PVDF-55 | Film | Solution-casting method | 1.56 × 10−4 (65 °C, hydrated in H2O) | 0.167 | 68 | |
| H3BTC | MOF-808 | 3D | Solvothermal synthesis | 8.97 × 10−6 (80 °C, 98% RH) | 0.37 | 69 |
| H3BTC | MOF-808-EDTA | 3D | Post-synthetic modification | 1.31 × 10−4 (80 °C, 98% RH) | 0.15 | 69 |
| MOF-808-ox | 3D | Post-synthetic modification | 4.25 × 10−4 (80 °C, 98% RH) | 0.14 | 69 | |
| MOF-808-ox@PVA-3 | Film | Solution-casting method | 2.03 × 10−5 (80 °C, hydrated in H2O) | — | 69 | |
| H3SNDC | VNU-17 | 3D | Solution reaction | 6.65 × 10−6 (70 °C, 98% RH) | 0.47 | 72 |
| Him9@VNU-17 | 3D | Post-treatment method | 1.53 × 10−4 (70 °C, 98% RH) | 0.44 | 72 | |
| Him11@VNU-17 | 3D | Post-treatment method | 5.93 × 10−3 (70 °C, 98% RH) | 0.27 | 72 | |
| H4TSNDC | VNU-23 | 3D | Solution reaction | 1.54 × 10−4(70 °C, 90% RH) | - | 73 |
| His8.2@VNU-23 | 3D | Post-treatment method | 1.79 × 10−2 (95 °C, 85% RH) | 0.27 | 73 | |
| H2TBNDC | Zr-BTNDC | 3D | Solvothermal synthesis | 7.88 × 10−5 (90 °C, 95% RH) | 0.17 | 74 |
| Zr-BTNDC-ox | 3D | Post-oxidation method | 4.03 × 10−3 (95 °C, 95% RH) | 0.22 | 74 | |
| H@Zr-TBNDC-ox | 3D | Post-acidification method | 3.16 × 10−2 (90 °C, 95% RH) | 0.25 | 74 | |
| H2ox | ((Me)2NH2)2[Li2(H2O)4Zr(ox)4] | 3D | Phase transition | 3.9 × 10−5 (17 °C, 67% RH) | 0.64 | 75 |
| L-asp | MIP-202(Zr) | 3D | Reflux with ambient pressure | 0.011 (90 °C, 95% RH) | 0.22 | 76 |
| H2fum | MOF-801 | 3D | Solvothermal synthesis | 1.88 × 10−3 (25 °C, 98% R H) | 0.256 | 77 |
| MOF-801@PP-60 | Film | Solution-casting method | 1.84 × 10−3 (52 °C, 98% R H) | — | 77 | |
| H2fum | C-SPAEKS/Him-MOF-801-4 | Film | Solution-casting method | 0.128 (90 °C, 100% RH) | — | 78 |
| C-SPAEKS/him@MOF-801-4 | Film | Solution-casting method | 0.068 (90 °C, 100% RH) | — | 78 | |
| 3-H3SPP | Zr(HPO4)0.65(3-HSPP)1.35·nH2O | 2D | Solution reaction | 0.04 (100 °C, 70% RH) | — | 79 |
| 3-H3SPP | 10 wt%SPEESK(DS76%)/ZrSPP | Film | Solution-casting method | 0.393 (120 °C, 100%RH) | — | 80 |
| 30 wt%SPEESK(DS34.6%)/ZrSPP | Film | Solution-casting method | 0.23 (160 °C, 100%RH) | — | 80 | |
| 4-H3SPP | Zr(HPO4)0.7(HO3SC6H4PO3)1.3 | 2D | Solution reaction | 0.063 (100 °C, 90% RH) | — | 82 |
| H8CDTP | 1_lp@H | 3D | Post-treatment method | 5.4 × 10−5 (80 °C, 95% RH) | — | 83 |
| 1_np@H | 3D | Post-treatment method | 6.6 × 10−5 (80 °C, 95% RH) | — | 83 | |
| Glyphosate | G1 | 1D | Solution reaction | ∼10−3 (140 °C, 95% RH) | — | 84 |
| Glyphosine | G2 | 2D | Solution reaction | ∼10−3 (140 °C, 95% RH) | 0.1 | 84 |
| Glyphosine | G3 | 3D | Solution reaction | ∼10−4 (140 °C, 95% RH) | — | 84 |
| Glyphosine | ZPGly | 2D | Solution reaction | 1 × 10−3 (140 °C, 95% RH) | 0.15 | 85 |
| H6FBTP | PCMOF20 | 2D | Phase transformation | 1 × 10−2 (80 °C, 95% RH) | 0.2 | 86 |
| H6TzGal | MIL-163 | 3D | Solvothermal synthesis | 2.1 × 10−3 (90 °C, 95% RH) | 0.25 | 90 |
| THPP | ZrPP-1 | 3D | Solvothermal synthesis | 8.0 × 10−3 (25 °C, 98% RH) | 0.21 | 91 |
| THBPP | ZrPP-2 | 3D | Solvothermal synthesis | 4.2 × 10−3 (25 °C, 98% RH) | 0.23 | 91 |
2. Proton conductive carboxylate Zr-MOFs
2.1. UiO-66 series MOFs
The three-dimensional porous MOF UiO-66 is constituted of [Zr6O4(OH)4] clusters with 1,4-benzenedicarboxylic acid (p-H2BDC) linkages,40 in which the pore structure is composed of a regular octahedral cage at about 11 Å and a regular tetrahedral cage at about 8 Å connected by a triangular window at about 6 Å. The dense structural units make the whole structure stable. At the same time, the Zr atom is highly oxyphilic, so the strong Zr–O bond also increases the stability of the structure. Therefore, UiO-66 has high hydrothermal stability and chemical stability as well as mechanical stability. It can be stable at 500 °C, and can maintain its stable structure in water, DMF, benzene or acetone, and has strong acid stability and some alkalinity stability. These structural characteristics and outstanding structural stability are extremely beneficial for the research of proton conductivity.Based on the research of the influence of ligand defects on the σ value of UiO-66-based MOFs, the same group further explored the role of a 3D ordered defect sublattice on the acidity of a zirconium 2-sulfoterephthalate MOF.45 The author first changed monosodium 2-sulfoterephthalic acid p-H2BDC-SO3H through ion exchange, and then reacted it with ZrCl4 in H2O to produce a MOF, Zr6O4(OH)8(p-BDC-SO3H)4.2·67H2O. Afterward, there is an ordered defect sublattice in the structure of the MOF by means of ICP-AES, CHN analysis, PXRD and ICP analysis and so on, and theoretical calculation. At the same time, they pointed out that the proton trapping nature of the defective sites of zirconium oxohydroxy clusters may cause the σ value of Zr6O4(OH)8(p-BDC-SO3H)4.2·67H2O to change little with RH and the value is ordinary. So they asserted that adding a certain amount of acids would saturate these proton capture sites and thus improve the proton conductivity of the resulting MOFs. Therefore, in the process of preparing Zr6O4(OH)8(p-BDC-SO3H)4.2·67H2O, they added a certain amount of acetic acid and sulfoacetic acid respectively before reflux to synthesize MOFs, Zr6O4(OH)8(p-BDC-SO3H)4·65H2O and Zr6O4(OH)8(p-BDC-SO3H)4·80H2O. And, they soaked Zr6O4(OH)8(p-BDC-SO3H)4.2·67H2O in 0.1 M H2SO4 aqueous solution for a day and got the MOF Zr6O4(OH)8(p-BDC-SO3H)3.8·153H2O. PXRD determination verified that the four MOFs have similar structures. AC impedance analysis of them manifested that Zr6O4(OH)8(p-BDC-SO3H)4·65H2O treated with a weaker acid, CH3COOH, had a σ value of 2.4 × 10−3 S cm−1, which is slightly higher than that of pristine Zr6O4(OH)8(p-BDC-SO3H)4.2·67H2O (1.93 × 10−3 S cm−1) at 65 °C and 95% RH. Interestingly, MOFs Zr6O4(OH)8(p-BDC-SO3H)4·80H2O and Zr6O4(OH)8(p-BDC-SO3H)3.8·153H2O treated with highly acidic HO3SCH2CO2H and H2SO4, respectively, have greatly enhanced σ values of 5.62 × 10−3 and 3.46 × 10−3 S cm−1, respectively, at 65 °C and 95% RH. All the four MOFs displayed very similar Ea values around 0.25 eV implying that the proton transport obeys the hopping mechanism. Their research provides useful guidance for people to use defects in ZrMOFs and to regulate the proton conductivity through defect control.
Also in 2015, C. S. Hong and co-workers first used microwave-assisted solvothermal synthesis to form a sulfhydryl (–SH) modified functional UiO-66 MOF, UiO-66-(SH)2, and then they used H2O2 post-oxidation to oxidize it to a sulfonic acid unit modified MOF, UiO-66-(SO3H)2.51 Powder X-ray diffraction (PXRD) verified that both UiO-66-(SO3H)2 and UiO-66-(SH)2 have the same phase as UiO-66. That is to say, the three compounds have basically the same structure. Like UiO-66, UiO-66-(SO3H)2 and UiO-66-(SH)2 maintain their thermal stability at 400 °C and show excellent water stability by dipping in H2O for thirty days and reflexing in H2O. The N2 adsorption test at −196 °C confirmed that the Brunauer–Emmett–Teller (BET) surface areas of these MOFs decreased with the increase of the substituent volume. For instance, BET areas are 897, 308 and 35 m2 g−1 for UiO-66, UiO-66-(SH)2 and UiO-66-(SO3H)2, respectively. Consequently, they determined the σ values of these MOFs by using a pelletized sample from 25–80 °C under 90% RH. The maximum σ value of UiO-66-(SO3H)2 is 8.4 × 10−2 S cm−1 at 80 °C and 90% RH, which is far greater than that of UiO-66-(SH)2 (2.5 × 10−5 S cm−1) and of UiO-66 (4.3 × 10−6 S cm−1) under the same conditions. This can be interpreted by the hydroscopicity of the MOFs following the introduction of different substituents. UiO-66-(SO3H)2 with acidic SO3H units exhibits strong hydrophilicity, which will lead to the formation of hydrophilic channels in the framework for proton transport. In contrast, UiO-66 and UiO-66-(SH)2 have lower water absorption ability. In addition, the high density of sulfonic acid groups in UiO-66-(SO3H)2 is also one of the reasons for its super high proton conductivity. Note that the σ value of UiO-66-(SO3H)2 at 25 °C and 90% RH (1.4 × 10−2 S cm−1) is higher than that (3.4 × 10−3 S cm−1) of UiO-66-SO3H at 30 °C and ∼97% RH.50
In 2020, J. Banys et al. employed dielectric spectroscopy to investigate the water dynamics of two functionalized UiO-66-NH2 and UiO-66-F4 MOFs,52 and discovered that both hydrated MOFs exhibit a tremendous dielectric dispersion, which can be divided into three overlapping processes. The three processes disappeared in the dehydrated sample, which suggests that the adsorbed H2O units in the MOFs are the source of these three processes. This again indicates that although –NH2 and –F groups have certain hydrophobicity, they can form H-bonds with the adsorbed H2O units and thus enhance the proton conductivity of the MOFs.
In 2019 S. K. Das et al.53 reported two homologous MOFs, PSM1 and PSM2, that were prepared by modifying UiO-66-NH2 through the sulfolactone reaction (Fig. 1). After PSM 1, PSM 2 and UiO-66-NH2 were immersed in boiling water for seven days, respectively, they have similar PXRD patterns suggesting that they have similar structures and maintain their structural integrity even after treatment with boiling water. Moreover, both PSM 1 and PSM 2 had good thermal stability. As shown in Fig. 1, PSM 1 and PSM 2 have basically the same structure, except that the side chain length of the –SO3H group is different, and the former is one less –CH2 than the latter. Although it is only a small difference, the σ value of the two MOFs is fairly different. For example, at 80 °C and 95% RH, PSM 1 has the highest σ value of 1.64 × 10−1 S cm−1, while PSM 2 only reaches 4.6 × 10−3 S cm−1 under the same conditions.
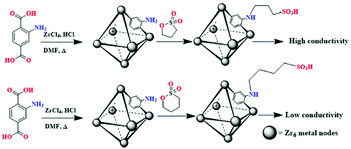 | ||
| Fig. 1 The post-preparation routes of PSM 1 (top) and PSM 2 (bottom). Reproduced with permission from ref. 53. Copyright 2019, American Chemical Society. | ||
Apparently, different side chain lengths of –SO3H groups may lead to different pKa values. The pKa value of the –SO3H group in PSM 1 (3.47) is smaller than that of the –SO3H group in PSM 2 (4.91), which may be the main reason why the σ value of PSM 1 is greater than that of PSM 2. The lower pKa constant causes the protons in the compound to dissociate more easily, resulting in higher proton conductivity. Because the spectral shifts of the N 1s XPS spectra of the two MOFs are the same, the influence of difference hydrogen bond networks inside the frameworks on the proton conductivity can be overlooked. They further analyzed theoretically the difference in the degree of dissociation of protons from sulfonic acid units in the two compounds, PSM 1 and PSM 2, by the molecular electrostatic potential. The lower Ea values of PSM 1 and PSM 2 imply that proton transport within the two compounds follows a water-assisted hopping mechanism (also called the Grotthuss mechanism). Finally, it was found that the two compounds exhibited good stability and cycling usability after 48 hours of continuous AC impedance tests and five consecutive AC impedance tests with heating and cooling cycles.
In the same year, Y. Q. Lan and co-workers also used UiO-66-NH2 as the starting material to obtain two kinds of modified MOFs, UiO-66-AS and IM-UO-66-AS (Fig. 2), which kept the framework structure of UiO-66-NH2 and had good thermal and water stability.54 As illustrated in Fig. 2, UiO-66-AS can be acquired by replacing part of 2-amino-terephthalic acid in UiO-66-NH2 with sodium 2-sulfoterephthalate, which has one more proton source and one more hopping site than UiO-66-NH2 (0 proton source, 1 hopping site). Furthermore, the uncoordinated amino group in UiO-66-AS was covalently connected with imidazole-2-carboxaldehyde through the Schiff base reaction to produce the MOF IM-UiO-66-AS, which had one more proton source and two more hopping sites than UiO-66-AS. Obviously, IM-UiO-66-AS must exhibit an excellent σ value because it has the most proton sources and proton hopping sites (2 proton sources and 4 proton hopping sites). The results of AC impedance determinations also confirmed this conclusion. At 80 °C and 98% RH, the σ value of IM-UiO-66-AS is 1.54 × 10−1 S cm−1, which is almost 3 and 5 orders of magnitude higher than UiO-66-AS (1.7 × 10−4 S cm−1) and UiO-66-NH2 (3 × 10−6 S cm−1), respectively, under the same conditions. Note that the σ value of IM-UiO-66-AS remained essentially unchanged after continuous testing at 80 °C and 98% RH up to 100 hours. In addition, the PXRD determinations also showed that the structure of the samples remained unchanged before and after the electrochemical test. These fully manifest the electrochemical stability of IM-UiO-66-AS, which offers a good foundation for future application.
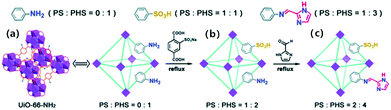 | ||
| Fig. 2 Structure of UiO-66-NH2 (a); the preparation routes of post-modification UiO-66-AS (b) and IM-UO-66-AS (c) indicating the proton source (PS) and the proton hopping site (PHS). Reproduced with permission from ref. 54. Copyright 2019, Royal Society of Chemistry. | ||
The authors further doped the microcrystalline sample of IM-UiO-66-AS into a PVDF-PVP composite carrier to make a hybrid matrix film (called IM-UiO-66-AS@PP), and measured its proton conductivity and application in H2/O2 fuel cells. They discovered that the composite membrane revealed good stability, flexibility and σ value. When the weight of the doped MOF in the membrane is 60%, its σ value can be 1.19 × 10−2 S cm−1 at 80 °C and 98% RH. Nevertheless, the higher doping amount of the MOF did not bring the continuous improvement of the proton conductivity of IM-UiO-66-AS@PP. The composite film was applied to a H2/O2 fuel cell and its highest open circuit voltage (OCV) and power density were 0.78 V and 17.5 mW cm−2, respectively (80 °C and 98% RH).
In 2014, the S. Devautour-Vinot group adopted broadband dielectric spectroscopy and molecular dynamics simulations to explore the water dynamics inside three Zr-MOFs, UiO-66, UiO-66-COOH and UiO-66-(COOH)2.55 Because the UiO-66 framework is quite hydrophobic, there is a relatively weak interaction between water molecules and pore walls, which means that charge carriers are less likely to be generated in this solid, so UiO-66 shows a low proton conductivity. However, molecular dynamics simulations indicated that the H2O units can form clusters in the cages of UiO-66, which just absorbed very little water units. In contrast, UiO-66-COOH and UiO-66-(COOH)2 exhibit hydrophilicity due to the regulation of the polar –COOH groups. Accordingly, the σ value of UiO-66-COOH and UiO-66-(COOH)2 improves. The authors showed for the first time that the σ value of a MOF is related to water adsorption and the density of free carboxylic acids within the framework.
Later, the same group further studied the proton conduction mechanism of UiO-66-(COOH)2 at the molecular level by using the advanced quasi-elastic neutron scattering method (QNES) and aMS-EVB3 molecular dynamics simulations.42 The QENS experiment shows that all protons are dynamically equivalent, and proton diffusion in the “clouds” around the oxygen atoms is caused by jumps between proton clouds. No diffusion of the O atom can be found. In addition, this research group also carried out aMS-EVB3 molecular dynamics simulation for UiO-66-(COOH)2 combining with QENS experiment, and verified that proton transport is mainly dominated by the Grotthuss mechanism, which is consistent with the calculated Ea value of 0.17 eV. Molecular dynamics simulations reveal that adsorbed water molecules within the framework can join the tetrahedron cages and neighbouring octahedral cages through hydrogen bonding bridges, namely by forming water hydrogen bonding networks between these cages, allow redundant proton hopping from a cage to another cage, to ensure that the excess protons can transfer for long distances, and thus ensure a higher σ value. For the first time, it has been expressed at the molecular level that long distance proton transport in a hydrated MOF is accomplished through a hydrogen-bonding network formed by water.
In 2017, P. Y. Wu and co-workers doped separately or simultaneously two MOFs, UiO-66-NH2 and UiO-66-SO3H, into Nafion and studied the σ value of the resulting composite films.57 They found that the composite membrane containing both UiO-66-NH2 and UiO-66-SO3H (denoted as UiO-66-NH2 + UiO-66-SO3H/Nafion-0.6) showed better performance than the single doped composite film (UiO-66-SO3H/Nafion-0.6 or UiO-66-NH2/Nafion-0.6) in σ value, mechanical strength and methanol permeability. For example, at 90 °C and 95% RH, the proton conductivity of UiO-66-NH2 + UiO-66-SO3H/Nafion-0.6 (0.6 presenting the weight percentage of the incorporated MOFs on the basis of Nafion) is 0.256 S cm−1 being about 1.17 times higher than that of the recast Nafion. The mechanism of these composite films was studied by water vapor adsorption and atomic force microscopy (AFM) and so on. The water vapor adsorption determination displayed that the co-doped composite film had the highest water absorption capacity under the synergistic action of the two hydrophilic functionalized Zr-MOFs. This indicates that denser hydrophilic channels can be formed in the composite membrane to facilitate proton transport. The AEM photos indicated that the interaction between the two MOFs, UiO-66-NH2 and UiO-66-SO3H, with minor particle size and the ionic clusters in the membrane also resulted to the enhancement of the σ value. Meanwhile, as both complexes have the ability to capture methanol molecules, the anti-methanol permeability of the composite film is greatly enhanced, which lays a foundation for the future application in DMFCs. Eventually, under extreme conditions (90 °C and 95% RH), the σ value of the membrane UiO-66-NH2 + UiO-66-SO3H/Nafion-0.6 remained basically constant after continuous testing for 50 hours, showing superior durability.
Later, S. Q. Zang and co-workers again observed the synergistic effect of acidic and alkaline MOFs, UiO-66-SO3H and UiO-66-NH2 with isomorphous structures on proton conductivity in the polymer chitosan (CS) in 2018.58 Using the methods similar to those mentioned above, the proton conductivity of composite films for undoped and doped single MOF (UiO-66-SO3H or UiO-66-NH2) and doped two MOFs (UiO-66-SO3H and UiO-66-NH2) was compared. They discovered that the composite film bearing both UiO-66-SO3H and UiO-66-NH2 manifested a remarkable σ value under hydrous (5.2 × 10−2 S cm−1 at 100 °C and 98% RH) and anhydrous conditions (3.78 × 10−3 S cm−1 at 120 °C) (Table 1). Note that the original σ value of UiO-66-SO3H and UiO-66-NH2 at 100 °C and 98% RH was 3.4 × 10−3 and 1.4 × 10−5 S cm−1, respectively, neither of which was extremely ideal. However, when they were mixed with CS and prepared into a composite membrane, CS/UiO-66-SO3H-6 + UiO-66-NH2-15, the effect of 1 + 1 > 2 was produced, which indicated that both the MOFs and CS played an important role in the transmission of protons. As shown in Fig. 3, the SO3H and NH2 units from UiO-66-SO3H and UiO-66-NH2 can interact with OH and NH3 or NH2 units of CS to establish abound H-bonded networks inside the composite film. Moreover, sulfonic acid groups can be used as proton sources in the absence or presence of H2O, and NH2 units can also be used as proton transport sites. In addition, NH2 groups can form acid–base pairs with the sulfonic group of adjacent MOFs, which is very helpful for proton conductivity. The performance of the composite film in the H2/O2 fuel cell is that the OCV and power density are 1.0 V and 10.6 mW cm−2, respectively.
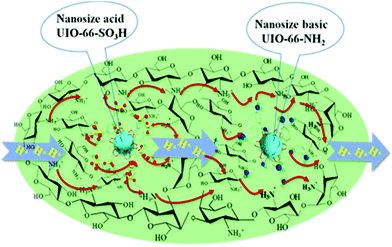 | ||
| Fig. 3 Proposed schematic diagram of proton transport in the composite membrane, CS/UiO-66-SO3H-6+UiO-66-NH2-15. Nanosize UiO-66-SO3H and UiO-66-NH2 were simplified as a Zr6O8 cluster (solid blue ball) surrounded by 12 sulfonated benzoic acid and 12 amido benzoic acid, respectively. The pair of embedded MOFs in the CS matrix is interconnected with functional groups of CS-facilitated proton transfer. Reproduced with permission from ref. 58. Copyright 2018, American Chemical Society. | ||
In 2017, P. Y. Wu's group examined the σ value of UiO-66-NH2 combined with graphene oxide (GO) to get a composite membrane doped with Nafion.59 The authors first chemically modified GO by coating the surface with polydopamine groups to facilitate the anchoring of UiO-66-NH2 by the Michael addition and Schiff base reactions. Subsequently, GO@UiO-66-NH2 dispersed in DMF solution was mixed with Nafion solution in DMF to obtain a GO@UiO-66-NH2/Nafion composite film. Through the comparative study of these membranes, GO/Nafion-0.6, UiO-66-NH2/Nafion-0.6 and GO +UiO-66-NH2/Nafion-0.6, the author observed that compared with the recast Nafion membranes, the σ value of these composite membranes is improved, but still cannot catch up with the proton conductivity of the GO@UiO-66-NH2/Nafion-0.6 film (σ: 0.303 S cm−1 at 90 °C and 95% RH) under the same test conditions. Moreover, GO@UiO-66-NH2/Nafion-0.6 can exhibit the highest anhydrous σ value of 3.403 × 10−3 S cm−1 at 120 °C. This indicates that the compound GO@UiO-66-NH2 obtained by the chemical reaction shows significant structural advantages after doping into the Nafion membrane. Obviously, homogeneous anchoring UiO-66-NH2 on the surface of GO is intensely beneficial to form a continuous proton transport channel. Moreover, the SO3H units from Nafion and the –NH2 units from UiO-66-NH2 can construct acid/base pairs providing the proton hopping sites. Additionally, UiO-66-NH2 has strong hydrophilicity. Thus, the above synergy leads to the composite film GO@UiO-66-NH2/Nafion-0.6 at high humidity or low humidity; even under anhydrous conditions it can efficiently transport protons. In addition, the authors also disclosed that when the doping amount of GO@UiO-66-NH2 is 0.6 wt%, the σ value of the composite membrane is the best; a lower or higher doping amount will lead to the performance degradation. The composite film GO@UiO-66-NH2/Nafion-0.6 also shows good methanol resistance and remarkable durability up to 54 hours.
Three year later, Y. Zheng and his colleagues made a covalent-ionically cross-linked SPENs/UiO-66-NH2 [SPENs = sulfonated poly(arylene ether nitrile)s] composite film and inspected its performance on the σ value, stability and MeOH permeability.60 As expected, the composite membrane displayed wonderful thermal and dimensional stability due to the crosslinking effect and high stability of Zr-MOFs. Naturally, the NH2 unit of UiO-66-NH2 is a good proton acceptor and donor, and thus the proton conductivity of the composite film can be greatly reinforced combining the interactions of NH2 units with SO3H and COOH of SPENs. For instance, the σ value of SPEN/UiO-66-NH2-5 can reach 1.351 × 10−1 S cm−1 at 80 °C in H2O, which is higher than that of the recast SPEN. The MeOH permeability of the composite film can be suppressed because of the barrier effect of cross-linking and UiO-66-NH2-x.
In 2020, W. Kang's group described that the MOF UiO-66-NH2 was firstly anchored with sulfonated poly(ether sulfone) (SPES) to prepare the UiO-66-NH2@NF nanofibers by the blend electrospinning approach.61 And then, they found that inside the nanofibers, –SO3H groups from SPES may form coherent proton transport channels with coordination of UiO-66-NH; in addition, the acid–base interaction between the –NH2 units from UiO-66-NH and the –SO3H units from SPES forms a channel-like ion cluster, which further promotes proton transport. These outstanding structural and performance advantages prompted the authors to further dope these nanofibers in the Nafion membrane to obtain a novel composite membrane with better performance. According to a series of determinations, such as SEM, TEM, PXRD, water adsorption, swelling ratio and AC impedance, the authors believed that the composite film UiO-66-NH2@NFs-8/Nafion with the loading amount of the MOF at 8% shows the best performance, and the σ value can reach 0.27 S cm−1 at 80 °C and 100% RH and excellent MeOH tolerability. The authors considered that the affinity between the nanofibers and Nafion membranes and the ability of the Zr-based MOF to capture methanol lead to the good resistance of the composite membrane to methanol permeability. Consequently, the composite film was applied to DMFC, and its OCV and the highest power density were 0.817 V and 95.49 mW cm−2, respectively. These results indicate that the method of introducing Zr-MOFs to anchor nanofibers is worthy of further reference in the preparation of high-performance composite membranes in the future.
P. Y. Wu and co-workers first prepared the hybrid nanosheets GO@UiO-66-SO3H by a simple method of in situ growth and thereafter doped it into the organic polymer poly(ether ether ketone) (SPEEK) to prepare the composite film in 2017.62 As expected, the best σ value of the SPEEK/UiO-66-SO3H@GO-10 membrane can be 0.268 S cm−1 at 70 °C and 95% RH, which is 2.6 times higher than that of the recast SPEEK. Like the above doped Zr-MOF-based composite films,43–45 the film SPEEK/UiO-66-SO3H@GO-10 shows strong mechanical properties and excellent methanol tolerance. As presented in Fig. 4, the authors proposed the feasible proton conduction mechanism.
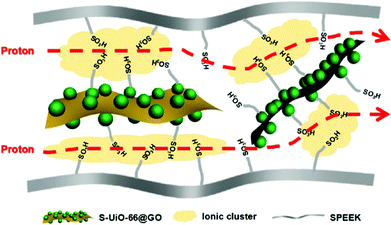 | ||
| Fig. 4 Schematic diagram of the enhanced transport properties of GO@UiO-66-SO3H/SPEEK. Reproduced with permission from ref. 62. Copyright 2017, American Chemical Society. | ||
First, the in situ growth method ensures uniform distribution of UiO-66-SO3H on the GO surface, which provides a stable and continuous proton transport channel. Second, numerous sulfonic acid groups from SPEEK and UiO-66-SO3H interact with each other to construct a denser and richer network of H-bonds with adsorbed H2O units. Eventually, under the interaction of hydrophilic and hydrogen bonding between UiO-66-SO3H@GO nanosheets and SPEEK, ion clusters and nanochannels are expanded and perfected, which is also very conducive to proton transport. To sum up, under the synergistic action of these functional units, the proton conduction efficiency of the composite membrane is greatly improved. Finally, MeOH crossover of the composite film was well reduced because of the barrier effect of UiO-66-SO3H@GO nanosheets.
In 2017, the effects of the crystal size and filling amount of UiO-66 and its sulfonated product UiO-66-SO3H on the properties of the Nafion membrane were examined by F. Costantino et al.63 For the UiO-66/Nafion film, they found that the composite film bearing a larger crystal (about 200 nm) in a low filling amount of 2% can attain the highest σ value of 0.207 S cm−1 at 110 °C and 95% RH. The proton conductivity is reduced if the doped crystal is in small size (e.g. 20 nm) or if the filling amount is less than or greater than 2%. This conclusion is contrary to the conclusions of the previous papers,43–48 which indicates the complexity of the research on proton conductivity in composite membranes. For the UiO-66-SO3H/Nafion film, its σ value can reach 0.189 S cm−1 at 110 °C and 95% RH when the filler loading is 2%, which is lower that of UiO-66/Nafion-2 and slightly higher than that of pure Nafion (0.162 S cm−1). This is also an anomaly, as the sulfonic acid functionalized zirconium complex UiO-66-SO3H in the Nafion membrane should be more conducive to the formation of rich H-bonded networks. The authors believed that UiO-66 acted as a modifier of the ionomer structural characteristics, which makes the proton conductivity enhance, and UiO-66-SO3H may affect the σ value of the corresponding composite membrane in terms of its hydrophilicity and functional groups.
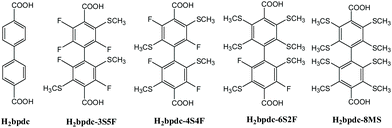
2.2. UiO-67 series MOFs
Because the MOF UiO-67 has a similar crystal structure to UiO-66, except that it employs biphenyl-4,4′-dicarboxylic acid (H2bpdc) replacing p-H2BDC, like UiO-66, UiO-67 has a mediocre proton conductivity. The reported proton conductive functionalized UiO-67 MOFs are very limited. Scheme 2 gives organic linkages in the UiO-67-based MOFs.In 2015, L. Liu's group synthesized the MOF UiO-67 in accordance with the previously reported method,48 and secondarily introduced imidazole (Him) units into the channels of this MOF by the evaporation method.65 The imidazole molecules were filled in the micropores of the MOF and the framework structure of Him@UiO-67 is similar to UiO-67. The results showed that the σ value of UiO-67 was negligible in the measurement temperature range, and Him@UiO-67 indicated a high σ value under anhydrous conditions, which may be because the introduction of imidazole increased the concentration of the proton carrier. Thus the σ value is positively related to the imidazole loading ratio and increasing temperature. The doped MOF Him@UiO-67 attains a maximum σ value of 1.52 × 10−3 S cm−1 at 130 °C under anhydrous conditions and a lower Ea value of 0.36 eV. Nevertheless, as the temperature is higher than 130 °C, the σ value began to decrease, which is possibly because the imidazole released from the micropores under high temperature conditions.
Obviously, the imidazole group in the framework not only provides the proton source, but also forms a hydrogen bond network with the framework component or constitutes a hydrogen bond network with each other to promote the proton hopping.
In 2020, J. He and co-workers obtained four boiling-water-stable modified UiO-67-based MOFs, Zr-bpdc-3S5F, Zr-bpdc-4S4F, Zr-bpdc-6S2F and Zr-bpdc-8MS through solvothermal synthesis with a systematic region-specific sulfur substituent (Scheme 2) and ZrCl4 as starting materials.66 Among them, a single crystal appropriate for X-ray diffraction for Zr-bpdc-4S4F is acquired. The framework of Zr-bpdc-4S4F is basically the same as UiO-67, in which the secondary building unit (SBU) is a square-antiprismatic geometry formed by Zr4+ and the oxygen atoms in μ3-O, μ3-OH, –COOH units. Each SBU is connected to others through the ligand H2bpdc-4S4F to form a ccp structure. The four MOFs exposed to air and dipped in boiling water were subjected to PXRD and gas adsorption measurements. The results depicted that they all showed excellent stability.
The researchers used 30% H2O2 to oxidize Zr-bpdc-4S4F to Zr-bpdc-4SO2Me4F, in which four sulfide groups can be converted to sulfone functional groups confirmed by IR, 1H and 19F NMR determinations. Note that the framework of Zr-bpdc-4S4F was not damaged. The AC impedance of the two MOFs is measured. The results exhibit that the proton conductivity of Zr-bpdc-4SO2Me4F is superior to its prototype. For example, Zr-bpdc-4SO2Me4F has a σ value of 1.75 × 10−4 S cm−1 at 80 °C and 90% RH, which is about 1000 times higher than that of Zr-bpdc-4S4F. Apparently, a higher σ value of Zr-bpdc-4SO2Me4F may be due to the increased hydrophilicity of these sulfone groups.
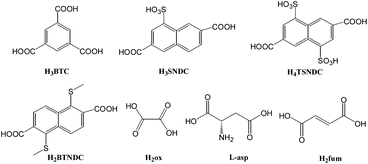
2.3. Other carboxylate-based Zr-MOFs
The proton conductivity of zirconium MOFs constructed from other carboxyl compounds (Scheme 3) is rarely studied, so it is summarized in this chapter.In 2017, X. M. Ren's group used H3BTC to prepare MOF-80868 in terms of the reported method.67 The authors found that the compound remained structurally stable after immersion in ambient water or DMF for seven days, but decreased crystallinity after immersion in 80 °C hot water for four hours, indicating less stability in hot water. The proton conduction data of MOF-808 at 99% RH and between 17 and 54 °C were tested by using a pressed sample tablet. The σ value of the MOF increases between 17 and 42 °C with the increase of temperature, and decreases above 42 °C. This phenomenon is obviously related to the structural instability of MOF-808 accompanied by guest water molecules at high temperature. At 42 °C and 99% RH, the σ value is 7.58 × 10−3 S cm−1. Interestingly, this compound displayed a high σ value at low temperatures. As an example, at 25 °C and 99% RH, its σ value can be 3.14 × 10−3 S cm−1. Subsequently, the researchers further measured the effect of humidity on the proton conductivity of MOF-808 at different humidity at 25 °C. The experimental data showed that the σ value has a serious dependence on humidity. Obviously, the adsorbed H2O units in the lattice could establish a stable H-bonded network providing an effective way for proton transmission. Additionally, the Ea value of MOF-808 is 0.37 eV showing that proton transport is mainly dominated by the Grotthuss mechanism.
Subsequently, this group fabricated a composite film MOF-808@PVDF-x (x denoting the mass percentage of MOF-808) by the casting method of mixing poly(vinylidene fluoride) (PVDF) and MOF-808 in different mass percentages. PXRD and SEM analysis disclosed that the crystal structure of MOF-808 remained well and was distributed evenly in the membrane. MOF-808@PVDF-x (x = 10, 25, 40, and 55) was immersed in deionized H2O to measure its σ values. It was observed that the σ value of the composite membrane has certain temperature dependence, and also improved with the increase of the mass percentage of MOF-808. When the mass percentage was 55 wt%, the proton conductivity reached a maximum value of 1.56 × 10−4 S cm−1 at 65 °C. In addition, by comparing the σ value of the composite membrane with that of the pure PVDF membrane under the same conditions, it can also be discovered that the addition of MOF-808 improves the σ value of the composite membrane. After immersion of MOF-808@PVDF-55 in deionized H2O for five days, it can still maintain high proton conductivity, indicating that the hybrid membrane has good durability. In addition, the Ea value of MOF-808@PVDF-x (x = 10, 25, 40, and 55) is less than 0.40 eV implying a Grotthuss mechanism.
Two years later, H. N. Wang and co-workers modified MOF-808 with organic acids, ethylenediaminetetraacetic acid (EDTA) and H2ox by the post-synthesis method and compared the proton conductivity of MOF-808, MOF-808-EDTA and MOF-808-ox.69 What puzzled us was that the σ value of MOF-808 they reported was much lower than that reported by Ren's group;68 for example, the σ value of MOF-808 reported by Wang's group is 1.25 × 10−6 S cm−1 at 30 °C and 98% RH, and the value reported by Ren's group is 3.14 × 10−3 S cm−1 at 25 °C and 98% RH. In addition, Wang's group believed that the σ value of MOF-808 increased with the increase of temperature, but Ren et al. believed that the σ value decreased when the temperature was higher than 42 °C. We hypothesized that this may be due to different test methods or sample treatment methods, but the very different conductivity values remind us that the performance study of Zr-MOFs is complex and requires more care. Let's take a look at the results of comparing the investigation of MOF-808, MOF-808-EDTA and MOF-808-ox.55 by Wang's group. They found that after anchoring EDTA of H2ox units, the proton conductivity can be augmented. For instance, at 80 °C and 98% RH, the proton conductivities of MOF-808, MOF-808-EDTA and MOF-808-ox are 0.897 × 10−5, 1.31 × 10−4 and 4.25 × 10−4 S cm−1, respectively. Additionally, the Ea values for the latter two modified MOFs are lower than that of MOF-808. Obviously, the two organic acids introduced into the framework can constitute hydrogen bond networks with adsorbed H2O units to facilitate proton conduction; especially rigid oxalic acid can build up more efficient hydrogen bonded transport channels. In conclusion, the authors provide an effective method to optimize the proton conduction of the Zr-MOFs through post-synthesis, which is worthy of reference. They also studied the properties of the composite film, MOF-808-ox@PVA-x, and noticed that as x = 3, the σ value (2.03 × 10−5 S cm−1) is the highest at 80 °C in water.
In 2016, H. A. Patel et al.70 reported that the sulfonated MOF-808 (SZM) that was synthesized by a literature approach,71 was mixed with Nafion by the casting method. The composite film is denoted as Naf-SZM, in which the loading amount of SZM is assumed to be 1, 5, 7.5 and 10 wt%. From the SEM images of the composite films, it was found that when the SZM concentration exceeded 5 wt%, pinholes and cracks appeared in the film, and the compatibility of SZM and Nafion would also decrease. Finally, it was found that 1 wt% was the optimal concentration of SZM, and the composite film under this condition also had good stability at 300 °C. When the humidity is 35% RH, the composite membrane shows better proton conductivity and higher performance stability than Nafion. They believed that SZM's superacidity sites improve the water uptake in the film and thus are helpful for long-distance proton conduction. By monitoring the OCV 24 hours, they discovered that the composite film Naf-1SZM showed high performance stability at low humidity (35% RH) and 80 °C in fuel cells. This experimental result once again proves that the acidic sites introduced by post-treatment can boost the σ value of the Zr-MOFs and the performance of the corresponding composite membranes, which is very beneficial for practical application in the future.
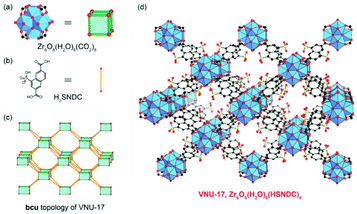 | ||
| Fig. 5 The crystal structure of VNU-17 is built by eight-connected, cubic Zr6O8(H2O)8(COO)8 clusters (a) joined by linear, ditopic HSNDC2− linkers (b) to construct a structure showing the bcu topology (c, view from the [001] plane). The framework of VNU-17, Zr6O8(H2O)8(HSNDC)4, is depicted (d). Atom colors: Zr, blue polyhedra; C, black; O, red; S, yellow. All H atoms are omitted for clarity. Reproduced with permission from ref. 72. Copyright 2017, the Partner Organisations. | ||
Furthermore, ac impedance spectroscopy measurements indicated that at a fixed temperature of 70 °C, the σ values of the three MOFs are humidity dependent, and increase with the increasing RH. Among them, the σ value of Him11@VNU-17 is the largest, which attains a maximum value of 5.93 × 10−3 S cm−1 under 98% RH, and the smallest is VNU-17. Note that over all RH ranges, Him11@VNU-17 with higher loading always has a higher σ value than Him9@VNU-17 with lower loading. Another thing to note is that the proton conductivity of Him11@VNU-17 is 900 times higher than that of the parent complex VNU-17 under 70 °C and 98% RH. Although sulfonic acid groups provide protons more easily than imidazolium ions, the high polarity of imidazolium ions completely hydrates the entire pores and grain boundaries and promotes the formation of a proton conductive network, so the proton conductivity of anchored MOFs is greater than that of unanchored MOFs. Note that the σ value of Him11@VNU-17 can be maintained for at least 40 hours and the structure does not change, which proves that its stability and durability of proton conductivity are excellent. In addition, the calculated Ea values of Him11@VNU-17, Him9@VNU-17 and VNU-17 are 0.27, 0.44 and 0.47 eV, respectively. This shows once again that Him11@VNU-17 with a high loading of Him is more likely to form a dense hydrogen bond network, which is conducive to proton hopping.
Later, the same group prepared a similar MOF [Zr6O8(H2O)8(H2TSNDC)4] namely VNU-23 by using 4,8-disulfonaphthalene-2,6-dicarboxylic acid (H4TSNDC) and ZrOCl2·8H2O,73 whose structure is similar to VNU-17, and the Zr6O8(H2O)8(COO)8 cluster is connected by TSNDC4− linkages. By using a simple method similar to that described above, VNU-23 was immersed in 0.5 M histamine (His) methanol solution for three days. Subsequently, His was anchored on VNU-23 to obtain His8.2@VNU-23 confirmed by 1H NMR, EA and single crystal X-ray diffraction. The protonated histamine can interact with SO3H units as well as coordinate with H2O units to constitute extended hydrogen bond networks. The σ value of His8.2@VNU-23 is greater than that of VNU-23, and the maximum value is 1.79 × 10−2 S cm−1 at 90 °C and 85% RH. When exploring the effect of temperature on the proton conductivity of His8.2@VNU-23, it was found that the σ value did not continue to decrease at 90–30 °C, but there was a stepwise increase at 60–50 °C. This situation may be related to the protonated histamine rearrangement. His8.2@VNU-23 has a lower Ea value of 0.27 eV suggesting that the Grotthuss mechanism can be observed.
In 2020, by adopting 1,5-bis(methylthio)naphthalene-2,6-dicarboxylic acid (H2BTNDC) and ZrCl4, J. He and co-workers solvothermally synthesized a porous MOF {[Zr6O8(H2O)8(BTNDC)4]·9DMF}n, namely Zr-BTNDC,74 in which 8-connected Zr6O8(H2O)8(COO)8 clusters were connected by BTNDC2− ligands forming a three-dimensional network with 6 Å pore channels. The oxidized product Zr-BTNDC-ox was obtained by stirring Zr-BTNDC in a solution of 30% H2O2 aqueous solution for one day. 1H NMR spectra confirmed that SCH3 units were oxidized to sulfoxide or sulfone units. The PXRD pattern of the oxidized product is almost the same as that of Zr-BTNDC, indicating that the structure has not changed. Consequently, Zr-BTNDC-ox was put into 0.05 M sulfuric acid solution for seven hours to get the acidic framework, H@Zr-BTNDC-ox. The PXRD test indicates that the structure of the acidized product has not changed but the crystallinity has decreased. Further electrochemical measurements showed that the post-treatment method (oxidation and acidification) enhanced the σ value of the resulting MOFs. It should be noted that the proton conductivity of the three complexes improves with the rise of temperature or humidity. Under similar conditions, the σ value of H@Zr-BTNDC-ox is 3.16 × 10−2 S cm−1 (90 °C and 95% RH), which is almost 10 times higher than that of Zr-BTNDC-ox (4.03 × 10−3 S cm−1 at 95 °C and 95% RH), and is 400 times higher than that of Zr-BTNDC (7.88 × 10−5 S cm−1 at 90 °C and 95% RH). At the same time, the Ea values of the three complexes are lower than 0.4 eV at both 85% and 95% RH, indicating that proton conduction in the frameworks follows the Grotthuss mechanism. From the structural analysis, it is easy to understand this phenomenon. In the acidification and oxidation of MOFs, H2O units, sulfones, sulfoxide and sulfate groups can all act as proton carriers and interact to form more complex hydrogen bond networks, which are more conducive to proton transport. In pristine Zr-BTNDC, only coordinated H2O and absorbed H2O units can take part in the proton transport.
In 2020, considering that the pore size (7.4 Å) of MOF-801 is suitable for the introduction of imidazole units (4.3 × 3.7 Å2), Z. Zhang and co-workers introduced imidazole into this framework in two different ways (impregnation and in situ methods) to prepare two related MOFs, Him@MOF-801 with free imidazole units incorporating inside the pores and Him-MOF-801 with imidazole coordinating with the zirconium atoms.78 PXRD determinations demonstrated that the structures of the three compounds are basically the same, although the imidazole introduced has a slight effect on the strength and position of some diffraction peaks. Moreover, after soaking in 80 °C H2O and 1 M hydrochloric acid aqueous solution for seven days, the two compounds Him@MOF-801 and Him-MOF-801 remained structurally rigid. This lays a material foundation for their application in membrane systems.
Subsequently, this group employed the solution casting approach to make hybrid membranes with Him@MOF-801 or Him-MOF-801 as fillers and sulfonated poly(arylene ether ketone sulfone) containing carboxyl groups (C-SPAEKS) as the organic matrix. The composite membrane can be expressed as C-SPAEKS/Him@MOF-801-X and C-SPAEKS/Him-MOF-801-X (X presents the mass percentage of MOFs). Since the doped MOFs are combined with C-SPAEKS through hydrogen bonding, the stability of the hybrid membrane is higher than that of the pure C-SPAEKS film. Meanwhile, the addition of MOFs increases the water absorption of the hybrid membrane and has a positive correlation with the mass percentage of MOFs. On this basis, the σ value of the hybrid membrane was explored. At 100% RH and 30–90 °C, the σ value of the hybrid membrane increases with increasing temperature. Comparing with the σ value of pure C-SPAEKS under the same testing conditions, the addition of MOFs improved the proton conductivity of the hybrid membrane, and 4% is the optimal filling amount of MOFs. At 100% RH and 90 °C, C-SPAEKS/Him-MOF-801-4 reached the maximum σ value of 0.128 S cm−1, which was twice that of C-SPAEKS/Him@MOF-801-4. In general, the two composite films exhibited high proton conductivity. This manifests that both the free imidazole groups in the pores and the coordination imidazole groups in the frameworks play a key role in improving the proton conductivity. The difference in proton conductivity between the two composite membranes is that the imidazole involved in coordination is more likely to provide protons than coordination H2O units. A C-SPAEKS/Him-MOF-801-4 film was used in DMFC to determine its performance. The OCV at 80 °C was 0.75 V, and the maximum power reached 15.4 mW cm−2.
In one word, through the introduction of imidazole units into Zr-MOFs (UiO-67,65VNU-1772 or MOF-80176), we can find that both free imidazole groups in the pores and imidazole units involved in the coordination of zirconium ions, all the resulting MOFs indicate a greatly improved proton conductivity under hydrous or anhydrous conditions. It can be seen that this is an efficient strategy to strengthen the proton conductivity of zirconium-based MOFs.
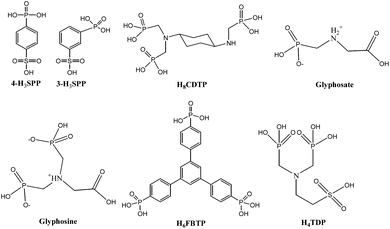
2.4. Phosphate-based MOFs
The organic ligands containing phosphate groups usually exhibit a strong coordination ability and various coordination modes by protonated species, so a large number of phosphate-based MOFs can be constructed. L. M. Zheng's group has reviewed the proton conductive phosphate-based MOFs.32 Nevertheless, the research of proton conduction in zirconium-based phosphate MOFs is very limited. Scheme 4 presents phosphate ligands used in proton conducting Zr-MOFs.In 2005, G. Alberti and his colleagues prepared a series of Zr-MOFs with the general formula Zr(HPO4)2−x(3-HSPP)x·nH2O (3-HSPP = 3-sulfophenyl phosphoric acid; x in the range 0.4–1.35).79 From the PXRD patterns of these MOFs, they speculated that these compounds were layered structures. Proton conductivity investigations indicated that as x = 1.35, the MOF had a maximum σ value of 0.04 S cm−1 at 100 °C and 70% RH. Obviously, phosphate groups present are responsible for the high σ value of the MOF Zr(HPO4)0.65(3-HSPP)1.35·nH2O.
Five years later, Z. Li and co-workers prepared SPPESK/ZrSPP composite membranes composed of sulfonated poly(phthalate ether sulfone ketone) (SPPESK) and the MOF ZrSPP bearing 3-H3SPP ligands by solution casting.80ZrSPP exhibits a layered structure,81 is insoluble in H2O, has good thermal stability, and is an excellent alcohol barrier. ZrSPP can disperse uniformly in SPPESK, and strong H-bonds can be formed between them. The TGA curve indicated that the composite membrane has high enough stability to be used in DMFCs. The AC impedance experiment was performed on composite membranes with different ZrSPP loadings. As the temperature increased, the σ value of the composite membranes increased significantly (>80 °C). Compared with the pure SPPESK membrane, the σ value of the composite membrane is higher, and the σ value of the composite membrane decreases with the increase of the ZrSPP loading. When the temperature was lower than 80 °C, the proton conductivity of SPPESK(DS34.6%)/ZrSPP (DS means degrees of sulfonation of SPPESK) is lower than that of SPPESK(DS76%)/ZrSPP, indicating that SPPESK plays a major role in proton conduction at low temperature, and a higher DS will have more sulfonic acid units support proton transfer. As the temperature continued to rise, it was found that the maximum value appeared at 120 °C, which may be due to the hydrophilicity of SO3H, and the composite membrane with a high DS was easy to expand, so its structure could not be maintained for a long time at high temperature. In addition, the researchers found that with the addition of ZrSPP, the elasticity of the composite membrane will become weaker, but at the same time it will also increase the oxidation resistance of the composite membrane. In general, a low DS of SPPESK and a high concentration of ZrSPP are beneficial to improve the thermal stability of the composite membrane. The hybrid membrane prepared by this group not only has a good σ value and low MeOH permeability, but also can increase the thermal stability of the membrane by adjusting the amount of raw materials, laying a foundation for future practical applications.
In 2010, V. Zima's group82 successfully synthesized two proton conductive layered MOFs, Zr(HO3SC6H4PO3)2·2H2O and Zr(HPO4)0.7(HO3SC6H4PO3)1.3·2H2O under hydrothermal conditions by using a similar ligand 4-sulfophenyl phosphoric acid (4-H3SPP) and ZrOCl2·8H2O. Adopting PXRD patterns, they also speculated that the two compounds were layered structures, in which an interlayer distance of about 19.9 Å can be calculated, and the Zr4+ and six oxygen atoms of the phosphonate group form an octahedral geometry. The σ value of the two MOFs is sensitive to humidity and temperature variations. That is, their proton conductivity rises with temperature or humidity. It's important to note that the σ value of Zr(HPO4)0.7(HO3SC6H4PO3)1.3·2H2O is much higher than that of Zr(HO3SC6H4PO3)2·2H2O under the same test conditions. They suggested that the presence of phosphate groups in Zr(HPO4)0.7(HO3SC6H4PO3)1.3·2H2O leads to structural disorder that increases the number of “labile” protons and changes their behavior compared to Zr(HO3SC6H4PO3)2·2H2O, and that Zr(HPO4)0.7(HO3SC6H4PO3)1.3·2H2O can absorb more water molecules than Zr(HO3SC6H4PO3)2·2H2O, which results in an increase in the σ value. Nevertheless, both the two MOFs not only show high proton conductivities, but also are insoluble in H2O and have high thermal stability. They are all expected to become a component of the proton exchange membrane of the fuel cell.
In 2012, F. Costantino and co-workers reported two open framework Zr-MOFs, Zr(H4CDTP)2Na2H2·5H2O or Zr(H4CDTP)2(NH4)2H2·5H2O (denoted as 1_lp@Na and 1_lp@NH4, respectively; lp means large pore) (H8CDTP = cyclohexyl-N,N,N′,N′-diamino tetraphosphoric acid, Scheme 4) under solvothermal conditions.83 The flexible MOF has rectangular channels of size 12 × 5 Å, which are occupied by five H2O units and two Na+ or NH4+ cations per formula unit, and with eight PO3C tetrahedra pointing to the inner space. The fully protonated phase 1_lp@H could be acquired by putting 1_lp@Na and 1_lp@NH4 into 0.2 M HCl aqueous solution. Then the authors found that there was a phase transition process: when 1_lp@H is heated above 150 °C, an anhydrous phase (hereafter 1_an) can be obtained. However, when 1_lp@Na or 1_lp@NH4 was heated, 1_an could not be obtained. This is because the cations in 1_lp@Na or 1_lp@NH4 can retain H2O and template the framework. Interestingly, after dipping 1_an into H2O for a few minutes or putting aside in air for 3–4 days, a new phase 1_np@H (np means narrow pore) can be produced. After heating 1_np@H up to 150 °C, phase 1_an can be recovered. They adopted PXRD determinations and Rietveld refinements to explain the transformation mechanisms. As indicated in Fig. 6, in the compounds 1_lp@H and 1_np@H, the position of crystallization H2O units in the cavity and the hydrogen bond formed changed obviously.
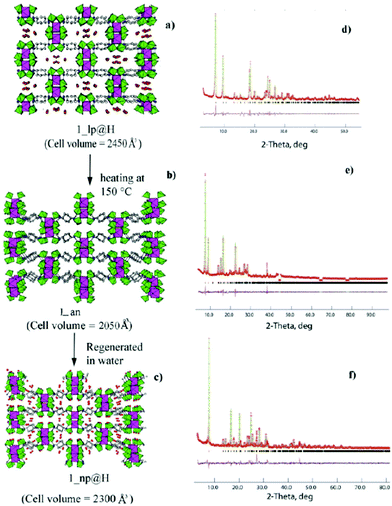 | ||
| Fig. 6 Structure representation and the corresponding Rietveld plots for 1_lp@H (a), 1_an (b), and 1_np@H (c). Note that 1_lp@H and 1_np@H are full crystallographic structures whereas 1_an is only a possible structural model based on the cell parameters. Reproduced with permission from ref. 83. Copyright 2012, American Chemical Society. | ||
Consequently, they further explored the proton conductivity of 1_lp@H and 1_np@H, and found that at 95% RH, the σ value of 1_lp@H changed from 2.6 × 10−5 (30 °C) to 5.4 × 10−5 S cm−1 (80 °C), and the value of 1_np@H varied from 1.5 × 10−6 (30 °C) to 6.6 × 10−6 (80 °C) S cm−1. They believed that there is a strong hydrogen bond system in 1_lp@H and the stretching along two directions is conducive to the hopping of protons, while the hydrogen bond in 1_np@H is weak and has only one direction, resulting in a weaker conductivity of 1_np@H than 1_lp@H.
In 2013, three MOFs, ZrF[H3(O3PCH2NHCH2COO)2] (G1), Zr3H8[(O3PCH2)2NCH2COO]4·2H2O (G2), and Zr[(O3PCH2)(HO3PCH2)NHCH2COOH]2·2H2O (G3) with different structures were prepared by using two phosphono-amino acid ligands (glyphosine and glyphosate).84 The crystal structures of G1 and G2 were obtained from ab initio PXRD data. The crystal structure of G3 was obtained by single crystal X-ray diffraction. Structural analyses display that G1 is a 1D ribbon-like framework, while G2 shows a layered structure and G3 indicates a 3D framework. The trend of the proton conductivity of the three MOFs with temperature change was measured at 95% RH. It was found that the σ value of G2 increased slightly with temperature rise, while that of G1 and G3 increased significantly, increasing by 4 and 10 times, respectively. The σ value of G3 is at least an order of magnitude lower than that of G1 and G2. The authors believed that the large surface area of G1 and G2 in comparison with G3 may lead to their higher σ values. During the measurement, it was found that the proton conductivity and hydration of G2 had a weak dependence on temperature. Using the Arrhenius equation, the calculated Ea value of G2 was 0.10 eV, and the conduction mechanism was the Grotthuss mechanism. Note that the highest σ value of G1 and G2 reaches up to 10−3 S cm−1 at 140 °C and 95% RH. As they have a good σ value, and high thermal stability, they are expected to be good proton conductive materials.
In 2014, the same group still used glyphosine as a starting material to synthesize a new MOF under mild reaction conditions, whose molecular formula is Zr2(PO4)H5((O3PCH2)2NCH2COO)2·H2O (ZPGly).85 Its structure was also obtained by the calculation method as previously reported.82 The layered structure of ZPGly is composed of a ZrO6 octahedron built by tetradentate PO4 groups and Zr4+ atoms. The uncoordinated –COOH and P–OH units are exposed to the sheet surface. Obviously, the large number of these hydrophilic groups between layers will contribute greatly to proton conduction. As expected, the σ value of this MOF is highly dependent on RH and can attain the highest value of 1 × 10−3 S cm−1 at 140 °C and 95% RH. In addition, the researchers investigated the hydration of this MOF in different humidity ranges and found that the overall hydration of ZPGly has little effect on the σ value, and the change in conductivity only reflects the change in the hydration of the crystal surface. It can be said that the σ value is mainly determined by surface proton transmission. Using the Arrhenius equation to calculate its Ea being 0.15 eV, they think that its proton conduction obeys a Grotthuss mechanism.
In 2017, by employing a rigid triangulated phosphate ligand 2,4,6-tris(4-phosphonophenyl)pyridine (H6FBTP), Z. H. Fard and co-workers solvothermally prepared a dense MOF, (DMA)3[Zr(HFBTP)F2] (DMA = dimethylammonium),86 in which the ZrO4F2 octahedron and PO3C tetrahedron are joined by organic ligands to build up a double-layer structure. The double-layer extends along the a-axis and stacks along the c-axis in AAA sequence. AC impedance determinations showed that the σ value of (DMA)3[Zr(HFBTP)F2] had a certain dependence on temperature, reaching a maximum value of 1 × 10−2 S cm−1 at 80 °C and 95% RH. Note that the initial σ value of this MOF is about 10−5 S cm−1 under 20 °C and 95% RH. Continuous heating/cooling cycles indicated that the σ value of this MOF only can be 3 × 10−3 S cm−1 under the same conditions, which never get back to the original number of 10−5 S cm−1. Thus, the researchers suggested that the phase change happened to this MOF from 20–70 °C and the phase was named PCMOF20. To study PCMOF20 more deeply, the authors re-prepared PCMOF20, and found that PCMOF20 has higher porosity, thermal stability and water stability. In addition, the structure of PCMOF20 had been determined, whose coordination environment is similar to (DMA)3[Zr(HFBTP)F2], and the distance and direction of aromatic hydrocarbons changed relative to (DMA)3[Zr(HFBTP)F2]. The migration of protons in PCMOF20 is highly dependent on water molecules, and there is almost no conductivity under anhydrous conditions, but the proton conductivity will increase with increasing humidity. The ultrahigh proton conductivity and stability of PCMOF20 indicate that it has great application prospects in the field of fuel cells.
Recently, K. Melánová et al.87 successfully prepared a series of mixed phosphate organophosphonate MOFs, Zr(PO4)-(H2PO4)1–2x(H2TDP)x·yH2O (x = 0.15, 0.34, 0.45; y = 2, 1.5) by controlling the γ-ZrP/H4TDP reaction ratio (γ-ZrP denotes the γ-modification compound Zr(PO4)(H2PO4)·2H2O, which was synthesized by a previous literature method;88 H4TDP = 2-bis(phosphonomethyl)amino-ethan-1-sulfonic acid). The layered structure of γ-ZrP includes ZrO6 octahedra placed in two different planes and connected to each other with a tetradentate PO4 inside and H2PO4 units outside these planes. For the parent γ-ZrP, the σ value is derived from the hydrogen bonding network formed by the interlayer H2O units and the OH units of the external H2PO4− group. As the H2TDP2− units replaced some of the H2PO4− units, the hydrogen bonding network was damaged, and at the same time, the mobility of protons between the layers increased, resulting in an increase in σ values. When x is equal to 0.15, the proton conductivity of Zr(PO4)(H2PO4)0.70(H2TDP)0.15·2H2O is the highest. In contrast, if the H2PO4− units are continually replaced, its proton conductivity will decrease, because the concentration and fluidity of unstable protons will decrease at this time. Overall, this series of MOFs shows negligible proton conductivity of about 10−5 S cm−1.
2.5. Phenolic hydroxyl group-based MOFs
Up to now, there are few reports on Zr-MOFs constructed from phenolic hydroxyl ligands (Scheme 5) in the literature, but these compounds often show super high structural stability, which is very helpful for conducting proton research.In 2016, P. G. M. Mileo et al. synthesized [{Zr2(H2-TzGal)2}·(solvent)n, solvent = DMA and H2O, H6TzGal = 5,5′-(1,2,4,5-tetrazine-3,6-diyl)bis(benzene-1,2,3-triol)] (denoted as MIL-163) according to the reported method,89 and explored its proton conductivity by experimental-modeling methods.90 MIL-163 is a 3D open structure with square channels (aperture = 12 Å). Each Zr4+ ion is coordinated with eight oxygen atoms from four different H2TzGal4− anions to form a ZrO8 polyhedron with shared edges, and these polyhedra extend along the c-axis into the ZrO8 chain. These chains are further connected by H2TzGal4− anions to build up a 3D structure. The proton conductivity of MIL-163 is very low under anhydrous conditions, which may be due to the lack of a conductive medium and the ineffective conduction of charge carriers. After increasing the humidity, the proton conductivity has been greatly improved, reaching the maximum value of 2.1 × 10−3 S cm−1 at 90 °C and 95% RH, indicating that the σ value of this MOF has a strong dependence on RH. In addition, the Ea value of MIL-163 is 0.25 eV. Monte Carlo simulation of MIL-163 found that a 3D H-bonding network can be formed inside the square channels, thereby generating multiple proton transport pathways, offering the best solution for the H2O-mediated proton transport provided by phenol in the organic linker. At the same time, guest DMA units tend to form a H-bonded network with H2O units inside the channels. These all contribute to the transport of protons, so MIL-163 has good proton conductivity.
In 2017, E.-X. Chen et al.91 reported two MOFs, Zr2(THPP)·(solvent) (namely ZrPP-1) and Zr2(THBPP)·(solvent) (namely ZrPP-2) (THPP = 5,10,15,20-tetrakis-(3,4,5-trihydroxyphenyl)porphyrin, THBPP = 5,10,15,20-tetrakis(3,4,5-trihydroxybiphenyl)porphyrin, and solvent = NH(Me)2 and H2O. The structures of the two MOFs are three-dimensional frameworks with nbo topology. ZrIV-pyrogallate chains are running along the c-direction. Moreover, these rod-like chains are connected across porphyrinic spacers forming an nbo-type bearing elliptical pores (aperture ≈8 × 4 Å2). The two MOFs have good chemical stability and can resist the contact of wet and even saturated NaOH aqueous solution. The σ value of the two MOFs was determined at various temperatures and humidity. The results showed that the σ value increased with the increase of humidity, attaining the best value at 25 °C and 98% RH (for ZrPP-1: σ = 8.0 × 10−3 S cm−1; for ZrPP-2: σ = 4.2 × 10−3 S cm−1). Note that their good proton conductivity can be repeated at least in two successive measurements without significant changes. The high σ value of the two MOFs may be due to the existence of a large number of proton sources, such as acidic groups –OH, dimethylamine cations, and lattice water molecules. In addition, the calculated Ea values of MOFs are less than 0.40 eV (ZrPP-1 and ZrPP-2 being 0.21 and 0.23 eV, respectively) demonstrating a proton conducting Grotthuss mechanism.
In conclusion, the remarkable stability and high proton conductivity demonstrated by such compounds suggest their great promise in the field of proton conductivity.
3. Conclusions
Recently, a great deal of work have been carried out on the proton conductivity of crystalline MOF materials, but some of the shortcomings of MOFs, such as low stability and unstable bonding, have led to the bottleneck of the research. Therefore, zirconium-based MOFs with high structural stability, such as super water stability, high acid–base tolerance and high thermal stability, have attracted much attention. In this review, we classify and summarize Zr-based MOFs according to the type of organic ligand used (aromatic carboxylic acid, fatty carboxylic acid, phosphoric acid, sulfonic acid, etc.). Their structural characteristics, synthesis strategies, structural stability, and proton conductivity characteristics, especially the conductive mechanism, are summarized and discussed in detail. Hopefully, this review will help researchers to have a comprehensive understanding of the latest developments in proton conducting zirconium MOFs. For future research, we have the following suggestions: first, people should choose as many kinds of organic ligands as possible (such as asymmetric carboxylic acids, ferrocene-based carboxylic acids, etc.) to construct more Zr-MOFs, so as to facilitate the research of proton conductivity. Second, so far, there are few studies on proton conductivity using single crystal Zr-MOFs, which limits the in-depth understanding of the conduction mechanism. It is hoped that more single crystal products can be obtained for proton conduction research in the next step. Third, it is hoped that more Zr-MOFs can be applied to composite membranes and fuel cells to study their application values. In a word, Zr-MOFs are promising proton conducting crystalline materials with great application prospects, which are worthy of further study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the National Science Foundation of China (21571156).References
- V. F. Valdes-Lopez, T. Mason, P. R. Shearing and D. J. L. Brett, Carbon monoxide poisoning and mitigation strategies for polymer electrolyte membrane fuel cells - A review, Prog. Energy Combust. Sci., 2020, 79, 100842 CrossRef.
- M. Shabani, H. Younesi, M. Pontie, A. Rahimpour, M. Rahimnejad and A. A. Zinatizadeh, A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery, J. Cleaner Prod., 2020, 264, 121446 CrossRef CAS.
- R. S. R. Rafidah, W. Rashmi, M. Khalid, W. Y. Wong and J. Priyanka, Recent progress in the development of aromatic polymer-based proton exchange membranes for fuel cell applications, Polymers, 2020, 12, 1061 CrossRef.
- M. Fang, C. Montoro and M. Semsarilar, Metal and covalent organic frameworks for membrane applications, Membranes, 2020, 10, 107 CrossRef CAS.
- D. W. Kang, M. Kang and C. S. Hong, Post-synthetic modification of porous materials: superprotonic conductivities and membrane applications in fuel cells, J. Mater. Chem. A, 2020, 8, 7474–7494 RSC.
- L. Zanchet, L. G. da Trindade, W. Bariviera, K. M. N. Borba, R. D. M. Santos, V. A. Paganin, C. P. de Oliveira, E. A. Ticianelli, E. M. A. Martini and M. O. de Souza, 3-Triethylammonium propane sulfonate ionic liquids for Nafion-based composite membranes for PEM fuel cells, J. Mater. Sci., 2020, 55, 11794–11795 CrossRef CAS.
- K. A. Mauritz and R. B. Moore, State of understanding of Nafion, Chem. Rev., 2004, 104, 4535–4586 CrossRef CAS.
- X. Liang, K. Cai, F. Zhang, J. Liu and G. Zhu, A proton-conductive lanthanide oxalatophosphonate framework featuring unique chemical stability: stabilities of bulk phase and surface structure, J. Mater. Chem. A, 2017, 5, 25350–25358 RSC.
- X.-X. Xie, Z. Zhang, J. Zhang, L. Hou, Z. Li and G. Li, Impressive proton conductivities of two highly stable metal-organic frameworks constructed by substituted imidazole dicarboxylates, Inorg. Chem., 2019, 58, 5173–5182 CrossRef CAS.
- X.-L. Sun, W.-H. Deng, H. Chen, H.-L. Han, J. M. Taylor, C.-Q. Wan and G. Xu, A metal–organic framework impregnated with a binary ionic liquid for safe proton conduction above 100 °C, Chem. – Eur. J., 2017, 23, 1248–1252 CrossRef CAS.
- Z. Sun, S. Yu, L. Zhao, J. Wang, Z. Li and G. Li, A highly stable two-dimensional copper(II)-organic framework for proton conduction and ammonia impedance sensing, Chem. – Eur. J., 2018, 24, 10829–10839 CrossRef CAS.
- T. He, Y.-Z. Zhang, H. Wu, X.-J. Kong, X.-M. Liu, L.-H. Xie, Y. Dou and J.-R. Li, Functionalized base-stable metal–organic frameworks for selective CO2 adsorption and proton conduction, ChemPhysChem, 2017, 18, 3245–3252 CrossRef CAS.
- D. Gui, X. Dai, Z. Tao, T. Zheng, X. Wang, M. A. Silver, J. Shu, L. Chen, Y. Wang, T. Zhang, J. Xie, L. Zou, Y. Xia, J. Zhang, J. Zhang, L. Zhao, J. Diwu, R. Zhou, Z. Chai and S. Wang, Unique proton transportation pathway in a robust inorganic coordination polymer leading to intrinsically high and sustainable anhydrous proton conductivity, J. Am. Chem. Soc., 2018, 140, 6146–6155 CrossRef CAS.
- S. M. Elahi, S. Chand, W.-H. Deng, A. Pal and M. C. Das, Polycarboxylate-templated coordination polymers: role of templates for superprotonic conductivities of up to 10−1 Scm−1, Angew. Chem., 2018, 130, 6772–6776 CrossRef.
- H. Chen, S.-Y. Han, R.-H. Liu, T.-F. Chen, K.-L. Bi, J.-B. Liang, Y.-H. Deng and C.-Q. Wan, High conductive, long-term durable, anhydrous proton conductive solid-state electrolyte based on a metal-organic framework impregnated with binary ionic liquids: Synthesis, characteristic and effect of anion, J. Power Sources, 2018, 376, 168–176 CrossRef CAS.
- W.-H. Xing, H.-Y. Li, X.-Y. Dong and S.-Q. Zang, Robust multifunctional Zr-based metal–organic polyhedra for high proton conductivity and selective CO2 capture, J. Mater. Chem. A, 2018, 6, 7724–7730 RSC.
- H. Zhong, Z.-H. Fu, J. M. Taylor, G. Xu and R.-H. Wang, Inorganic acid-impregnated covalent organic gels as high-performance proton-conductive materials at subzero temperatures, Adv. Funct. Mater., 2017, 27, 1701465 CrossRef.
- X. Liang, B. Li, M. Wang, J. Wang, R. Liu and G. Li, Effective approach to promoting the proton conductivity of metal−organic frameworks by exposure to aqua−ammonia vapor, ACS Appl. Mater. Interfaces, 2017, 9, 25082–25086 CrossRef CAS.
- F. Guo, C. Chen, K. Wang, Q. Zhang and Z. Lin, Supramolecular templating approach for the solvent-free synthesis of open-framework metal oxalates, Inorg. Chem., 2016, 55, 7817–7819 CrossRef CAS.
- R. Liu, L. Zhao, W. Dai, C. Yang, X. Liang and G. Li, A comparative investigation on proton conductivities for two metal-organic frameworks under water and aqua-ammonia vapors, Inorg. Chem., 2018, 57, 1474–1482 CrossRef CAS.
- S. Chand, S. M. Elahi, A. Pal and M. C. Das, Metal–organic frameworks and other crystalline materials for ultrahigh superprotonic conductivities of 10−2 Scm−1 or Higher, Chem. – Eur. J., 2019, 25, 6259–6269 CrossRef CAS.
- Z.-C. Guo, Z.-Q. Shi, X.-Y. Wang, Z.-F. Li and G. Li, Proton conductive covalent organic frameworks, Coord. Chem. Rev., 2020, 422, 213465 CrossRef CAS.
- T. Jadhav, Y. Fang, C.-H. Liu, A. Dadvand, E. Hamzehpoor, W. Patterson, A. Jonderian, R. S. Stein and D. F. Perepichka, Transformation between 2D and 3D covalent organic frameworks via reversible [2+2] cycloaddition, J. Am. Chem. Soc., 2020, 142, 8862–8870 CrossRef CAS.
- B. Zhou, J. Le, Z. Cheng, X. Zhao, M. Shen, M. Xie, B. Hu, X. Yang, L. Chen and H. Chen, Simple transformation of covalent organic frameworks to highly proton-conductive electrolytes, ACS Appl. Mater. Interfaces, 2020, 12, 8198–8205 CrossRef CAS.
- A. Karmakar, R. Illathvalappil, B. Anothumakkool, A. Sen, P. Samanta, A. V. Desai, S. Kurungot and S. K. Ghosh, Hydrogen-bonded organic frameworks (HOFs): a new class of porous crystalline proton-conducting materials, Angew. Chem., Int. Ed., 2016, 55, 10667–10671 CrossRef CAS.
- Z.-B. Sun, Y.-L. Li, Z.-H. Zhang, Z.-F. Li, B. Xiao and G. Li, A path to improve proton conductivity: from a 3D hydrogen-bonded organic framework to a 3D copper-organic framework, New J. Chem., 2019, 43, 10637–10644 RSC.
- W. Yang, F. Yang, T.-L. Hu, S.-C. King, H. Wang, H. Wu, W. Zhou, J.-R. Li, H. D. Arman and B. L. Chen, Microporous diaminotriazine-decorated porphyrin-based hydrogen bonded organic framework: permanent porosity and proton conduction, Cryst. Growth Des., 2016, 16, 5831–5835 CrossRef CAS.
- Y. Qin, T.-L. Gao, W.-P. Xie, Z.-F. Li and G. Li, Ultrahigh proton conduction in two highly stable ferrocenyl carboxylate frameworks, ACS Appl. Mater. Interfaces, 2019, 11, 31018–31027 CrossRef CAS.
- Z.-Q. Shi, N.-N. Ji, M.-H. Wang and G. Li, A comparative study of proton conduction between a 2D Zinc(II) MOF and its corresponding organic ligand, Inorg. Chem., 2020, 59, 4781–4789 CrossRef CAS.
- Y. Qin, X. Wang, W. Xie, Z. Li and G. Li, Structural effect on proton conduction in two highly stable disubstituted ferrocenyl carboxylate frameworks, Inorg. Chem., 2020, 59, 10243–10252 CrossRef CAS.
- S. Kanda, K. Yamashita and K. Ohkawa, A proton conductive coordination polymer. I. [N,N′-bis(2-hydroxyethyl)dithiooxamido]copper(II), Bull. Chem. Soc. Jpn., 1979, 52, 3296–3301 CrossRef CAS.
- S.-S. Bao, G. K. H. Shimizu and L.-M. Zheng, Proton conductive metal phosphonate frameworks, Coord. Chem. Rev., 2019, 378, 577–594 CrossRef CAS.
- X.-X. Xie, Y.-C. Yang, B.-H. Dou, Z.-F. Li and G. Li, Proton conductive carboxylate-based metal-organic frameworks, Coord. Chem. Rev., 2020, 403, 213100 CrossRef CAS.
- W.-H. Li, W.-H. Deng, G.-E. Wang and G. Xu, Conductive MOFs, EnergyChem, 2020, 2, 100029 CrossRef.
- J. Ha, J. Hwa Lee and H. Moon, Alterations to secondary building units of metal–organic frameworks for the development of new functions, Inorg. Chem. Front., 2020, 7, 12–27 RSC.
- H.-N. Wang, X. Meng, L.-Z. Dong, Y. Chen, S.-L. Li and Y.-Q. Lan, Coordination polymer-based conductive materials: ionic conductivity vs. electronic conductivity, J. Mater. Chem. A, 2019, 7, 24059–24091 RSC.
- D. W. Lim, M. Sadakiyo and H. Kitagawa, Proton transfer in hydrogen-bonded degenerate systems of water and ammonia in metal–organic frameworks, Chem. Sci., 2019, 10, 16–33 RSC.
- Y. Ye, L. Gong, S. Xiang, Z. Zhang and B. Chen, Metal–organic frameworks as a versatile platform for proton conductors, Adv. Mater., 2020, 32, 1907090 CrossRef CAS.
- X. Meng, H.-N. Wang, S.-Y. Song and H.-J. Zhang, Proton-conducting crystalline porous materials, Chem. Soc. Rev., 2017, 46, 464–480 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef.
- Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Zr-based metal–organic frameworks: design, synthesis, structure, and applications, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- D. R. Sun and Z. H. Li, Robust Ti- and Zr-based metal-organic frameworks for photocatalysis, Chin. J. Chem., 2017, 35, 135–147 CrossRef CAS.
- T. N. Tu, M. V. Nguyen, H. L. Nguyen, B. Yuliarto, K. E. Cordova and S. Demir, Designing bipyridine-functionalized zirconium metal-organic frameworks as a platform for clean energy and other emerging applications, Coord. Chem. Rev., 2018, 364, 33–50 CrossRef CAS.
- J. M. Taylor, S. Dekura, R. Ikeda and H. Kitagawa, Defect control to enhance proton conductivity in a metal−organic framework, Chem. Mater., 2015, 27, 2286–2289 CrossRef CAS.
- J. M. Taylor, T. Komatsu, S. Dekura, K. Otsubo, M. Takata and H. Kitagawa, The role of a three dimensionally ordered defect sublattice on the acidity of a sulfonated metal–organic framework, J. Am. Chem. Soc., 2015, 137, 11498–11506 CrossRef CAS.
- S. Biswas, J. Zhang, Z. Li, Y.-Y. Liu, M. Grzywa, L. Sun, D. Volkmer and P. V. D. Voort, Enhanced selectivity of CO2 over CH4 in sulphonate-, carboxylate- and iodo-functionalized UiO-66 frameworks, Dalton Trans., 2013, 42, 4730–4737 RSC.
- Q. Yang, S. Vaesen, F. Ragon, A. D. Wiersum, D. Wu, A. Lago, T. Devic, C. Martineau, F. Taulelle, P. L. Llewellyn, H. Jobic, C. Zhong, C. Serre, G. D. Weireld and G. Maurin, A water stable metal–organic framework with optimal features for CO2 capture, Angew. Chem., 2013, 125, 10506–10510 CrossRef.
- M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, A facile synthesis of UiO-66, UiO-67 and their derivatives, Chem. Commun., 2013, 49, 9449–9451 RSC.
- W. Zhang, H. Huang, C. Zhong and D. Liu, Cooperative effect of temperature and linker functionality on CO2 capture from industrial gas mixtures in metal–organic frameworks: a combined experimental and molecular simulation study, Phys. Chem. Chem. Phys., 2012, 14, 2317–2325 RSC.
- F. Yang, H. Huang, X. Wang, F. Li, Y. Gong, C. Zhong and J.-R. Li, Proton conductivities in functionalized UiO-66: tuned properties, thermogravimetry mass, and molecular simulation analyses, Cryst. Growth Des., 2015, 15, 5827–5833 CrossRef CAS.
- W. J. Phang, H. Jo, W. R. Lee, J. H. Song, K. Yoo, B. Kim and C. S. Hong, Superprotonic conductivity of a UiO-66 framework functionalized with sulfonic acid groups by facile postsynthetic oxidation, Angew. Chem., Int. Ed., 2015, 54, 5142–5146 CrossRef CAS.
- S. Balčiunas, D. Pavlovaitè, M. Kinka, J. Y. Yeh, P. C. Han, F. K. Shieh, K. C. W. Wu, M. Šimènas, R. Grigalaitis and J. Banys, Dielectric spectroscopy of water dynamics in functionalized UiO-66 metal-organic frameworks, Molecules, 2020, 25, 1962 CrossRef.
- S. Mukhopadhyay, J. Debgupta, C. Singh, R. Sarkar, O. Basu and S. K. Das, Designing UiO-66-based superprotonic conductor with the highest metal−organic framework based proton conductivity, ACS Appl. Mater. Interfaces, 2019, 11, 13423–13432 CrossRef CAS.
- X.-M. Li, J. Liu, C. Zhao, J.-L. Zhou, L. Zhao, S.-L. Li and Y.-Q. Lan, Strategic hierarchical improvement of superprotonic conductivity in a stable metal–organic framework system, J. Mater. Chem. A, 2019, 7, 25165–25171 RSC.
- A. Planchais, S. Devautour-Vinot, F. Salles, F. Ragon, T. Devic, C. Serre and G. Maurin, A joint experimental/computational exploration of the dynamics of confined water/Zr-based MOFs systems, J. Phys. Chem. C, 2014, 118, 14441–14448 CrossRef CAS.
- D. D. Borges, S. Devautour-Vinot, H. Jobic, J. Ollivier, F. Nouar, R. Semino, T. Devic, C. Serre, F. Paesani and G. Maurin, Proton transport in a highly conductive porous zirconium-based metal–organic framework: molecular insight, Angew. Chem., Int. Ed., 2016, 55, 3919–3924 CrossRef CAS.
- R. Zhuang, B.-B. Tang and P.-Y. Wu, Proton conductivity of proton exchange membrane synergistically promoted by different functionalized Metal-Organic Frameworks, ACS Appl. Mater. Interfaces, 2017, 9, 22597–22603 CrossRef.
- X.-Y. Dong, J.-H. Wang, S.-S. Liu, Z. Han, Q.-J. Tang, F.-F. Li and S.-Q. Zang, Synergy between isomorphous acid and basic metal-organic frameworks for anhydrous proton conduction of low-cost hybrid membranes at high temperatures, ACS Appl. Mater. Interfaces, 2018, 10, 38209–38216 CrossRef CAS.
- Z. Rao, K. Feng, B.-B. Tang and P.-Y. Wu, Construction of well interconnected metal-organic framework structure for effectively promoting proton conductivity of proton exchange membrane, J. Membr. Sci., 2017, 533, 160–170 CrossRef CAS.
- P.-L. Zheng, Q.-Y. Liu, D.-H. Wang, Z.-K. Li, Y.-W. Meng and Y. Zheng, Preparation of covalent-ionically cross-linked UiO-66-NH2/sulfonated aromatic composite proton exchange membranes with excellent performance, Front. Chem., 2020, 8, 56 CrossRef.
- L. Wang, N. Deng, Y. Liang, J. Ju, B. Cheng and W. Kang, Metal-organic framework anchored sulfonated poly(ether sulfone) nanofibers as highly conductive channels for hybrid proton exchange membranes, J. Power Sources, 2020, 450, 227592 CrossRef CAS.
- H.-Z. Sun, B.-B. Tang and P.-Y. Wu, Rational design of S–UiO-66@GO hybrid nanosheets for proton exchange membranes with significantly enhanced transport performance, ACS Appl. Mater. Interfaces, 2017, 9, 26077–26087 CrossRef CAS.
- A. Donnadio, R. Narducci, M. Casciola, F. Marmottini, R. D'Amato, M. Jazestani, H. Chiniforoshan and F. Costantino, Mixed membrane matrices based on Nafion/UiO-66/SO3H–UiO-66 nano-MOFs: revealing the effect of crystal size, sulfonation, and filler loading on the mechanical and conductivity properties, ACS Appl. Mater. Interfaces, 2017, 9, 42239–42246 CrossRef CAS.
- S. Neelakandan, R. Ramachandran, M. Fang and L. Wang, Improving the performance of sulfonated polymer membrane by using sulfonic acid functionalized heterometallic metal-organic framework for DMFC applications, Int. J. Energy Res., 2020, 44, 1673–1684 CrossRef CAS.
- S. Liu, Z. Yue and Y. Liu, Incorporation of imidazole within the metal–organic framework UiO-67 for enhanced anhydrous proton conductivity, Dalton Trans., 2015, 44, 12976–12980 RSC.
- W.-R. Xian, Y. He, Y. Diao, Y.-L. Wong, H.-Q. Zhou, S.-L. Zheng, W.-M. Liao, Z. Xu and J. He, A bumper crop of boiling-water-stable metal−organic frameworks from controlled linker sulfuration, Inorg. Chem., 2020, 59, 7097–7102 CrossRef CAS.
- H. Furukawa, F. Gandara, Y. B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, Water adsorption in porous metal−organic frameworks and related materials, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS.
- H.-B. Luo, M. Wang, S.-X. Liu, C. Xue, Z.-F. Tian, Y. Zou and X.-M. Ren, Proton conductance of a superior water-stable metal–organic framework and its composite membrane with poly(vinylidenefluoride), Inorg. Chem., 2017, 56, 4169–4175 CrossRef CAS.
- X. Meng, H.-N. Wang, L.-S. Wang, Y.-H. Zou and Z.-Y. Zhou, Enhanced proton conductivity of a MOF-808 framework through anchoring organic acids to the zirconium clusters by post-synthetic modification, CrystEngComm, 2019, 21, 3146–3150 RSC.
- H. A. Patel, N. Mansor, S. Gadipelli, D. J. L. Brett and Z. Guo, Superacidity in Nafion/MOF hybrid membranes retains water at low humidity to enhance proton conduction for fuel cells, ACS Appl. Mater. Interfaces, 2016, 8, 30687–30691 CrossRef CAS.
- J.-C. Jiang, F. Gandara, Y.-B. Zhang, K. Na, O. M. Yaghi and W. G. Klemperer, Superacidity in sulfated metal-organic framework-808, J. Am. Chem. Soc., 2014, 136, 12844–12847 CrossRef CAS.
- T. H. N. Lo, M. V. Nguyen and T. N. Tu, An anchoring strategy leads to enhanced proton conductivity in a new metal–organic framework, Inorg. Chem. Front., 2017, 4, 1509–1516 RSC.
- M. V. Nguyen, T. H. N. Lo, L. C. Luu, H. T. T. Nguyen and T. N. Tu, Enhancing proton conductivity in a metal–organic framework at T > 80 °C by an anchoring strategy, J. Mater. Chem. A, 2018, 6, 1816–1821 RSC.
- Q. Zeng, W.-R. Xian, Y.-H. Zhong, L.-H. Chung, W.-M. Liao and J. He, Highly enhanced hydrated proton conductivity by combination of post-synthetic oxidation and acidification in a zirconium-organic framework, J. Solid State Chem., 2020, 285, 121234 CrossRef CAS.
- S. Tominaka, F. Coudert, T. D. Dao, T. Nagao and A. K. Cheetham, Insulator-to-proton-conductor transition in a dense metal–organic framework, J. Am. Chem. Soc., 2015, 137, 6428–6431 CrossRef CAS.
- S.-J. Wang, M. Wahiduzzaman, L. Davis, A. Tissot, W. Shepard, J. Marrot, C. Martineau-Corcos, D. Hamdane, G. Maurin, S. Devautour-Vinot and C. Serre, A robust zirconium amino acid metal-organic framework for proton conduction, Nat. Commun., 2018, 9, 4937 CrossRef.
- J. Zhang, H.-J. Bai, Q. Ren, H.-B. Luo, X.-M. Ren, Z.-F. Tian and S.-F. Lu, Extra water- and acid-stable MOF-801 with high proton conductivity and its composite membrane for proton-exchange membrane, ACS Appl. Mater. Interfaces, 2018, 10, 28656–28663 CrossRef CAS.
- Z. Zhang, J. Ren, J. Xu, Z. Wang, W. He, S. Wang, X. Yang, X. Du, L. Meng and P. Zhao, Adjust the arrangement of imidazole on the metal-organic framework to obtain hybrid proton exchange membrane with long-term stable high proton conductivity, J. Membr. Sci., 2020, 607, 118194 CrossRef CAS.
- G. Alberti, M. Casciola, A. Donnadio, P. Piaggio, M. Pica and M. Sisani, Preparation and characterisation of a-layered zirconium phosphate sulfophenylenphosphonates with variable concentration of sulfonic groups, Solid State Ionics, 2005, 176, 2893–2898 CrossRef CAS.
- Z. Li, F. Dong, L. Xu, S. Wang and X. Yu, Preparation and properties of medium temperature membranes based on zirconium sulfophenylphosphate/sulfonated poly(phthalazinone ether sulfone ketone) for direct methanol fuel cells, J. Membr. Sci., 2010, 351, 50–57 CrossRef CAS.
- E. W. Stein, S. A. Clearfield and M. A. Subramanianm, Conductivity of group IV metal sulfophosphonates and a new class of interstratified metal amine-sulfophosphonates, Solid State Ionics, 1996, 83, 113–124 CrossRef.
- V. Zima, J. Svoboda, K. Melánová, L. Beneš, M. Casciola, M. Sganappa, J. Brus and M. Trchová, Synthesis and characterization of new zirconium 4-sulfophenylphosphonates, Solid State Ionics, 2010, 18, 705–713 CrossRef.
- F. Costantino, A. Donnadio and M. Casciola, Survey on the phase transitions and their effect on the ion-exchange and on the proton-conduction properties of a flexible and robust zr phosphonate coordination polymer, Inorg. Chem., 2012, 51, 6992–7000 CrossRef CAS.
- M. Taddei, A. Donnadio, F. Costantino, R. Vivani and M. Casciola, Synthesis, crystal structure, and proton conductivity of one-dimensional, two-dimensional, and three-dimensional zirconium phosphonates based on glyphosate and glyphosine, Inorg. Chem., 2013, 52, 12131–12139 CrossRef CAS.
- A. Donnadio, M. Nocchetti, F. Costantino, M. Taddei, M. Casciola, F. da Silva Lisboa and R. Vivani, A layered mixed zirconium phosphate/phosphonate with exposed carboxylic and phosphonic groups: x-ray powder structure and proton conductivity properties, Inorg. Chem., 2014, 53, 13220–13226 CrossRef CAS.
- Z. H. Fard, N. E. Wong, C. D. Malliakas, P. Ramaswamy, J. M. Taylor, K. Otsubo and G. K. H. Shimizu, Superprotonic phase change to a robust phosphonate metal−organic framework, Chem. Mater., 2018, 30, 314–318 CrossRef.
- K. Melánová, J. Brus, V. Zima, L. Beneš, J. Svoboda, L. Kobera and P. Kutálek, Formation of layered proton-conducting zirconium and titanium organophosphonates by topotactic reaction: physicochemical properties, proton dynamics, and atomic-resolution structure, Inorg. Chem., 2020, 59, 505–513 CrossRef.
- D. M. Poojary, B. Shpeizer and A. Clearfield, X-ray powder structure and rietveld refinement of gamma-zirconium phosphate, Zr(PO4)(H2PO4)·2H2O, J. Chem. Soc., Dalton Trans., 1995, 111–113 RSC.
- G. Mouchaham, L. Cooper, N. Guillou, C. Martineau, E. Elkaïm, S. Bourrelly, P. L. Llewellyn, C. Allain, G. Clavier, C. Serre and T. Devic, A robust infinite zirconium phenolate building unit to enhance the chemical stability of Zr MOFs, Angew. Chem., Int. Ed., 2015, 54, 13297–13301 CrossRef CAS.
- P. G. M. Mileo, S. Devautour-Vinot, G. Mouchaham, F. Faucher, N. Guillou, A. Vimont, C. Serre and G. Maurin, Proton-conducting phenolate-based Zr metal−organic framework: a joint experimental−modeling investigation, J. Phys. Chem. C, 2016, 120, 24503–24510 CrossRef CAS.
- E.-X. Chen, G. Xu and Q. Lin, Robust porphyrin-spaced zirconium pyrogallate frameworks with high proton conduction, Inorg. Chem., 2019, 58, 3569–3573 CrossRef CAS.
| This journal is © the Partner Organisations 2020 |