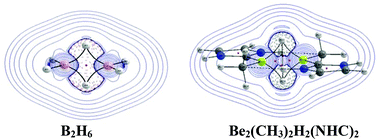
The structure and bonding of diberyllium hydride complexes Be2(CH3)2H2L2, where L = H− (2), CO (3), NHC (4) and CN− (5) were studied in detail and compared with isostructural diborane (1). The bonding in Be2(CH3)2H2L2 was analyzed considering the interaction of two Be(CH3)L fragments with the bridging (μ-H)2 fragment. The geometrical and molecular orbital (MO) analyses indicate that the extent of back donation from Be→L (L = CO, NHC and CN−) is less in Be2(CH3)2H2L2 as compared to that in Be(CH3)L fragments. The NBO, MO and QTAIM analyses indicate a significant charge concentration at the bridging (μ-H)2 in Be2(CH3)2H2L2, whereas charge depletion is observed at the (μ-H)2 fragment in diborane. The accumulation of electron density at the bridging (μ-H)2 is duly reflected in the detection of a (μ-H)−(μ-H) bond path in Be2(CH3)2H2L2, in spite of having longer (μ-H)−(μ-H) distances. The interaction between the bridging H-atoms in diberyllium hydride complexes can be considered as homopolar hydride–hydride dihydrogen bonding. The direction of the charge flow is from (μ-H)2 to BH2 in diborane, whereas from Be(CH3)L to (μ-H)2 in Be2(CH3)2H2L2. Hence, the bridging (μ-H)2 fragments in Be2(CH3)2H2L2 are electron rich as compared to the (μ-H)2 fragment in diborane. The experimental report of the ring opening of the ArNHC (Ar = 2,4,6-trimethylphenyl) ligand through hydride transfer from [Be(CH3)H(ArNHC)]2 corroborates well with the presence of electron rich bridging (μ-H)2 in diberyllium hydride complexes.