Soft material nanoarchitectonics at interfaces: molecular assembly, nanomaterial synthesis, and life control
Katsuhiko
Ariga
 *ab,
Xiaofang
Jia
*ab,
Xiaofang
Jia
 a and
Lok Kumar
Shrestha
a and
Lok Kumar
Shrestha
 a
a
aWPI Research Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: ARIGA.Katsuhiko@nims.go.jp
bGraduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8561, Japan
First published on 14th January 2019
Abstract
A new conceptual paradigm that combines nanotechnology and other research disciplines, called “nanoarchitectonics”, has been established. Nanoarchitectonics can be achieved with contributions of atomic/molecular level manipulation, chemical synthesis/modification, self-organization/self-assembly, field-controlled arrangement, material fabrication, and bio-related techniques together with advanced knowledge and skills in nanoscience and nanotechnology. In particular, the dynamic nature of the nanoarchitectonics concept accords with soft material functionalization. Therefore, the nanoarchitectonics concept provides rational strategies to create functional materials with deep relations with molecular system designs that are especially useful for the development of novel functions of soft materials. In this review article, recent research efforts on soft material nanoarchitectonics at interfacial media are mainly focused. The presented examples are classified according to the following processes: (i) from molecules to two-dimensional material systems at the air–water interface; (ii) from interfacial assembly to hierarchical nanostructures with layer-by-layer assembly; (iii) from interfacial assembly to hierarchical nanostructures at the liquid–liquid interface; (iv) from material systems to life control at the interface.
Design, System, ApplicationLimitation of motional freedom by reducing the system dimension from three-dimensions to two-dimensions is advantageous in several cases. Therefore, this feature is useful for soft materials that have high possibilities of changes in their conformations, shapes, motions, orientations and arrangements. Nanoarchitectonics in surface media such as solid surfaces, air–water interfaces, and liquid–liquid interfaces would be an attractive strategy for preparation of functional materials with soft material components. For these strategies, molecules and materials are designed to promote specific interactions such as hydrogen bonding in interfacial environments. The presented examples are classified according to the following processes: (i) from molecules to two-dimensional material systems at the air–water interface; (ii) from interfacial assembly to hierarchical nanostructures with layer-by-layer assembly; (iii) from interfacial assembly to hierarchical nanostructures at the liquid–liquid interface: (iv) from material systems to life control at the interface. These examples strikingly demonstrate utilities of the soft material nanoarchitectonics at interfaces in various applications such as sensing and cell culture as well as forefront applications of molecular machine controls. |
1. Introduction
Preparation and fabrication of functional materials and systems are central issues in the development of multiple functions and applications including material synthesis and production,1 sensing and separation,2 environmental matters,3 energy conversion and storage,4 advanced devices,5 and biological and biomedical applications.6 With the current development of nanoscience and nanotechnology, synthesis of functional materials has been done with higher contributions of designs of nanoscale materials. In particular, rational construction of functional structures is widely performed using hard inorganic nanomaterials,7 because the latter components have fewer possibilities in structural uncertainties and fluctuations. In contrast, fabrication of materials with soft and flexible components, i.e. soft material syntheses, often include uncontrollable aspects in material construction, although such soft nature of materials is in turn advantageous for dynamic functions such as stimuli-responsive properties.8 Soft material syntheses with nanoscale units often require contributions of other scientific disciplines including organic syntheses,9 supramolecular chemistry with self-assembly/self-organization,10 template syntheses,11 and bio-nanotechnologies such as DNA origami technology,12 in addition to well-known nanotechnological fabrication.13 Therefore, a new conceptual paradigm that combines nanotechnology and these research disciplines has to be established. A novel concept, “nanoarchitectonics”,14 has been proposed as possibly the most appropriate for this demand.In the transition period from the 20th century to the 21st century, the necessity for the architectonics concept with nanoscale component materials was realized.15 In 1999, an article entitled “Architectonic Quantum Dot Solids” was published by Heath and co-workers.16 The exact term, nanoarchitectonics, was first used in the conference entitled: “the 1st International Symposium on Nanoarchitectonics using Suprainteractions” in Tsukuba, by Masakazu Aono in Japan in 2000.17 It would be the first example to use the term nanoarchitectonics in this meaning in scientific communities. In 2001, the National Institute of Advanced Industrial Science and Technology initiated a research centre for Interfacial Nanoarchitectonics under the leadership of Toshimi Shimizu. A research center named Functional Engineered Nano Architectonics was established at UCLA in 2013. The first scientific paper with the term nanoarchitectonics, “Welding, organizing, and planting organic molecules on substrate surfaces - Promising approaches towards nanoarchitectonics from the bottom up”, was published by Stefan Hecht in 2003.18 In 2007, Masakazu Aono started to direct a research center, World Premier International Research Center for Materials Nanoarchitectonics (WPI-MANA), in Tsukuba, Japan.
Nanoarchitectonics can be achieved with contributions of atomic/molecular level manipulation, chemical synthesis/modification, self-organization/self-assembly, field-controlled arrangement, material fabrication, and bio-related techniques together with advanced knowledge and skills in nanoscience and nanotechnology (Fig. 1).19 The nanoarchitectonics concept is very general and is applicable to a wide range of scientific and technological disciplines from molecular system design to engineering including preparation of functional materials,20 fabrication of advanced supramolecular assemblies,21 target detection and sensors,22 energy-related applications (batteries and capacitors),23 ecological and environmental issues,24 catalysts,25 devices,26 and biological/biomedical applications.27
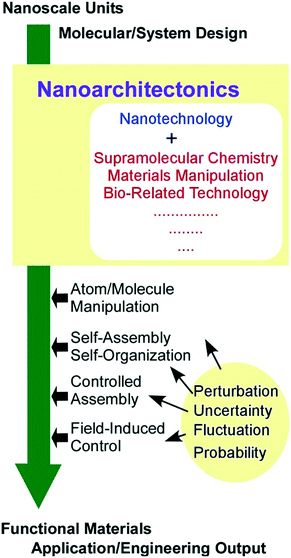 | ||
| Fig. 1 Outline of the nanoarchitectonics concept with related research fields, actions, and factors. | ||
The dynamic nature of the nanoarchitectonics concept especially accords with soft material functionalization. One of the distinct features of the nanoarchitectonics concept would be harmonization of processes and working principles.28 Effective size regions of nanoarchitectonics range from nanometers to micrometers in which quantum effects, static distributions, and thermal fluctuations may be uncontrollably involved in the architecting processes together with unexpected mutual interactions between components. Therefore, balanced harmonization between various factors becomes practically important compared to perfect and precise control of possible factors, as the latter may be virtually impossible. In many cases, the nanoarchitectonics process is supposed to dynamically manage various interactions possibly with their unpredictable uncertainties in balanced harmonization, which is an important feature for soft material fabrication.
Because nanoarchitectonics processes have to harmonize rather complicated interactions, limitation of the motional freedom by reducing the system dimension from three-dimensions to two-dimensions is advantageous in several cases.29 In particular, this feature is useful for soft materials that have high possibilities of changes in their conformations, shapes, motions, orientations and arrangements. Nanoarchitectonics in surface media such as solid surfaces, air–water interfaces, and liquid–liquid interfaces would be an attractive strategy for the preparation of functional materials with soft material components. Immobilization of components on a solid surface would provide opportunity for high-resolution observation of nanoarchitectonics processes. In contrast, the highly dynamic and flexible nature of liquid interfaces provides certain freedom of component diffusion, rearrangements, and mixing, but the two-dimensional (2D) confined nature of the interfacial media prevents the components and architected materials from limitlessly dispersing. In addition, molecular interactions in interfacial media with discontinuous changes of dielectric nature are highly modulated, resulting in efficient and direction-specific molecular association.30
In this review article, recent research efforts on soft material nanoarchitectonics in interfacial media are exemplified. These examples are classified according to the following processes: (i) from molecules to 2D material systems at the air–water interface; (ii) from interfacial assembly to hierarchical nanostructures with layer-by-layer (LbL) assembly; (iii) from interfacial assembly to hierarchical nanostructures at the liquid–liquid interface: (iv) from material systems to life control at the interface. Finally, future technologies such as control of molecular machines and nanocars in interfacial media are also discussed in the conclusion section.
2. From molecules to 2D material systems at the air–water interface
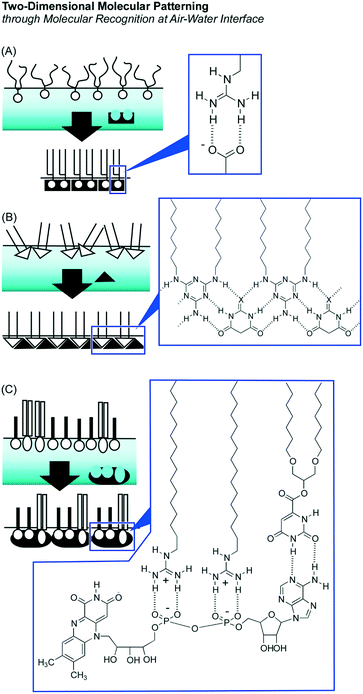
Dynamic interfaces such as the air–water interface are appropriate media for softly assembling molecules to architect functional materials in ultrathin film forms and 2D molecular patterns. At the air–water interface, component molecules preserve their high motional freedom in a heterogeneous 2D environment between two phases with totally different dielectric constants. Therefore, the component molecules possess high opportunities of free contact in both lateral and vertical directions avoiding limitless dispersion in the bulk phase.In particular, monolayer films at the air–water interface provide rational encountering points between synthetic molecules (monolayer) and biomolecules from the aqueous subphase.31 In biological systems, molecular recognition between target signal molecules and receptor biomolecules, specific hybridization of nucleic acid strands, and selection of substrates by enzymes actually happen in aqueous interfacial media such as cell membrane surfaces, macromolecular interfaces, and pockets of protein insides. The air–water interface works as an appropriate medium and as a model of aqueous bio-interfaces for molecular recognition and assembly. Striking enhancement of molecular interaction at the air–water interface was proved both experimentally and theoretically (Fig. 2).32 While binding constants of electrostatic hydrogen-bonding pairs between guanidinium and phosphate were assigned to 1.4 M−1 at molecular dispersion in the aqueous phase33 and 102–104 M−1 in aqueous mesoscopic interfaces of micelles and lipid bilayers,34 the corresponding constants are enhanced to 106–107 M−1 at the air–water interface.35 The experimentally observed striking enhancement of molecular recognition efficiency can be also proved by quantum chemical calculations in interfacial media between the low dielectric phase (lipid) and high dielectric phase (water).36 The contribution of favourable characteristics for electrostatic hydrogen-bonding in the low dielectric phase is significant at the less-disturbed aqueous interface. According to these discoveries, efficient molecular recognitions for various biomolecules including sugars,37 amino acids,38 nucleotides,39 nucleic acid bases,40 and peptides41 have been accomplished at the air–water interface.
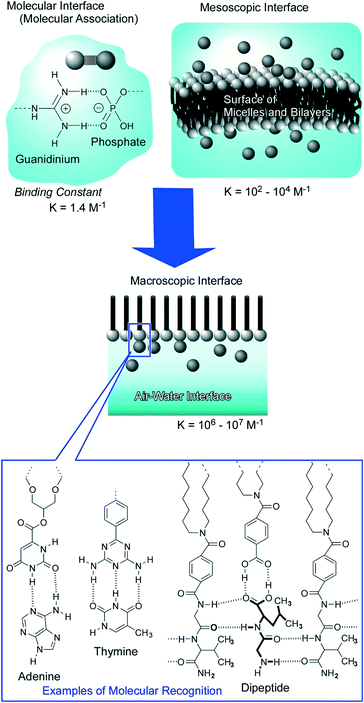 | ||
| Fig. 2 Molecular recognition at the air–water interface: striking enhancement of the binding constant and rich variety of guest selection with examples of recognition motifs. | ||
These enhanced natures of molecular interactions at the air–water interface are advantageous for the preparation of 2D pattern structures in molecular-level precision through molecular assemblies within a 2D medium with the aid of aqueous components. This methodology, so-called 2D molecular patterning,42 utilizes aqueous template molecules with multiple binding sites to make defined arrangements of amphiphiles through specific molecular recognition at the air–water interface (Fig. 3). Specific binding of an aqueous dicarboxylate template to guanidinium amphiphiles resulted in a regular 2D molecular pattern in the crystal lattice depending on the length of the spacer between two carboxylate groups in the template.43 Regular patterns were prepared through formation of supramolecular polymers of dialkylmelamine amphiphiles and aqueous barbituric acid molecules.44 Binding of one aqueous flavin adenine dinucleotide template to two guanidinium amphiphiles and one orotate amphiphile can create 2D molecular patterns with sub-nanometer level regular structures.45
The importance of regulation of molecular arrangements within 2D environments is well recognized in lipid raft formation at biological cell membranes, which is deeply related to various biological functions. However, driving forces for the formation of lipid rafts have not been fully understood. In a recent review by Mukai and Regen,46 they have investigated in detail the lipid raft formation with the nearest-neighbour recognition concept and suggested important roles of polyunsaturated phospholipids in the lipid raft formation.
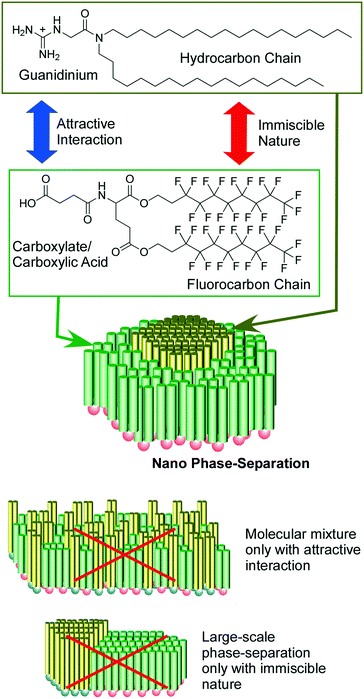
As raft-like nano-domain formation by artificially designed molecules, 2D nanoarchitectonics with fluorocarbon–hydrocarbon diblock molecules based upon the intrinsic immiscible nature between fluorocarbon and hydrocarbon were introduced in a recent review article by Krafft and co-workers.47 When these diblock molecules spread at the air–water interface, circular mesoscopic nano-domains were unusually formed even at zero surface pressure. Further compression of the monolayer of fluorocarbon–hydrocarbon diblock molecules resulted in the formation of regular hexagonal arrays. Oishi and co-workers reported controlled formation of regular domains within the mixed monolayer of fluorocarbon carboxylic acid and hydrocarbon guanidinium on the basis of balances of repulsive interaction between fluorocarbon and hydrocarbon and attractive force between carboxylate and guanidinium (Fig. 4).48 Their domain sizes and shapes can be controlled from the nanometer to the micrometer scale by various external factors including temperature, pH, pressure, composition, and ionic strength.
Regularly assembled molecules in interfacial media often show interesting properties because they possibly have unique orientation, anisotropy, and aggregation. Seki summarized the motions of photo-active molecules and macromolecules in their assemblies at interfaces in his recent review article.49 Spatial–temporal motional control of soft materials in sub-micrometer level precision can be accomplished with sufficient repeatability and durability. Conversion of photonic energies to mechanical functions would open a new paradigm of soft material nanoarchitectonics.
Sakakibara et al. demonstrated regulation of photo-energy conversion and transfer processes of the assembled structures of an oligo(p-phenylenevinylene)-derived π-gelator.50 This molecule assembled into supramolecular fibers of an entangled network upon drop-casting its solution on a solid surface (conventional technique). In the formed network fibers, oligo(p-phenylenevinylene) segments are arranged perpendicularly to the long axis of the fiber. The orientation is favorable for the long-range excitation energy transfer. On the other hand, spreading the same molecule at the air–water interface creates aligned nanorods in which the oligo(p-phenylenevinylene) segments are mainly oriented in parallel to the long axis of the nanorods. In the latter case, fluorescence enhancement would be significantly induced, which is appropriate for charge transport.
Molecular recognition at the air–water interface is not limited to complex formation between separate component species. Continuous formation of molecular recondition structures resulted in two-dimensionally developed materials. As discussed in a recent review article by Sakamoto,51 this strategy is appropriate for the preparation of low-dimensional materials through well-defined molecular interactions such as coordination and hydrogen bonding. In fact, interfacial environments such as the air–water interface provide opportunities to form 2D nanosheets of coordination polymers and metal–organic frameworks. Similarly, hydrogen bonding-based supramolecular polymerization between amphiphilic melamine and barbituric acid to form a linear polymer-like extended one-dimensional structure was accomplished on the basis of enhanced efficiency of hydrogen bonding at the air–water interface.44,52
Supramolecular polymerization of DNA origami pieces at the air–water interface was recently reported by Yonamine et al. who first demonstrated the formation of Langmuir films and Langmuir–Blodgett (LB) films of DNA origami (Fig. 5).53 The DNA origami pieces used in this research possessed a rectangular shape (90 nm × 65 nm in dimension), which was complexed with a cationic lipid to give sufficient solubility in organic solvents such as chloroform. A chloroform solution of the rectangular DNA origami was spread at the air–water interface to form its Langmuir monolayer. Although the DNA origami pieces kept their rectangular shape at the air–water interface, repeated cycles of lateral mechanical compression and expansion of the DNA origami monolayer activated the motions of the DNA origami in the 2D plane. This dynamic process induced one-dimensional supramolecular polymerization of the DNA origami pieces through hydrogen bond formation at unpaired DNA strands at two opposite sides of the rectangle piece. While the width and thickness of the formed belt-shaped supramolecular polymers kept the same, their lengths were increased to 130 nm, 680 nm and 2240 nm after the first, second and third compression, respectively. Yonamine et al. also demonstrated lipid-fueled transfer of DNA wheels at the solid interface from a hydrophilic surface to a hydrophobic surface.54 DNA wheels are basically hydrophilic, but addition of lipid to the system makes them hydrophobic, resulting in 2D transfer of the DNA wheels. This transfer is made through the solution space accompanied by a flip-flop motion.
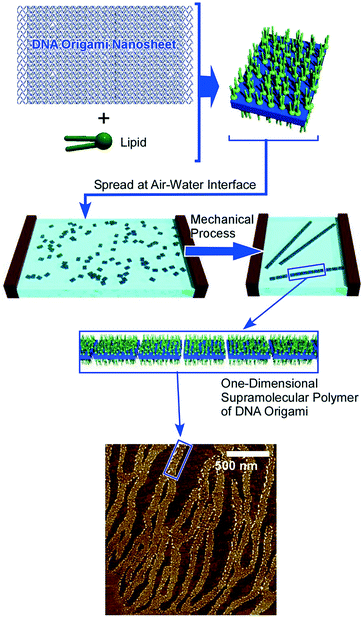 | ||
| Fig. 5 Formation of Langmuir films and LB films of DNA origami with one-dimensional supramolecular polymerization of the DNA origami upon 2D mechanical actions. | ||
Materialization of assembled structures at the air–water interface leads to bottom-up synthesis as recently demonstrated by Mori et al. who successfully prepared carbon nanosheets through carbonization of two-dimensionally assembled carbon nanorings at the air–water interface with vortex flow.55 In this approach, the anisotropic carbon nanoring molecule, 9,9′,10,10′-tetra-butoxy-cyclo[6]-paraphenylene[2]-3,6-phenanthrenylene, was spread onto the water surface with a constant vortex flow (Fig. 6). The self-assembly process with dynamic motion resulted in the formation of molecular nanosheets. Heat-treatment of the assembled nanosheets under a constant inert gas flow resulted in ultrathin uniform carbon nanosheets with nanopore structures maintaining the original 2D morphology of the molecular assembly but with a sufficient increase of electric conductivity. Fabrication of nitrogen-doped carbon nanosheets was also conducted by simply mixing pyridine with the original monolayer components. The nitrogen-doped carbon nanosheets have exhibited further increased electrical conductivity and are also expected as a highly efficient catalyst for oxygen reduction reactions for high-performance fuel cells.
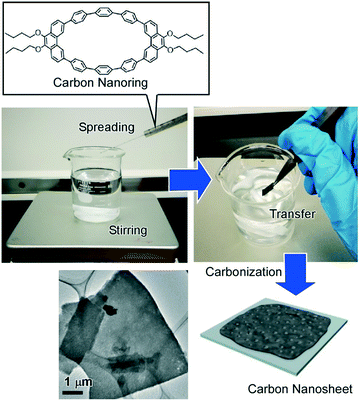 | ||
| Fig. 6 Preparation of carbon nanosheets through carbonization of two-dimensionally assembled carbon nanorings at the air–water interface with vortex flow (vortex LB method). | ||
3. From interfacial assembly to hierarchic nanostructures with layer-by-layer assembly
Layered nanostructures are capable of immobilizing functional units within defined structures, which is useful for the preparation of materials with various functions.56 For example, Oliveira Jr and co-workers used alternate layer structures of a monoclonal antibody and chitosan/gold nanoparticles as immuno-sensors for a biomarker of venous thromboembolism.57 Ruiz-Hitzky et al. demonstrated sensing application for the detection of alkali ions by montmorillonite layered silicates with dimethylsila-14-crown-5 and dimethylsila-17-crown-6 macrocyclic polyethers.58In addition to the conventional functionalization of pre-formed layered structures, the construction of layered structures from unit layers is also a powerful strategy to create functional materials with various components in defined structures. Conversion of interfacial assembly to hierarchical nanostructures can be accomplished through constructing unit monolayers into layered structures by appropriate methods such as the LB method59 and LbL assembly.60 While the LB method provides well-packed precisely controlled thin films, less-organized flexible layered structures can be obtained from a wide range of materials by the LbL assembly as so-called fuzzy nano-assembly (Fig. 7).61 The basic mode of the LbL assembly is based on electrostatic interaction with charge neutralization and charge re-saturation, but other interactions such as hydrogen bonding,62 coordination,63 supramolecular inclusion,64 bio-specific interaction,65 charge transfer interaction,66 and stereo-complex formation67 can also be used as driving forces for the assembly. Procedures for the LbL assembly are very simple and can be done simply with beakers and tweezers in most cases. Coupling with other techniques such as spin-coating,68 automatic dipping,69 and spray-coating70 is also possible. In addition, the LbL assembly is applicable to microscopic colloidal templates for the formation of hollow microspheres.71 The LbL assembly is one of the highly appropriate procedures in soft material nanoarchitectonics in hierarchical layered motifs.
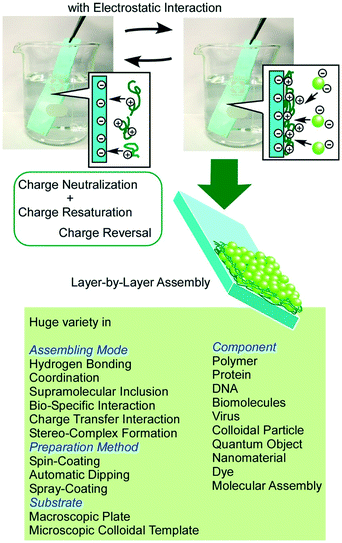 | ||
| Fig. 7 Outline of LbL assembly. A huge variety of component materials and assembling mechanisms can be involved. | ||
Various functions including electrochemical energy-related applications are expected from 2D materials such as graphene and MoS2 nanosheets.72 Not limited to the typical applications, 2D materials can work as atomically flat structural components in nanoarchitectonics structure fabrication. For example, the 2D ultra-narrow gap filled with an ionic liquid between highly π-conjugated nanosheets of reduced graphene oxide was fabricated with the aid of the LbL assembly technique.73 In this approach, graphene oxide nanosheets were first dispersed and then converted to reduced graphene oxides in the presence of an ionic liquid (imidazolium salts). The resulting reduced graphene oxide nanosheets were efficiently covered by imidazolium cations to keep them dispersed in water. The formed cationic complexes have alternate layered structures of graphene nanosheets and an ultrathin ionic liquid phase, which were further nanoarchitected into LbL structures with an anionic polyelectrolyte, poly(sodium styrenesulfonate), on an electrode surface of a quartz crystal microbalance (QCM) sensor. Exposure of the surface modified QCM sensor to vapours of organic solvents resulted in QCM frequency shifts upon adsorption of the tested organic vapours depending on their affinity to graphene–ionic liquid layered hybrids. Greater responses for vapour detection were obtained for aromatic molecules such as benzene and toluene. A sensitivity to aromatic benzene vapour that is more than 10 times higher than that to aliphatic cyclohexane was observed, although these two molecules have similar molecular sizes, molecular weights, and vapour pressures. The liquid phase of imidazolium salts between graphene nanosheets would be a highly π-electron rich 2D phase, where aromatic molecules would have a higher partition coefficient.
Hierarchical nanoarchitectures can be fabricated through nanoarchitecting materials with internal nanostructures such as mesoporous carbon into layered motifs by the LbL method.74 Standard mesoporous carbon, CMK-3, was first partially surface oxidized and then assembled into layered structures by the LbL assembly method together with polyelectrolytes, poly(diallyldimethlyammonium chloride) and poly(sodium styrenesulfonate) on the QCM sensor plate. Detection of various tea components such as tannic acid, catechin and caffeine was investigated after immersing the QCM sensor with CMK-3 LbL films in water. The detection sensitivity to tannic acid well exceeded those observed for catechin and caffeine because of size matching of the guest and nanopore inside. Interestingly, sigmoidal behaviors of adsorption amounts of tannic acids on the CMK-3 LbL film as a function of the concentration of tannic acid were observed. The highly cooperative adsorption of tannic acid within the confined space of carbon nanochannels would be a plausible reason of the higher sensitivity to tannic acid.
A higher level of hierarchical structures was nanoarchitected by LbL assembly of mesoporous carbon capsules with a counterionic polyelectrolyte (Fig. 8).75 Mesoporous carbon capsules, with dimensions of 1000 × 700 × 300 nm3 and a thickness of 35 nm with a uniform pore diameter distribution centered at 4.3 nm and a specific surface of 918 m2 g−1, were synthesized using zeolite crystals as templates. After being coated with the charged surfactant, the mesoporous carbon capsules were assembled with appropriate polyelectrolytes into a LbL motif on the QCM sensor plates. Their sensor capabilities were examined by exposures of sensors to organic vapours, resulting in higher sensitivity to aromatic species on the basis of favourable π–π interactions. Further modifications of the sensing affinities of these sensing systems can be done with impregnation of the second sensing components. Impregnation with lauric acids altered the sensing affinity to aliphatic amine guests. In contrast, highly sensitive detection toward acetic acid vapour was achieved by doping dodecyl amine as the second sensing component to the mesoporous capsules.
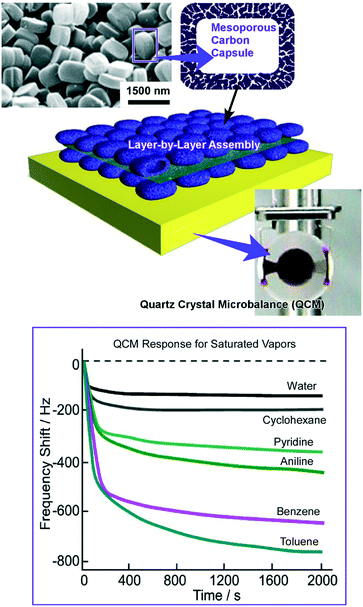 | ||
| Fig. 8 LbL assembly of mesoporous carbon capsules on a QCM sensor plate for sensing organic vapours with high sensitivity towards aromatic species. | ||
Similar hierarchical LbL structures can be fabricated with silica-based mesoporous capsules.76 In the latter cases, the prepared hierarchical LbL structures can be used for material delivery and drug delivery systems with unusual material release profiles. The hierarchical structural features lead to auto-modulated stimuli-free on–off release of molecules trapped within capsules.
Recently, Leong and co-workers reported the LbL assembly of DNA modified 2D MoS2 nanosheets.77 Good biocompatibility can be expected for the transition metal dichalcogenide MoS2 nanosheets. The tagged DNA sequences were designed to be assembled only with complementary combinations, resulting in alternate layers of MoS2 nanosheets and DNA. Desirable drugs such as doxorubicin can be loaded within the spaces between the sheets. Further, superstructures guided by a linker aptamer with recognition capabilities towards ATP can be nanoarchitected. The drugs were safely shielded when the LbL structures were preserved. Exposure to ATP in cancer cells induces disassembly of the LbL structures to release drug molecules. According to the hierarchical design, doxorubicin, as an anticancer drug, can be released in the highly metabolically active environment of cancer cells.
4. From interfacial assembly to hierarchical nanostructures at the liquid–liquid interface
As demonstrated in recent various examples, hierarchical nanoarchitectonics of functional molecules and ionic species is capable of constructing various functional systems. Tanaka and co-workers proposed a method for the preparation of programmable hetero-atom ions using supramolecular arrays of porphyrin and phthalocyanine.78 Haketa and Maeda recently reviewed methods to nanoarchitect dimension-regulated π-electronic ion-pairing assemblies,79 which are useful for ion-responsive functional materials. Akamatsu et al. reported optical imaging of cesium ions on the basis of an optode membrane incorporating a calix[6]arene derivative and appropriate dye components.80 The proposed system can be down-sized into ca. 100 nm-sized nanoparticles. These sensing systems can be utilized for detection of cesium ions in conventional environmental media including domestic water supplies and seawater.As unique ion-related hierarchical assemblies, cubic-shaped three-dimensional crystals of an organometallic heteronanostructure between C60 and silver ions, so-called bucky cubes, could be fabricated.81 The bucky cube was precipitated at the liquid–liquid interface between benzene or toluene solution of C60 and ethanolic silver(I) nitrate solution. Washing the bucky cubes with low molecular weight aliphatic alcohols induced the growth of needle-like crystals from the cube surface, resulting in needle-on-cube heterostructures.
As demonstrated in the last example, liquid–liquid interfaces are powerful media to form assembled and crystallized materials in regulated shapes (Fig. 9).82 Various examples of nanoarchitectonics construction with fullerene (C60 and C70) molecules by the liquid–liquid interfacial precipitation method have been reported as seen in pioneering examples of one-dimensionally grown C60 nanowhiskers and nanotubes reported by Miyazawa and co-workers.83 At the interface between a good solvent and a poor solvent for fullerenes, supersaturation induces the formation of shape-defined nanostructures of fullerene molecules. The shapes, dimensions, and sizes of the formed fullerene nanoarchitectures can be controlled by selection of combinations of good and poor solvents together with other physical parameters. Regular-shaped 2D nanostructures such as C60 hexagon nanosheets were formed.84 Faint modification of the synthetic conditions in the liquid–liquid interfacial precipitation processes such as selection of poor solvents, fullerene concentrations, and volume ratios of both solvents resulted in the controlled nanoarchitectonics of fullerene crystals in one-, two-, and three-dimensional regular motifs.85
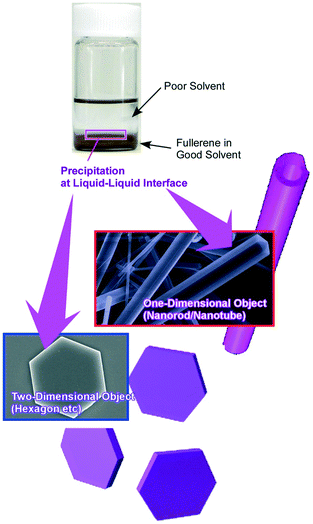 | ||
| Fig. 9 Various examples of nanoarchitectonics construction with fullerene (C60 and C70) molecules by using the liquid–liquid interfacial precipitation method. | ||
One of the successful fullerene nanoarchitectonics in three-dimensional motifs would be the formation of cubic assemblies from pure C70 molecules.86 These cubic objects can be formed using ultrasonic-agitated liquid–liquid interfacial precipitation between a mesitylene solution of C70 and tert-butyl alcohol. The nanoarchitected C70 cubic assemblies were then converted into hierarchical structures of needles-on-cube nanoarchitectures simply upon exposure of the cubes to isopropyl alcohol under ambient conditions. From the surface faces of the cubes, rearrangements of C70 assemblies resulted in vertical growth of needles of C70 assemblies, which further possessed nanoporous structures. The hierarchical needles-on-cube nanoarchitectures with nanopores with high-surface-areas are capable of high-performance sensing for toxic aromatic vapours. Enhanced interaction between sp2-featured fullerene assembly and aromatic gases, high-contact probability, and easy diffusion of the guest gases would be the causes of highly sensitive sensing. Like the antenna of insects, the formed needles-on-cube nanoarchitectures could sense targets selectively and sensitively.
Additional integrated three-dimensional nanoarchitectures of C70 assemblies are hole-in-cube assemblies of C70 molecules.87 The formation of hole-in-cube assemblies, which have one open microscale hole on each face of the cubic assembly of C70, was induced by mesitylene and tert-butyl alcohol treatment under an appropriate level of agitating conditions. Manipulations of the open hole structures including intentional hole closures and re-opening were operative by addition of extra C70 molecules and local irradiation of electron beams, respectively. The created holes on the cube surfaces can trap microscopic objects selectively. Based on the highly sp2-nature of the insides of holes, graphitic carbon microparticles were selectively trapped over polymer-resin microparticles with a similar size. Immobilization of hole-in-cube assemblies of C70 molecules on a QCM electrode would lead to fabrication of a sensor system useful for the selective detection of micron-sized pollutant particles in an environment with exhaust gas.
Time-programmed shape changes of fullerene assemblies like transformation from eggs to tadpoles were also demonstrated in liquid–liquid interfacial environments as defined by supramolecular differentiation (Fig. 10).88 This phenomenon has been realized through the co-assembly process of fullerene derivatives with two-different substituents, alkyl arms and phenyl-attachments. Egg-like spherical assemblies were first formed. Sufficient incubation under appropriate liquid–liquid interfacial conditions induces local phase separation at the surface of the egg spheres. The formed phase-separated domains work as seeds for further growth of tube-like structures. After the programmed time interval under appropriate conditions, the tube-like structures start to grow like transformation from eggs to tadpoles. It would be surprising that non-living and non-DNA-including pure material systems can imitate bio-like time-programmed shape transformation.
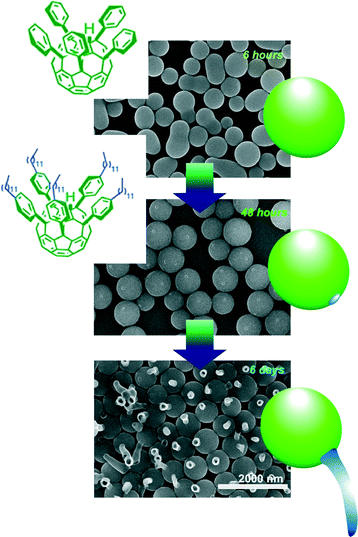 | ||
| Fig. 10 Time-programmed shape changes of fullerene assemblies like transformation from eggs to tadpoles were demonstrated in liquid–liquid interfacial environment as supramolecular differentiation. | ||
5. From material systems to life control at the interface
Not limited to organic molecules and inorganic substances, nanoarchitectonics of biomolecules can also create advanced functions in both basic sciences and engineering applications.89 In particular, bio-substances have intrinsic soft nature and thus can be an important subject in soft material nanoarchitectonics. Takahashi, Kamagata and co-workers proposed a DNA garden strategy for stretchable DNA arrays that can be used for single-molecule fluorescence imaging of DNA-binding proteins.90 Desirable DNA nanoarchitectonics arrangements are made with micro-contact printing techniques to immobilize biotinylated bovine serum albumin to the 2-methacryloyloxyethyl phosphorylcholine polymer on a coverslip. The subsequent tethering of neutravidin and biotinylated DNA achieved DNA gardening. The prepared DNA gardens can be used for single-molecule level observations on a tumor suppressor sliding along extended DNA chains and specification of restriction enzyme cleavage sites.He and Xu proposed a novel concept, an instructed-assembly to handle nanoarchitectonics of ordered molecular superstructures upon at least one trigger event including receptor–ligand interaction and some reactions.91 It provides spatiotemporal regulation on transformation from a starting equilibrium to another one, which can be possibly utilized for molecular imaging, tissue engineering, and cancer therapy. The instructed-assembly will be also available for dynamics of molecular processes to control the cell fate.
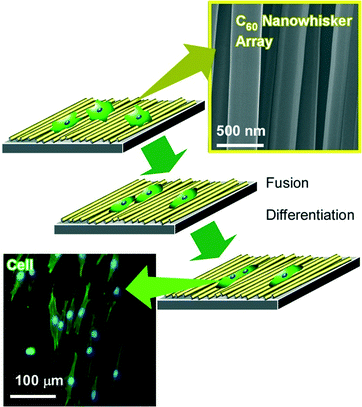
Using interfacial nanoarchitectonics to control the cell fate, Minami and co-workers reported the modulation of cell differentiations of mouse skeletal myoblast C2C12 cells on interfacially nanoarchitected arrays of super-hard materials of fullerene nanowhiskers (Fig. 11).92 Fullerene nanowhiskers are needle-like objects of one-dimensional fullerene assemblies that can be synthesized by the liquid–liquid interfacial precipitation method, be aligned at the air–water interface with appropriate lateral pressures, and be transferred onto a solid surface by the LB technique. The cells grew along with the aligned fullerene nanowhiskers with shape shifting. For example, myoblasts spread with elongated morphologies of distinctly high aspect ratios on the aligned nanowhisker array, while cells exhibited polygonal shape on the surface of random nanowhisker scaffold similar to that on a bare glass. In addition, large fusion index values were observed for the cells grown on the highly aligned arrays of fullerene nanowhiskers. Furthermore, the gene expression level of MyoD in the cells on the aligned fullerene nanowhiskers became 1.35 times higher than that observed for the cells on the surface of bare glass. The obtained results on the up-regulation of the myogenic genes indicated acceleration of the early and late stages of myogenic differentiation on the aligned fullerene nanowhiskers. Similar cell controls were also investigated on curvature-controlled alignments of fullerene nanowhisker arrays that were fabricated by the vortex LB method with circular liquid flow at the interface.93 In this case, preferential growth of human osteoblast MG63 cells along the direction of alignment nanowhiskers was again observed.
In contrast to arrays of super-hard materials, super-soft interfaces, liquid themselves, can be used as cell culture substrates. Rosenberg introduced for the first time the use of a fluid substrate for the growth of both transformed and anchorage-dependent cells in 1964.94 In 1983, Keese and Giaever subsequently demonstrated the use of perfluorocarbon–water interfaces for cell culture, and the use of additives for the formation of thin layers of protein at an interface as a substrate.95 These pioneering research efforts were then followed by several examples for interfacial cell culture at liquid–liquid interfaces.96 As an epoch-making finding, Minami and co-workers first demonstrated suppression of myogenic differentiation of mammalian cells caused at the liquid–liquid interface in 2017.97 In 2018, this discovery was expanded to stem cell cultures by Gautrot and co-workers.98
Minami and co-workers successfully demonstrated cell adhesion and growth of C2C12 myoblasts at the liquid interface between fluorocarbon fluid and culture medium where strains between the cell and substrate are expected to be significantly diminished (Fig. 12).97 On conventional super-hard substrates, C2C12 cells were clearly differentiated in differentiation medium as indicated by significant up-regulation of the muscle specific gene expression level (MHC; myosin heavy chain). In contrast, the liquid–liquid interface induced remarkable attenuation of expression of myogenin, a transcription factor in the early stages of differentiation of C2C12. The stiffness of a substrate is important in myogenic differentiation of C2C12 cells. Due to the super-soft nature of the liquid–liquid interface, traction forces exerted by cells are significantly weakened, and thereby myogenic differentiation of C2C12 myoblasts is suppressed. In clinical applications, adult stem cells easily lose their stemness accompanied by differentiation on a conventional plastic culture dish. The results indicated the potential applications of interfacial culture systems for the retention of stemness. Because cell culture at the liquid–liquid interface enables us to transfer living cells onto a solid substrate as LB films of living cells, this methodology would open a new avenue for tissue engineering. Architecting living cells into regulated structures can be regarded as an ultimate example of soft material nanoarchitectonics.
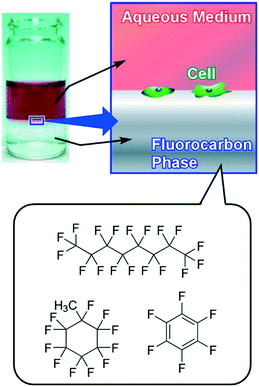 | ||
| Fig. 12 Culture of C2C12 myoblast cells at the super-soft liquid interface between fluorocarbon fluid and culture medium. | ||
6. Conclusion and perspective
In this review article, various research examples of soft material nanoarchitectonics are briefly explained in particular from viewpoints of science and technology in interfacial environments including interfacial molecular recognition, fabrication of 2D structures and materials, LbL material constructions, hierarchical structural formation, and cell cultures. Accomplishments of these processes surely and heavily depend on successful designs of unit molecules. In addition, the obtained nanoarchitected materials have a wide range of possibilities in engineering applications such as environmental sensing and remediation, and biomedical usages including tissue engineering and cell fate regulation. In particular, the soft nature of component materials and interfacial environments as working media are crucial keys of these nanoarchitectonics processes from molecular system design to future engineering applications.Common features of the exemplified research examples are dynamic natures for advanced functions. Therefore, development of methodologies for precise control of molecular dynamics in interfacial media would be the next-generation target in soft material nanoarchitectonics. Regulation of single molecules such as molecular machines and nanocars with precise observations and manipulations has become possible by sophisticated nanotechnology with advanced tools, mainly scanning tunnelling microscopy under ultra-high vacuum at lower temperature.99 Such efforts based on high-level nanotechnology performances would clarify the importance of molecular system designs on dynamic functions. It would be one future direction of soft material nanoarchitectonics.
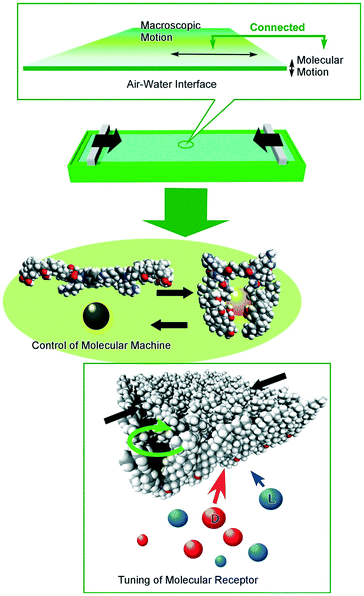
In contrast to the above-mentioned precise molecular control under ultra-clean conditions, molecular control under ambient conditions also had great contributions to dynamic functions in realistic material usages. We have been proposing important roles of interfacial media to control molecular motions and their functions by ambient and conventional external stimuli such as macroscopic mechanical motions (Fig. 13).100 Because dynamic interfaces such as the air–water interface can couple macroscopic ambient motions along lateral directions and precise molecular functions within nanoscale thickness, control of nanoscopic motions and functions of molecular machines and receptors embedded at the air–water interface is possible by mechanical motions, lateral compression and expansions of a single molecular film (Langmuir monolayer) in tens of centimeters.101 Various examples on regulations of molecular functions including capture and release of a guest molecule,102 pressure modulated enantioselective recognition of amino acids,103 precise discrimination between thymine and uracil derivatives,104 mechanically controlled indicator displacement assay,105 closure of molecular pliers in both the analogue mode106 and digital mode,107 and motional regulation of molecular rotors108 have been accomplished by macroscopic mechanical stimuli.
The nanoarchitectonics concept provides rational strategies to create functional materials with deep relationships with molecular system designs that are especially useful for the development of functions of soft materials. Including the essence of interfacial science would also supply an important factor of connection of molecular functions and ambient motions. Upon reconsidering important aspects as seen in molecular architectonics,109 self-assembly-based nanoarchitectonics,110 two-dimensional interfacial nanoarchitectonics,111 and bio-related nanoarchitectonics,112 various applications and engineering outputs in devices, materials, energy and bio-technology113 can be continuously created. We expect that molecular system designs can be rationally connected with realistic engineering outputs upon combining the above-mentioned methodologies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by JSPS KAKENHI Grant Number JP16H06518 (Coordination Asymmetry) and CREST JST Grant Number JPMJCR1665.Notes and references
- A. Vinu, P. Srinivasu, D. P. Sawant, T. Mori, K. Ariga, J.-S. Chang, S.-H. Jhung, V. V. Balasubramanian and Y. K. Hwang, Chem. Mater., 2007, 19, 4367 CrossRef CAS; K. K. R. Datta, S. B. V. Subba, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961 CrossRef PubMed; M. Hu, J. Reboul, S. Furukawa, N. L. Torad, Q. Ji, P. Srinivasu, K. Ariga, S. Kitagawa and Y. Yamauchi, J. Am. Chem. Soc., 2012, 134, 2864 CrossRef PubMed; N. Chatani, Bull. Chem. Soc. Jpn., 2018, 91, 211 CrossRef; S. Zhang, S. Guo, Z. Chen, Y. Wang, H. Gao, J. Gomez-Herrero, P. Ares, F. Zamora, Z. Zhu and H. Zeng, Chem. Soc. Rev., 2018, 47, 982 RSC; I. Saptiama, Y. V. Kaneti, Y. Suzuki, K. Tsuchiya, N. Fukumitsu, T. Sakae, J. Kim, Y.-M. Kang, K. Ariga and Y. Yamauchi, Small, 2018, 14, 1800474 CrossRef PubMed.
- B. Chen, Y. Ding, X. Li, W. Zhu, J. P. Hill, K. Ariga and Y. Xie, Chem. Commun., 2013, 49, 10136 RSC; W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev., 2017, 46, 3242 RSC; I. Stassen, N. Burtch, A. Talin, P. Falcaro, M. Allendorf and R. Ameloot, Chem. Soc. Rev., 2017, 46, 3185 RSC; Q. Ji, X. Qiao, X. Liu, H. Jia, J. S. Yu and K. Ariga, Bull. Chem. Soc. Jpn., 2018, 91, 391 CrossRef CAS; J. Sasaki, M. Suzuki and K. Hanabusa, Bull. Chem. Soc. Jpn., 2018, 91, 538 CrossRef.
- N. L. Torad, M. Hu, S. Ishihara, H. Sukegawa, A. Belik, M. Imura, K. Ariga, Y. Sakka and Y. Yamauchi, Small, 2014, 10, 2096 CrossRef CAS PubMed; F. C. Moreira, R. A. R. Boaventura, E. Brillas and V. J. P. Vilar, Appl. Catal., B, 2017, 202, 217 CrossRef; X. Liu, J. Iocozzia, Y. Wang, X. Cui, Y. Chen, S. Zhao, Z. Li and Z. Lin, Energy Environ. Sci., 2017, 10, 402 RSC; K. Ishimaki, T. Uchiyama, M. Okazaki, D. Lu, Y. Uchimoto and K. Maeda, Bull. Chem. Soc. Jpn., 2018, 91, 486 CrossRef; S. Yu, X. Wang, H. Pang, R. Zhang, W. Song, D. Fu, T. Hayat and X. Wang, Chem. Eng. J., 2018, 333, 343 CrossRef.
- W. Chaikittisilp, H.-S. Huang, M. Hu, T. Fujita, K. C.-W. Wu, L.-C. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259 RSC; D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361 CrossRef CAS; Y. Yamada, T. Yamada and Y. Kanemitsu, Bull. Chem. Soc. Jpn., 2017, 90, 1129 CrossRef; S. Stauss and I. Honma, Bull. Chem. Soc. Jpn., 2018, 91, 492 CrossRef; P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, Angew. Chem., Int. Ed., 2018, 57, 102 CrossRef PubMed; T. Miyasaka, Bull. Chem. Soc. Jpn., 2018, 91, 1058 CrossRef; M. Watanabe, K. Dokko, K. Ueno and M. L. Thomas, Bull. Chem. Soc. Jpn., 2018, 91, 1660 CrossRef; D. R. Dekel, J. Power Sources, 2018, 375, 158 CrossRef.
- K. Krishnan, M. Muruganathan, T. Tsuruoka, H. Mizuta and M. Aono, Adv. Funct. Mater., 2017, 27, 1605104 CrossRef CAS; F. Yang, M. Suda and H. M. Yamamoto, Bull. Chem. Soc. Jpn., 2017, 90, 1259 CrossRef; F. Yao, P. Gui, Q. Zhang and Q. Lin, Mol. Syst. Des. Eng., 2018, 3, 702 RSC; Y. Kobayashi, Bull. Chem. Soc. Jpn., 2018, 91, 467 CrossRef; A. Yamamura, S. Watanabe, M. Uno, M. Mitani, C. Mitsui, J. Tsurumi, N. Isahaya, Y. Kanaoka, T. Okamoto and J. Takeya, Sci. Adv., 2018, 4, eaao5758 CrossRef.
- Y. Yoshida and S. Yamanaka, Circ. Res., 2017, 120, 1958 CrossRef CAS PubMed; I. Saptiama, Y. V. Kaneti, Y. Suzuki, Y. Suzuki, K. Tsuchiya, T. Sakae, K. Takai, N. Fukumitsu, Z. A. Alothman, M. S. A. Hossain, K. Ariga and Y. Yamauchi, Bull. Chem. Soc. Jpn., 2017, 90, 1174 CrossRef; S. Ufaz, A. Balter, C. Tzror, S. Einbender, O. Koshet, J. Shainsky-Roitman, Z. Yaari and A. Schroeder, Mol. Syst. Des. Eng., 2018, 3, 38 RSC; A. Harada and K. Kataoka, Polym. J., 2018, 50, 95 CrossRef; I. Saptiama, Y. V. Kaneti, H. Oveisi, Y. Suzuki, K. Tsuchiya, K. Takai, T. Sakae, S. Pradhan, M. S. A. Hossain, N. Fukumitsu, K. Ariga and Y. Yamauchi, Bull. Chem. Soc. Jpn., 2018, 91, 195 CrossRef.
- C. Tan, X. Cao, X.-J. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han, G.-H. Nam, M. Sindoro and H. Zhang, Nat. Rev. Cancer, 2017, 17, 20 CrossRef CAS PubMed; T. Kitamura and Y. Niidome, Bull. Chem. Soc. Jpn., 2017, 90, 161 CrossRef; H. Shirai, M. T. Nguyen, D. Cempel, H. Tsukamoto, T. Tokunaga, Y.-C. Liao and T. Yonezawa, Bull. Chem. Soc. Jpn., 2017, 90, 279 CrossRef; S. Dang, Q.-L. Zhu and Q. Xu, Nat. Rev. Mater., 2018, 3, 17075 CrossRef; K. Zheng, M. I. Setyawati, D. T. Leong and J. Xie, Coord. Chem. Rev., 2018, 357, 1 CrossRef.
- M. Wei, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127 RSC; Y. Wang and T. Michinobu, Bull. Chem. Soc. Jpn., 2017, 90, 1388 CrossRef CAS; W. Lu, X. Le, J. Zhang, Y. Huang and T. Chen, Chem. Soc. Rev., 2017, 46, 1284 RSC; M. Irie and M. Morimoto, Bull. Chem. Soc. Jpn., 2018, 91, 237 CrossRef; K. Sada, Bull. Chem. Soc. Jpn., 2018, 91, 1282 CrossRef; P. Weis and S. Wu, Macromol. Rapid Commun., 2018, 39, 1700220 CrossRef PubMed.
- G. Povie, Y. Segawa, T. Nishihara, Y. Miyauchi and K. Itami, Science, 2017, 356, 172 CrossRef CAS PubMed; J. Yamaguchi and K. Itami, Bull. Chem. Soc. Jpn., 2017, 90, 367 CrossRef; K. Takimiya and M. Nakano, Bull. Chem. Soc. Jpn., 2018, 91, 121 CrossRef; Y. Koga, T. Kaneda, Y. Saito, K. Murakami and K. Itami, Science, 2018, 359, 435 CrossRef PubMed; S. Hiroto, Bull. Chem. Soc. Jpn., 2018, 91, 829 CrossRef; Z. Sun, T. Matsuno and H. Isobe, Bull. Chem. Soc. Jpn., 2018, 91, 907 CrossRef; T. Sugahara, J.-D. Guo, T. Sasamori, S. Nagase and N. Tokitoh, Angew. Chem., Int. Ed., 2018, 57, 3499 CrossRef PubMed.
- K. Katagiri, M. Hashizume, K. Ariga, T. Terashima and J. Kikuch, Chem. – Eur. J., 2007, 13, 5272 CrossRef CAS PubMed; K. Ariga, J. P. Hill, M. V. Lee, A. Vinu, R. Charvet and S. Acharya, Sci. Technol. Adv. Mater., 2008, 9, 014109 CrossRef PubMed; K. Ariga, M. Naito, Q. Ji and D. Payra, CrystEngComm, 2016, 18, 4890 RSC; T. Sagami, S. Umemoto, Y. O. Tahara, M. Miyata, Y. Yonamine, D. Ishikawa, T. Mori, K. Ariga, H. Miyake and S. Shinoda, Bull. Chem. Soc. Jpn., 2017, 90, 739 CrossRef; V. F. Korolovych, A. J. Erwin, A. Stryutsky, E. K. Mikan, V. V. Shevchenko and V. V. Tsukruk, Bull. Chem. Soc. Jpn., 2017, 90, 919 CrossRef; S. Cherumukkil, B. Vedhanarayanan, G. Das, V. K. Praveen and A. Ajayaghosh, Bull. Chem. Soc. Jpn., 2018, 91, 100 CrossRef; T. Shimizu, Bull. Chem. Soc. Jpn., 2018, 91, 623 CrossRef; S. Dhiman and S. J. George, Bull. Chem. Soc. Jpn., 2018, 91, 687 CrossRef; S. Hiraoka, Bull. Chem. Soc. Jpn., 2018, 91, 957 CrossRef.
- W. Chaikittisilp, K. Ariga and Y. Yamauchi, J. Mater. Chem. A, 2013, 1, 14 RSC; V. Malgras, Q. Ji, Y. Kamachi, T. Mori, F.-K. Shieh, K. C.-W. Wu, K. Ariga and Y. Yamauchi, Bull. Chem. Soc. Jpn., 2015, 88, 1171 CrossRef CAS; L. Yu, H. B. Wu and X. W. D. Lou, Acc. Chem. Res., 2017, 50, 293 CrossRef PubMed; G. Lai and T.-Y. Luh, Bull. Chem. Soc. Jpn., 2018, 91, 262 CrossRef; L. Wang, X. Jiao, P. Liu, Y. Ouyang, X. Xia, W. Lei and Q. Hao, Appl. Surf. Sci., 2018, 427, 174 CrossRef.
- F. Hong, F. Zhang, Y. Liu and H. Yan, Chem. Rev., 2017, 117, 12584 CrossRef CAS PubMed; K. Matsuura, Bull. Chem. Soc. Jpn., 2017, 90, 873 CrossRef; K. V. Presnell and H. S. Alper, Mol. Syst. Des. Eng., 2018, 3, 13 RSC; Y. Goto and H. Suga, Bull. Chem. Soc. Jpn., 2018, 91, 410 CrossRef; T. Sawada and T. Serizawa, Bull. Chem. Soc. Jpn., 2018, 91, 455 CrossRef; N. C. Seeman and H. F. Sleiman, Nat. Rev. Mater., 2018, 3, 17068 CrossRef.
- L. Syedmoradi, M. Daneshpour, M. Alvandipour, F. A. Gomez, H. Hajghassem and K. Omidfar, Biosens. Bioelectron., 2017, 87, 373 CrossRef CAS PubMed; M. Kiguchi and S. Fujii, Bull. Chem. Soc. Jpn., 2017, 90, 1 CrossRef; M. Taniguchi, Bull. Chem. Soc. Jpn., 2017, 90, 1189 CrossRef; R. Chen, J. Kang, M. Kang, H. Lee and H. Lee, Bull. Chem. Soc. Jpn., 2018, 91, 979 CrossRef; X. Chen and L. Zhang, Sens. Actuators, B, 2018, 254, 648 CrossRef.
- K. Ariga, Y. Yamauchi and M. Aono, APL Mater., 2015, 3, 061001 CrossRef CAS; K. Ariga, Q. Ji, W. Nakanishi, J. P. Hill and M. Aono, Mater. Horiz., 2015, 2, 406 RSC; M. Aono and K. Ariga, Adv. Mater., 2016, 28, 989 CrossRef PubMed.
- K. Ariga and M. Aono, Jpn. J. Appl. Phys., 2016, 55, 1102A6 CrossRef.
- G. Markovich, C. P. Collier, S. E. Henrichs, F. Remacle, R. D. Levine and J. R. Heath, Acc. Chem. Res., 1999, 32, 415 CrossRef CAS.
- K. Ariga, Q. Ji, J. P. Hill, Y. Bando and M. Aono, NPG Asia Mater., 2012, 4, e17 CrossRef.
- S. Hecht, Angew. Chem., Int. Ed., 2003, 42, 24 CrossRef CAS PubMed.
- K. Ariga, M. Li, G. J. Richards and J. P. Hill, J. Nanosci. Nanotechnol., 2011, 11, 1 CrossRef CAS PubMed.
- K. Ariga, A. Vinu, Y. Yamauchi, Q. Ji and J. P. Hill, Bull. Chem. Soc. Jpn., 2012, 85, 1 CrossRef CAS; W. Nakanishi, K. Minami, L. K. Shrestha, Q. Ji, J. P. Hil and K. Ariga, Nano Today, 2014, 9, 378 CrossRef; M. Osada and T. Sasaki, Dalton Trans., 2018, 47, 2841 RSC; D. Sangian, Y. Ide, Y. Bando, A. E. Rowan and Y. Yamauchi, Small, 2018, 14, 1800551 CrossRef PubMed.
- M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2013, 15, 10580 RSC; L. K. Shrestha, K. M. Strzelczyk, R. G. Shrestha, K. Ichikawa, K. Aramaki, J. P. Hill and K. Ariga, Nanotechnology, 2015, 26, 204002 CrossRef CAS PubMed; L. Zhang, T. Wang, Z. Shen and M. Liu, Adv. Mater., 2016, 28, 1044 CrossRef PubMed; K. Ariga, V. Malgras, Q. Ji, M. B. Zakaria and Y. Yamauchi, Coord. Chem. Rev., 2016, 320–321, 139 CrossRef; A. Lorenzo, M. L. Cortez, A. Lorenzo, W. A. Marmisolle, C. von Bilderling, E. Maza, L. Pietrasanta, F. Battaglini, M. Ceolin and O. Azzaroni, Soft Matter, 2018, 14, 1939 RSC; W. A. Marmisolle, E. M. Maza, M. Ceolin and O. Azzaroni, Phys. Chem. Chem. Phys., 2018, 20, 7570 RSC.
- S. Ishihara, J. Labuta, W. Van Rossom, D. Ishikawa, K. Minami, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2014, 16, 9713 RSC; K. Ariga, K. Minami and L. K. Shrestha, Analyst, 2016, 141, 2629 RSC; M. Pandeeswar, S. P. Senanayak and T. Govindaraju, ACS Appl. Mater. Interfaces, 2016, 8, 30362 CrossRef CAS PubMed; M. Komiyama, T. Mori and K. Ariga, Bull. Chem. Soc. Jpn., 2018, 91, 1075 CrossRef; J. A. Jackman, N.-J. Cho, M. Nishikawa, G. Yoshikawa, T. Mori, L. K. Shrestha and K. Ariga, Chem. – Asian J., 2018, 13, 3366 CrossRef PubMed.
- K. Takada, N. Ohta and Y. Tateyama, J. Inorg. Organomet. Polym. Mater., 2015, 25, 205 CrossRef CAS; Q. Zou, K. Liu, M. Abbas and X. Yan, Adv. Mater., 2016, 28, 1031 CrossRef PubMed; J. Kim, J. H. Kim and K. Ariga, Joule, 2017, 1, 739 CrossRef; L. K. Shrestha, R. G. Shrestha, S. Joshi, R. Rajbhandari, N. Shrestha, M. P. Adhikari, R. R. Pradhananga and K. Ariga, J. Inorg. Organomet. Polym., 2017, 27(Suppl 1), S48 CrossRef.
- K. Ariga, S. Ishihara, H. Abe, M. Li and J. P. Hill, J. Mater. Chem., 2012, 22, 2369 RSC; K. Ariga, J. A. Jackman, N.-J. Cho, S.-H. Hsu, L. K. Shrestha, T. Mori and J. Takeya, Chem. Rec. DOI:10.1002/tcr.201800103 , in press.
- H. Abe, J. Liu and K. Ariga, Mater. Today, 2016, 19, 12 CrossRef CAS; K. Ariga, S. Ishihara and H. Abe, CrystEngComm, 2016, 18, 6770 RSC; H. Wang, S. Yin, K. Eid, Y. Li, Y. Xu, X. Li, H. Xue and L. Wang, ACS Sustainable Chem. Eng., 2018, 6, 11768 CrossRef.
- K. Ariga, S. Watanabe, T. Mori and J. Takeya, NPG Asia Mater., 2018, 10, 90 CrossRef; A. Nayak, S. Unayama, S. Tai, T. Tsuruoka, R. Waser, M. Aono, I. Valov and T. Hasegawa, Adv. Mater., 2018, 30, 1703261 CrossRef PubMed; Y. Yan, J. Ye, K. Wang, J. Yao and Y. S. Zhao, Small, 2018, 14, 1702698 CrossRef PubMed.
- K. Ariga, Q. Ji, M. J. McShane, Y. M. Lvov, A. Vinu and J. P. Hill, Chem. Mater., 2012, 24, 728 CrossRef CAS; K. Ariga, Q. Ji, T. Mori, M. Naito, Y. Yamauchi, H. Abe and J. P. Hill, Chem. Soc. Rev., 2013, 42, 6322 RSC; K. Ariga, K. Kawakami, M. Ebara, Y. Kotsuchibashi, Q. Ji and J. P. Hill, New J. Chem., 2014, 38, 5149 RSC; E. Stulz, Acc. Chem. Res., 2017, 50, 823 CrossRef PubMed; K. Ariga, D. L. Leong and T. Mori, Adv. Funct. Mater., 2018, 28, 1702905 CrossRef.
- K. Ariga, J. Li, J. Fei, Q. Ji and J. P. Hill, Adv. Mater., 2016, 28, 1251 CrossRef CAS PubMed; K. Ariga, Mater. Chem. Front., 2017, 1, 208 RSC.
- K. Ariga, M. V. Lee, T. Mori, X.-Y. Yu and J. P. Hill, Adv. Colloid Interface Sci., 2010, 154, 20 CrossRef CAS PubMed; K. Ariga, T. Mori and J. P. Hill, Langmuir, 2013, 29, 8459 CrossRef PubMed; K. Ariga, Q. Ji, W. Nakanishi and J. P. Hill, J. Inorg. Organomet. Polym. Mater., 2015, 25, 466 CrossRef.
- K. Ariga, T. Mori and J. Li, Langmuir DOI:10.1021/acs.langmuir.8b01434 , in press.
- K. Ariga, H. Ito, J. P. Hill and H. Tsukube, Chem. Soc. Rev., 2012, 41, 5800 RSC.
- K. Ariga and T. Kunitake, Acc. Chem. Res., 1998, 31, 371 CrossRef CAS; K. Ariga, ChemNanoMat, 2016, 2, 333 CrossRef.
- B. Springs and P. Haake, Bioorg. Chem., 1977, 6, 181 CrossRef CAS.
- M. Onda, K. Yoshihara, H. Koyano, K. Ariga and T. Kunitake, J. Am. Chem. Soc., 1996, 118, 8524 CrossRef CAS.
- D. Y. Sasaki, K. Kurihara and T. Kunitake, J. Am. Chem. Soc., 1991, 113, 9685 CrossRef CAS; D. Y. Sasaki, K. Kurihara and T. Kunitake, J. Am. Chem. Soc., 1992, 114, 10994 CrossRef.
- M. Sakurai, H. Tamagawa, Y. Inoue, K. Ariga and T. Kunitake, J. Phys. Chem. B, 1997, 101, 4810 CrossRef CAS; H. Tamagawa, M. Sakurai, Y. Inoue, K. Ariga and T. Kunitake, J. Phys. Chem. B, 1997, 101, 4817 CrossRef.
- K. Kurihara, K. Ohto, Y. Tanaka, Y. Aoyama and T. Kunitake, J. Am. Chem. Soc., 1991, 113, 444 CrossRef CAS; K. Ariga, K. Isoyama, O. Hayashida, Y. Aoyama and Y. Okahata, Chem. Lett., 1998, 1007 CrossRef.
- Y. Ikeura, Y. Honda, K. Kurihara and T. Kunitake, Chem. Lett., 1990, 169 CrossRef CAS; Y. Ikeura, K. Kurihara and T. Kunitake, J. Am. Chem. Soc., 1991, 113, 7342 CrossRef.
- K. Taguchi, K. Ariga and T. Kunitake, Chem. Lett., 1995, 701 CrossRef CAS; K. Ariga, A. Kamino, H. Koyano and T. Kunitake, J. Mater. Chem., 1997, 7, 1155 RSC.
- T. Kawahara, K. Kurihara and T. Kunitake, Chem. Lett., 1992, 1839 CrossRef CAS.
- X. Cha, K. Ariga, M. Onda and T. Kunitake, J. Am. Chem. Soc., 1995, 117, 11833 CrossRef CAS; X. Cha, K. Ariga and T. Kunitake, J. Am. Chem. Soc., 1996, 118, 9545 CrossRef; K. Ariga, A. Kamino, X. Cha and T. Kunitake, Langmuir, 1999, 15, 3875 CrossRef.
- K. Ariga, J. Nanosci. Nanotechnol., 2004, 4, 23 CrossRef CAS PubMed; K. Sakakibara, J. P. Hill and K. Ariga, Small, 2011, 7, 1288 CrossRef PubMed.
- Y. Oishi, T. Kato, M. Kuramori, K. Suehiro, K. Ariga, A. Kamino, H. Koyano and T. Kunitake, Chem. Commun., 1997, 1357 RSC.
- H. Koyano, K. Yoshihara, K. Ariga, T. Kunitake, Y. Oishi, O. Kawano, M. Kuramori and K. Suehiro, Chem. Commun., 1996, 1769 RSC.
- Y. Oishi, Y. Torii, M. Kuramori, K. Suehiro, K. Ariga, K. Taguchi, A. Kamino and T. Kunitake, Chem. Lett., 1996, 411 CrossRef CAS; Y. Oishi, Y. Torii, T. Kato, M. Kuramori, K. Suehiro, K. Ariga, K. Taguchi, A. Kamino, H. Koyano and T. Kunitake, Langmuir, 1997, 13, 519 CrossRef.
- M. Mukai and S. L. Regen, Bull. Chem. Soc. Jpn., 2017, 90, 1083 CrossRef CAS.
- X. Liu, J. G. Riess and M. P. Krafft, Bull. Chem. Soc. Jpn., 2018, 91, 846 CrossRef CAS.
- Y. Oishi, T. Kato, T. Narita, K. Ariga and T. Kunitake, Langmuir, 2008, 24, 1682 CrossRef CAS PubMed.
- T. Seki, Bull. Chem. Soc. Jpn., 2018, 91, 1026 CrossRef CAS.
- K. Sakakibara, P. Chithra, B. Das, T. Mori, M. Akada, J. Labuta, T. Tsuruoka, S. Maji, S. Furumi, L. K. Shrestha, J. P. Hill, S. Acharya, K. Ariga and A. Ajayaghosh, J. Am. Chem. Soc., 2014, 136, 8548 CrossRef CAS PubMed.
- R. Sakamoto, Bull. Chem. Soc. Jpn., 2017, 90, 272 CrossRef CAS.
- H. Koyano, P. Bissel, K. Yoshihara, K. Ariga and T. Kunitake, Langmuir, 1997, 13, 5426 CrossRef CAS; H. Koyano, P. Bissel, K. Yoshihara, K. Ariga and T. Kunitake, Chem. – Eur. J., 1997, 3, 1077 CrossRef.
- Y. Yonamine, K. Cervantes-Salguero, K. Minami, I. Kawamata, W. Nakanishi, J. P. Hill, S. Murata and K. Ariga, Phys. Chem. Chem. Phys., 2016, 18, 12576 RSC.
- Y. Yonamine, K. Cervantes-Salguero, W. Nakanishi, I. Kawamata, K. Minami, H. Komatsu, S. Murata, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2015, 17, 32122 RSC.
- T. Mori, H. Tanaka, A. Dalui, N. Mitoma, K. Suzuki, M. Matsumoto, N. Aggarwal, A. Patnaik, S. Acharya, L. K. Shrestha, H. Sakamoto, K. Itami and K. Ariga, Angew. Chem., Int. Ed., 2018, 57, 9679 CrossRef CAS PubMed.
- T. Low, A. Chaves, J. D. Caldwell, A. Kumar, N. X. Fang, P. Avouris, T. F. Heinz, F. Guinea, L. Martin-Moreno and F. Koppens, Nat. Mater., 2017, 16, 182 CrossRef CAS PubMed; K. Takase, H. Nishizawa, K. Imamura, A. Onda, K. Yanagisawa and S. Yin, Bull. Chem. Soc. Jpn., 2018, 91, 1546 CrossRef; M. Matsumoto, Y. Mizutani and M. Aoki, Bull. Chem. Soc. Jpn., 2018, 91, 1205 CrossRef.
- V. C. Rodrigues, M. L. Moraes, J. C. Soares, A. C. Soares, R. Sanfelice, E. Deffune and O. N. Oliveira Jr., Bull. Chem. Soc. Jpn., 2018, 91, 891 CrossRef CAS.
- E. Ruiz-Hitzky, A. Gómez-Avilés, M. Darder and P. Aranda, Bull. Chem. Soc. Jpn., 2018, 91, 608 CrossRef CAS.
- S. Acharya, J. P. Hill and K. Ariga, Adv. Mater., 2009, 21, 2959 CrossRef CAS; K. Ariga, Y. Yamauchi, T. Mori and J. P. Hill, Adv. Mater., 2013, 25, 6477 CrossRef PubMed.
- K. Ariga, Y. M. Lvov, K. Kawakami, Q. Ji and J. P. Hill, Adv. Drug Delivery Rev., 2011, 63, 762 CrossRef CAS PubMed; K. Ariga, Y. Yamauchi, G. Rydzek, Q. Ji, Y. Yonamine, K. C.-W. Wu and J. P. Hill, Chem. Lett., 2014, 43, 36 CrossRef; G. Rydzek, Q. Ji, M. Li, P. Schaaf, J. P. Hill, F. Boulmedais and K. Ariga, Nano Today, 2015, 10, 138 CrossRef.
- G. Decher, Science, 1997, 277, 1232 CrossRef CAS.
- W. B. Stockton and M. F. Rubner, Macromolecules, 1997, 30, 2717 CrossRef CAS; L. Wang, Z. Wang, X. Zhang, J. Shen, L. Chi and H. Fuchs, Macromol. Rapid Commun., 1997, 18, 509 CrossRef.
- H. Lee, L. J. Kepley, H.-G. Hong and T. E. Mallouk, J. Am. Chem. Soc., 1988, 110, 618 CrossRef CAS; S. W. Keller, H.-N. Kim and T. E. Mallouk, J. Am. Chem. Soc., 1994, 116, 8817 CrossRef.
- A. Ikeda, T. Hatano, S. Shinkai, T. Akiyama and S. Yamada, J. Am. Chem. Soc., 2001, 123, 4855 CrossRef CAS.
- W. Muller, H. Ringsdorf, E. Rump, G. Wildburg, X. Zhang, L. Angermaier, W. Knoll, M. Liley and J. Spinke, Science, 1993, 262, 1706 CrossRef CAS PubMed; Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, J. Chem. Soc., Chem. Commun., 1995, 2313 RSC.
- Y. Shimazaki, M. Mitsuishi, S. Ito and M. Yamamoto, Langmuir, 1997, 13, 1385 CrossRef CAS.
- T. Serizawa, K.-i. Hamada, T. Kitayama, N. Fujimoto, K. Hatada and M. Akashi, J. Am. Chem. Soc., 2000, 122, 1891 CrossRef CAS; T. Serizawa, K. Hamada and M. Akashi, Nature, 2004, 429, 52 CrossRef PubMed.
- J. Cho, K. Char, J.-D. Hong and K.-B. Lee, Adv. Mater., 2001, 13, 1076 CrossRef CAS; S.-S. Lee, J.-D. Hong, C. H. Kim, K. Kim, J. P. Koo and K.-B. Lee, Macromolecules, 2001, 34, 5358 CrossRef.
- S. L. Clark and P. T. Hammond, Adv. Mater., 1998, 10, 1515 CrossRef CAS; S. S. Shiratori and M. Yamada, Polym. Adv. Technol., 2000, 11, 810 CrossRef.
- M. Lefort, G. Popa, E. Seyrek, R. Szamocki, O. Felix, J. Hemmerlé, L. Vidal, J.-C. Voegel, F. Boulmedais, G. Decher and P. Schaaf, Angew. Chem., Int. Ed., 2010, 49, 10110 CrossRef CAS PubMed.
- G. B. Sukhorukov, E. Donath, S. Davis, H. Lichtenfeld, F. Caruso, V. I. Popov and H. Möhwald, Polym. Adv. Technol., 1998, 9, 759 CrossRef CAS; E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem., Int. Ed., 1998, 37, 2201 CrossRef PubMed; F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef PubMed; G. B. Sukhorukov, M. Brumen, E. Donath and H. Möhwald, J. Phys. Chem. B, 1999, 103, 6434 CrossRef.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS; A. H. Khan, S. Ghosh, B. Pradhan, A. Dalui, L. K. Shrestha, S. Acharya and K. Ariga, Bull. Chem. Soc. Jpn., 2017, 90, 627 CrossRef; Y. Wang, C. C. Mayorga-Martinez and M. Pumera, Bull. Chem. Soc. Jpn., 2017, 90, 847 CrossRef; C. Sengottaiyan, R. Jayavel, P. Bairi and R. G. Shrestha, Bull. Chem. Soc. Jpn., 2017, 90, 955 CrossRef; S. Zhong and Q. Xu, Bull. Chem. Soc. Jpn., 2018, 91, 1606 CrossRef.
- Q. Ji, I. Honma, S.-M. Paek, M. Akada, J. P. Hill, A. Vinu and K. Ariga, Angew. Chem., Int. Ed., 2010, 49, 9737 CrossRef CAS PubMed.
- K. Ariga, A. Vinu, Q. Ji, O. Ohmori, J. P. Hill, S. Acharya, J. Koike and S. Shiratori, Angew. Chem., Int. Ed., 2008, 47, 7254 CrossRef CAS PubMed.
- Q. Ji, S. B. Yoon, J. P. Hill, A. Vinu, J.-S. Yu and K. Ariga, J. Am. Chem. Soc., 2009, 131, 4220 CrossRef CAS PubMed.
- Q. Ji, M. Miyahara, J. P. Hill, S. Acharya, A. Vinu, S. B. Yoon, J.-S. Yu, K. Sakamoto and K. Ariga, J. Am. Chem. Soc., 2008, 130, 2376 CrossRef CAS PubMed; Q. Ji, S. Acharya, J. P. Hill, A. Vinu, S. B. Yoon, J.-S. Yu, K. Sakamoto and K. Ariga, Adv. Funct. Mater., 2009, 19, 1792 CrossRef.
- B. L. Li, M. I. Setyawati, L. Chen, J. Xie, K. Ariga, C.-T. Lim, S. Garaj and D. T. Leong, ACS Appl. Mater. Interfaces, 2017, 9, 15286 CrossRef CAS PubMed.
- N. Mihara, Y. Yamada and K. Tanaka, Bull. Chem. Soc. Jpn., 2017, 90, 427 CrossRef CAS.
- Y. Haketa and H. Maeda, Bull. Chem. Soc. Jpn., 2018, 91, 420 CrossRef CAS.
- M. Akamatsu, H. Komatsu, A. Matsuda, T. Mori, W. Nakanishi, H. Sakai, J. P. Hill and K. Ariga, Bull. Chem. Soc. Jpn., 2017, 90, 678 CrossRef CAS.
- L. K. Shrestha, M. Sathish, J. P. Hill, K. Miyazawa, T. Tsuruoka, N. M. Sanchez-Ballester, I. Honma, Q. Ji and K. Ariga, J. Mater. Chem. C, 2013, 1, 1174 RSC.
- M. Sathish, K. Miyazawa, J. P. Hill and K. Ariga, J. Am. Chem. Soc., 2009, 131, 6372 CrossRef CAS PubMed; L. K. Shrestha, R. G. Shrestha, J. P. Hill, T. Tsuruoka, Q. Ji, T. Nishimura and K. Ariga, Langmuir, 2016, 21, 12511 CrossRef PubMed.
- K. Miyazawa, Sci. Technol. Adv. Mater., 2015, 16, 013502 CrossRef PubMed.
- L. K. Shrestha, Y. Yamauchi, J. P. Hill, K. Miyazawa and K. Ariga, J. Am. Chem. Soc., 2013, 135, 586 CrossRef CAS PubMed.
- L. K. Shrestha, Q. Ji, T. Mori, K. Miyazawa, Y. Yamauchi, J. P. Hill and K. Ariga, Chem. – Asian J., 2013, 8, 1662 CrossRef CAS PubMed.
- P. Bairi, K. Minami, W. Nakanishi, J. P. Hill, K. Ariga and L. K. Shrestha, ACS Nano, 2016, 10, 6631 CrossRef CAS PubMed.
- P. Bairi, K. Minami, J. P. Hill, K. Ariga and L. K. Shrestha, ACS Nano, 2017, 11, 7790 CrossRef CAS PubMed.
- P. Bairi, K. Minami, J. P. Hill, W. Nakanishi, L. K. Shrestha, C. Liu, K. Harano, E. Nakamura and K. Ariga, ACS Nano, 2016, 10, 8796 CrossRef CAS PubMed; K. Ariga, T. Mori and L. K. Shrestha, Chem. Rec., 2018, 18, 676 CrossRef PubMed.
- E. Ruiz-Hitzky, M. Darder, P. Aranda and K. Ariga, Adv. Mater., 2010, 22, 323 CrossRef CAS PubMed; A. Vinu, M. Miyahara and K. Ariga, J. Nanosci. Nanotechnol., 2006, 6, 1510 CrossRef PubMed; M. Komiyama, K. Yoshimoto, M. Sisido and K. Ariga, Bull. Chem. Soc. Jpn., 2017, 90, 967 CrossRef; J. Sun, D. Zhang, W. Zhao, Q. Ji and K. Ariga, Bull. Chem. Soc. Jpn. DOI:10.1246/bcsj.20180201 , in press.
- C. Igarashi, A. Murata, Y. Itoh, D. R. G. Subekti, S. Takahashi and K. Kamagata, Bull. Chem. Soc. Jpn., 2017, 90, 34 CrossRef CAS.
- H. He and B. Xu, Bull. Chem. Soc. Jpn., 2018, 91, 900 CrossRef CAS PubMed.
- K. Minami, Y. Kasuya, T. Yamazaki, Q. Ji, W. Nakanishi, J. P. Hill, H. Sakai and K. Ariga, Adv. Mater., 2015, 27, 4020 CrossRef CAS PubMed.
- V. Krishnan, Y. Kasuya, Q. Ji, M. Sathish, L. K. Shrestha, S. Ishihara, K. Minami, H. Morita, T. Yamazaki, N. Hanagata, K. Miyazawa, S. Acharya, W. Nakanishi, J. P. Hill and K. Ariga, ACS Appl. Mater. Interfaces, 2015, 7, 15667 CrossRef CAS PubMed.
- M. D. Rosenberg, Cellular control mechanisms and cancer, ed. P. Emmelot and O. Mohbock, Elsevier, Amsterdam, Netherlands, 1964, pp. 146–164 Search PubMed.
- I. Giaever and C. R. Keese, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 219 CrossRef CAS; C. R. Keese and I. Giaever, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 5622 CrossRef; C. R. Keese and I. Giaever, Exp. Cell Res., 1991, 195, 528 CrossRef PubMed.
- Y. Shiba, T. Ohshima and M. Sato, Kagaku Kogaku Ronbunshu, 1998, 24, 343 CrossRef CAS; Y. Shiba, T. Ohshima and M. Sato, Biotechnol. Bioeng., 1998, 57, 583 CrossRef PubMed; M. Sato, T. Shinozawa, H. Ueno and M. Sadakata, Kagaku Kogaku Ronbunshu, 1991, 17, 671 CrossRef; J. Ando, S. M. Albelda and E. M. Levine, Dev. Biol., 1991, 7A, 525 Search PubMed; M. Pilarek, I. Grabowska, M. A. Ciemerych, K. Dabkowska and K. W. Szewczyk, Biotechnol. Lett., 2013, 35, 1387 CrossRef PubMed.
- K. Minami, T. Mori, W. Nakanishi, N. Shigi, J. Nakanishi, J. P. Hill, M. Komiyama and K. Ariga, ACS Appl. Mater. Interfaces, 2017, 9, 30553 CrossRef CAS PubMed.
- D. Kong, W. Megone, K. D. Q. Nguyen, S. di Cio, M. Ramstedt and J. E. Gautrot, Nano Lett., 2018, 18, 1946 CrossRef CAS PubMed; D. Kong, L. Peng, S. Di Cio, P. Novak and J. E. Gautrot, ACS Nano, 2018, 12, 9206 CrossRef PubMed.
- Y. Shirai, K. Minami, W. Nakanishi, Y. Yonamine, C. Joachim and K. Ariga, Jpn. J. Appl. Phys., 2016, 55, 1102A2 CrossRef CAS; W.-H. Soe, Y. Shirai, C. Durand, Y. Yonamine, K. Minami, X. Bouju, M. Kolmer, K. Ariga, C. Joachim and W. Nakanishi, ACS Nano, 2017, 11, 10357 CrossRef PubMed; K. Ariga, T. Mori and W. Nakanishi, Chem. – Asian J., 2018, 13, 1266 CrossRef PubMed.
- K. Ariga, T. Mori and J. P. Hill, Adv. Mater., 2012, 24, 158 CrossRef CAS PubMed; K. Ariga, Anal. Sci., 2016, 32, 1141 CrossRef PubMed; K. Ariga, T. Mori, W. Nakanishi and J. P. Hill, Phys. Chem. Chem. Phys., 2017, 19, 23658 RSC; L. K. Shrestha, T. Mori and K. Ariga, Curr. Opin. Colloid Interface Sci., 2018, 35, 68 CrossRef.
- K. Ariga, T. Mori, S. Ishihara, K. Kawakami and J. P. Hill, Chem. Mater., 2014, 26, 519 CrossRef CAS; K. Ariga, K. Minami, M. Ebara and J. Nakanishi, Polym. J., 2016, 48, 371 CrossRef.
- K. Ariga, Y. Terasaka, D. Sakai, H. Tsuji and J. Kikuchi, J. Am. Chem. Soc., 2000, 122, 7835 CrossRef CAS; K. Ariga, T. Nakanishi, Y. Terasaka, H. Tsuji, D. Sakai and J. Kikuchi, Langmuir, 2005, 21, 976 CrossRef PubMed.
- T. Michinobu, S. Shinoda, T. Nakanishi, J. P. Hill, K. Fujii, T. N. Player, H. Tsukube and K. Ariga, J. Am. Chem. Soc., 2006, 128, 14478 CrossRef CAS PubMed; T. Michinobu, S. Shinoda, T. Nakanishi, J. P. Hill, K. Fujii, T. N. Player, H. Tsukube and K. Ariga, Phys. Chem. Chem. Phys., 2011, 13, 4895 RSC.
- T. Mori, K. Okamoto, H. Endo, J. P. Hill, S. Shinoda, M. Matsukura, H. Tsukube, Y. Suzuki, Y. Kanekiyo and K. Ariga, J. Am. Chem. Soc., 2010, 132, 12868 CrossRef CAS PubMed; T. Mori, K. Okamoto, H. Endo, K. Sakakibara, J. P. Hill, S. Shinoda, M. Matsukura, H. Tsukube, Y. Suzuki, Y. Kanekiyo and K. Ariga, Nanoscale Res. Lett., 2011, 6, 304 CrossRef PubMed.
- K. Sakakibara, L. A. Joyce, T. Mori, T. Fujisawa, S. H. Shabbir, J. P. Hill, E. V. Anslyn and K. Ariga, Angew. Chem., Int. Ed., 2012, 51, 9643 CrossRef CAS PubMed.
- D. Ishikawa, T. Mori, Y. Yonamine, W. Nakanishi, D. Cheung, J. P. Hill and K. Ariga, Angew. Chem., Int. Ed., 2015, 54, 8988 CrossRef CAS PubMed.
- T. Mori, D. Ishikawa, Y. Yonamine, Y. Fujii, J. P. Hill, I. Ichinose, K. Ariga and W. Nakanishi, ChemPhysChem, 2017, 18, 1470 CrossRef CAS PubMed.
- T. Mori, H. Komatsu, N. Sakamoto, K. Suzuki, J. P. Hill, M. Matsumoto, H. Sakai, K. Ariga and W. Nakanishi, Phys. Chem. Chem. Phys., 2018, 20, 3073 RSC.
- M. B. Avinash and T. Govindaraju, Acc. Chem. Res., 2018, 51, 414 CrossRef CAS PubMed.
- K. Ariga, M. Nishikawa, T. Mori, J. Takeya, L. K. Shrestha and J. P. Hill, Sci. Technol. Adv. Mater. DOI:10.1080/14686996.2018.1553108 , in press.
- T. Govindaraju and M. B. Avinash, Nanoscale, 2012, 4, 610 RSC.
- M. B. Avinash and T. Govindaraju, Nanoscale, 2014, 6, 13348 RSC; M. Pandeeswar, S. P. Senanayak and T. Govindaraju, ACS Appl. Mater. Interfaces, 2016, 8, 30362 CrossRef CAS PubMed.
- K. Tahara, T. Kadowaki, J. Kikuchi, Y. Ozawa, S. Yoshimoto and M. Abe, Bull. Chem. Soc. Jpn., 2018, 91, 1630 CrossRef CAS; H. Asanuma, K. Murayama, Y. Kamiya and H. Kashida, Bull. Chem. Soc. Jpn., 2018, 91, 1739 CrossRef; Y. Einaga, Bull. Chem. Soc. Jpn., 2018, 91, 1752 CrossRef; T. Yonezawa, D. Čempel and M. T. Nguyen, Bull. Chem. Soc. Jpn., 2018, 91, 1781 CrossRef; F. Peng, M. I. Setyawati, J. K. Tee, X. Ding, J. Wang, M. E. Nga, H. K. Ho and D. T. Leong, Nat. Nanotechnol DOI:10.1038/s41565-018-0356-z , in press.
| This journal is © The Royal Society of Chemistry 2019 |