Degradable gold core–mesoporous organosilica shell nanoparticles for two-photon imaging and gemcitabine monophosphate delivery†
Saher
Rahmani‡
ab,
Arnaud
Chaix‡
a,
Dina
Aggad
c,
Phuong
Hoang
a,
Basem
Moosa
a,
Marcel
Garcia
c,
Magali
Gary-Bobo
c,
Clarence
Charnay
b,
Abdulaziz
AlMalik
d,
Jean-Olivier
Durand
 *b and
Niveen M.
Khashab
*b and
Niveen M.
Khashab
 *a
*a
aSmart Hybrid Materials Laboratory, Advanced Membranes and Porous Materials Center, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
bInstitut Charles Gerhardt Montpellier, UMR-5253 CNRS-UM-ENSCM, cc 1701, Place Eugène Bataillon, 34095 Montpellier cedex 05, France. E-mail: durand@univ-montp2.fr
cInstitut des Biomolécules Max Mousseron, UMR 5247, Université de Montpellier, Avenue Charles Flahault, 34093 Montpellier cedex 05, France
dLife Sciences and Environment Research Institute, Center of Excellence in Nanomedicine (CENM), King Abdulaziz City for Science and Technology (KACST), Riyadh 11461, Saudi Arabia
First published on 12th September 2017
Abstract
The synthesis of degradable gold core–mesoporous organosilica shell nanoparticles is described. The nanoparticles were very efficient for two-photon luminescence imaging of cancer cells and for in vitro gemcitabine monophosphate delivery, allowing promising theranostic applications in the nanomedicine field.
Design, System, ApplicationThe design of core–shell (gold core–mesoporous organosilica shell) nanoparticles from two molecular precursors, bis(triethoxysilylpropyl)tetrasulfide and bis(triethoxysilyl)ethane, is described. The precursors bring ordered mesoporosity when combined with the cationic surfactant cetyltrimethylammonium bromide during the sol–gel procedure while bis(triethoxysilylpropyl)tetrasulfide brings biodegradability to the structure. The molecular precursors allowed the construction of small core–shell nanoparticles of 280 nm diameter compatible with the endocytosis of the nanoparticles by cancer cells. A functional ordered mesoporosity was obtained. Indeed, the interactions of gemcitabine monophosphate with the pores made by the molecular precursors allowed the encapsulation of a high amount of this drug (35% weight). The drug was not released at physiological pH, but led to a high cancer cell killing effect of 60%. The highly functionalized nanoparticles we designed allowed performing two-photon imaging of cancer cells thanks to the gold core of the nanoparticles. Combined with gemcitabine monophosphate delivery, the nanoparticles we proposed could led to a very precise treatment of cancers in the future, with reduced side effects and enhanced efficiency. Therefore, these nanoparticles are very promising for theranostics and nanomedicine applications. |
Introduction
Organosilica nanoparticles have attracted much attention for biological applications in the last decade and this field has been recently reviewed.1,2 The organic part of these nanoparticles represents 80–90% of their structure thus leading to unique features which make them very suitable for theranostic applications. Furthermore, porous systems have been reported3–5 which allow loading of drugs such as doxorubicin with a very high efficiency. However, these nanoparticles are very stable in simulated biological fluids6,7 which could be problematic for their elimination and clearance. Therefore, combining degradability8,9 with organosilica nanomaterials would be of high interest to avoid accumulation of these nanoparticles in the body with elimination through renal clearance, while providing excellent loading capacities and targeting properties for cancer applications. We and others9 have recently described mesoporous organosilica nanostructures incorporating disulfide and tetrasulfide bridges which are degraded by glutathione reduction. For imaging applications, two-photon excitation presents attractive features such as high spatial resolution with 3D reconstruction, low scattering losses, low auto-fluorescence and high depth penetration in tissues.10 Gold nanoparticles are particularly suitable for two-photon excited luminescence and their penetration in 3D spheroids has been studied using this technique.11 Following our work on gold core–periodic mesoporous organosilica shell nanoparticles for two-photon excitation,12,13 we decided to investigate degradable gold core–mesoporous organosilica shell nanoparticles based on tetrasulfide bridges for two-photon imaging of cancer cells and drug delivery. We decided to study a highly hydrophilic drug: gemcitabine monophosphate (GMP). Nanoparticle formulation with GMP is very challenging; covalent systems such as squalenization,14,15 formulations with ion coordination (MOFs,16 Zn2+ (ref. 17–19) or Gd3+ (ref. 20)), and calcium phosphate-based systems21–25 have been therefore developed. We anticipated that the interactions of GMP with the organosilica matrix would stabilize the complex, allowing an efficient delivery of this drug in cancer cells. Herein, we report the first synthesis of degradable core–shell nanoparticles Au@BTSE-BTSPS, BTSE: 1,2-bis(triethoxysilyl)ethane and BTSPS: bis[3-(triethoxysilyl)propyl] tetrasulfide, composed of gold nanoparticles as the core and degradable tetrasulfide-based organosilica as the shell. The luminescence of the core allowed tracking of the nanoparticles in cancer cells with two-photon excitation, and the porous shell allowed loading of GMP with a very high efficiency and delivery of GMP in cancer cells.Results and discussion
The nanoparticles (Au@E-TS), composed of 13 nm gold cores inside 280 nm organosilica spheres, were synthesized in two steps. At first, the gold nanoparticles were prepared using the Frens method.26 They were then stabilized with APTES in order to incorporate them in a micellar solution of CTAB, and to subsequently perform the hydrolytic polycondensation of the organosilane precursors. The co-condensation of 1,2-bis(triethoxysilyl)ethane (E) and bis[3-(triethoxysilyl)propyl]tetrasulfide (TS) precursors (Fig. 1) was thus performed at 50 °C for 2 h under mild conditions.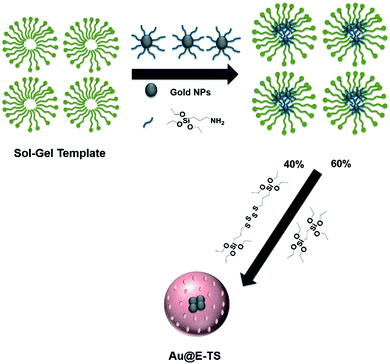 | ||
| Fig. 1 Sol–gel synthesis of Au@E-TS nanoparticles through the co-condensation of 1,2-bis(triethoxysilyl)ethane and bis[3-(triethoxysilyl)propyl]tetrasulfide precursors. | ||
The core–shell nanoparticles were isolated through centrifugation and the surfactant was removed from the pores using an ammonium nitrate/95% EtOH solution. Au@E-TS nanoparticles were then characterized via transmission electron microscopy (TEM), which depicted spherical core–shell NPs composed of several gold cores inside porous organosilica spheres with diameters ranging from 250 to 300 nm (Fig. 2A and B). The distribution of the NPs confirmed the nanoscale size of the carriers (Fig. S2†). Besides, as shown by N2-adsorption desorption analysis, the nanoparticles present a specific surface area of 413 m2 g−1 (Fig. 2C). The optical properties of Au and Au@E-TS NPs were then analyzed. By comparing the UV-visible absorption spectrum of Au@E-TS NPs with the UV-vis spectrum of Au NPs (Fig. 2D), an important red shift with an important enlargement of the plasmon band was observed due to the aggregation of the NP gold core inside the organosilica shell. The morphology of the core–shell system was investigated by using dark-field scanning TEM (DF-STEM) (Fig. S3A†) and DF-STEM mapping (Fig. S3B–E†), whereas the gold core crystal structure of Au@E-TS NPs was studied by electron diffraction (Fig. S3F†). The distribution of the elements in the NPs was studied by DF-STEM and electron energy-loss spectroscopy (Fig. S3B–E†). A homogeneous distribution of oxygen, carbon, sulfur and silicon atoms was observed which shows that no phase demixing occurred during the sol–gel procedure. This verification is particularly important as the efficient degradation of the shell depends on the homogenous dispersion of sulfur within the NP matrix. The X-ray diffraction (XRD) patterns in Fig. S4.A† of Au@E-TS nanoparticles at small angles display the presence of peaks at 2.03° corresponding to regular repetitions of the mesopores. Besides, the wide angle X-ray diffraction pattern in Fig. S4.B† shows a broad peak at 23°, which corresponds to non-regular repetitions within the siloxane framework, with two peaks at 37.4° and 43.5° which correspond to gold metal.
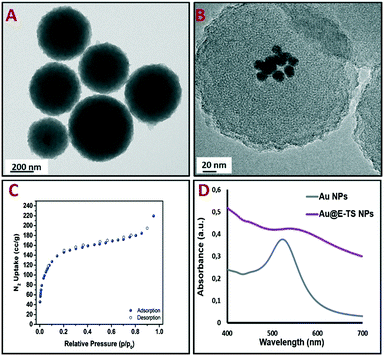 | ||
| Fig. 2 TEM images of Au@E-TS NPs (A and B). N2-adsorption–desorption isotherm (C). UV-vis spectra of the Au NPs and Au@E-TS NPs (D). | ||
After checking the morphology and the composition of Au@E-TS NPs, we then investigated their biocompatibility as well as their capabilities for two-photon excited confocal luminescence imaging of cancer cells. Before the imaging experiments, MDA-MB-231 cancer cells were washed twice, then Au@E-TS NPs were incubated in cancer cells for 24 hours at 80 μg mL−1 and the cell membranes were stained with CellMask Orange for 15 minutes at 1 μL mL−1. As shown in Fig. 3, the intracellular two-photon excited luminescence of the nanoparticles confirmed the successful endocytosis of the nanoparticles. Note that the presence of gold as a core in the NPs contributed to the detection of Au@E-TS NPs, showing that these nanocarriers were efficient for TPE, probably due to the aggregation of the gold NPs into the organosilica matrix thus leading to plasmon resonance in the NIR region.27
After having shown the endocytosis of Au@E-TS NPs, we then examined the biodegradable core–shell NPs as vectors for GMP delivery. The loading of these nanoparticles with GMP was examined in water at room temperature. The loading capacity was obtained using the UV-vis absorption spectra of the supernatant, and was 35 wt%. After that, the release of the drug was then examined. Drug release experiments were first carried out at pH 7 in ultrapure water, with GMP loaded nanoparticles. No drug release was observed under these conditions showing the important interactions between the drug and the organosilica matrix. The release of GMP was shown when the pH was adjusted to 5.5 (lysosomal pH, Fig. S5†).
The delivery of GMP was tested in MDA-MB-231 cancer cells (Fig. 4). No significant cytotoxicity of the empty nanoparticles was observed up to a concentration of 50 μg mL−1 showing the biocompatibility of the biodegradable core–shell NPs. Furthermore, the GMP delivery and cancer killing effect were highly efficient, with the cell survival rate down to 40% at only 10 μg mL−1 of the nanomaterials.
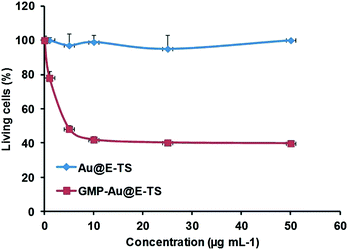 | ||
| Fig. 4 GMP delivery in MDA-MB-231 cancer cells with Au@E-TS NPs. Cytotoxicity study of a range from 0.1 to 50 μg mL−1. Au@E-TS NPs were incubated for 72 h in MDA-MB-231 cancer cells. | ||
Conclusions
In summary, we have designed mixed gold core–shell organosilica nanoparticles by co-condensation of bis(triethoxysilyl)ethane and bis[3-(triethoxysilyl) propyl]tetrasulfide. The morphology and compositions were fully characterized by various techniques. These nanoparticles were tested in vitro with MDA-MB-231 cancer cells for two-photon luminescence imaging and for gemcitabine monophosphate delivery, showing promising potential for biomedical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank King Abdullah University for Science and Technology (KAUST) and King Abdulaziz City for Science and Technology (KACST) for the financial support. The Erasmus Mundus grant for S. R. is gratefully acknowledged. M. Gary-Bobo thanks the Région Languedoc Roussillon for “Chercheur d'Avenir, grant 2013”. The RIO imaging platform is acknowledged for technical assistance. The authors thank Dr. G. Mouchaham for nitrogen adsorption/desorption analysis.Notes and references
- J. G. Croissant, X. Cattoen, J.-O. Durand, M. Wong Chi Man and N. M. Khashab, Nanoscale, 2016, 8, 19945–19972 RSC.
- M. Nakamura, Nanotechnol. Rev., 2012, 1, 469–491 CAS.
- X. Du, X. Li, L. Xiong, X. Zhang, F. Kleitz and S. Z. Qiao, Biomaterials, 2016, 91, 90–127 CrossRef CAS PubMed.
- Y. Chen and J. Shi, Adv. Mater., 2016, 28, 3235–3272 CrossRef CAS PubMed.
- J. G. Croissant, X. Cattoen, M. W. C. Man, J.-O. Durand and N. M. Khashab, Nanoscale, 2015, 7, 20318–20334 RSC.
- S. Datz, H. Engelke, C. V. Schirnding, L. Nguyen and T. Bein, Microporous Mesoporous Mater., 2016, 225, 371–377 CrossRef CAS.
- C. Urata, H. Yamada, R. Wakabayashi, Y. Aoyama, S. Hirosawa, S. Arai, S. Takeoka, Y. Yamauchi and K. Kuroda, J. Am. Chem. Soc., 2011, 133, 8102–8105 CrossRef CAS PubMed.
- D. Cassano, M. Santi, V. Cappello, S. Luin, G. Signore and V. Voliani, Part. Part. Syst. Charact., 2016, 33, 818–824 CrossRef CAS.
- J. G. Croissant, Y. Fatieiev and N. M. Khashab, Adv. Mater., 2017, 29 DOI:10.1002/adma.201604634.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244–3266 CrossRef CAS PubMed.
- T. D. Rane and A. M. Armani, PLoS One, 2016, 11, 1–13 Search PubMed.
- J. Croissant, D. Salles, M. Maynadier, O. Mongin, V. Hugues, M. Banchard-Desce, X. Cattoen, M. W. C. Man, A. Gallud, M. Garcia, M. Gary-Bobo, L. Raehm and J.-O. Durand, Chem. Mater., 2014, 26, 7214–7220 CrossRef CAS.
- R. Liu and R. D. Priestley, J. Mater. Chem. A, 2016, 4, 6680–6692 CAS.
- A. Maksimenko, J. Caron, J. Mougin, D. Desmaele and P. Couvreur, Int. J. Pharm., 2015, 482, 38–46 CrossRef CAS PubMed.
- J. Caron, E. Lepeltier, L. H. Reddy, S. Lepetre-Mouelhi, S. Wack, C. Bourgaux, P. Couvreur and D. Desmaele, Eur. J. Org. Chem., 2011, 2615–2628, DOI:10.1002/ejoc.201100036.
- V. Rodriguez-Ruiz, A. Maksimenko, V. Agostoni, P. Couvreur, R. Gref, R. Anand, M. Lampropoulou, K. Yannakopoulou and S. Monti, J. Drug Targeting, 2015, 23, 759–767 CrossRef CAS PubMed.
- C. Poon, C. He, D. Liu, K. Lu and W. Lin, J. Controlled Release, 2015, 201, 90–99 CrossRef CAS PubMed.
- C. Poon, X. Duan, C. Chan, W. Han and W. Lin, Mol. Pharmaceutics, 2016, 13, 3665–3675 CrossRef CAS PubMed.
- C. He, C. Poon, C. Chan, S. D. Yamada and W. Lin, J. Am. Chem. Soc., 2016, 138, 6010–6019 CrossRef CAS PubMed.
- L. Li, R. Tong, M. Li and D. S. Kohane, Acta Biomater., 2016, 33, 34–39 CrossRef CAS PubMed.
- Y. Zhang, N. M. J. Schwerbrock, A. B. Rogers, W. Y. Kim and L. Huang, Mol. Ther., 2013, 21, 1559–1569 CrossRef CAS PubMed.
- Y. Zhang, L. Peng, R. J. Mumper and L. Huang, Biomaterials, 2013, 34, 8459–8468 CrossRef CAS PubMed.
- Y. Zhang, W. Y. Kim and L. Huang, Biomaterials, 2013, 34, 3447–3458 CrossRef CAS PubMed.
- L. Miao, S. Guo, J. Zhang, W. Y. Kimb and L. Huang, Adv. Funct. Mater., 2014, 24, 6601–6611 CrossRef CAS PubMed.
- J. Zhang, L. Miao, S. Guo, Y. Zhang, L. Zhang, A. Satterlee, W. Y. Kim and L. Huang, J. Controlled Release, 2014, 182, 90–96 CrossRef CAS PubMed.
- G. Frens, Nature Phys. Sci., 1973, 241, 20–22 CrossRef CAS.
- C. Jiang, T. Zhao, P. Yuan, N. Gao, Y. Pan, Z. Guan, N. Zhou and Q.-H. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 4972–4977 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Size distribution, elemental mapping analyses, XRD, drug release analysis, and experimental details for in vitro cell cultures. See DOI: 10.1039/c7me00058h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |

