Multichromophoric di-anchoring sensitizers incorporating a ruthenium complex and an organic triphenyl amine dye for efficient dye-sensitized solar cells†
Pei-Yang
Su
a,
Jun-Min
Liu
*a,
Xue-Lian
Lin
a,
Yi-Fan
Chen
a,
Yong-
Shen
a,
Li-Min
Xiao
b,
Dai-Bin
Kuang
a and
Cheng-Yong
Su
 *a
*a
aMOE Laboratory of Bioinorganic and Synthetic Chemistry, State Key Laboratory of Optoelectronic Materials and Technologies, Lehn Institute of Functional Materials, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: liujunm@mail.sysu.edu.cn; cesscy@mail.sysu.edu.cn; Fax: +86-20-84115178; Tel: +86-20-84115178
bSchool of Computer Science and Engineering, Beihang University, Beijing 100191, China
First published on 14th September 2015
Abstract
Multichromophoric dye-sensitized solar cells (DSSCs) based on the dual sensitization with a metal–organic combinatorial dye comprised of a ruthenium complex and an organic triphenyl amine dyad have been constructed, which convert light into electrical energy with a higher power conversion efficiency and incident-photon-to-current efficiency than the devices made of individual inorganic and organic dyes.
Dye-sensitized solar cells (DSSCs) have gained considerable attention due to their relatively low manufacturing costs, high cell performance, and good mechanical flexibility.1 Sensitizers, as crucial components in DSSCs, are responsible for light harvesting and electron injection. The key tunable factors to obtain high efficiency dyes are to maximize the light-harvesting efficiency as well as to reduce charge recombination.2 Despite convenient structural modifications, most of the dyes developed thus far consist of uniform chromophore units, either metal complexes or metal-free organic dyes, that can absorb light only in limited wavelength regions, leaving the rest of the visible light essentially unused for energy conversion.3 To circumvent this problem, co-sensitization strategies were investigated by a combination of two or more dyes sensitized together on a semiconductor film.4 Many co-sensitization systems are reported to show enhanced photovoltaic performance relative to their individual single-dye systems.5 However, the physical mixing of different dyes in DSSCs may cause complexity in cell assembly and low cell stability, limiting their practical application.
To improve the efficiency of DSSCs, there are some successful cases using multi-anchoring organic dyes with bridging groups to link up several D–π–A chains.6 Recently, we have developed a new class of calix[4]arene-based dyes containing four light-harvesting units and four anchoring groups per molecule, which enhanced molar extinction coefficients, promoted electron transfer between the dye and the surrounding TiO2, and increased the stability of the dyes adsorbed on the TiO2 film.7 Wang et al. reported di-anchoring organic dyes with bridging aromatic units and found that the introduction of the bridging unit could suppress the charge recombination and minimize the dye aggregation.8 Therefore, such a new design approach becomes a useful direction for improving the light-harvesting ability and electron injection efficiency.
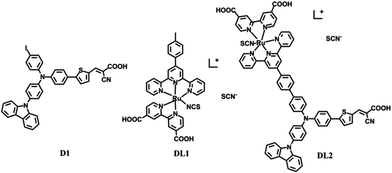
In this study, a novel di-anchoring sensitizer DL2 (Scheme 1) containing a benzene spacer which bridges one starburst triarylamine-based organic dye and one terpyridine-based ruthenium sensitizer was designed and synthesized. The analogous ruthenium sensitizer DL1 and organic dye D19 were also synthesized for comparison. The cell study results indicate that the di-chromophoric and di-anchoring dyes DL2 can produce a significantly higher value of short-circuit current density (Jsc), thus giving a higher conversion efficiency (η). This new combinatorial dye exhibits higher light-harvesting efficiencies than individual dyes, which have been verified by UV-vis spectra and incident photon-to-current conversion efficiency (IPCE) measurements.
The synthetic details of DL1 and DL2 are described in the ESI (Scheme S1†). The precursors with one terpyridine and one aldehyde group were synthesized through a Suzuki coupling reaction. Then, the di-anchoring groups were introduced via a Knoevenagel condensation reaction followed by reacting RuCl3·3H2O and 4,4′-dicarboxylic acid-2,2′-bipyridine (dcppyH2). The chemical structures were characterized by 1H NMR, 13C NMR, and ESI-MS.
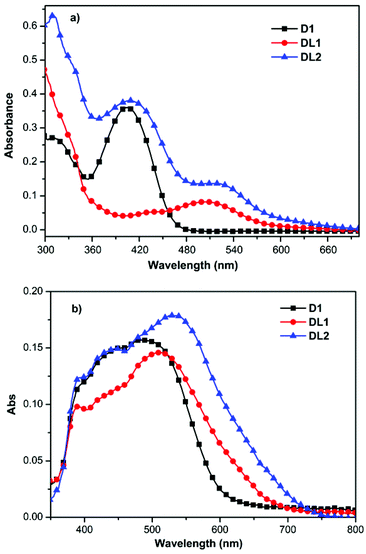
As expected, the absorption spectrum of DL2 in DMF (Fig. 1a) exhibits two distinct broad absorption peaks in the wavelength ranges of 350–450 nm and 450–600 nm, which is essentially a linear combination of the D1 and DL1 spectra, indicating that the HOMO–LUMO gaps of the individual dyes remain virtually unchanged in the dyad. DL2 containing two light-harvesting units exhibits broader absorption than D1 and DL1, which can improve its light-harvesting ability. In addition, the dye DL2 shows higher molar absorption coefficients than D1 and DL1. Upon adsorption onto the nanocrystalline TiO2 films, the absorption peaks of the three dyes (Fig. 1b) are significantly broadened, which is favorable for light harvesting. The broader absorption of DL2 compared with D1 and DL1 confirmed anchoring of the dyes onto TiO2/FTO surfaces, which is consistent with their absorption spectra in DMF. Furthermore, since DL2 has a much larger molecular size than D1 and DL1, the dye loading amounts for DL2 are slightly lower.
To elucidate the electronic properties of DL2 further, we have performed theoretical calculations using both density functional theory (DFT) for the ground-state geometries and time-dependent DFT (TDDFT) for the excited-state energies and properties (see computational details in the ESI†). The computed data are listed in Table 1 and the relevant occupied and unoccupied Kohn–Sham molecular orbitals of DL2 involved in the transitions are shown in Fig. 2. It is found that the first lowest-lying electronic transition, S1 (mainly HOMO−1 → LUMO), is directly charged from the ruthenium-NCS unit to the carboxy pyridine ligand bound to the TiO2 surface in the inorganic part of DL2. In contrast, S2 (mainly HOMO → LUMO+1) shows apparent intramolecular charge transfer (ICT) between the triphenylamine-based donor and the cyanoacrylic acid in the organic part of DL2. This is consistent with the experimental absorption spectrum results.
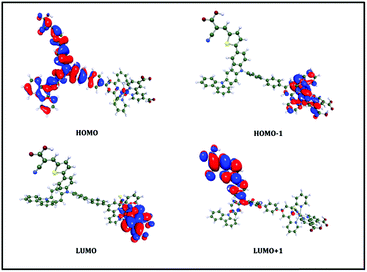 | ||
| Fig. 2 Computed isosurface plots of selected Kohn–Sham molecular orbitals (from HOMO−1 to LUMO+1) of DL2. | ||
| State (nm) | Characters | ||
|---|---|---|---|
| Occupied orbitals | Empty orbitals | Contributions (%) | |
| S1 (520) | HOMO−1 | LUMO | 91.9% |
| S2 (415) | HOMO | LUMO+1 | 96.1% |
The FT-IR spectra of the DL2 dyes have been recorded to study the binding ability of the dye attached to the TiO2 surface.10 Fig. S1† shows the FTIR spectra of the dye powders and the dyes adsorbed on TiO2. For the powders of DL2, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands of carboxylic acids (COOH) in 4-picolinic acids and 2-cyanoacrylic acids were observed at 1720 and 1654 cm−1, respectively. When the dyes were adsorbed on the TiO2 surface, the C
O stretching bands of carboxylic acids (COOH) in 4-picolinic acids and 2-cyanoacrylic acids were observed at 1720 and 1654 cm−1, respectively. When the dyes were adsorbed on the TiO2 surface, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands at 1720 and 1654 cm−1 disappeared and instead two new peaks at 1713 and 1644 cm−1 were found. This indicates deprotonation of two types of COOH groups in DL2 dyes taking place on the TiO2 surface.
O stretching bands at 1720 and 1654 cm−1 disappeared and instead two new peaks at 1713 and 1644 cm−1 were found. This indicates deprotonation of two types of COOH groups in DL2 dyes taking place on the TiO2 surface.
Cyclic voltammetry was performed for D1, DL1 and DL2 (Fig. S2†) and the energy levels of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) derived from the oxidation potentials and absorption/emission data (Fig. S3†) of three dyes are summarized in Table S1.† All the HOMO levels of the dyes are within −5.93 to −5.79 eV, which are much higher than the energy level of the conduction band of TiO2 (−4.4 eV), providing the possibility of electron injection. The LUMO levels were observed to be within the range of −3.86 to −3.11 eV, which are lower than the iodide/triiodide redox potential value (−4.9 eV), indicating that the oxidized dyes could be regenerated by the electrolyte.
DSSCs were fabricated using D1, DL1, and DL2 as the sensitizers, with an active area of 0.16 cm2, nanocrystalline anatase TiO2 particles, and the electrolyte composed of 0.6 M 1-methyl-3-propylimidazolium iodide, 0.10 M guanidinium thiocyanate, 0.03 M I2, 0.5 M tert-butylpyridine in acetonitrile and valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v). The current density–voltage (J–V) curves (Fig. 3) of the DSSCs fabricated from these dyes were measured under simulated AM 1.5 G irradiation (100 mW cm−2), with the photovoltaic parameters summarized in Table 2. The DL2-based device displays much higher Jsc (11.75 mA cm−2) and η (5.84%) than those comprising D1 and DL1 dyes (Table 2). This comparison clearly shows that the DL2 dyad converts light into electricity much more efficiently than individual dyes. However, the dye DL2-sensitized cell has a lower open-circuit voltage (Voc) than that of D1, for DSSCs using the DL2 dye show a shorter electron lifetime, as described in the following paragraph.
15, v/v). The current density–voltage (J–V) curves (Fig. 3) of the DSSCs fabricated from these dyes were measured under simulated AM 1.5 G irradiation (100 mW cm−2), with the photovoltaic parameters summarized in Table 2. The DL2-based device displays much higher Jsc (11.75 mA cm−2) and η (5.84%) than those comprising D1 and DL1 dyes (Table 2). This comparison clearly shows that the DL2 dyad converts light into electricity much more efficiently than individual dyes. However, the dye DL2-sensitized cell has a lower open-circuit voltage (Voc) than that of D1, for DSSCs using the DL2 dye show a shorter electron lifetime, as described in the following paragraph.
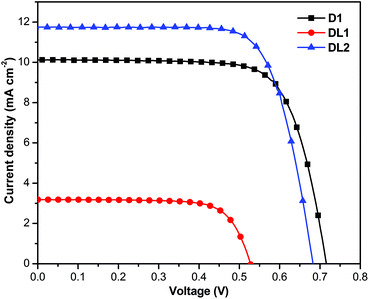 | ||
| Fig. 3 Current–voltage characteristics of D1-, DL1-, and DL2-sensitized solar cells under AM 1.5 G simulated solar light (100 mW cm−2). | ||
| Dye | J sc/mA cm−2 | V oc/mV | FF | η (%) |
|---|---|---|---|---|
| D1 | 10.13 | 752 | 0.75 | 5.45 |
| DL1 | 3.18 | 536 | 0.73 | 1.23 |
| DL2 | 11.75 | 682 | 0.73 | 5.84 |
To elucidate the difference between the Jsc values, the incident photon-to-current conversion efficiencies (IPCEs) for the DSSCs based on the three dyes are shown in Fig. 4. The IPCEs of D1 and DL1 dyes show a band in the region of 380–620 nm with values of 82% at 410 nm and 20% at 510 nm, respectively. It is interesting that DL2 with the D1 and DL1 dye combination displays a broader and red-shifted band with a plateau between 400 and 560 nm with an IPCE value of over 79%, indicating that incorporation of a ruthenium complex and an organic triphenyl amine dye can effectively lead to enhanced DSSC cell currents.
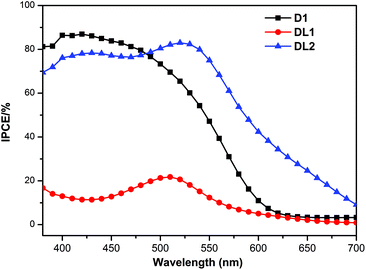 | ||
| Fig. 4 Photocurrent action spectra of D1-, DL1-, and DL2-sensitized solar cells under AM 1.5 G simulated solar light (100 mW cm−2). | ||
Electrochemical impedance spectroscopy (EIS) analysis was performed to explain the different Voc values obtained for the DSSCs based on D1, DL1 and DL2. The interfacial charge transfer resistance (Rct) and the electron lifetime (τ) could be acquired by fitting the EIS spectra.11 As shown in the Nyquist plots (Fig. 5a), the Rct values decrease in the sequence of D1 > DL2 > DL1, and the fitted τ values decrease in the order of D1 > DL2 > DL1, indicating the same order of decreasing charge recombination rate and increasing electron lifetime, which is consistence with that of the Voc values. The Bode phase plots shown in Fig. 5b likewise support the differences in the electron lifetime for TiO2 films based on the three dyes. The increase in the lifetime of the TiO2 film is associated with a pronounced rise in the charge transfer resistance, which depends on the dye structure and adsorption number on TiO2.12 The Voc of D1 is higher than that of DL1, because the hydrophobic groups, such as carbazole, triarylamine, and thiophene units, might be beneficial for retarding the electron transfer from TiO2 to the electrolyte, which would increase the electron lifetime and enhance the open circuit voltage. The lower Voc for the DL2 dyad was attributed mostly to the lower packing density of dyes on TiO2 compared to D1 dyes, which would result in the higher I3− concentration in the vicinity of the TiO2 surface and smaller charge recombination resistance.
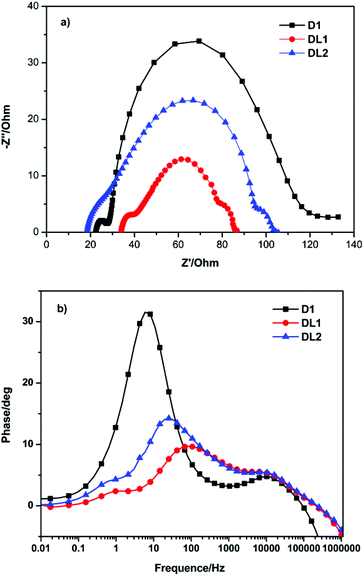 | ||
| Fig. 5 Electrochemical impedance spectra (a) Nyquist plot and (b) Bode plot of DSSCs for D1, DL1, and DL2 dyes. | ||
Intensity modulated photocurrent spectroscopy (IMPS) and intensity modulated photovoltage spectroscopy (IMVS) were carried out to further investigate the different photovoltaic behaviors of the three dyes. Fig. 6a shows the recombination time (τr) of DSSCs based on D1, DL1, and DL2 dyes at various incident light intensities. The results indicate that the recombination time of the devices displays a systematic trend D1 > DL2 > DL1, consistent with the EIS results described above. On the other hand, the electron transport time (τd) of DSSCs based on the three dyes increases in the order DL1 > DL2 > D1 as shown in Fig. 6b. Considering the interaction with the dye and the electrolyte, we deduced it could be the reason that DL1 and DL2 having the [Ru(L)(dcbpyH2)NCS]+ structure might limit the access to the surface by Li+ ions which are known to assist the electron diffusion along the TiO2 network, resulting in slow photo-injected electron transport to FTO glass.13
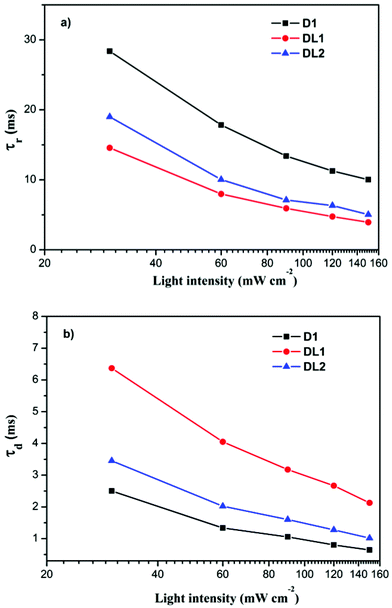 | ||
| Fig. 6 (a) Incident light intensity dependent recombination time constants, (b) incident light intensity dependent transport time constants for DSSCs for D1, DL1, and DL2 dyes. | ||
To compare the physical co-sensitization effects of DL1 and D1 with our chemically integrated combinatorial dye on the cell performance, the photoanode TiO2 films were first immersed in the dye solution of DL1 for 6 h. Then, they were immersed in D1 dye solutions for 6 h, 9 h, and 12 h, respectively. The co-sensitized devices produce the photovoltaic efficiency of 3.78%, 4.43%, and 4.94%, respectively, which are significantly deceased with respect to D1 dye alone under the same conditions (Table S2†), respectively. The results indicate that the physical co-sensitization of DL1 and D1 is not able to maximize the device's performance as the chemically combinatorial dye does, implying that the synergistic co-sensitization effect from the dye combination is complicated, and chemical integration of metal–organic dyes might provide an alternative way for efficient dye-sensitized solar cells.
Conclusions
We have demonstrated that DSSCs based on a metal–organic combinatorial dye can be constructed by introducing multichromophoric di-anchoring dyads DL2 as the light-harvesting materials. On account of broad absorption cross-sections, DL2-based DSSCs display higher Jsc, η, and IPCE under standard conditions than those made of individual dyes. To the best of our knowledge, this is the first example of a chemically combinatorial dye incorporating a ruthenium complex and an organic triphenyl amine dye for efficient dye-sensitized solar cells. The combination strategy of inorganic and organic dyes offers an alternative way for the construction of more efficient DSSCs in which multiple electron donors, acceptors, and chromophores can be organized in an orderly fashion to facilitate red-shifted absorptions as well as high absorptivity for increased device efficiency.Acknowledgements
This work was supported by the NSF of China (21272292, 61370059, 21572280, 61232009, 91222201), NSF of Guangdong Province (S2013030013474), Beijing Natural Science Foundation (4152030), and Special Research Fund for the Doctoral Program of Higher Education (20120171130006).Notes and references
- (a) B. O’ Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef PubMed; (b) B. O’ Regan and J. R. Durrant, Acc. Chem. Res., 2009, 42, 1799–1808 CrossRef PubMed; (c) H. Ren, H. Shao, L. Zhang, D. Guo, Q. Jin, R. Yu, L. Wang, Y. Li, Y. Wang, H. Zhao and D. Wang, Adv. Energy Mater., 2015, 5, 1500296–1500302 Search PubMed; (d) Z. Dong, H. Ren, C. M. Hessel, J. Wang, R. Yu, Q. Jin, M. Yang, Z. Hu, Y. Chen, Z. Tang, H. Zhao and D. Wang, Adv. Mater., 2014, 26, 905–909 CrossRef CAS PubMed; (e) L. Yi, Y. Liu, N. Yang, Z. Tang, H. Zhao, G. Ma, Z. Su and D. Wang, Energy Environ. Sci., 2013, 6, 835–840 RSC.
- (a) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed; (b) S. Ahmad, E. Guillèn, L. Kavan, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2013, 6, 3439–3466 RSC; (c) L. Martín-Gomis, F. Fernández-Lázaro and Á. Sastre-Santos, J. Mater. Chem. A, 2014, 2, 15672–15682 RSC; (d) F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 132, 10720–10728 CrossRef PubMed.
- (a) A. J. Mozer, P. Wagner, D. L. Officer, G. G. Wallace, W. M. Campbell, M. Miyashita, K. Sunahara and S. Mori, Chem. Commun., 2008, 4741–4743 RSC; (b) C. Huang, S. Barlow and S. Marder, J. Org. Chem., 2011, 76, 2386–2407 CrossRef CAS PubMed; (c) B. E. Hardin, H. J. Snaith and M. D. McGhee, Nat. Photonics, 2012, 6, 162 CrossRef CAS PubMed.
- (a) S. Chang, H. Wang, Y. Hua, Q. Li, X. Xiao, W. K. Wong, W. Y. Wong, X. Zhu and T. Chen, J. Mater. Chem. A, 2013, 1, 11553–11558 RSC; (b) X. Sun, Y. Wang, X. Li, H. Ågren, W. Zhu, H. Tian and Y. Xie, Chem. Commun., 2014, 50, 15609–15612 RSC.
- (a) C. M. Lan, H. P. Wu, T. Y. Pan, C. W. Chang, W. S. Chao, C. T. Chen, C. L. Wang, C. Y. Lin and E. W. G. Diau, Energy Environ. Sci., 2012, 5, 6460–6464 RSC; (b) C. L. Wang, J. Y. Hu, C. H. Wu, H. H. Kuo, Y. C. Chang, Z. J. Lan, H. P. Wu, E. W. G. Diau and C. Y. Lin, Energy Environ. Sci., 2014, 7, 1392–1396 RSC.
- (a) C.-H. Siu, L. T. L. Lee, P.-Y. Ho, P. Majumdar, C.-L. Ho, T. Chen, J. Zhao, H. Lie and W.-Y. Wong, J. Mater. Chem. C, 2014, 2, 7086–7095 RSC; (b) D. Cao, J. Peng, Y. Hong, X. Fang, L. Wang and H. Meier, Org. Lett., 2011, 13, 1610–1613 CrossRef CAS PubMed.
- L.-L. Tan, J.-M. Liu, S.-Y. Li, L.-M. Xiao, D.-B. Kuang and C.-Y. Su, ChemSusChem, 2015, 8, 280–287 CrossRef CAS PubMed.
- X. Ren, S. Jiang, M. Cha, G. Zhou and Z.-S. Wang, Chem. Mater., 2012, 24, 3493–3499 CrossRef CAS.
- L.-L. Tan, H.-Y. Chen, L.-F. Hao, Y. Shen, L.-M. Xiao, J.-M. Liu, D.-B. Kuang and C.-Y. Su, Phys. Chem. Chem. Phys., 2013, 15, 11909–11917 RSC.
- Z.-S. Wang, K. Hara, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, H. Arakawa and H. Sugihara, J. Phys. Chem. B, 2005, 109, 3907–3914 CrossRef CAS PubMed.
- L.-L. Tan, J.-F. Huang, Y. Shen, L.-M. Xiao, J.-M. Liu, D.-B. Kuang and C.-Y. Su, J. Mater. Chem. A, 2014, 2, 8988–8994 CAS.
- Z. Ning, Y. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170–1181 CAS.
- S. Kambe, S. Nakade, T. Kitamura, Y. Wada and S. Yanagida, J. Phys. Chem. B, 2002, 106, 2967–2972 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures of D1, DL1 and DL2, FTIR spectra of dye powders and dyes adsorbed on TiO2 nanoparticles for DL2, their cyclic voltammetry curves, absorption and electrochemical data, and photovoltaic parameters of the co-sensitization DSSCs. See DOI: 10.1039/c5qi00095e |
| This journal is © the Partner Organisations 2015 |