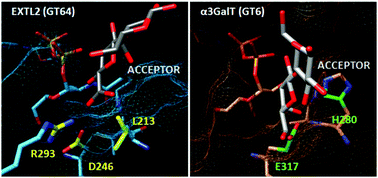
Glycosyltransferases are enzymes that catalyze a monosaccharide transfer reaction from a donor to an acceptor substrate with the synthesis of a new glycosidic bond. They are highly substrate specific and regioselective, even though the acceptor substrate often presents multiple reactive groups. Currently, many efforts are dedicated to the development of biocatalysts for glycan synthesis and, therefore, a better understanding of how natural enzymes achieve this goal can be of valuable help. To gain a deeper insight into the catalytic strategies used by retaining glycosyltransferases, the wild type EXTL2 (CAZy family GT64) and four mutant forms (at positions 293 and 246) were studied using QM(DFT)/MM calculations and molecular dynamics simulations. Existing hypotheses on the roles of Arg293, an enigmatic residue in the CAZy family GT64 that seemed to contradict a mechanism through an oxocarbenium intermediate, and of Asp246 have been tested. We also provide a molecular interpretation for the results of site-directed mutagenesis experiments. Moreover, we have investigated why an Asp, and not a Glu like in the family GT6, is found on the β-face of the transferred GlcNAc. It is predicted that an Asp246Glu mutant of EXTL2 would be unable to catalyze the α-1,4 transfer. The results herein presented clarify the roles that Arg293, Asp246 and Leu213 have at different stages of the catalytic process (for binding but also for efficient chemical reaction). Altogether, we provide a molecular view that connects the identity and conformation of these residues to the substrate specificity and regioselectivity of the enzyme, illustrating a delicate interplay between all these aspects.