Sterically demanding aryl–alkyl Suzuki–Miyaura coupling†
Chengxi
Li
a,
Guolan
Xiao
a,
Qing
Zhao
a,
Huimin
Liu
b,
Tao
Wang
b and
Wenjun
Tang
*a
aState Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, 345 Ling Ling Rd, Shanghai 200032, P. R. China. E-mail: tangwenjun@sioc.ac.cn
bAutoChem Department, Mettler-Toledo International Trading Co. Ltd, 589 Guiping Road, Shanghai 200233, P. R. China
First published on 17th March 2014
Abstract
Efficient sterically demanding aryl–alkyl Suzuki–Miyaura coupling between di-ortho-substituted aryl halides and secondary alkylboronic acids has been achieved with a Pd-AntPhos catalyst that has shown high reactivity and a broad substrate scope with unprecedented steric hindrance. The methodology has facilitated the synthesis of molecular gears such as by cross-coupling.
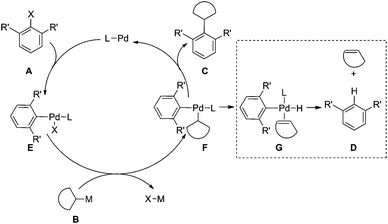
Despite the recent advances in the area of aryl–aryl Suzuki–Miyaura coupling reactions,1,2 the development of efficient aryl–alkyl cross-couplings is more challenging and has been relatively unexplored.3 In contrast to the sp2-hybridized arylboronic acids, the sp3-hybridized alkylboronic acids are relatively bulkier and slower for transmetallation. Moreover, the presence of β-hydrogen in alkylboronic acid allows the β-hydride elimination pathway and facilitates the reduction of aryl halides during cross-coupling (Fig. 1). The formation of the β-hydride elimination–reduction side-product increases significantly with growing steric bulk on the substrates. The development of sterically demanding aryl–alkyl Suzuki–Miyaura coupling remains a significant challenge, particularly of di-ortho-substituted aryl halide substrates. Herein we report an efficient method for Suzuki–Miyaura coupling between di-ortho-substituted aryl halides and secondary alkylboronic acids, and a cross-coupling product was formed for the first time between di-ortho-secondary-alkyl aryl bromides and cyclic alkylboronic acids. The methodology has facilitated the synthesis of molecular gears such as hexaalkylbenzenes by cross-coupling.
 | ||
| Fig. 1 Pd-catalyzed sterically demanding aryl–alkyl coupling and the β-hydride elimination–reduction side pathway. | ||
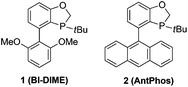
The steric and electronic properties of the phosphorus ligands have a significant impact on the reactivity and outcome of palladium-catalyzed cross-coupling reactions.4 During our study on sterically hindered aryl–aryl Suzuki–Miyaura coupling,5 we observed the excellent reactivity of ligand 1 (BI-DIME) and ligand 2 (AntPhos)6 for tetra-ortho-substituted biaryl coupling. In particular, an interesting coordination between the Pd center and the anthracenyl moiety of AntPhos was revealed in the X-ray structure of the Pd(0)-AntPhos complex. Although there is no information on whether such coordination still exists during the catalytic cycle of cross-coupling, the coordination ability of the rigid anthracenyl moiety should make the formation of G unfavorable (Fig. 1) during the aryl–alkyl cross-coupling and thus inhibit the β-hydride elimination–reduction pathway. We thus envisioned that AntPhos would be advantageous for sterically demanding aryl–alkyl Suzuki–Miyaura coupling and further broaden the synthetic utility of Suzuki–Miyaura coupling (Fig. 2).
While most known palladium catalysts are applied only for the coupling between mono-ortho-substituted aryl halides and secondary alkylboronic acids or the coupling between di-ortho-substituted aryl halides and primary alkylboronic acids, only two examples7 are available for the coupling between di-ortho-substituted aryl halides and secondary alkylboronic acids or zinc reagents with a limited substrate scope. We applied ligands 1 and 2 for the Suzuki–Miyaura coupling between 2,4,6-triisopropylphenyl bromide and cyclohexylboronic acid, an extremely sterically demanding and unexplored cross-coupling system. The reactions were performed under nitrogen at 110 °C in toluene for 12 h at 1 mol% palladium loading. To our delight, the desired coupling product was isolated in 31% yield with BI-DIME (1) as the ligand along with the reduction side-product 7 (Table 1, entry 1). The yield increased significantly when AntPhos (2) was applied as the ligand (entries 2). Elevation of the reaction temperature by using xylenes as the solvent provided 6 in 63% isolated yield. To the best of our knowledge, this is the most sterically demanding aryl–alkyl cross-coupling product to date. To investigate whether other commercially available ligands are also applicable, a series of mono- or bis-phosphorus ligands were also employed for comparison (entries 4–12). Except that S-Phos provided a low yield (3%, entry 8) of 6, no other ligands were applicable for this system, indicating the uniqueness and power of AntPhos for the success of this reaction.
| Entriesa | Ligand | Conv.b (%) | Yield of 6c (%) | 7 (%) |
|---|---|---|---|---|
| a The reactions were performed in toluene under nitrogen at 110 °C for 24 h in the presence of 1 mol% Pd(OAc)2 and 2 mol% ligand with K3PO4 as the base unless otherwise specified. b Conversions were analyzed by reversed phase HPLC on a C-18 column. c Isolated yields. d The reaction was run in xylenes at 140 °C for 12 h. | ||||
| 1 | 1 (BI-DIME) | 100 | 31 | 69 |
| 2 | 2 (AntPhos) | 100 | 58 | 42 |
| 3d | 2 | 100 | 63 | 37 |
| 4 | DPPF | 54 | 0 | 54 |
| 5 | DPPE | 100 | 0 | 98 |
| 6 | DPPP | 100 | 0 | 97 |
| 7 | DPPB | 100 | 0 | 98 |
| 8 | S-Phos | 100 | 3 | 97 |
| 9 | X-Phos | 46 | 0 | 46 |
| 10 | Ru-Phos | 52 | 0 | 52 |
| 11 | PCy3 | 100 | 0 | 97 |
| 12 | PPh3 | 83 | 0 | 83 |
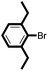
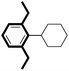
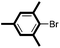
We then looked into the substrate scope of this sterically hindered cross-coupling reaction. As can be seen in Table 2, a series of di-ortho-substituted aryl bromides were successfully coupled with 3-, 4-, 5-, and 6-membered cyclic alkyl boronic acids with a Pd-AntPhos catalyst to form the corresponding coupling products in good to excellent yields (70–99%). In general, the yields followed the trend of the ring size of the cyclic alkyl boronic acids: 3 > 4 > 5 > 6 (entries 12, 14, 16 vs.Table 1, entry 3). High yields were achieved on reactions with cyclopropyl boronic acids (entries 15 and 16 due to its relatively small size as well as its inability to undergo the β-hydride elimination pathway). The β-hydride elimination–reduction side-product formation became more severe with the increase of the ring size, as indicated by diminished coupling yields. The reactions proceeded well with di-ortho-methyl, ethyl, or isopropyl substituents (entries 1, 5, 6, and 13–16). 9-Anthracenyl, 2-methylnaphthyl bromide, and 2-methoxynaphthyl bromide were also applicable (entries 2–4 and 7–9). Functionalities such as methoxy, nitro, and cyano groups were well tolerable (entries 9–11). When isopropylboronic acid was employed to couple with 2-bromo-1,3,5-trimethylbenzene, the major product was unfortunately the n-propyl substituted arene (entry 17) derived from isomerization-coupling. No formation of tert-butyl substituted arene was observed when tert-butylboronic acid was used as the starting material.
| Entrya | Aryl halide | Boronic acid | Product | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried in toluene under nitrogen at 110 °C for 24 h in the presence of 1 mol% Pd(OAc)2 and 2 mol% AntPhos with K3PO4 as the base. b Isolated yields. c The reactions were run in xylenes at 140 °C for 12 h. d The major coupling product is n-propyl arene (82%), along with isopropyl arene (6%) and the reduction side-product (12%). | ||||
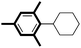
| 1 |
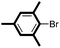
|
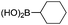
|
|
92 |
| 2c |
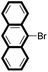
|
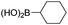
|
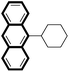
|
74 |
| 3 |
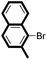
|
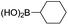
|
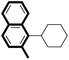
|
82 |
| 4 |
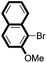
|
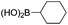
|
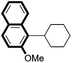
|
70 |
| 5c |
|
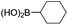
|
|
80 |
| 6 |
|
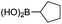
|
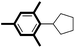
|
93 |
| 7 |
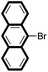
|
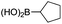
|
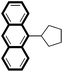
|
80 |
| 8 |
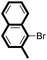
|
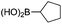
|
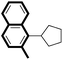
|
87 |
| 9 |
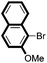
|
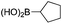
|
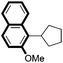
|
75 |
| 10 |
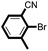
|
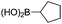
|
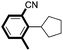
|
79 |
| 11c |
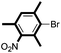
|
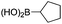
|
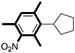
|
79 |
| 12 |
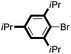
|
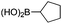
|
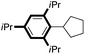
|
81 |
| 13 |
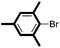
|
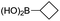
|
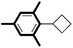
|
92 |
| 14 |
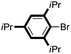
|
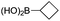
|
|
90 |
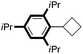
| 15 |
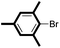
|
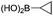
|
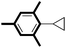
|
99 |
| 16 |
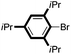
|
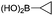
|
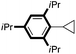
|
99 |
| 17d |
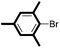
|
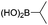
|
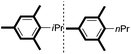
|
6/82 |
In order to gain kinetic insight into this cross-coupling methodology, the coupling of 2-bromo-5-methoxy-1,3-dimethylbenzene (8) and cyclohexylboronic acid (5) was performed in xylenes in the presence of 1 mol% Pd(OAc)2 and 2 mol% AntPhos, monitored by ReactIR. As shown in Scheme 1, the amount of bromide 8 and the coupling product 9 was easily traced by their IR absorbance at 1163 cm−1 and 1149 cm−1 (symmetrical C–O–C bond stretching), respectively. The reaction proceeded rapidly when the reaction temperature was elevated over 80 °C and bromide 8 was completely consumed in only 25 min at 130 °C, demonstrating the high reactivity of the Pd-AntPhos catalyst for this reaction. The desired product 9 was isolated in 82% yield, along with 15% reduction side-product.
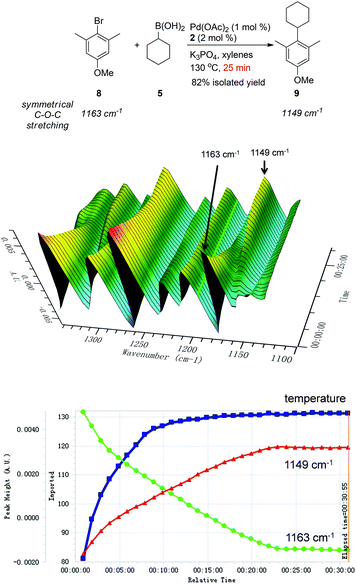 | ||
| Scheme 1 Reaction profile of the cross-coupling between 2-bromo-5-methoxy-1,3-dimethylbenzene (8) and cyclohexylboronic acid (5) by ReactIR: (a) 3D-FTIR profiles. (b) Component profiles. | ||
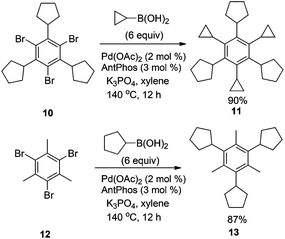
Hexaalkylbenzenes are molecular gears8 that have unique static and dynamic behaviors due to restricted rotation of each substituent on the benzene ring. These compounds are traditionally accessed by trimerization of disubstituted alkynes,9 and a few reports are available on their syntheses by metal-catalyzed cross-coupling.10 We believe that this methodology provides a facile and versatile method to synthesise these interesting compounds. Thus, the coupling of 1,3,5-tribromo-2,4,6-tricyclopentylbenzene (10) with cyclopropylboronic acid provided the coupling product 11 in 90% yield (Scheme 2). In a similar manner, 1,3,5-tricyclopentyl-2,4,6-trimethylbenzene (13) can be synthesized from 1,3,5-tribromo-2,4,6-trimethylbenzene (12) and cyclopentaneboronic acid in 87% yield by using this methodology. Such structures, which are difficult to access by cyclotrimerization of alkynes, could provide interesting physical organic properties.
In summary, efficient sterically demanding aryl–alkyl Suzuki–Miyaura couplings between di-ortho-substituted aryl halides and secondary alkylboronic acids have been achieved with a Pd-AntPhos catalyst that has shown a high reactivity and a broad substrate scope with unprecedented steric hindrance. The unique structure of AntPhos plays a major role in its reactivity and allows overcoming the β-hydride elimination–reduction side pathway. The methodology has been applied successfully for efficient synthesis of molecular gears such as hexaalkylbenzenes by cross-coupling. A further study on sterically demanding aryl–alkyl Suzuki–Miyaura coupling and the application of the Pd-AntPhos catalyst in broadening the scope of metal-catalyzed cross-coupling is currently ongoing in our laboratory.
Notes and references
- Selected reviews: (a) A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS; (b) N. Miyaura, Top. Curr. Chem., 2002, 219, 11 CrossRef CAS; (c) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (d) N. Miyaura, Top. Curr. Chem., 2002, 219, 11 CrossRef CAS; (e) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS PubMed; (f) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS; (g) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176 CrossRef CAS; (h) F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 2419 CAS; (i) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (j) F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047 CrossRef CAS PubMed; (k) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004, vol. 2 Search PubMed; (l) Modern Arylation Methods, ed. L. Ackermann, Wiley-VCH, Weinheim, 2009 Search PubMed; (m) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed; (n) R. J. Lundgren and M. Stradiotto, Chem.–Eur. J., 2012, 18, 9758 CrossRef CAS PubMed; (o) S. M. Wong, C. M. So and F. Y. Kwong, Synlett, 2012, 1132 CAS.
- Recent papers on aryl–aryl Suzuki–Miyaura couplings: (a) W. Shen, Tetrahedron Lett., 1997, 38, 5575 CrossRef CAS; (b) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 1998, 37, 3387 CrossRef CAS; (c) A. F. Littke, C. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020 CrossRef CAS; (d) A. Zapf, A. Ehrentraut and M. Beller, Angew. Chem., Int. Ed., 2000, 39, 4153 CrossRef CAS; (e) J. P. Stambuli, R. Kuwano and J. F. Hartwig, Angew. Chem., Int. Ed., 2002, 41, 4746 CrossRef CAS PubMed; (f) C. A. Fleckenstein and H. Plenio, Chem.–Eur. J., 2007, 13, 2701 CrossRef CAS PubMed; (g) C. A. Fleckenstein and H. Plenio, J. Org. Chem., 2008, 73, 3236 CrossRef CAS PubMed; (h) C. M. So, C. P. Lau and F. Y. Kwong, Angew. Chem., Int. Ed., 2008, 47, 8059 CrossRef CAS PubMed; (i) C. M. So, W. K. Chow, P. Y. Choy, C. P. Lau and F. Y. Kwong, Chem.–Eur. J., 2010, 16, 7996 CrossRef CAS PubMed; (j) S. C. To and F. Y. Kwong, Chem. Commun., 2011, 47, 5079 RSC; (k) W. Tang, N. D. Patel, G. Xu, X. Xu, J. Savoie, S. Ma, M.-H. Hao, S. Keshipeddy, A. G. Capacci, X. Wei, Y. Zhang, J. Gao, W. Li, S. Rodriguez, B. Lu, N. Yee and C. H. Senanayake, Org. Lett., 2012, 14, 2258 CrossRef CAS PubMed; (l) G. Xu, W. Fu, G. Liu, C. H. Senanayake and W. Tang, J. Am. Chem. Soc., 2014, 136, 570 CrossRef CAS PubMed.
- For a recent review: (a) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed. Recent papers on aryl–alkyl Suzuki–Miyaura couplings: (b) N. Kataoka, Q. Shelby, J. P. Stambuli and J. F. Hartwig, J. Org. Chem., 2002, 67, 5553 CrossRef CAS PubMed; (c) A. van den Hoogenband, J. H. M. Lange, J. W. Terpstra, M. Koch, G. M. Visser, M. Visser, T. J. Korstanje and J. T. B. H. Jastrzebski, Tetrahedron Lett., 2008, 49, 4122 CrossRef CAS PubMed; (d) S. Imao, B. W. Glasspoole, V. S. Laberge and C. M. Crudden, J. Am. Chem. Soc., 2009, 131, 5024 CrossRef PubMed; (e) T. Ohmura, T. Awano and M. Suginome, Chem. Lett., 2009, 38, 664 CrossRef CAS; (f) T. Ohmura, T. Awano and M. Suginome, J. Am. Chem. Soc., 2010, 132, 13191 CrossRef CAS PubMed; (g) D. L. Sandrock, L. Jean-Gérard, C.-y. Chen, S. D. Dreher and G. A. Molander, J. Am. Chem. Soc., 2010, 132, 17108 CrossRef CAS PubMed; (h) J. C. H. Lee, R. McDonald and D. G. Hall, Nat. Chem., 2011, 3, 894 CrossRef CAS PubMed; (i) T. Awano, T. Ohmura and M. Suginome, J. Am. Chem. Soc., 2011, 133, 20738 CrossRef CAS PubMed; (j) G. A. Molander and S. R. Wisniewski, J. Am. Chem. Soc., 2012, 134, 16856 CrossRef CAS PubMed.
- (a) G. Liu, G. Xu, R. Luo and W. Tang, Synlett, 2013, 2465 CAS; (b) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed.
- (a) W. Tang, A. G. Capacci, X. Wei, W. Li, A. White, N. D. Patel, J. Savoie, J. J. Gao, S. Rodriguez, B. Qu, N. Haddad, B. Z. Lu, D. Krishnamurthy, N. K. Yee and C. H. Senanayake, Angew. Chem., Int. Ed., 2010, 49, 5879 CrossRef CAS PubMed; (b) Q. Zhao, C. Li, C. H. Senanayake and W. Tang, Chem.–Eur. J., 2013, 19, 2261 CrossRef CAS PubMed.
- For the application of AntPhos for sterically demanding Miyaura borylation, see: W. Tang, S. Keshipeddy, Y. Zhang, X. Wei, J. Savoie, N. D. Patel, N. K. Yee and C. H. Senanayake, Org. Lett., 2011, 13, 1366 CrossRef CAS PubMed.
- (a) S. D. Dreher, P. G. Dormer, D. L. Sandrock and G. A. Molander, J. Am. Chem. Soc., 2008, 130, 9257 CrossRef CAS PubMed; (b) C. Han and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 7532 CrossRef CAS PubMed.
- (a) J. Siegel, A. Gutierrez, W. B. Schweizer, O. Ermer and K. Mislow, J. Am. Chem. Soc., 1986, 108, 1569 CrossRef CAS; (b) H. Iwamura and K. Mislow, Acc. Chem. Res., 1988, 21, 175 CrossRef CAS.
- (a) S. Kotha, E. Brahmachary and K. Lahiri, Eur. J. Org. Chem., 2005, 4741 CrossRef CAS; (b) F. Fabris, L. Zambrini, E. Rosso and O. De Lucchi, Eur. J. Org. Chem., 2004, 3313 CrossRef CAS.
- Y. Yu, A. D. Bond, P. W. Leonard, U. J. Lorenz, T. V. Timofeeva, K. P. C. Vollhardt, G. D. Whitener and A. A. Yakovenko, Chem. Commun., 2006, 2572 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures of cross-coupling reactions, characterization data, and spectra. See DOI: 10.1039/c4qo00024b |
| This journal is © the Partner Organisations 2014 |