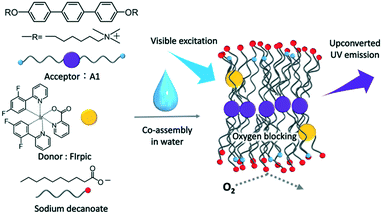
Visible-to-UV photon upconversion in air-saturated water by multicomponent co-assembly†
Yusuke
Kawashima
a,
Hironori
Kouno
a,
Kana
Orihashi
a,
Koki
Nishimura
a,
Nobuhiro
Yanai
 *ab and
Nobuo
Kimizuka
*ab and
Nobuo
Kimizuka
 *a
*a
aDepartment of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: yanai@mail.cstm.kyushu-u.ac.jp; n-kimi@mail.cstm.kyushu-u.ac.jp
bPRESTO, JST, Honcho 4-1-8, Kawaguchi, Saitama 332-0012, Japan
First published on 5th February 2020
Abstract
Air-stable, visible-to-UV photon upconversion based on triplet–triplet annihilation (TTA-UC) in water has been an outstanding issue despite its importance in a wide range of applications. This is achieved by giving oxygen barrier properties to cationic acceptor self-assemblies through ion complex formation with anionic fatty acids, i.e., supramolecular crowding.
Design, System, ApplicationIon-paring-based multicomponent self-assembly is proposed as a means to realize visible (vis)-to-ultraviolet (UV) triplet–triplet annihilation-based photon upconversion (TTA-UC) in air-saturated water. Generally, the photoexcited triplet state is deactivated by molecular oxygen, and therefore TTA-UC emission is quenched in air-saturated water. The development of a methodology to achieve air-stable vis-to-UV TTA-UC is highly desired to boost sunlight-powered renewable energy production such as water-splitting photocatalysis. An amphiphilic cationic acceptor is designed by modifying a typical vis-to-UV TTA-UC acceptor p-terphenyl with alkyl chain spacers and quaternary ammonium groups. This cationic acceptor is co-assembled with an anionic fatty acid through ionic and hydrophobic interactions, and a hydrophobic triplet donor is incorporated in the co-assemblies. The dense packing of alkyl moieties prevents the intrusion of oxygen molecules, resulting in the air-stable vis-to-UV TTA-UC emission. The current work offers an important rational strategy not only for TTA-UC but also for other functions based on air-sensitive photoexcited triplet states. |
The efficient utilization of solar energy is the key to solve current energy problems. Water-splitting photocatalysts can convert solar energy into molecular hydrogen, which is anticipated as a clean energy source. Highly efficient water-splitting reactions occur under the illumination of ultraviolet (UV) light, however, the portion of UV light is limited to only ca. 5% in the whole solar irradiance.1 Therefore, the visible (vis)-to-UV photon energy conversion is expected to expand the available wavelength range for water-splitting photocatalysis.
Photon upconversion (UC) is a method to convert lower-energy photons to higher-energy photons by combining the energy of multiple photons. For solar energy applications, photon upconversion based on triplet–triplet annihilation (TTA-UC) is particularly promising since it can operate under low-intensity light comparable to sunlight.2–23 As summarized in Fig. S1,† TTA-UC is composed of two kinds of chromophores, a donor (triplet sensitizer) and an acceptor (emitter). Donor triplets are generated via intersystem crossing (ISC) from photoexcited singlets. Triplet energy transfer (TET) from the donor to the acceptor generates acceptor triplets. Two acceptor triplets generate a higher-energy singlet state of the acceptor through TTA, which results in the upconverted delayed fluorescence. As noted, the vis-to-UV TTA-UC is expected to boost the efficiency of photocatalytic reactions.24–39 However, TTA-UC has a fatal problem where the photoexcited triplets are easily quenched by molecular oxygen dissolved in water. A common strategy to avoid oxygen quenching is to employ viscous droplets or polymers,5,40,41 which inevitably makes the diffusion of large TTA chromophores slow.
As an alternative strategy, we have reported that the self-assembly of amphiphilic chromophores enables TTA-UC even in air-saturated water.15,42–44 In this mechanism, TTA-UC occurs via triplet energy migration in densely self-assembled chromophore arrays and is not dependent on the conventional molecular diffusion. High oxygen blocking ability was achieved by co-assembly of cationic amphiphilic acceptors with anionic fatty acids, which increased the molecular density around the chromophores, the so-called supramolecular crowding.44 Meanwhile, the demonstration of this strategy has been limited to the model vis-to-vis (green-to-blue) TTA-UC.
Here, we report the first example of air-stable vis-to-UV TTA-UC in water by generalizing the supramolecular design concept. We designed a novel UV-emitting bola-type amphiphile A1 in which a p-terphenyl chromophore was introduced as an acceptor to perform vis-to-UV TTA-UC (Fig. 1).31 Alkyl chains and quaternary ammonium groups are attached to the p-terphenyl skeleton as hydrophobic spacers and hydrophilic groups, respectively. Following our successful design for air-stable green-to-blue TTA-UC,44 cationic A1 was co-assembled with anionic decanoate (Dec) whose alkyl chain length is close to the alkyl spacer of A1. By introducing a visible light absorbing triplet donor FIrpic,45 the ternary co-assemblies showed stable vis-to-UV TTA-UC even in air-saturated water.
The new amphiphilic acceptor A1 was synthesized and characterized by 1H NMR and elemental analysis (Scheme S1†). A1 was molecularly dispersed in methanol, as confirmed by its concentration dependence of absorption spectra (Fig. S2†). The molecularly-dispersed A1 in methanol ([A1] = 0.25 mM) showed a fluorescence peak at 358 nm (Fig. S3†) and a fluorescence quantum yield of 86%.
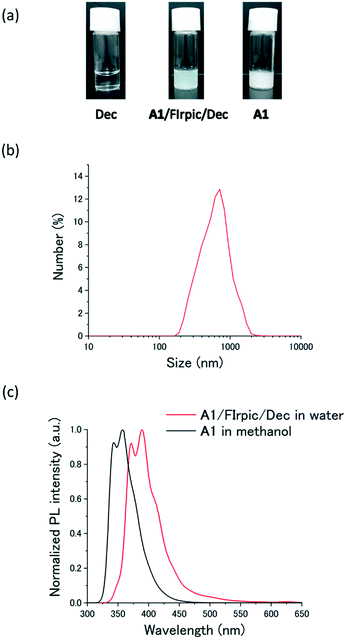
To form the vis-to-UV upconverting ternary molecular system, A1 was co-assembled with FIrpic and Dec. The three components, A1, FIrpic and Dec, were mixed by dissolving in methanol and the ternary mixture was obtained by removing the solvent under reduced pressure. After adding water, ultrasonication and heating treatment of the mixture gave a pale-yellow translucent dispersion (Fig. 2a, [A1] = 10 mM, [FIrpic] = 100 μM and [Dec] = 80 mM). The appearance of this A1–FIrpic–Dec ternary dispersion is totally different from aqueous Dec and A1 (Fig. 2a). Since the concentration of Dec is below its critical micellar concentration (cmc) of 86 mM, Dec provided a transparent solution.46A1 was poorly soluble in water, and some precipitates were observed. However, a stable dispersion was obtained when Dec (80 mM) was added in excess as compared to the concentration of A1 (10 mM). Apparently, the co-assembly of A1 with Dec through electrostatic and hydrophobic interactions improved the dispersibility of A1 in water.
Dynamic light scattering (DLS), zeta potential, UV-vis absorption, fluorescence, and scanning electron microscopy (SEM) measurements were performed to obtain further information about the ternary assembly. The DLS profile of the ternary dispersion of A1–FIrpic–Dec showed a particle size of 871 ± 28 nm (Fig. 2b) that was close to that of the binary system A1–Dec (Fig. S4†). The SEM images of A1–FIrpic–Dec also showed a particle size of around 1 μm and a featureless morphology (Fig. S5†). The co-assembly of A1 with excess Dec anions was further supported by the negative zeta potential of −24.7 mV observed for A1–FIrpic–Dec. Compared with the fluorescence peak at 358 nm of the molecularly-dispersed A1 in methanol, a fluorescence spectrum of the A1–FIrpic–Dec ternary dispersion showed a redshift to 391 nm, which is still in the UV range (Fig. 2c). We also observed red-shifts in the excitation spectrum of aqueous A1–FIrpic–Dec as compared to that of A1 in methanol (Fig. S6†). These results suggest the presence of excitonic interactions among the terphenyl chromophores in the aqueous co-assemblies.
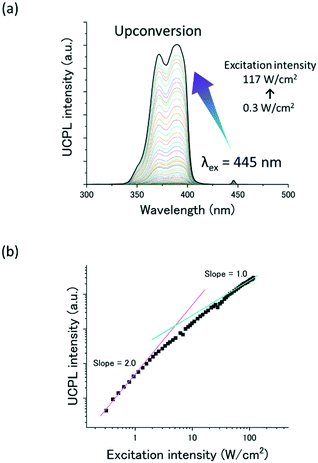
The TTA-UC properties of the A1–FIrpic–Dec ternary co-assemblies were then characterized in deaerated water. The aqueous dispersion of A1–FIrpic–Dec ([A1] = 10 mM, [FIrpic] = 100 μM and [Dec] = 80 mM) was deaerated by repeated freeze–pump–thaw cycles. Under the irradiation of a visible laser at 445 nm, the aqueous dispersion showed upconverted UV emission at around 390 nm (Fig. 3a). The double logarithmic plots of the UC emission intensity at 390 nm against the excitation intensity showed a slope change from 2 to 1, which is a typical characteristic of TTA-UC (Fig. 3b).47–49 Furthermore, the upconverted emission was observed for the microsecond scale, supporting the delayed fluorescence mechanism via the long-lived triplet state (Fig. S7†). Although the TTA-UC efficiency was not high (0.1% at 10 W cm−2) compared with previous vis-to-UV TTA-UC systems,24–39 this is partly due to a reduced fluorescence quantum yield (∼26%) in the aqueous co-assemblies. It is also possible that the deactivation of triplets occurred at strongly interacting sites.50 The optimization of the chemical structure of amphiphilic acceptors and the co-assembly conditions to improve the TTA-UC efficiency is an important future task. It is necessary to satisfy the balance between the optimized interchromophore interactions for the high TTA-UC efficiency and the high molecular packing density for oxygen blocking as mentioned below.
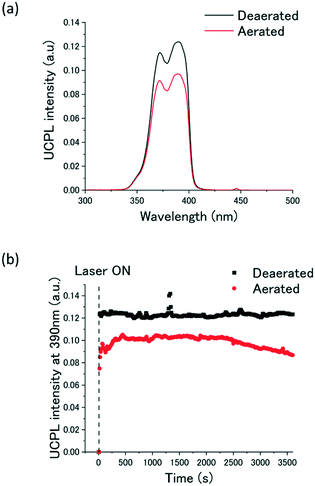
Significantly, the ternary A1–FIrpic–Dec co-assemblies showed a stable vis-to-UV TTA-UC emission even in air-saturated water (Fig. 4a). The oxygen-barrier efficiency (ΦOB) was estimated by the time dependence of the UC emission intensity at 390 nm. It was found that the UC emission was maintained for more than one hour even in aerated water (Fig. 4b). By comparing the time-averaged UC emission intensity of the degassed and aerated samples, the ΦOB was calculated as ΦOB = IUC,aerated/IUC,deaerated = 80%, which is comparable to our previous aqueous co-assembled system showing air-stable green-to-blue TTA-UC.44 In our previous work, we have shown that the oxygen blocking ability can be provided by enhancing the packing density of chromophores and alkyl chains in the aqueous co-assemblies. While it is difficult to study the effect of alkyl chain length in the current study due to the poor dispersibility of the co-assemblies with longer alkyl chains, our previous work also showed that the longer alkyl chain of the counter anions leads to a higher oxygen-barrier efficiency. It is notable that such a supramolecular crowding strategy which takes advantage of ion-paring-based co-assembly is generalized for single-chained, bola-type acceptor amphiphiles that lead to the aqueous vis-to-UV TTA-UC even under the aerated conditions.
Conclusions
In this work, we showed the first example of air-stable vis-to-UV TTA-UC in water. The novel anionic amphiphilic acceptor A1 was co-assembled with the donor Flrpic and the cationic lipid Dec in water. The ternary aqueous co-assemblies showed a high oxygen barrier efficiency ΦOB of 80%. The current system clarified the generality of our previous supramolecular crowding strategy in which the enhancement in molecular packing density in aqueous co-assemblies provided the remarkable oxygen blocking ability.44 This work provides important design guidelines to protect air-sensitive species in water for various applications, including visible-light-driven water splitting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI grant number JP17H04799, JP16H06513, and the Sumitomo Foundation.References
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Phys. Rev. Lett., 2006, 97, 143903 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- J. Zhao, S. Ji and H. Guo, RSC Adv., 2011, 1, 937–950 RSC.
- J. H. Kim and J. H. Kim, J. Am. Chem. Soc., 2012, 134, 17478–17481 CrossRef CAS PubMed.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322–4332 RSC.
- Y. C. Simon and C. Weder, J. Mater. Chem., 2012, 22, 20817–20830 RSC.
- K. Börjesson, D. Dzebo, B. Albinsson and K. Moth-Poulsen, J. Mater. Chem. A, 2013, 1, 8521–8524 RSC.
- S. H. C. Askes, A. Bahreman and S. Bonnet, Angew. Chem., Int. Ed., 2014, 53, 1029–1033 CrossRef CAS PubMed.
- R. Andernach, H. Utzat, S. D. Dimitrov, I. McCulloch, M. Heeney, J. R. Durrant and H. Bronstein, J. Am. Chem. Soc., 2015, 137, 10383–10390 CrossRef CAS PubMed.
- M. Häring, R. Pérez-Ruiz, A. J. von Wangelin and D. D. Díaz, Chem. Commun., 2015, 51, 16848–16851 RSC.
- T. F. Schulze and T. W. Schmidt, Energy Environ. Sci., 2015, 8, 103–125 RSC.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- C. Fan, W. Wu, J. J. Chruma, J. Zhao and C. Yang, J. Am. Chem. Soc., 2016, 138, 15405–15412 CrossRef CAS PubMed.
- N. Yanai and N. Kimizuka, Chem. Commun., 2016, 52, 5354–5370 RSC.
- S. P. Hill and K. Hanson, J. Am. Chem. Soc., 2017, 139, 10988–10991 CrossRef CAS PubMed.
- Z. Huang and M. L. Tang, J. Am. Chem. Soc., 2017, 139, 9412–9418 CrossRef CAS PubMed.
- C. Duan, L. Liang, L. Li, R. Zhang and Z. P. Xu, J. Mater. Chem. B, 2018, 6, 192–209 RSC.
- C. Kerzig and O. S. Wenger, Chem. Sci., 2018, 9, 6670–6678 RSC.
- W. Xu, W. Liang, W. Wu, C. Fan, M. Rao, D. Su, Z. Zhong and C. Yang, Chem. – Eur. J., 2018, 24, 16677–16685 CrossRef CAS PubMed.
- D. Yang, P. Duan and M. Liu, Angew. Chem., Int. Ed., 2018, 57, 9357–9361 CrossRef CAS PubMed.
- L. Huang, E. Kakadiaris, T. Vaneckova, K. Huang, M. Vaculovicova and G. Han, Biomaterials, 2019, 201, 77–86 CrossRef CAS PubMed.
- Z. Wang, J. Zhao, M. Di Donato and G. Mazzone, Chem. Commun., 2019, 55, 1510–1513 RSC.
- W. Zhao and F. N. Castellano, J. Phys. Chem. A, 2006, 110, 11440–11445 CrossRef CAS PubMed.
- T. N. Singh-Rachford, R. R. Islangulov and F. N. Castellano, J. Phys. Chem. A, 2008, 112, 3906–3910 CrossRef CAS PubMed.
- F. Deng, J. Blumhoff and F. N. Castellano, J. Phys. Chem. A, 2013, 117, 4412–4419 CrossRef CAS PubMed.
- P. Duan, N. Yanai and N. Kimizuka, Chem. Commun., 2014, 50, 13111–13113 RSC.
- M. Majek, U. Faltermeier, B. Dick, R. Pérez-Ruiz and A. J. von Wangelin, Chem. – Eur. J., 2015, 21, 15496–15501 CrossRef CAS PubMed.
- X. Jiang, X. Guo, J. Peng, D. Zhao and Y. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 11441–11449 CrossRef CAS PubMed.
- J. Peng, X. Guo, X. Jiang, D. Zhao and Y. Ma, Chem. Sci., 2016, 7, 1233–1237 RSC.
- N. Yanai, M. Kozue, S. Amemori, R. Kabe, C. Adachi and N. Kimizuka, J. Mater. Chem. C, 2016, 4, 6447–6451 RSC.
- K. A. El Roz and F. N. Castellano, Chem. Commun., 2017, 53, 11705–11708 RSC.
- V. Gray, P. Xia, Z. Huang, E. Moses, A. Fast, D. A. Fishman, V. I. Vullev, M. Abrahamsson, K. Moth-Poulsen and M. Lee Tang, Chem. Sci., 2017, 8, 5488–5496 RSC.
- M. Barawi, F. Fresno, R. Pérez-Ruiz and V. A. de la Peña O'Shea, ACS Appl. Energy Mater., 2018, 2, 207–211 CrossRef.
- Q. Chen, Y. Liu, X. Guo, J. Peng, S. Garakyaraghi, C. M. Papa, F. N. Castellano, D. Zhao and Y. Ma, J. Phys. Chem. A, 2018, 122, 6673–6682 CrossRef CAS PubMed.
- S. He, X. Luo, X. Liu, Y. Li and K. Wu, J. Phys. Chem. Lett., 2019, 10, 5036–5040 CrossRef CAS PubMed.
- S. Hisamitsu, J. Miyano, K. Okumura, J. K. H. Hui, N. Yanai and N. Kimizuka, ChemistryOpen, 2019, 8, 1–5 CrossRef.
- H. L. Lee, M. S. Lee, H. Park, W. S. Han and J. H. Kim, Korean J. Chem. Eng., 2019, 36, 1791–1798 CrossRef CAS.
- K. Okumura, N. Yanai and N. Kimizuka, Chem. Lett., 2019, 48, 1347–1350 CrossRef CAS.
- J. H. Kim, F. Deng, F. N. Castellano and J. H. Kim, Chem. Mater., 2012, 24, 2250–2252 CrossRef CAS.
- Q. Liu, B. Yin, T. Yang, Y. Yang, Z. Shen, P. Yao and F. Li, J. Am. Chem. Soc., 2013, 135, 5029–5037 CrossRef CAS PubMed.
- H. Kouno, T. Ogawa, S. Amemori, P. Mahato, N. Yanai and N. Kimizuka, Chem. Sci., 2016, 7, 5224–5229 RSC.
- R. Haruki, H. Kouno, M. Hosoyamada, T. Ogawa, N. Yanai and N. Kimizuka, Chem. – Asian J., 2019, 14, 1723–1728 CrossRef CAS PubMed.
- H. Kouno, Y. Sasaki, N. Yanai and N. Kimizuka, Chem. – Eur. J., 2019, 25, 6124–6130 CrossRef CAS PubMed.
- R. J. Holmes, S. R. Forrest, Y. J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Appl. Phys. Lett., 2003, 82, 2422–2424 CrossRef CAS.
- F. E. Stanley, A. M. Warner, E. Schneiderman and A. M. Stalcup, J. Chromatogr. A, 2009, 1216, 8431–8434 CrossRef CAS PubMed.
- A. Monguzzi, J. Mezyk, F. Scotognella, R. Tubino and F. Meinardi, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 195112 CrossRef.
- Y. Y. Cheng, T. Khoury, R. G. C. R. Clady, M. J. Y. Tayebjee, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, Phys. Chem. Chem. Phys., 2010, 12, 66–71 RSC.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, J. Phys. Chem. Lett., 2012, 3, 299–303 CrossRef CAS.
- S. Hisamitsu, N. Yanai, H. Kouno, E. Magome, M. Matsuki, T. Yamada, A. Monguzzi and N. Kimizuka, Phys. Chem. Chem. Phys., 2018, 20, 3233–3240 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, TTA-based UC mechanism, absorption and emission spectra. See DOI: 10.1039/d0me00003e |
| This journal is © The Royal Society of Chemistry 2020 |