Open Access Article
Open Access ArticleEnantioselective dearomative [3 + 2] cycloadditions of indoles with azomethine ylides derived from alanine imino esters†
Anthony L.
Gerten
and
Levi M.
Stanley
*
Department of Chemistry, Iowa State University, Ames, IA 50011, USA. E-mail: lstanley@iastate.edu
First published on 4th January 2016
Abstract
Catalytic, enantioselective [3 + 2] cycloadditions of azomethine ylides derived from alanine imino esters with 3-nitroindoles are reported. The dearomative cycloaddition reactions occur in the presence of a catalyst generated in situ from Cu(OTf)2 and (R)-Difluorphos to form exo′-pyrroloindoline cycloadducts and establish four contiguous stereogenic centers, two of which are fully substituted. The exo′-pyrroloindoline products are formed in moderate-to-good yields (39–85%) with high diastereoselectivities (up to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and enantioselectivities (up to 96% ee).
1 dr) and enantioselectivities (up to 96% ee).
Introduction
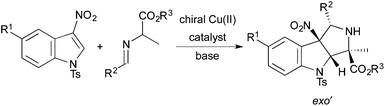
The development of dearomatization reactions offers the potential to rapidly generate molecular complexity and new molecular frameworks.1 In recent years, [2 + 2],2 [3 + 2],3 [4 + 2],4 and [5 + 2]5 dearomative cycloadditions have emerged as promising strategies to construct polycyclic carbocycles and heterocycles from arenes and heteroarenes. Despite the steady development of new dearomative cycloadditions reactions, examples of catalytic, enantioselective dearomative cycloadditions are limited.2a,3e,g–iAmong the promising classes of dearomative cycloadditions for further development are intermolecular [3 + 2] cycloadditions of nitrogen-containing 1,3-dipoles with arenes and heteroarenes. In particular, cycloadditions of azomethine ylides with arenes and heteroarenes have emerged as a viable approach to generate polycyclic nitrogen heterocycles.6 Gribble and co-workers initially reported cycloadditions of unstabilized azomethine ylides with 2- and 3-nitroindoles to form racemic pyrroloindolines.7 More recently, Chataingner, Piettre, and co-workers showed that cycloadditions of unstabilized azomethine ylides with electron-deficient arenes and heteroarenes form a wide variety of racemic, polycyclic cycloadducts in good-to-excellent yields.8
In 2014, Awata and Arai reported the first catalytic, enantioselective dearomative cycloadditions of stabilized azomethine ylides with 3-nitroindoles.9 Cycloadditions of a wide range of azomethine ylides derived from glycine imino esters occur with excellent enantioselectivity and nearly perfect exo′-selectivity when the reactions are run in the presence of a complex prepared from Cu(OTf)2 and a chiral PyBidine ligand. However, catalytic, enantioselective dearomative cycloadditions of azomethine ylides derived from alanine imino esters have not been reported. Herein, we report the first examples of diastereo- and enantioselective dearomative cycloadditions of alanine-derived azomethine ylides with 3-nitroindoles. These reactions generate pyrroloindolines with four contiguous stereogenic centers, two of which are fully substituted (Scheme 1).
Results and discussion
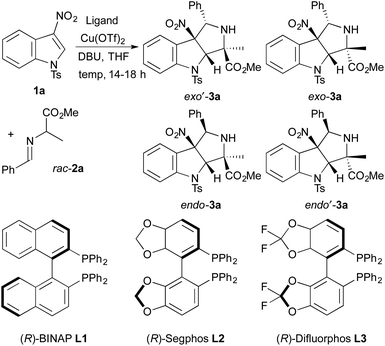
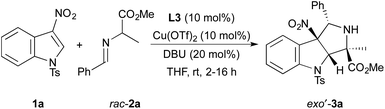
To identify a diastereo- and enantioselective catalyst for dearomative cycloadditions of alanine-derived azomethine ylides, we evaluated the model reaction of N-tosyl-3-nitroindole 1a with racemic imino ester 2a (Table 1). Initially, we found that copper(II) triflate complexes of the chiral bisphosphine ligands (R)-BINAP, (R)-Segphos, and (R)-Difluorphos (10 mol%) catalyzed the cycloadditions of 1a with 2a. These reactions formed a diastereomeric mixture of cycloadducts 3a with exo′-3a generated as the major diastereomer (entries 1–3). The reaction of 1a and 2a occurred with the highest diastereoselectivity (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 exo′-3a
4 exo′-3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo-3a
exo-3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo-3a) with the copper(II) triflate complex of (R)-difluorophos as catalyst (entry 3).10 The cycloadduct exo′-3a was isolated in 73% yield with 86% ee. The high enantioselectivity and diastereoselectivity observed for the model reaction led us to explore additional reaction parameters using the combination of Cu(OTf)2 and (R)-difluorphos as catalyst.
endo-3a) with the copper(II) triflate complex of (R)-difluorophos as catalyst (entry 3).10 The cycloadduct exo′-3a was isolated in 73% yield with 86% ee. The high enantioselectivity and diastereoselectivity observed for the model reaction led us to explore additional reaction parameters using the combination of Cu(OTf)2 and (R)-difluorphos as catalyst.
| Entry | Ligand | Temp (°C) | Equiv. 2a | dr | Yield exo′-3ac (%) | ee |
|---|---|---|---|---|---|---|
exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endob endob |
exo′-3ad (%) | |||||
a Reaction conditions: 1a (0.20 mmol), rac-2a (0.20–0.40 mmol), Cu(OTf)2 (0.02 mmol, 10 mol%), ligand (0.02 mmol, 10 mol%), DBU (0.04 mmol, 20 mol%), THF (1.0 mL), 14–18 h.
b Determined by 1H NMR spectroscopy of the crude reaction mixture.
c Isolated yield of exo′-3a with >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr.
d Determined by chiral HPLC analysis.
e Reaction conducted with 5 mol% of the Cu catalyst. 5 dr.
d Determined by chiral HPLC analysis.
e Reaction conducted with 5 mol% of the Cu catalyst.
|
||||||
| 1 | L1 | rt | 1.0 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
35 | 60 |
| 2 | L2 | rt | 1.0 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
60 | 82 |
| 3 | L3 | rt | 1.0 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
73 | 86 |
| 4 | L3 | 0 | 1.0 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
51 | 92 |
| 5 | L3 | −20 | 1.0 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
34 | 93 |
| 6e | L3 | rt | 1.0 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
58 | 76 |
| 7 | L3 | rt | 1.2 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
75 | 89 |
| 8 | L3 | rt | 1.5 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
78 | 88 |
| 9 | L3 | rt | 2.0 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
72 | 90 |
By lowering the reaction temperature to 0 °C and −20 °C, we observed higher enantioselectivity for exo′-3a (92–93% ee) with lower diastereoselectivity (entries 4 and 5). For example, the reaction of 1a with 2a forms a 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 mixture of exo′
44 mixture of exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo diastereomers when the reaction is carried out at −20 °C. The endo selectivity of the reaction carried out at −20 °C led us to postulate that the model cycloaddition is endo-selective, but epimerization of endo-3a to exo′-3a occurs at higher temperatures through a retro-Mannich/Mannich addition pathway and leads to the observed exo′-selectivity.11
endo diastereomers when the reaction is carried out at −20 °C. The endo selectivity of the reaction carried out at −20 °C led us to postulate that the model cycloaddition is endo-selective, but epimerization of endo-3a to exo′-3a occurs at higher temperatures through a retro-Mannich/Mannich addition pathway and leads to the observed exo′-selectivity.11
Lowering the loading of the copper catalyst also proved detrimental to the diastereo- and enantioselectivity of the model reaction. The reaction of 1a with 2a in the presence of 5 mol% of the copper catalyst formed 3a as a 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 mixture of exo′
18 mixture of exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo diastereomers with exo′-3a isolated in 76% ee (entry 6). The decrease in enantioselectivity and diastereosectivity observed at lower catalyst loading suggests: (1) the rate of uncatalyzed background reaction is likely competitive with the rate of the catalyzed process; and (2) the copper complex may catalyze the epimerization of endo-3a to exo′-3a.
endo diastereomers with exo′-3a isolated in 76% ee (entry 6). The decrease in enantioselectivity and diastereosectivity observed at lower catalyst loading suggests: (1) the rate of uncatalyzed background reaction is likely competitive with the rate of the catalyzed process; and (2) the copper complex may catalyze the epimerization of endo-3a to exo′-3a.
Varying the concentration of imino ester 2a has minimal impact on the yield and selectivity of the model reaction (compare entries 3 and 7–9). The diastereoselectivity remains essentially unchanged when varying the amounts of imino ester 2a from 1.0–2.0 equivalents. However, a slight excess (1.2 equivalents, entry 7) of 2a leads to a modest increase in enantioselectivity, but this trend does not hold when the concentration of 2a is further increased (entries 8 and 9).
To develop a better understanding of the rates of the catalyzed reaction and the uncatalyzed background reaction and the evolution of the diastereoselectivity over time, we conducted cycloadditions of 1a with 2a in the presence and absence of copper catalyst and monitored the yield and diastereoselectivity over time (Table 2). The catalyzed reaction of 1a with 2a occurs to approximately 90% conversion after 2 h (entry 1) and is complete after 6 h (entry 3), while the uncatalyzed reaction occurs to approximately 50% and 80% conversion over the same time periods (entries 2 and 4). Although the uncatalyzed background reaction is slower than the catalyzed process, this data shows that the rate of uncatalyzed cycloaddition is sufficient to negatively impact the enantioselectivity of the cycloadditions and likely explains the poor enantioselectivity observed when the catalyst loading is lowered.
| Entry | Cu(II) catalyst | Time (h) | dr | NMR yieldb (%) | ee |
|---|---|---|---|---|---|
exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endob endob |
exo′-3ac (%) | ||||
| a Catalysed reaction conditions: 1a (0.20 mmol), rac-2a (0.24 mmol), Cu(OTf)2 (0.02 mmol), L3 (0.02 mmol), DBU (0.04 mmol), THF (1.0 mL), 2–16 h. b Determined by 1H NMR spectroscopy of the crude reaction mixture using dibromomethane as an internal standard. Conversion of 1a is listed in parentheses. c Determined by chiral HPLC analysis after purification of exo′-3a by column chromatography. Isolated yields of exo′-3a were 65% (entry 1), 75% (entry 3), and 75% (entry 5). | |||||
| 1 | Yes | 2 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
84 (91) | 87 |
| 2 | No | 2 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
49 (52) | — |
| 3 | Yes | 6 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
99 (99) | 90 |
| 4 | No | 6 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
78 (82) | — |
| 5 | Yes | 16 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
99 (99) | 89 |
The evolution of diastereoselectivity over time is greatly influenced by the presence or absence of the copper catalyst. The uncatalyzed model reaction forms a 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 ratio of exo′
35 ratio of exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo diastereomers after 2 h, and this ratio does not change significantly over an additional 4 h (Table 2, entries 2 and 4). In contrast, the diastereoselectivity of the copper-catalyzed cycloaddition changes markedly with reaction time. The catalyzed reaction forms a 69
endo diastereomers after 2 h, and this ratio does not change significantly over an additional 4 h (Table 2, entries 2 and 4). In contrast, the diastereoselectivity of the copper-catalyzed cycloaddition changes markedly with reaction time. The catalyzed reaction forms a 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 ratio of exo′
26 ratio of exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo diastereomers after 2 h (entry 1); a ratio that is similar to that observed in the uncatalyzed reaction. However, the diastereomeric ratio improves to 77
endo diastereomers after 2 h (entry 1); a ratio that is similar to that observed in the uncatalyzed reaction. However, the diastereomeric ratio improves to 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 (entry 3) after 6 h and to 93
14 (entry 3) after 6 h and to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (entry 5) after 16 h. The dramatic decrease in the amount of endo-3a and increase in the amount of exo′-3a indicates that the complex generated from Cu(OTf)2 and L3 catalyzes the epimerization of endo-3a to exo′-3a which leads to the high exo′ selectivity of the cycloaddition reaction.
4 (entry 5) after 16 h. The dramatic decrease in the amount of endo-3a and increase in the amount of exo′-3a indicates that the complex generated from Cu(OTf)2 and L3 catalyzes the epimerization of endo-3a to exo′-3a which leads to the high exo′ selectivity of the cycloaddition reaction.
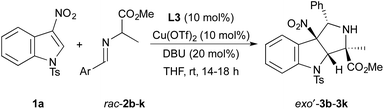
With a practical set of reaction conditions and a diastereo- and enantioselective catalyst identified, we evaluated cycloadditions of N-tosyl-3-nitroindole 1a with a variety of imino esters 2b–k derived from alanine methyl ester and an array of aromatic aldehydes. Results of these reactions are summarized in Table 3. In general, cycloadditions of 1a with imino esters containing 4-substituted aryl groups occur to form exo′-3 in >70% yield with >90% ee and >90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 exo′
3 exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo diastereoselectivity (entries 1–5). The notable exceptions to these typical yields and selectivities include the reactions of imino esters 2b (Ar = 4-F3CC6H4) and 2c (Ar = 4-ClC6H4). The cycloaddition of 2b with 1a occurs in high yield with excellent diastereoselectivity (entry 1). However, exo′-3b is isolated with 80% ee, possibly due to a faster rate of uncatalyzed background reaction with an azomethine ylide containing an electron-withdrawing 4-trifluoromethyl group on the arene moiety. This hypothesis is supported by a positive correlation between enantioselectivity of the reaction and increasing electron-donating ability of substituents at the 4-position on the aryl ring of the dipoles. The cycloaddition of 2c with 1a forms exo′-3b in 70% yield with 88% ee, but the reaction occurs with modest diastereoselectivity (entry 2).
endo diastereoselectivity (entries 1–5). The notable exceptions to these typical yields and selectivities include the reactions of imino esters 2b (Ar = 4-F3CC6H4) and 2c (Ar = 4-ClC6H4). The cycloaddition of 2b with 1a occurs in high yield with excellent diastereoselectivity (entry 1). However, exo′-3b is isolated with 80% ee, possibly due to a faster rate of uncatalyzed background reaction with an azomethine ylide containing an electron-withdrawing 4-trifluoromethyl group on the arene moiety. This hypothesis is supported by a positive correlation between enantioselectivity of the reaction and increasing electron-donating ability of substituents at the 4-position on the aryl ring of the dipoles. The cycloaddition of 2c with 1a forms exo′-3b in 70% yield with 88% ee, but the reaction occurs with modest diastereoselectivity (entry 2).
| Entry | rac-2 (Ar) | exo′-3 | dr | Yield exo′-3c (%) | ee |
|---|---|---|---|---|---|
exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endob endob |
exo′-3d (%) | ||||
a Reaction conditions: 1a (0.20 mmol), 2 (0.24 mmol), Cu(OTf)2 (0.02 mmol), L3 (0.02 mmol), DBU (0.04 mmol), THF (1.0 mL), 14–18 h.
b Determined by 1H NMR spectroscopy of the crude reaction mixture.
c Isolated yield of exo′-3a with >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr.
d Determined by chiral HPLC analysis. 5 dr.
d Determined by chiral HPLC analysis.
|
|||||
| 1 | 2b (4-F3CC6H4) | 3b | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 | 80 |
| 2 | 2c (4-ClC6H4) | 3c | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
70 | 88 |
| 3 | 2d (4-BrC6H4) | 3d | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 | 90 |
| 4 | 2e (4-H3CC6H4) | 3e | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
76 | 91 |
| 5 | 2f (4-MeOC6H4) | 3f | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 | 96 |
| 6 | 2g (3-MeOC6H4) | 3g | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
72 | 89 |
| 7 | 2h (3-BrC6H4) | 3h | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
40 | 80 |
| 8 | 2i (2-MeOC6H4) | 3i | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
39 | 82 |
| 9 | 2j (2-FC6H4) | 3j | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
51 | 87 |
| 10 | 2k (2-ClC6H4) | 3k | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
85 | 79 |
Cycloadditions of imino esters 2g–k containing 2- and 3-substituted aryl groups with 1a generally occur with slightly lower yields and selectivities (entries 6–10) than imino esters 2b–f. While the cycloaddition of 3-MeO-substituted imino ester 2g occurs to form exo′-3g in high yield with high diastereo- and enantioselectivity (entry 6), the cycloaddition of 3-Br-substituted imino ester 2h formed exo′-3h in only 40% yield with modest diastereoselctivity, likely due to a relatively slow epimerization of endo-3g to exo′-3g, and slightly lower enantioselectivity (entry 7). Cycloadditions of 2-MeO-, 2-F-, and 2-Cl-substituted imino esters 2i–k formed exo′-3i–k in 39–85% yield with 79–87% ee and with diastereoselectivities ranging from 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 to 88
27 to 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 exo′
4 exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo (entries 8–10).
endo (entries 8–10).
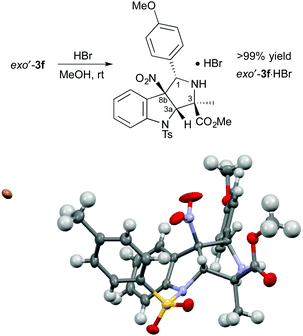
The absolute configuration of exo′-3f was determined after treatment with HBr to form exo′-3f·HBr in >99% yield (Scheme 2). The absolute configuration of exo′-3f·HBr was determined to be (1S,3R,3aS,8bS) by X-ray crystallographic analysis.
Although our studies focused on reactions of N-tosyl-3-nitroindole 1a with imino esters derived from alanine methyl ester, we have also demonstrated that cycloadditions involving an imino ester derived from alanine isopropyl ester and N-tosyl-5-bromo-3-nitroindole occur in high yields with excellent stereoselectivity (Scheme 3). For example, the reaction of N-tosyl-3-nitroindole 1a with imino ester 2l derived from alanine isopropyl ester occurs with 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 exo′
7 exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo diastereoselectivity and forms pyrroloindoline exo′-3l in 71% yield with 94% ee (Scheme 3a). The endo diastereomer was not observed after the 18 h reaction time. The cycloaddition of imino ester 2a with N-tosyl-5-bromo-3-nitroindole 1b occurs with nearly perfect exo′ selectivity (>98
exo diastereoselectivity and forms pyrroloindoline exo′-3l in 71% yield with 94% ee (Scheme 3a). The endo diastereomer was not observed after the 18 h reaction time. The cycloaddition of imino ester 2a with N-tosyl-5-bromo-3-nitroindole 1b occurs with nearly perfect exo′ selectivity (>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exo′
1 exo′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo
exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo, Scheme 2b). Pyrroloindoline exo′-3m was isolated in 79% yield with 91% ee.
endo, Scheme 2b). Pyrroloindoline exo′-3m was isolated in 79% yield with 91% ee.
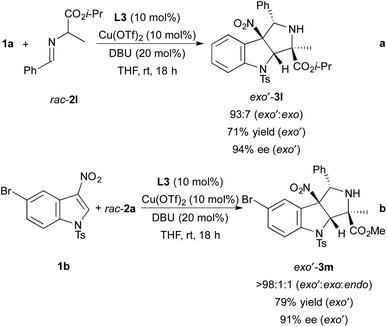 | ||
| Scheme 3 Dearomative cycloadditions of indole 1a with imino ester rac-2l and indole 1b with imino ester rac-2a. | ||
Conclusions
In summary, we have developed the first catalytic, enantioselective dearomative cycloadditions of alanine-derived imino esters with 3-nitroindoles. These exo′-selective dearomative cycloadditions form a variety of highly enantioenriched pyrroloindolines with four contiguous stereogenic centers, two of which are fully substituted, when the reactions are conducted in the presence of a catalyst generated from Cu(OTf)2 and (R)-difluorphos. The high diastereoselectivities observed favoring the formation of the exo′-cycloadduct result from a Cu(OTf)2/(R)-difluorphos-catalyzed epimerization of the endo-cycloadduct to the exo′-cycloadduct during the course of the reaction. Studies to develop new catalytic, enantioselective dearomative cycloadditions with additional classes of aromatic and heteroaromatic dipolarophiles are ongoing in our laboratory.Acknowledgements
We thank Iowa State University and the Iowa State University Center for Catalysis for financial support of this work. We thank Dr Akady Ellern (ISU) for X-ray diffraction data collection and structure determination.Notes and references
- For reviews, see: (a) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; (b) S. P. Roche and J. A. Porco Jr., Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (c) J. M. Keane and W. D. Harman, Organometallics, 2005, 24, 1786 CrossRef CAS; (d) A. R. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917 CrossRef CAS PubMed.
- (a) M. Jia, M. Monari, Q. Yang and M. Bandini, Chem. Commun., 2015, 51, 2320 RSC; (b) D. Zhau, J. Zhang and Z. Xie, J. Am. Chem. Soc., 2015, 137, 9423 CrossRef PubMed; (c) L. Zhang, J. Am. Chem. Soc., 2005, 127, 16804 CrossRef CAS PubMed.
- For selected examples, see: (a) C. Venkatesh, P. Singh, H. Ila and H. Junjappa, Eur. J. Org. Chem., 2006, 5378 CrossRef CAS; (b) J. Zhang, Z. Chen, H. Wu and J. Zhang, Chem. Commun., 2012, 48, 1817 RSC; (c) T. Miura, Y. Funakoshi and M. Murakami, J. Am. Chem. Soc., 2014, 136, 2272 CrossRef CAS PubMed; (d) M. Yonekawa, Y. Koyama, S. Kuwata and T. Takata, Org. Lett., 2012, 14, 1164 CrossRef CAS PubMed; (e) B. M. Trost, V. Ehmke, B. M. O'Keefe and D. A. Bringley, J. Am. Chem. Soc., 2014, 136, 8213 CrossRef CAS PubMed; (f) H. Li, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2014, 136, 6288 CrossRef CAS PubMed; (g) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed; (h) L. M. Repka, J. Ni and S. E. Reisman, J. Am. Chem. Soc., 2010, 132, 14418 CrossRef CAS PubMed; (i) J. Barluenga, E. Tudela, A. Ballesteros and M. Tomás, J. Am. Chem. Soc., 2009, 131, 2096 CrossRef CAS PubMed; (j) A. Acharya, D. Anumandla and C. S. Jeffrey, J. Am. Chem. Soc., 2015, 137, 14858 CrossRef CAS PubMed; (k) M. C. DiPoto, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2015, 137, 14861 CrossRef CAS PubMed; (l) H. Li, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2014, 136, 6288 CrossRef CAS PubMed; (m) H. Li and J. Wu, Synthesis, 2015, 22 Search PubMed.
- (a) M. Kawano, T. Shoko, S. Negishi, H. Tanaka, T. Hoshikawa and J. Matsuo, Angew. Chem., Int. Ed., 2013, 52, 906 CrossRef CAS PubMed; (b) N. Chopin, H. Gerard, I. Chataigner and S. R. Piettre, J. Org. Chem., 2009, 74, 1237 CrossRef CAS PubMed; (c) M. Andreini, M. De Paolis and I. Chataigner, Catal. Commun., 2015, 63, 15 CrossRef CAS.
- G. Mei, H. Yuan, Y. Gu, W. Chen, L. Chung and C. Li, Angew. Chem., Int. Ed., 2014, 53, 11051 CrossRef CAS PubMed.
- For a review see: J. H. Ryan, ARKIVOC, 2015, 160 Search PubMed.
- S. Roy, T. L. S. Kishbaugh, J. P. Jasinski and G. W. Gribble, Tetrahedron Lett., 2007, 48, 1313 CrossRef CAS.
- (a) S. Lee, I. Chataingner and S. R. Piettre, Angew. Chem., Int. Ed., 2011, 50, 472 CrossRef CAS PubMed; (b) S. Lee, S. Diab, P. Queval, M. Sebban, I. Chataingner and S. R. Piettre, Chem. – Eur. J., 2013, 19, 7181 CrossRef CAS PubMed.
- (a) A. Awata and T. Arai, Angew. Chem., Int. Ed., 2014, 53, 10462 CrossRef CAS PubMed.
- The formation of endo′-3a was not observed in cycloadditions of 1a and 2a catalyzed by complexes of Cu(OTf)2 and chiral bisphosphine ligands L1–L3.
- A. M. Sarotti, R. A. Spanavello, A. G. Suárez, G. A. Echeverría and O. E. Piro, Org. Lett., 2012, 14, 2556 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data for new compounds, and crystallographic data (CIF) for compound 3f·HBr. CCDC 1434459. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00346f |
| This journal is © the Partner Organisations 2016 |