Simultaneous colorimetric detection of improvised explosive compounds using microfluidic paper-based analytical devices (μPADs)
Kelley L.
Peters
a,
Inge
Corbin
a,
Lindsay M.
Kaufman
a,
Kyle
Zreibe
a,
Lucas
Blanes
b and
Bruce R.
McCord
*a
aFlorida International University, 11200 SW 8th Street, Miami, FL 33199, USA. E-mail: mccordb@fiu.edu; Fax: +1-305-348-3772
bCentre for Forensic Science, University of Technology, Broadway, Sydney, NSW, Australia
First published on 22nd July 2014
Abstract
In this paper the development of microfluidic paper-based analytical devices (μPADs) is described for rapid, on-site detection of improvised explosives. Five lane μPADs were designed and printed using wax ink on chromatography paper to create hydrophobic channels. Each channel contains colorimetric reagents capable of reacting with one or more explosive compounds resulting in a specific colorimetric reaction. Two devices were prepared, each capable of performing five simultaneous analyses on a single μPAD. The first μPAD was developed to detect inorganic explosives such as black powder, flash powder, and ammonium nitrate. It detects nitrate, nitrite, chlorate, and perchlorate oxidizers, as well as ammonium. The second μPAD was developed to detect military explosives such as TNT and RDX along with other high explosives like urea nitrate. It also detects organic peroxides such as TATP and its precursor hydrogen peroxide. All experiments were performed by dissolving the explosives in deionized water or 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 acetone/H2O as transport solvents with a detection time of around 5 minutes. Detection limits ranged from 0.39–19.8 μg of explosive compound. These two customized μPAD devices permit the on-site forensic testing of unknown explosives, thereby supplying law enforcement and military personnel with a resource for fast, easy detection of military, commercial, and homemade explosive components at low cost.
50 acetone/H2O as transport solvents with a detection time of around 5 minutes. Detection limits ranged from 0.39–19.8 μg of explosive compound. These two customized μPAD devices permit the on-site forensic testing of unknown explosives, thereby supplying law enforcement and military personnel with a resource for fast, easy detection of military, commercial, and homemade explosive components at low cost.
Introduction
In recent years there has been a dramatic increase in the use of improvised explosive devices (IEDs) due to improved controls placed on commercial and military explosives.1 IEDs were once limited to war zones, but have become an increasing concern for law enforcement officials who may encounter terrorists manufacturing homemade explosives. In these situations, fast and accurate identification of the explosive material is paramount.Commonly used materials for improvised explosive preparations include fertilizers and industrial chemicals containing oxidizers such as chlorates, perchlorates, and nitrates as well as other less stable compounds, such as peroxides.2 These materials encompass a wide range of properties, such as volatility, polarity, and composition, which require a variety of different analytical techniques to identify the explosive materials. For example, the combination of gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry is often used to identify organic compounds, while ion chromatography and capillary electrophoresis are used to determine inorganic ions.3–6 Metals can be detected using scanning electron microscopy/elemental diffraction spectroscopy or X-ray diffraction.7,8 These devices are large, expensive pieces of instrumentation that are not portable, so the sample must be sent to a laboratory for testing. This cumbersome process increases the amount of time before any analytical information on the identity of the explosive can be provided to on-site personnel.
On-site analytical instrumentation, such as ion mobility spectrometry, Fourier transform infrared spectroscopy, and Raman spectroscopy, can be used to detect explosives in the field;9 however, these devices commonly rely on the detection of volatile components and can be costly and bulky, making them unavailable in many situations. These portable instruments also require a power source such as a battery, which can be drained before the on-site work is finished.12 Colorimetric and immunoassay based tests have also been developed, but the current procedures are not multiplexed and may require multiple tests and reagents, extending the time of analysis and increasing the amount of sample that is needed if an unknown explosive is present.10,11 Therefore, the development of a simpler, cheaper, and quicker on-site detection method for multiple explosive compounds is needed.
Paper has become an increasingly attractive substrate for on-site microfluidic testing since it is cheap, compatible with many chemical applications, and does not require the use of external pumps to transport liquids. Microfluidic paper-based analytical devices (μPADs) permit the development of inexpensive analytical devices through the fabrication of hydrophobic patterns on chromatography paper.13,28 There are many different ways of fabricating μPADs such as photolithography, plotting, inkjet etching, plasma etching, cutting, and wax printing.16 One of the most effective ways to produce μPADs is wax printing on chromatography paper due to the ease of application and minimal instrumentation required (commercially available printer and laminator). The wax channels can be used to compartmentalize chemical reactions and direct the liquid samples toward individual sections of the paper containing test reagents.17
μPADs have been previously designed for point-of-care testing in medical diagnostics16 and to test substandard pharmaceuticals29 in third world countries. There have also been a number of previous attempts to produce μPADs for the analysis of explosives. For example, pyrene excited with UV light was utilized for the determination of organic explosives,14 a system for detection of organic peroxides and nitrobenzenes was developed,15 and a method was published to detect trinitroaromatic explosives on paper.27 However, these procedures tend to focus on a small subset of explosives, and none of them address the important issue of detecting improvised explosives, particularly those developed from fertilizers and pyrotechnic materials.
In this project we have developed two different μPADs for the analysis of the widest possible range of both military and improvised explosives. Both inorganic and organic explosives are detected. In contrast to previous research, this article demonstrates the capability of multiplexing the analysis of these explosives. Thus it is possible to detect mixtures of different components on the same device. Furthermore, the procedure illustrates an interesting application of organized sequential chemical reactions on μPADs and a single eluent reservoir that is used to extract the explosives and transport them to the test areas using capillary action.
Experimental
Chemicals
All reagents and chemicals were analytical grade. Explosive samples such as TNT, RDX, and urea nitrate were prepared as dilute solutions from law enforcement sources. Potassium chlorate, ammonium nitrate, potassium nitrite, and potassium perchlorate were all purchased from Sigma-Aldrich (St. Louis, MO, USA). 30% aqueous hydrogen peroxide solution was purchased from Fisher Scientific (Fair Lawn, NJ, USA).The handling of explosives can be hazardous and should be performed with appropriate laboratory safeguards. All materials were stored as dilute solutions in sealed plastic vials in an explosion proof freezer. All experiments were conducted with appropriate protection such as face shield, gloves, and lab coat. Laboratory hoods were used when appropriate.
μPAD fabrication
The paper microfluidic devices were designed using Microsoft paint (Microsoft; Redmond, WA, USA) and printed on Whatman no. 1 chromatography paper (GE Healthcare, UK) using a wax-based printer (Xerox ColorQube 8750; Xerox, US). The paper was then placed into an aluminum foil carrier and run through a laminator at 160 °C, speed 1 (Tah Hsin Industrial Corp, TCC-600). This process was repeated twice and the μPADs were cut to the appropriate size for use. Two microliters of each colorimetric reagent were spotted onto the μPADs and allowed to dry for 1 minute. This process was used for all colorimetric reagents for both the single lane μPADs and five lane μPADs.Inorganic μPAD
The five lane inorganic explosives detection μPAD included a test for chlorate, nitrate, ammonium, nitrite, and perchlorate in each respective lane. To detect chlorate, an aniline sulfate reagent (Fisher Scientific; Fair Lawn, NJ, USA) was spotted at the midpoint of the sample lane and 50% H2SO4 (Fisher Scientific; Fair Lawn, NJ, USA) was spotted at the top of the sample lane. For the nitrate test, 3 steps were involved: (1) a solid reducing mixture consisting of 0.08 g sulfanilic acid (Sigma-Aldrich; St. Louis, MO, USA), 1.87 g sodium acetate (Fisher Scientific; Fair Lawn, NJ, USA), and 0.37 g zinc powder (Alfa Aesar; Ward Hill, MA, USA) was made into a slurry using a saturated trehalose (Fisher Scientific; Fair Lawn, NJ, USA) solution in ethanol (Fisher Scientific; Fair Lawn, NJ, USA) and pressed into the bottom of the sample lane using a small metal spatula; (2) 20% H2SO4 was spotted midway up the lane; (3) 2.5% 1-napthol (Sigma-Aldrich; St. Louis, MO, USA) in ethanol was spotted at the top of the sample lane. In order to detect ammonium, Nessler's reagent (La-Mar-Ka, Inc.; Baton Rouge, LA, USA) was spotted at the top of the sample lane. For the nitrite test, a Griess reagent was prepared by spotting 0.5% aqueous 1-napthylamine (Sigma-Aldrich; St. Louis, MO, USA) midway up the sample lane and 0.1% aqueous sulfanilic acid at the top of the sample lane. To detect perchlorate, 0.05% aqueous methylene blue (Fisher Scientific; Fair Lawn, NJ, USA) solution was spotted at the top of the sample lane. This μPAD was run using deionized water as the solvent.High/organic μPAD
The five lane organic/high explosives μPAD includes tests for RDX/HMX/PETN, TNT/TNB/tetryl, urea nitrate, nitrate, and hydrogen peroxide. For the detection of RDX, three steps were utilized. (1) A solid reducing mixture of zinc powder was made into a paste using 50% acetic acid (Fisher Scientific; Fair Lawn, NJ, USA) and pressed into the bottom of the sample lane using a small metal spatula; (2) midway up the sample lane 0.05% sulfanilic acid was spotted; (3) 0.1% 1-napthylamine was spotted at the top of the sample lane. To detect TNT, 1.5 M potassium hydroxide (Fisher Scientific; Fair Lawn, NJ, USA) was spotted at the top of the sample lane. For the urea nitrate test, 0.023 M para-dimethylaminocinnamaldehyde (Acros Organics; NJ, USA) in ethanol was spotted at the top of the sample lane. To test for hydrogen peroxide, 1 M aqueous ammonium titanyl oxalate (Acros Organics; NJ, USA) was spotted at the top of the sample lane. This μPAD was run using 50% acetone/50% water as the solvent.Portable testing system
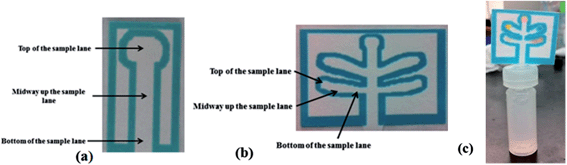
One of each μPAD can be generated in less than 15 minutes. These devices can be stored in the open at ambient temperature for 1 month before slight color changes start to occur. The portable testing system involved the use of a plastic 1 ml vial (Agilent #5182-0567) or reduced volume 250 μL (Agilent #5188-2788) vial with a slit cut in the lid (Agilent #5042-6491), allowing the insertion of the μPAD device into the solvent (Fig. 1c). To perform the analysis, a small amount of unknown material is placed into the respective solvent and allowed to dissolve. The μPAD was then inserted through the slit in the lid and the lid is placed on the vial. The bottom tip of the paper device in the solvent allows capillary action to carry the solvent and analytes up the μPAD into lanes containing the colorimetric test reagents. This entire process, including run time, takes approximately 5 minutes to complete.Interference testing
Gold Bond blue powder, Gold Bond yellow powder, Gold Bond white powder, Publix baking powder, Publix baking soda, salt, iodized salt, Publix powdered whip topping, Publix laundry detergent, Publix flour, Crystal Light Pink Lemonade mix, and Crystal Light Iced Tea mix were purchased from supermarkets in Miami, FL, United States. Codeine, ephedrine, methamphetamine, and cocaine were obtained in powder form from the International Forensic Research Institute at Florida International University. One thousand ppm samples were prepared for each interferant in the appropriate solvent depending on which μPAD was being tested to determine if any of these commonly encountered substances produced interferences.Real samples
The Hodgdon Pyrodex and Triple Seven black powder substitutes were both obtained from Hodgdon Powder Company, Inc (Shawnee Mission, KS, USA). GOEX black powder was obtained from GOEX (Minden, LA, USA) and Red Dot smokeless powder was obtained from Alliant Powder (Radford, VA, USA). American Pioneer Powder FFG and Jim Shockey's Gold were obtained from American Pioneer Powder, Inc. (Whitewater, CO, USA). The Lemon Drop firework was obtained from a retail store.Results and discussion
The goal of this project was to develop a set of μPADs capable of detecting a wide range of improvised explosive compositions. For detection purposes, explosives can be divided into two main groups: inorganic pyrotechnic compositions and organic explosives. Therefore two different devices were created: the first μPAD was designed to detect inorganic materials including the important oxidizers used in pyrotechnic manufacturing such as nitrates, perchlorates, and chlorates. In addition, this μPAD also contained test lanes for ammonium, to detect the common fertilizer based explosive ammonium nitrate and a lane for nitrite, which is also a post blast reaction product that appears following the deflagration of nitrate salts. The second μPAD was designed for the detection of military explosives such as TNT, RDX and PETN, as well as urea nitrate and peroxide based explosives.Each device was designed using a five lane template to allow multiple tests to be performed on a single device with minimal run time. The external size of these devices during developmental stages was 45 mm × 38 mm with lane sizes of 13 mm × 4 mm (Fig. 1b). This size was later reduced to 24 mm × 17 mm to increase speed of analysis. Single lane μPADs were also designed to allow for the development and testing of individual colorimetric tests (Fig. 1a).
The printing of the μPAD was completed using a wax based printer on Whatman no. 1 chromatography paper. This type of paper is an ideal substrate for fabricating μPADs due to faster transfer of solutions, better analytical performance, and high color intensities produced for colorimetric tests compared to filter paper and other thicker subtrates.18 To ensure that the wax ink was fully embedded into the paper, the μPADs were run through the laminator twice.
The μPADs were originally printed using black ink, but significant bleeding of the ink occurred due to the effect of the organic solvents that were used. Therefore a comparison of the effects of the wax ink colors and solvent composition was performed to optimize the devices. Solvents chosen were also selected based on their ability to maintain the solubility of the explosive compounds being detected. The optimal wax color chosen was bright blue, since none of the subsequent colorimetric tests generated this color and this color produced minimal solvent induced bleeding. White ink was not an option since this is not readily available and the lighter colors (light blue, light pink, lavender, light grey) did not provide a sufficiently solid barrier to solvent flow.
Deionized water was used as the optimal solvent for all experiments using the inorganic explosives μPAD since all of the inorganic explosives are soluble in water. For detection with the high/organic explosives μPAD, multiple solvents were tested including acetone, acetonitrile, methanol, ethanol, deionized water, 50% DMSO/50% water, 50% acetone/50% water, 75% acetone/25% water, 50% methanol/50% water, and 75% methanol/25% water. If the percentage of organic solvent was increased above 50%, a noticeable increase in the bleeding of the wax ink occurred due to dissolution of the dye affecting visualization of the color changes. The optimal solvent for this μPAD was determined to be 50% acetone/50% deionized water, in order to maintain the solubility of all tested compounds and minimize the bleeding of the wax ink. The optimized solvent and wax color were used for all further experiments using the high/organic explosives μPAD.
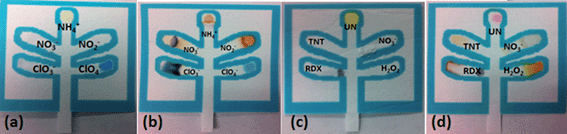
The first μPAD was developed for the detection of inorganic explosives such as pyrotechnic mixtures, black powders, and ammonium nitrate (Fig. 2a and b) while the second was developed to detect high explosives such as trinitroaromatics, nitro amines, nitrate esters, and organic peroxides (Fig. 2c and d).
Table 1 lists the relevant expected color changes for each lane in the two developed μPADs.
| Inorganic explosives μPAD | Organic/high explosives μPAD | ||||
|---|---|---|---|---|---|
| Compound targeted | Color change | Results blank/sample | Compound targeted | Color change | Results blank/sample |
| Chlorate19 (ClO3−) | Colorless to dark green |

|
RDX/HMX/PETN24 | Colorless to pink/red |

|
| Nitrate21,22 (NO3−) | Colorless to orange |

|
Trinitrotoluene (TNT), TNB, tetryl19 | Colorless to orange/red |

|
| Ammonium20 (NH4+) | Pale yellow to brown |

|
Urea nitrate25 (UN) | Yellow to red |

|
| Nitrite19 (NO2−) | Colorless to orange/brown |

|
Nitrate21,22 (NO3−) | Colorless to orange |

|
| Perchlorate20 (ClO4−) | Blue to purple |

|
Hydrogen peroxide23 (H2O2) | Colorless to yellow |

|
Initially the tests performed on these μPADs were chosen based on a literature study of previously developed liquid based colorimetric tests used in qualitative analysis. However, many of these original colorimetric tests required acid concentrations that were high enough to digest paper. Therefore, reagents were modified to permit the development of distinctive color changes without the use of strong acids. For example, the first colorimetric test for chlorate utilized concentrated sulfuric acid and an aniline sulfate solution. In order to adapt this test for use with a μPAD, dilution of concentrated sulfuric acid was varied from 0 to 80% and the colorimetric test was performed in a test tube to determine the minimum acid concentration that could be used while still being able to detect a color change for chlorate. The optimal level was determined to be about 50% sulfuric acid and then tested on the μPAD. Fifty percent concentrated H2SO4 was spotted onto multiple μPADs and allowed to sit for approximately one month. After one month, there was no visible degradation of the μPADs and the resulting colorimetric test successfully detected chlorate.
For the detection of nitrate, a sequential 3 step test was chosen based on the Griess test.21,22 A solid mixture consisting of sulfanilic acid, sodium acetate, and zinc powder was used to reduce the nitrate to nitrite prior to the colorimetric reaction. A saturated trehalose solution was used to make a paste with the solid reducing mixture for two reasons: (1) it facilitated an easier application to the paper devices and (2) trehalose slows down the migration of the liquid sample on the μPAD allowing more time for nitrate to interact with the solid reducing mixture and be converted to nitrite. The paste was placed at the bottom of the sample lane, allowing for an initial reduction of any nitrates present in the sample. Following reduction to nitrite, the liquid sample continued to travel up the lane where the reduced nitrate reacts with 20% sulfuric acid that was placed mid-way up the sample lane and sulfanilic acid to produce a diazonium salt. This diazonium salt continued to travel up the sample lane to 1-napthol spotted at the end of the sample lane. The reaction between the diazonium salt and 1-napthol produced an azo dye, resulting in formation of an orange color. An additional test lane was developed that was specific for nitrite and did not include a reducing agent. This procedure utilized an alternative version of the Griess test where visualization occurs with 1-napthylamine instead of 1-napthol.21
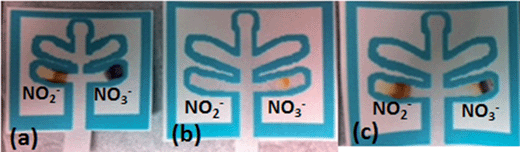
Following the development of these two lanes, a study was performed to determine if nitrate could be differentiated from nitrite utilizing this single μPAD. As shown in Fig. 3, when only nitrite was present, the nitrite channel appeared orange/brown and the nitrate channel was purple, presumably due to reduction of nitrite. When only nitrate was present, an orange color appeared only in the nitrate channel and the nitrite channel was blank. When both salts are present, both channels appeared colored, with an orange/brown color visible in the nitrite lane, and purple and orange colors in the nitrate lane. Therefore, the device successfully differentiated between nitrate and nitrite based explosive compositions as well as detected the presence of nitrite in post blast samples resulting from the reduction of nitrate.
Nitroaromatics such as TNT were detected through the use of 1.5 M potassium hydroxide deposited on the paper, and the subsequent formation of a reddish-orange Meisenheimer complex.19 Hydrogen peroxide was detected using ammonium titanyl oxalate with the formation of a yellow color.23 This test will also produce a weak orange color in the presence of triacetone triperoxide (TATP). The overall composition of the organic explosives μPAD is shown in Table 1 along with the color changes observed for a positive result.
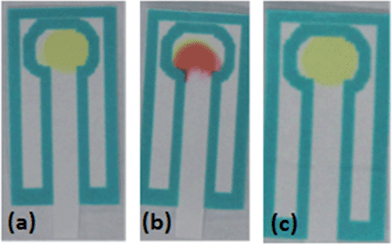
The p-DMAC colorimetric test for urea nitrate detects the presence of the uronium ion25 through a red color change produced by uronium addition to the dye complex; if only urea is present in the sample, no color change will be produced showing the specificity of this test for the detection of uronium (Fig. 4).
The addition of the nitrate test (described previously) on the five lane μPAD permits the user to distinguish between urea and ammonium nitrate. If the nitrate test is positive but the urea nitrate test is not, ammonium nitrate could be present. However, this test is not specific for ammonium nitrate and will show an orange color in the presence of any nitrate salt, while nitrite salts will appear purple. The nitrate test also permits the detection of nitrocellulose containing smokeless powders.
The test for military explosives RDX/HMX/PETN involves the use of a Griess test with sulfanilic acid and 1-napthylamine. Therefore, a study was done to determine if nitrite or nitrate would cause a false positive. It was determined that nitrite and nitrate will both produce an orange-brown color change, while RDX, HMX, and PETN will produce a pink color change allowing for the compounds to be readily differentiated.
Limits of detection were determined for these μPADs as the lowest concentration that a color change could still visibly be detected (Table 2). Instrumental limits of detection were determined through the use of a Camag Scanner 3 color densitometer plate reader or through a digital photograph with analysis by Image J software. It was then calculated by determining the concentration equal to three times the standard deviation of ten replicates at the lowest visible concentration.
| Compound being detected | Visually minimum detectable amount (μg) | Visible LOD (ppm) | Instrumental minimum detectable amount (μg) | Instrumental LOD (ppm) |
|---|---|---|---|---|
| Chlorate | 2.64 | 100 | 1.40 | 53 |
| Nitrite | 2.64 | 100 | 1.37 | 52 |
| Ammonium | 7.92 | 300 | 7.13* | 270* |
| Nitrate | 21.12 | 800 | 19.8 | 750 |
| Perchlorate | 10.56 | 400 | 8.18* | 310* |
| TNT | 1.31 | 50 | 1.31 | 50 |
| Hydrogen peroxide | 2.62 | 100 | 0.39* | 15* |
| RDX | 7.86 | 300 | 7.34* | 280* |
| Urea nitrate | 10.48 | 400 | 9.17 | 350 |
Three different procedures were used for the determination of limits of detection for the colorimetric tests. For detection by eye, single lane μPADs were run for each colorimetric test from 50 ppm to 1000 ppm and the lowest visible color change compared to the blank was determined. Two instrumental procedures were also utilized, the first involving a Camag TLC Scanner 3 and the second using a digital camera (Canon Rebel EOS T3i, 18-135 mm lens) followed by processing with Image J software.
For calculations using the Camag Scanner 3 color scanner, measurements were done through absorbance detection. The intensity of the color that develops in the test zone is a function of the concentration of the analyte and therefore the more analyte present the higher the intensity of the color and the higher the absorbance detected. The wavelength used for the analysis was determined by scanning a test zone area at 500 nm wavelength to determine the location of the test zone with the highest color intensity. This location was then fixed as wavelengths were scanned from 200 nm to 700 nm. The wavelength generating the highest absorbance at this location was used for all future measurements for that analyte.
For the calculations using Image J, measurements were based on the amount of pixels counted from pictures of μPADs using a specific analyte. μPADs were run from 50 ppm to 1000 ppm and a picture was taken of all these μPADs. This picture was then loaded into Image J and a pixel color range was determined for the measurements by determining the range in which the highest concentration generated the most pixels while the blank generated no pixel count in that specified range. This range was fixed for all measurements for the specified analyte and the pixel count was measured and plotted versus concentration.
Interference testing
These interferences were chosen since they are white powders similar in appearance to many explosive powders. It was determined that no false positive were produced. These powders were also run with each explosive compound individually and explosives mixtures; they did not produce false negatives for any of the colorimetric tests.Controlled substances such as methamphetamine, cocaine, codeine, and ephedrine were also tested as possible interferences for both μPADs. These substances were tested individually and mixed with explosive compounds. No false positives or false negatives were generated.
It was also determined that the limit of detection for these compounds was not affected when these interferences were present.
Real samples
Real samples were also tested using fireworks, black powder, black powder substitutes, and smokeless powders. These powders were tested pre-burned and post-burned to determine which compounds were present (Table 3) and the results were compared to those previously determined.23 All tests were done using 1000 ppm aqueous solutions of the corresponding explosive powders.| Powder name | Non-burned powder | Burned powder | Powder content25 |
|---|---|---|---|
| Hodgdon Pyrodex (The FFFG equivalent) | NO3−, ClO4− | NO3−, NO2− | KNO3, KClO4 |
| Alliant Powder Red Dot Smokeless Powder | NO3− | NO2−, NO3− | Nitrocellulose, nitroglycerin |
| FFFg GOEX Black Rifle Powder | NO3− | NO2−, NO3− | KNO3 |
| FFG Hodgdon Triple Seven | NO3−, ClO4− | NO2−, NO3− | KNO3, KClO4, 3-nitrobenzoic acid |
| American Pioneer | NO3−, ClO4− | NO2−, NO3− | KNO3, KClO4 |
| Jim Shockey's Gold FFG | NO3−, ClO4− | NO2−, NO3− | KNO3, KClO4 |
| Lemon Drop Firework | NO3−, ClO4− | ClO4−, NO2−, NO3− | KNO3, KClO4 |
Nitrite was only detected in the burned powders since it is produced when nitrate is burned. The Alliant Powder Red Dot smokeless powder produced a positive result for nitrate, which could be due to nitrocellulose, nitroglycerin, or both. A pure nitroglycerin or nitrocellulose sample was not able to be obtained at a suitable concentration for detection to identify which compound is causing this positive result. All of the results obtained for the μPADs correlated with previous analysis using varying analytical instrumentation.26
Influence of the μPAD dimensions in the reaction time
The μPAD size and set up was also adjusted in order to permit faster analysis times. The μPAD size was reduced from 45 mm × 38 mm to 24 mm × 17 mm for future testing analysis. This dropped analysis time from approximately 18 minutes to less than 5 minutes. The colorimetric changes were still clearly visible and the amount of solvent used was significantly reduced. Therefore, the decrease of the size reduced both analysis time and cost.Conclusion
Two different five lane μPADs were developed for the analysis of unknown suspected explosive materials. The first device is able to identify multiple components of inorganic explosives such as chlorate, perchlorate, nitrate, nitrite, and ammonium, using deionized water as the solvent. The second device is capable of identifying high/organic explosives such as TNT, RDX, hydrogen peroxide, and urea nitrate using 50% acetone/50% water as the solvent. Limits of detection ranged from 0.39–19.8 μg of explosive compound, making the devices well suited for the identification of unknown powders recovered from improvised explosive manufacturing sites. Total analysis time was 5 minutes with very few steps needed to process the μPADs.Compared to on-site detection techniques for explosive compound identification utilized today, these newly designed μPADs are simpler, smaller, and easily portable. They facilitate the identification of combinations of explosive compounds by permitting simultaneous multiplex testing. Therefore, these μPADs will provide law enforcement and military personnel with inexpensive and portable chemical tests for rapid determination of suspected explosive samples.
Acknowledgements
The authors would like to acknowledge Margie Phipps for technical support. This work was funded by the U.S. Department of Justice under Contract no. NIJ2012-DN-BX-K048. Points of view in the document are those of the authors and do not necessarily represent the official view of the U.S. Department of Justice. Lucas Blanes is supported by an Australian Research Council Linkage Project grant LP120200079.References
- The National Counterterrorism Center, 2011 Report on Terrorism, National Counterterrorism Center, US Government Printing Office, Washington, DC, 2012 Search PubMed.
- K. Yeager, Improvised Explosives Characteristics, Detection, and Analysis, in Forensic Investigation of Explosions, ed. A. Beveridge, CRC Press, Florida, 2nd edn, 2012, pp. 494–534 Search PubMed.
- A. Cagan, H. Schmidt, J. E. Rodriguez and G. A. Eiceman, Fast gas-chromatography-differential mobility spectrometry of explosives from TATP to tetryl without gas atmosphere modifiers, Int. J. Ion Mobility Spectrom., 2010, 13, 157–165 CrossRef CAS PubMed.
- G. Jiang, Simultaneous UHPLC/MS Analyses of Explosive Compounds, Application Note 51879, Thermo Fisher Scientific, San Jose, CA, 2010 Search PubMed.
- B. McCord, K. Hargadon, K. Hall and S. Burmeister, Forensic Analysis of explosives using ion chromatographic methods, Anal. Chim. Acta, 1994, 288, 43–56 CrossRef CAS.
- M. Pumera, Analysis of explosives via microchip electrophoresis and conventional capillary electrophoresis: A review, Electrophoresis, 2006, 27, 244–256 CrossRef CAS PubMed.
- R. Berk, Automated SEM/EDS Analysis of Airbag Residue. *I: Particle Identification, J. Forensic Sci., 2009, 54, 60–68 CrossRef CAS PubMed.
- G. Yang, F. Nie, H. Huang, L. Zhao and W. Pang, Preparation and Characterization of Nano-TATB Explosive, Propellants, Explosives, Pyrotechniques, 2006, vol. 31, pp. 390–394 Search PubMed.
- S. Singh, Sensors-An effective approach for the detection of explosives, J. Hazard. Mater., 2007, 144, 15–28 CrossRef CAS PubMed.
- J. Almog, Forensic Science Does Not Start in the Lab: The Concept of Diagnostic Field Tests, J. Forensic Sci., 2006, 51, 1228–1234 CrossRef PubMed.
- J. Yinon, Field detection and monitoring of explosives, Trends Anal. Chem., 2002, 21, 292–301 CrossRef CAS.
- H. Hill and G. Simpson, Capabilities and Limitation of Ion Mobility Spectrometry for Field Screening Applications, Field Anal. Chem. Technol., 1997, 1, 119–134 CrossRef.
- A. Martinez, S. Phillips, G. Whitesides and E. Carrilho, Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices, Anal. Chem., 2010, 82, 3–10 CrossRef CAS PubMed.
- R. Taudte, A. Beavis, L. Wilson-Wilde, C. Roux, P. Doble and L. Blanes, A portable explosive detector based on fluorescence quenching of pyrene deposited on colored wax-printed μPADs, Lab Chip, 2013, 13, 4164–4172 RSC.
- M. Salles, G. Meloni, W. de Araujo and T. Paixao, Explosive colorimetric discrimination using a smartphone, paper device, and chemometrical approach, Anal. Methods, 2014, 6, 2047–2052 RSC.
- X. Li, D. Ballerini and W. Shen, A perspective on paper-based microfluidics: Current status and future trends, Biomicrofluidics, 2012, 6, 011301-1–011301-13 Search PubMed.
- E. Carrilho, A. Martinez and G. Whitesides, Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics, Anal. Chem., 2009, 81, 7091–7095 CrossRef CAS PubMed.
- E. Evans, E. Gabriel, W. Coltro and C. Garcia, Rational selection of substrates to improve color intensity and uniformity on microfluidic paper-based analytical devices, Analyst, 2014, 139, 2127–2132 RSC.
- R. Houghton, Field Confirmation Testing for Suspicious Substances, CRC Press, Boca Raton, 2009 Search PubMed.
- E. Jungreis, Spot Test Analysis: Clinical, Environmental, Forensic, and Geochemical Applications, JohnWiley & Sons, Inc., New York, 1985 Search PubMed.
- K. Niki, Y. Kiso, T. Takeuchi, T. Hori, T. Oguchi, T. Yamada and M. Nagai, A spot test for nitrite and nitrate detection by color band length and number of colored zebra-bands formed in a mini-column, Anal. Methods, 2010, 2, 678–683 RSC.
- E. Fu, S. Ramsey, P. Kauffman, B. Lutz and P. Yager, Transport in two-dimensional paper networks, Microfluid. Nanofluid., 2011, 10, 29–35 CrossRef CAS PubMed.
- M. Xu, B. Bunes and L. Zang, Paper-Based Vapor Detection of Hydrogen Peroxide: Colorimetric Sensing with Tunable Interference, ACS Appl. Mater. Interfaces, 2011, 3, 642–647 CAS.
- T. Jenkins and M. Walsh, Development of field screening methods for TNT, 2,4-DNT, and RDX in soil, Talanta, 1992, 39, 419–428 CrossRef CAS.
- J. Almog, A. Klein, T. Tamiri, Y. Shloosh and S. Abramovich-Bar, A Field Diagnostic Test for the Improvised Explosive Urea Nitrate, J. Forensic Sci., 2005, 50, 1–5 Search PubMed.
- E. Bender and A. Beveridge, Investigation of Pipe Bombs, in Forensic Investigation of Explosions, ed. A. Beveridge, CRC Press, Florida, 2nd edn, 2012, pp. 431–481 Search PubMed.
- A. Pesenti, R. Taudte, B. McCord, P. Doble, C. Roux and L. Blanes, Coupling paper-based microfluidics and lab on a chip technologies for confirmatory analysis of trinitro-aromatic explosives, Anal. Chem., 2014 DOI:10.1021/ac403062y.
- A. Hatch, E. Garcia and P. Yager, Diffusion-based analysis of molecular interactions in microfluidic devices, Proc. IEEE, 2004, 92, 126–139 CrossRef CAS.
- A. Weaver, H. Reiser, T. Barstis, M. Benvenuti, D. Ghosh, M. Hunkler, B. Joy, L. Koenig, K. Raddell and M. Lieberman, Paper analytical devices for fast field screening of beta lactam antibiotics and antituberculosis pharmaceuticals, Anal. Chem., 2013, 85(13), 6453–6460 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |