 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Human exposure to aluminium
Christopher
Exley
The Birchall Centre, Lennard-Jones Laboratories, Keele University, Staffordshire, UK. E-mail: c.exley@keele.ac.uk; Fax: +44 (0)1782 712378; Tel: +44 (0)1782 734080
First published on 19th August 2013
Abstract
Human activities have circumvented the efficient geochemical cycling of aluminium within the lithosphere and therewith opened a door, which was previously only ajar, onto the biotic cycle to instigate and promote the accumulation of aluminium in biota and especially humans. Neither these relatively recent activities nor the entry of aluminium into the living cycle are showing any signs of abating and it is thus now imperative that we understand as fully as possible how humans are exposed to aluminium and the future consequences of a burgeoning exposure and body burden. The aluminium age is upon us and there is now an urgent need to understand how to live safely and effectively with aluminium.
Environmental impactThe aim of this critical review of human exposure to aluminium is to provide an holistic interpretation of aluminium's exposome in relation to humans. It should enable a change in our thinking about the myriad ways that humans are exposed to aluminium and importantly it provides a much more complete definition of the body burden of aluminium. The latter must now be the starting place for furthering our understanding of how this burden impacts upon human physiology and potentially its role in human disease. |
The aluminium age
Aluminium is the most abundant metal and the third most abundant element in the Earth's crust. In spite of the dynamic, ever changing and evolving nature of the Earth's crust the recycling of aluminium in the lithosphere is essentially complete and aluminium is effectively excluded from the biosphere.1 It has been the non-availability of biologically reactive aluminium throughout biochemical evolution which today explains its lack of essentiality in all extant biota.2 However, the geochemical cycle for aluminium has now become a biogeochemical cycle and primarily through interference due to human activities either indirectly, for example, the acidification of catchments by acid deposition of anthropogenic origin, or directly by the extraction of aluminium from its inert ores. It is now approximately 125 years since the advent of ‘The Aluminium Age’. The ability to separate aluminium metal from its ores on an industrial scale changed aluminium from being a largely decorative metal to the most widely used metal of the 21st century.3 Unfortunately the efficiency of extraction and use of this metal by the aluminium industry cannot match that of the geochemical cycling of aluminium since almost half of cast aluminium is destined to end up as waste.4 The aluminium industry is burgeoning with the majority of current and projected growth coming from newly extracted aluminium, not recycled aluminium as might be commonly perceived, and all of this aluminium has the potential, at least, to enter and accumulate within the biotic cycle. Once aluminium has entered the biotic cycle it has little prospect of a quick return to the lithospheric cycle and biota are now subject to an ever increasing burden of potentially biologically available aluminium. The consequences of a burgeoning burden of aluminium in the biotic cycle have already been manifested in the death of fish and trees in acidified surface waters and catchments respectively5 while the spread of acid soils is limiting plant growth on over 30% of the Earth's ice-free land.6 Human beings have placed themselves at the centre of the Earth's living cycle and humans are not immune from the burgeoning presence of aluminium in this cycle. It is now of critical and urgent importance that we understand human exposure to aluminium.7Aluminium is toxic
Aluminium's success as a modern material with myriad applications comes from a wide breadth of physical and chemical properties which combined with its ubiquity in nature make it an extremely cost effective natural resource. There is also, today, a perception that aluminium is a ‘safe’ metal with few if any significant implications for human health. This is a view which though seemingly convenient for the aluminium industry is neither supported by observation; for example, aluminium is the cause of dialysis encephalopathy,8 nor by decades of animal experimentation demonstrating intoxication. It is truly an anomaly that the perceived innocuousness of aluminium in humans has persisted through to the present day and to the extent that there is no legislation whatsoever limiting human's exposure to aluminium. There is a clear and unambiguous case for a more thorough understanding of human exposure to aluminium and the elucidation of aluminium's exposome.9What do we mean by ‘human exposure to aluminium’?
While there is a casual awareness that we might be exposed to aluminium through the use, for example, of aluminium saucepans or foil,10 the reality is that the majority of people are completely ignorant of their exposure to aluminium in their everyday lives. Much as a bee forages for nectar apparently oblivious to its additional bounty of aluminium11 we are also blind to the myriad ways that everyday life exposes us to aluminium. Our understanding of what constitutes ‘exposure’ is probably prejudiced by a focus upon aluminium in the diet. Research has documented human exposure to aluminium through both whole diet studies12 as well as the aluminium content of individual dietary components.13 Some regulatory authorities have even established tolerable weekly (daily) intakes (TWI) of aluminium which are meant to address safe limits for aluminium in the human diet.14 There are no such guidelines for exposure to aluminium via the skin or the nose or the lung or indeed a single guideline for an overall exposure to aluminium. There is also a tendency to ‘score’ exposures to aluminium based upon entry of aluminium into the systemic circulation15 with the underlying assumption that any remaining aluminium, which is usually the significant majority, is of minor importance. We clearly need a wider definition of human exposure to aluminium and one which is all encompassing and not simply convenient. Of paramount importance is that the definition reflects a potential for aluminium to react with human physiology and to elicit a response to its presence. It needs to incorporate the relationship between the body burden of aluminium and the consequences of any such body burden.What is ‘the body burden’ of aluminium?
The body burden of aluminium is the sum of aluminium atoms associated with the body at any one moment in time. It includes aluminium on the surface of the skin, aluminium in hair and nails, aluminium associated with external secretions/excretions in the mouth, nose, ear, lung, stomach, small intestine, urinary and reproductive tracts and aluminium in faeces in the large intestine. It also includes aluminium associated with all of the systemic compartments including endo/epithelia, blood, lymph, sweat, tears, humours, tissues, organs and bone. Aluminium has been found throughout the body16 and when or where it has not been found is a reflection, not of absence but of the lower limits of analytical detection of current methods and instrumentation. The true body burden of aluminium for an individual is clearly not yet a quantity which is accessible by conventional means,17 at least not for a living person. While measurements of body burden are available these are actually indirect estimates of the systemic body burden, for example, the aluminium content of urine.18–20 These measurements are particularly helpful in comparing relative changes in the body burden of aluminium between individuals or between populations. However, they are less informative about where aluminium is found in the body or its potential for systemic toxicity.Humans are exposed to aluminium!
The most recent analysis shows that to meet the current annual global demand for aluminium 11 kg of the metal must be cast for every person on Earth.4 This aluminium, extracted by industry from its inert edaphic stores, has the potential at least to impact upon biota including humans. If humans are directly exposed to, for example, only 0.1% of this potential then our current daily exposure equates to 30 mg of aluminium. On a similar basis our exposure to aluminium would have been 1 mg per day in 1950 and will be 100 mg per day by 2050. There would seem to be no escape from a burgeoning human exposure to aluminium! So, what are the main contributors to these average daily exposures to aluminium?The air that we breathe must be a significant contributor to the body burden of aluminium. Aluminium-based particulates of myriad sizes, shapes and compositions are primary components of aerosols whether over the more pristine regions of the planet, such as the Antarctic, or the industrialised centres of rapidly growing economies such as China. If we accept 100 ng Al per m3 as representative of clean air21 then our exposure to aluminium through normal breathing is approximately 1.4 μg per day. This is essentially a lowest possible exposure to aluminium from breathing and it would not be unrealistic to suggest that the majority of this aluminium would be retained in lung and olfactory epithelia. This value could easily be increased one thousand fold to 1.4 mg per day in many industrialised regions.22 Exposure to aluminium through breathing can also be significantly influenced by specific activities including industrial/workplace exposure23 and habitual exposure such as smoking of cigarettes and cannabis24 and use of cocaine25 and heroin.26 Aluminium is an important component of many aerosol formulations of cosmetics, and particularly antiperspirants, and these, especially through regular use, will contribute significantly to exposure to aluminium through breathing.27
Diet is another significant contributor to the body burden of aluminium. Measurements of the intake of aluminium in whole diets have varied from about 1 to more than 20 mg per day.28 However, such are often conservative estimates of mean daily intake and they do not always account for compounding factors such as contamination from cooking and cookware29 or specific products with unusually high burdens of aluminium.30 These data certainly do not take account of individual eating patterns where certain products or types of product, for example, fast or convenience foods,31 may constitute a significant proportion of an individual's overall diet. Dietary supplements, such as vitamins, whether ‘natural’ products or otherwise are never included in these estimates of aluminium intake despite being regular components of many people's diets and despite being widely contaminated with aluminium.32 It is worth noting that all dietary intake contributes to the body burden of aluminium until, of course, it is excreted.
Topically applied cosmetics and related skin, hair and hygiene products are often significant sources of aluminium either intentionally when aluminium is added to formulations or unintentionally when aluminium is present as a contaminant.33 Antiperspirants are arguably the most important single contributor to the body burden of aluminium as their use involves applying about 2 g of aluminium to the skin every day.27,34 This aluminium contributes towards the body burden until its residue is washed off the skin surface perhaps up to 24 h later. Similarly, the aluminium content of many sunscreens and sunblocks equates to up to 5 g of aluminium being applied to the skin over just one day on the beach.35 Similar data could be calculated for many other products which are applied to the skin surface such as body creams, tanning lotions and make-up including lip products.36 The regularity with which many of the products are applied to skin and hair must mean that they add substantially to the body burden of aluminium.
Aluminium is both an intentional and an unintentional component of many medicines including prescription and over-the-counter drugs.37,38 Intentional applications include antacid and buffered aspirin, where several grams of aluminium may be ingested on a daily basis, and adjuvant used in vaccination and allergy treatments where up to a milligram of aluminium is injected along with an antigen or allergen.39,40 Included in this category might also be the use of aluminium in prostheses in surgery41 and dentistry.42 Each of these applications of aluminium in medicine has the potential to add substantially to the body burden of aluminium and for the exposure to be over an extended period of time.
The body burden of aluminium
The body burden of aluminium is a dynamic entity and is the outcome of an individual's exposure and excretion in any given period. It is entirely unpredictable and must be estimated through measurements of each individual's aluminium exposome.9 Such is accessible through non-invasive measurements of the excretion of aluminium though such indices are probably only useful as indicators of possible intoxication by aluminium. The assumption being that a higher than ‘normal’ body burden of aluminium would render an individual as being more susceptible to aluminium and, additionally, to subsequent challenges by aluminium. Of course, specific effects of aluminium are often going to be associated with particular target sites, such as the brain, and the body burden per se may not accurately predict such toxicity. The understanding of aluminium's exposome and an individual's body burden of aluminium is a system's biology problem and will require data derived from environmental, in vivo and in silico approaches.43 In the meantime it is worthwhile to briefly examine how the human body is impacted by its everyday exposure to aluminium (Fig. 1).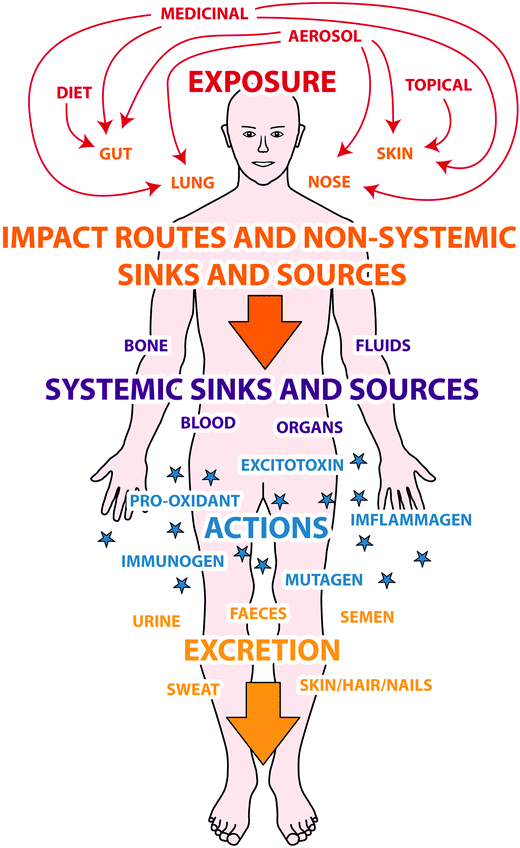 | ||
| Fig. 1 Aluminium's exposome. A schematic which explores relationships between exposure, immediate targets mediating exposure, sinks and sources of biologically available aluminium with putative mechanisms of action and finally excretion of aluminium. | ||
Impact routes and non-systemic sinks and sources
Each of the external surfaces of the body, primarily the skin, nose, lung and gastrointestinal tract, is coincidentally a route of uptake of aluminium into the body and a sink contributing directly to the overall body burden of aluminium.16 Historically these four major impact routes have only been considered as barriers to the absorption and subsequent systemic accumulation of aluminium whereas they are actually significant contributors to the overall body burden of aluminium and, importantly, they are also themselves targets for the biological activity and hence toxicity, of aluminium. Future research must elaborate upon how their myriad functions are influenced by the impact of their direct exposure to different forms of aluminium and how any subsequent changes in physiology manifest themselves in the human phenotype.The skin
The outer epidermis or stratum corneum of the skin is an enucleated layer of keratin-rich cells held within a predominantly lipid intercellular matrix. Transport of topically applied aluminium, such as an antiperspirant or a sunscreen, across this layer would involve passive diffusion by both trans- and paracellular routes (see Fig. 2) and is expected to be minimal.44 However, the intact stratum corneum is punctuated by apoeccrine and eccrine sweat ducts as well as hair follicles and these allow aluminium access to the epidermis, dermis and hypodermis.45 While the evidence to-date is that only a very small proportion of aluminium in topically-applied antiperspirant enters the bloodstream to ultimately be excreted via the kidney this observation does not preclude the persistence of such aluminium within the structures of the skin and neither does it preclude the entry of this aluminium into the lymphatic system. The nature of the aluminium compounds which are present in topical applications, the amount of aluminium which is often applied and the regularity of many such applications must mean that skin is a significant sink for aluminium and a persistent source of biologically available aluminium both locally and systemically (Fig. 3).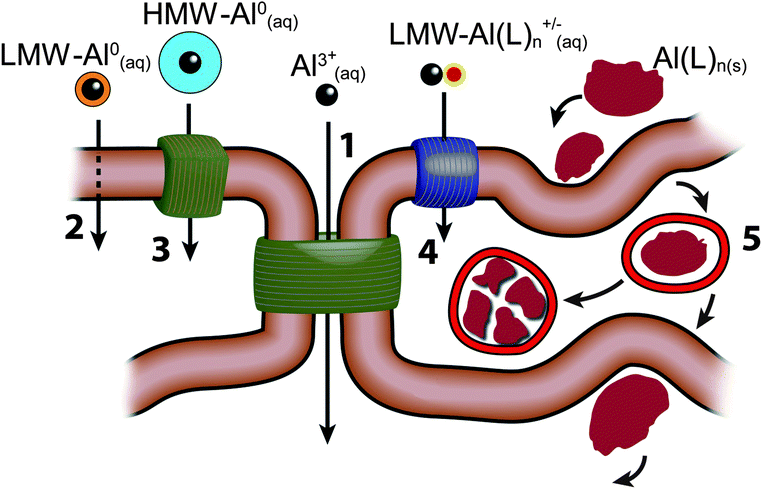 | ||
| Fig. 2 There are 5 major routes by which aluminium could be transported across cell membranes or cell epi-/endothelia; (1) paracellular; (2) transcellular; (3) active transport; (4) channels; (5) adsorptive or receptor-mediated endocytosis. There are 5 major classes of forms of aluminium which could participate in these transport routes. These are shown in the figure as; the free solvated trivalent cation (Al3+(aq)); low molecular weight, neutral, soluble complexes (LMW-Al0(aq)); high molecular weight, neutral, soluble complexes (HMW-Al0(aq)); low molecular weight, charged, soluble complexes (LMW-Al(L)n+/−(aq)); nano and micro-particulates (Al(L)n(s)). | ||
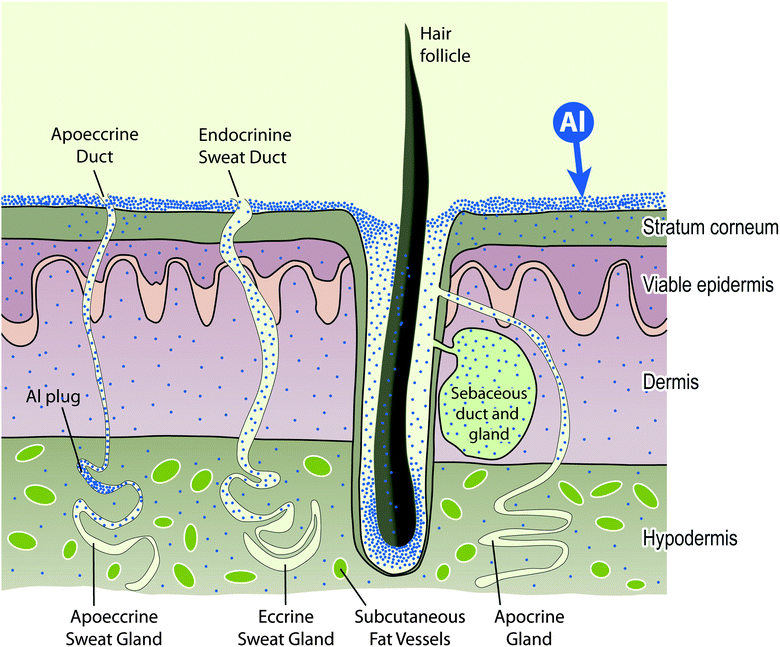 | ||
| Fig. 3 The skin is a sink for topically applied aluminium and will act as a source of biologically reactive aluminium both to structures within the skin and to the systemic circulation. | ||
The nose
When aluminium enters the nasal cavity, for example as an antiperspirant aerosol or as air-borne particulates, it is directed towards either the respiratory epithelium or the olfactory epithelium and neuronal supply to the nasal cavity. Aluminium impacting upon the respiratory epithelium will either dissolve into the mucus layers lining the epithelium or it will be transported, for example as a particulate, by cilia towards the back of the throat by mucociliary clearance. The latter will move aluminium to the gut while aluminium which permeates the mucus layers will remain within the respiratory epithelium and be both a local and systemic source of biologically available aluminium.46 The cilia of the olfactory epithelium are non-motile and aluminium impacting upon this surface will be presented with a large surface area for association with this surface and for dissolution into the mucus layer covering the epithelium. The olfactory epithelium is essentially continuous with the olfactory nerve and olfactory bulb and presents an uptake route for aluminium, as complexes or particulates, into the brain.47The lung
The lung, consisting of both conducting and respiratory airways, presents a considerable surface area for interactions with air-borne aluminium. Both airway and alveolar epithelia are ‘serviced’ by dynamic layers of mucus which may both help in removing aluminium from the lung and offer a substrate for the capture and dissolution of more labile forms of incipient aluminium. The lung epithelia are diverse in respect of their composition of different cell types and, in the alveolar epithelium in particular, myriad transport proteins and channels. The highly dynamic nature of the lung epithelium means that it must be a site for the accumulation of aluminium and a surface for the uptake of aluminium into lung tissues and access to the systemic circulation.48The gut
The gastrointestinal tract is the immediate recipient of ingested aluminium and aluminium which has been removed from the nose and lung by mucociliary clearance. It can be considered as a single layer of cells that presents a physical barrier to irritants and antigenic substances while also providing a surface for essential functions such as absorption and secretion. It is a continuously self-renewing epithelium which contains stem cells for a variety of cell types including mucous cells. The gastrointestinal tract presents a wide spectrum of exposure milieu for aluminium, which are significantly influenced by luminal pH differences, and thereby offers opportunities for its absorption, its retention in tissues or mucosa and its elimination in faeces.49Systemic sinks and sources
The blood is probably the main distribution network for systemic aluminium though this statement is made with the proviso that there are no reliable data on the aluminium content of lymph. There are data showing significant concentrations of aluminium in sweat50,51 and these suggest that the lymphatic system may have a role to play in aluminium transport throughout the body. Aluminium is found in blood associated with both serum and cell fractions.52 While thermodynamics predicts that in serum, aluminium is bound and transported by the iron transport protein transferrin, kinetic constraints suggest otherwise53,54 and implicate low molecular weight ligands such as citrate and phosphate in the distribution of aluminium between the blood and the tissues.43 This is further supported by research which questions whether the transferrin receptor binds the transferrin–aluminium complex.55,56 There are clearly more ways for aluminium to leave the blood than receptor-mediated endocytosis of the transferrin–aluminium complex (see Fig. 2) and these other mechanisms are responsible for driving the subsequent distribution of aluminium between all of the major tissues and organs including the brain. There are surprising few data relating to the aluminium content of human organs57 and only the brain has received significant recent attention.58,59 For a ‘reference’ population (i.e. not knowingly exposed to aluminium) the major organs acting as systemic sinks for aluminium appear to be lung and bone, up to 6 μg g−1 dry wt, followed by liver, kidney and brain, all less than 1 μg g−1 dry wt,57,60 though these averaged data should certainly be taken as equivocal in that there are no human tissues where the distribution of aluminium is expected to be homogeneous.58 Future research in this field should look to measure as many tissue replicates as possible as well as to identify focal deposits of aluminium within the various tissues. Aluminium has been measured in various body fluids including urine,61 cerebrospinal fluid,62 sweat50,51 and seminal fluids63 while data are absent for other important body fluids such as interstitial fluids and lymph. The reality is that today aluminium is omnipresent throughout the body with at least a few atoms of the element in every physical, chemical, biological compartment of the human body.7Actions attributed to aluminium
The toxicity of aluminium will not be reviewed herein. However, mention will be made of the major routes through which biologically available aluminium is known to exert biochemical effect.Pro-oxidant
Despite being described as ‘redox inactive’ aluminium is a potent pro-oxidant and may be exerting this activity through the formation of an aluminium superoxide semi-reduced radical cation, AlO2˙2+.64 The evidence to support both the formation of this complex and its redox activity in vivo is burgeoning65,66 and suggests that its pro-oxidant activity is significant at concentrations of aluminium which are commonly found throughout the body.Excitotoxin
Evidence of excitotoxic damage is common in animal models of aluminium intoxication and aluminium-induced excitotoxicity has been implicated in human neurodegenerative diseases. A common feature of these pathways is an elevated and sustained increase in intracellular free calcium67–69 which is a consistent feature of excitotoxicity in, for example, Alzheimer's disease.70Inflammagen
Human exposure to aluminium has been heavily linked to inflammatory cascades in a wide range of diseases.71–76 The inflammatory activity of aluminium is probably mediated through a similarly wide range of mechanisms including its activity as a pro-oxidant and mediator of myriad pro-inflammatory events and biomarkers.77,78Immunogen
The immunopotency of aluminium has been known for at least 100 years and still today forms the basis for the use of aluminium salts as adjuvants in vaccinations and allergy therapies. What is then surprising is the uncertainty regarding their mechanism of action40 and burgeoning evidence of their toxicity in potentially susceptible individuals.19,79,80Mutagen
Aluminium has been recognised as a mutagen for many years.81 However, specific research on its mutagenicity, carcinogenicity and teratogenicity in humans is extremely scarce with the majority of studies focussing upon effects in cell lines only.82 The situation may be about to change with a recent surge of interest into a potential role for aluminium exposure in breast cancer.83–85Excretion of aluminium
Aluminium is excreted from the body, and hence removed from the body burden, by a number of routes including via the faeces,86 urine,87 sweat,50 skin, hair, nails,87 sebum and semen.63 There are no data to support reliable comparisons between the relative contributions of these different modes of excretion though one can probably surmise that faeces is the major route for non-systemic aluminium and urine for systemic aluminium. Regarding the former, it is commonly cited that absorption of aluminium across the gastrointestinal tract is less than 1% of ingested aluminium16 and it is thus inferred that 99% must be excreted in the faeces. However, these data are not supported by a study which showed that excretion of aluminium in faeces in 8 men over 20 days varied between 74 and 96% of the ingested amount.86 Studies which measure the absorption of aluminium from the gastrointestinal tract are often based upon changes in the aluminium content of serum and sometimes whole blood88 and are liable to under-estimate absorption as they cannot account for the potentially rapid distribution of aluminium from the blood to the tissues. Clearly the majority of aluminium that enters the gastrointestinal tract will be excreted in faeces though whether this proportion is 99% or 90% of the ingested amount remains to be elucidated. There are similar ambiguities surrounding the urinary excretion of aluminium and in particular what might constitute the daily excretion for a healthy individual. For example, data compiled for Reference Man suggest 10–100 μg of aluminium are excreted in urine each day.87 Recent data for individuals presenting at a renal stones clinic suggest a median daily excretion of only 5 μg of aluminium89 while our own results for 20 young and healthy individuals give a mean urinary excretion of aluminium of 27 μg per 24 h.90 While urine has been considered as the major route of excretion of systemic aluminium there are intriguing new data on the aluminium content of sweat which implicate perspiration as an efficient and neglected mechanism of removal of aluminium from the body.50,51 If sweat is a major route for the elimination of systemic aluminium then we may need to consider this in the light of our everyday use of antiperspirants.91 As has been found for other areas of human physiology the data describing the excretion of aluminium from the body is incomplete and to the extent that any attempt to model human exposure to aluminium is likely to be premature.Addressing the body burden of aluminium
While there cannot be a ‘normal’ level of aluminium in the body, at least not in the terms of a requirement for the metal, it is useful to know if an individual is showing signs of an aluminium overload.92 There have been several attempts to define biomarkers for systemic aluminium overload and these have included measurements of total aluminium in blood, urine, hair, nails and sweat.50,87,88,93 These measurements have invariably been equivocal in defining aluminium overload in all but the most extreme examples of exposure to aluminium. Only blood and urine have been tested to any significant degree and both have their limitations. Whole blood is a better indicator than serum as it is less prone to temporal factors associated with the redistribution of aluminium between many potential compartments including the tissues.53 Urine samples are non-invasive and if they can be collected over a 24 h period then as a composite sample they are an accurate representation of an ultra-filterable fraction of aluminium in the blood over this same period.19 We have developed urinary aluminium excretion as an indirect method of comparing individuals' body burden of aluminium.90 Consenting individuals provide 24 h urine samples on 5 consecutive days. They then repeat this over a subsequent 5 day period except during this period they are asked to drink up to 1.5 L of a silicon-rich (>17 mg L−1 as Si) mineralwater each day. We know that silicon-rich mineral waters facilitate the excretion of systemic aluminium via the kidney and over extended time periods can help to eliminate aluminium from the body.18–20 The measurements of urinary aluminium excretion over the first period of 5 days give a preliminary assessment of the body burden of aluminium while the second set of measurements are used to both confirm the preliminary data and provide an indicator of the extent to which the individual might be suffering from aluminium overload. For example, in most individuals it is found that more aluminium is excreted following imbibition of the silicon-rich mineralwater and that the observed increase in aluminium excretion is indicative of the aluminium status of the individual. If the individual then continues to include up to 1.5 L of a silicon-rich mineral in their everyday diet then after, for example, 12 months, when the above measurements are repeated, one would expect to find a lower excretion of aluminium during the first 5 days and little or no change in this value following resumption of drinking of the mineral water. While drinking of a silicon-rich mineralwater may be a useful way to estimate the body burden of aluminium of an individual it may also be beneficial as a way to reverse a current burden or even prevent the establishment of an aluminium body burden with ageing. It is of interest that the therapeutic potential of silicon-rich waters has been muted for well over a century.94,95Conclusions
To fully understand or at least to appreciate human exposure to aluminium and its significance for human health we must take account of two aspects of the natural history of aluminium which when taken together render it unique amongst the elements of life. The first is that to the best of current knowledge it is completely non-essential with no extant organism requiring aluminium to complete its life cycle. The second is that under most exposure conditions aluminium is not overtly toxic in humans and whether it is or it becomes covertly toxic is the rational for examining human exposure to aluminium. It is significant that fish, in which aluminium is acutely toxic,96 are able to both sense and avoid, if possible, extremely low concentrations of aluminium.97 While they are not sensing aluminium per se, only its effects upon their physiology, it is of interest to speculate as to when and if such an avoidance or sensory mechanism might also be activated in humans. If we are to concede that human exposure to aluminium is a largely ‘unconscious’ event then it becomes of paramount importance to recognise the forms it takes and how each contribute to overall exposure?What is a body burden of aluminium? It is convenient to think of it as a balance between exposure and excretion. It must be dynamic as both exposure and excretion are continuously changing albeit usually within quite narrowly defined constraints. How is a body burden of aluminium related to biologically-available aluminium? The latter defines that fraction of the body burden which is, at any one time, a participant in biological events in the body98 and as such is probably a more accurate descriptor of human exposure to aluminium.9 Taking into account the two unique criteria for understanding human exposure to aluminium, briefly it is non-essential and not overtly toxic, we might surmise that all biologically available aluminium is exerting toxicity whereas much of the body burden of aluminium could be benign. The benign component of a burden may be both a source and a sink for biologically available aluminium and so it may be equally important to our understanding of human exposure as the aforementioned and biologically available fraction. Of course, the body burden of aluminium has no known function or role in human physiology and so no content of aluminium within any specific compartment anywhere in the body should be considered as normal. This then translates to the idea that all aluminium is toxic wherever it is biologically available throughout the body. Toxicity infers a negative contribution to a life-affirming process and suggests that there should be a toxic phenotype which results from exposure to biologically available aluminium. Human exposure to aluminium can and will result in toxicity7 though everyday exposure to aluminium is more often manifested as a ‘coping mechanism’ whereby the body must expend energy to deal with the omnipresence of biologically available, or perhaps better, biologically reactive aluminium. Furthering our understanding of how human exposure to aluminium might be manifested as human disease will require the identification of specific biological targets for aluminium. We need to determine if certain physiological systems might be more prone to an ‘attack’ by aluminium than others and to understand the nature of any enhanced vulnerability.
For the majority of metals which are both abundant in the environment and omnipresent in the human body there are recognisable guidelines which relate to how much of the metal constitutes, enough, not enough and too much. This is the problem of aluminium, knowing what might be a safe exposure and recognising what could be an unsafe exposure. While we are unable to make these judgements it would be wise to adopt a precautionary approach and to reduce human exposure to aluminium to a practicable minimum.
Acknowledgements
Andrew Lawrence at KUDIS is thanked for his invaluable help in preparing each of the figures.Notes and references
- C. Exley, J. Inorg. Biochem., 2003, 97, 1–7 CrossRef CAS.
- C. Exley, Trends Biochem. Sci., 2009, 34, 589–593 CrossRef CAS PubMed.
- F. Hachez-Leroy, Eur. Rev. Hist., 2013, 20, 217–236 CrossRef.
- J. M. Cullen and J. M. Allwood, Environ. Sci. Technol., 2013, 47, 3057–3064 CAS.
- N. M. Johnson, C. T. Driscoll, J. S. Eaton, G. E. Likens and W. H. McDowell, Geochim. Cosmochim. Acta, 1981, 45, 1421–1437 CrossRef CAS.
- L. V. Kochian, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1995, 46, 237–260 CrossRef CAS.
- C. Exley, in Molecular and Supramolecular Bioinorganic Chemistry: Applications in Medical Sciences, ed. A. L. R. Merce, J. Felcman and M. A. L. Recio, Nova Sci. Pub. Inc., New York, USA, 2008, pp. 45–68 Search PubMed.
- A. C. Alfrey, G. R. Legendre and W. D. Kaehny, N. Engl. J. Med., 1976, 294, 184–188 CrossRef CAS PubMed.
- C. Exley, Curr. Inorg. Chem., 2012, 2, 3–7 CrossRef CAS.
- V. Fekete, E. Deconinck, F. Bolle and J. Van Loco, Food Addit. Contam., Part A, 2012, 29, 1322–1333 CrossRef CAS PubMed.
- G. A. Meindl and T. L. Ashman, Environ. Pollut., 2013, 177, 78–81 CrossRef CAS PubMed.
- V. Fekete, S. Vandevijvere, F. Bolle and J. Van Loco, Food Chem. Toxicol., 2013, 55, 602–608 CrossRef CAS PubMed.
- N. Arnich, V. Sirot, G. Riviere, J. Jean, L. Noel, T. Guerin and J. C. Leblan, Food Chem. Toxicol., 2012, 50, 2432–2449 CrossRef CAS PubMed.
- European Food Safety Authority, EFSA J., 2011, 9, 2157 Search PubMed.
- U. Shafer and G. Jahreis, Trace Elem. Electrolytes, 2009, 26, 95–99 Search PubMed.
- C. Exley, E. Burgess, J. P. Day, E. H. Jeffery, S. Melethil and R. A. Yokel, J. Toxicol. Environ. Health, 1996, 48, 569–584 CrossRef CAS.
- P. O. Ganrot, Environ. Health Perspect., 1986, 65, 363–441 CAS.
- C. Exley, O. Korchazhkina, D. Job, S. Strekopytov, A. Polwart and P. Crome, J. Alzheimer's Dis., 2006, 10, 17–24 CAS.
- C. Exley, L. Swarbrick, R. Gherardi and F.-J. Authier, Med. Hypotheses, 2009, 72, 135–139 CrossRef CAS PubMed.
- S. Davenward, P. Bentham, J. Wright, P. Crome, D. Job, A. Polwart and C. Exley, J. Alzheimer's Dis., 2013, 33, 423–430 CAS.
- V. V. Goncharuk, V. B. Lapshin, M. A. Chichaeva, M. S. Matveeva, A. O. Samsoni-Todorov, V. V. Taranov and A. V. Syroezhkin, J. Water Chem. Tech., 2012, 34, 1–10 CrossRef.
- S. Polizzi, M. Ferrara, M. Bugiani, D. Barbero and T. Baccolo, J. Inorg. Biochem., 2007, 101, 1339–1343 CrossRef CAS PubMed.
- S. Polizzi, E. Pira, M. Ferrara, M. Bugiani, A. Papaleo, R. Albera and S. Palmi, Neurotoxicology, 2002, 23, 761–774 CrossRef CAS.
- C. Exley, A. Begum, M. P. Woolley and R. N. Bloor, Am. J. Med., 2006, 119, 276e9–276e11 CrossRef PubMed.
- F. Pechansky, F. H. P. Kessler, L. von Diemen, D. B. Bumaguin, H. L. Surratt and J. A. Inciardi, Rev. Bras. Psiquiatr., 2007, 29, 39–42 CrossRef PubMed.
- C. Exley, U. Ahmed, A. Polwart and R. N. Bloor, Addict. Biol., 2007, 12, 197–199 CrossRef CAS PubMed.
- C. Exley, Mol. Med. Today, 1998, 4, 107–109 CrossRef CAS.
- S. M. Bratakos, A. E. Lazou, M. S. Bratakos and E. S. Lazos, Food Addit. Contam., Part B, 2012, 5, 33–44 CrossRef CAS.
- G. Bassioni, F. S. Mohammed, E. Al Zubaidy and I. Kobrsi, Int. J. Electrochem. Sci., 2012, 7, 4498–4509 CAS.
- T. Stahl, H. Taschan and H. Brunn, Environ. Sci. Eur., 2011, 23, 37 CrossRef.
- F. F. Lopez, C. Cabrera, M. L. Lorenzo and M. C. Lopez, Sci. Total Environ., 2002, 3000, 69–79 CrossRef.
- U. Shafer and M. Seifert, Trace Elem. Electrolytes, 2006, 23, 150–161 Search PubMed.
- O. Al-Dayel, J. Hefne, T. Al-Ajyan and A. Al-Drahim, Asian J. Chem., 2011, 23, 3408–3412 CAS.
- A. Pineau, O. Guillard, B. Fauconneau, F. Favreau, M. H. Marty, A. Gaudin, C. M. Vincent, A. Marrauld and J. P. Marty, J. Inorg. Biochem., 2012, 110, 21–26 CrossRef CAS PubMed.
- S. Nicholson and C. Exley, Free Radical Biol. Med., 2007, 43, 1216–1217 CrossRef CAS PubMed.
- S. Liu, S. K. Hammond and A. Rojas-Cheatham, Environ. Health Perspect., 2013, 121, 705–710 CrossRef PubMed.
- C. M. Reinke, J. Breitkreutz and H. Leuenberger, Drug Saf., 2003, 26, 1011–1025 CrossRef CAS PubMed.
- D. Bohrer, D. C. Bertagnolli, S. M. R. de Oliveira, P. C. do Nascimento, L. M. de Carvalho and S. G. Pomblum, Nephrol., Dial., Transplant., 2007, 22, 605–611 CrossRef CAS PubMed.
- J. C. May, J. J. Progar and R. Chin, J. Biol. Stand., 1984, 12, 175–183 CrossRef CAS.
- C. Exley, P. Siesjö and H. Eriksson, Trends Immunol., 2010, 31, 103–109 CrossRef CAS PubMed.
- D. Zaffe, C. Bertoldi and U. Consolo, Biomaterials, 2004, 25, 3837–3844 CrossRef CAS PubMed.
- J. W. Nicholson and B. Czarnecka, J. Biomater. Appl., 2009, 24, 293–308 CrossRef CAS PubMed.
- J. Beardmore and C. Exley, J. Inorg. Biochem., 2009, 103, 205–209 CrossRef CAS PubMed.
- R. Flarend, T. Bin, D. Elmore and S. L. Hem, Food Chem. Toxicol., 2001, 39, 163–168 CrossRef CAS.
- T. Yanagishita, Y. Tamada, Y. Ohshima, K. Ito, Y. Akita and D. Watanabe, J. Dermatol. Sci., 2012, 69–71 CrossRef CAS PubMed.
- D. P. Perl and P. F. Good, Lancet, 1987, 1, 1028 CrossRef CAS.
- K. K. Divine, J. L. Lewis, P. G. Grant and G. Bench, Chem. Res. Toxicol., 1999, 12, 575–581 CrossRef CAS PubMed.
- V. Riihimaki and A. Aitio, Crit. Rev. Toxicol., 2012, 42, 827–853 CrossRef PubMed.
- J. J. Powell, R. Jugdaohsingh and R. P. H. Thompson, Proc. Nutr. Soc., 1999, 58, 147–153 CrossRef CAS.
- S. J. Genuis, D. Birkholz, I. Rodushkin and S. Beesoon, Arch. Environ. Contam. Toxicol., 2011, 61, 344–357 CrossRef CAS PubMed.
- D. Newton and R. J. Talbot, Hum. Exp. Toxicol., 2012, 31, 1195–1198 CAS.
- T. Tamada, Bunseki Kagaku, 2004, 53, 435–440 CrossRef CAS.
- C. Exley, J. Beardmore and G. Rugg, Int. J. Quantum Chem., 2007, 107, 275–278 CrossRef CAS.
- J. Beardmore, G. Rugg and C. Exley, J. Inorg. Biochem., 2007, 101, 1187–1191 CrossRef CAS PubMed.
- M. Hemadi, G. Miquel, P. H. Kahn and J. M. E. Chahine, Biochemistry, 2003, 42, 3120–3130 CrossRef CAS PubMed.
- T. Sakajiri, T. Yamamura, T. Kikuchi, K. Ichimura, T. Sawada and H. Yajima, Biol. Trace Elem. Res., 2010, 136, 279–286 CrossRef CAS PubMed.
- G. Roider and G. Drasch, Trace Elem. Electrolytes, 1999, 16, 77–86 CAS.
- E. House, M. Esiri, G. Forster, P. G. Ince and C. Exley, Metallomics, 2012, 4, 56–65 RSC.
- C. Exley and E. R. House, Monatsh. Chem., 2011, 142, 357–363 CrossRef CAS.
- S. Tang, P. J. Parsons and D. Perl, Biol. Trace Elem. Res., 1999, 68, 267–279 CrossRef CAS PubMed.
- S. Davenward, P. Bentham, J. Wright, P. Crome, D. Job, A. Polwart and C. Exley, J. Alzheimer's Dis., 2013, 33, 423–430 CAS.
- P. M. Roos, O. Vesterberg, T. Syversen, T. P. Flaten and M. Nordberg, Biol. Trace Elem. Res., 2013, 151, 159–170 CrossRef CAS PubMed.
- O. Hovatta, E. R. Venalainen, L. Kuusimaki, J. Heikkila and I. Reima, Hum. Reprod., 1998, 13, 115–119 CrossRef CAS.
- C. Exley, Free Radical Biol. Med., 2004, 36, 380–387 CrossRef CAS PubMed.
- J. I. Mujika, F. Ruiperez, I. Infante, J. M. Ugalde, C. Exley and X. Lopez, J. Phys. Chem. A, 2011, 115, 6717–6723 CrossRef CAS PubMed.
- F. Ruiperez, J. J. Mujika, J. M. Ugalde, C. Exley and X. Lopez, J. Inorg. Biochem., 2012, 117, 118–123 CrossRef CAS PubMed.
- J. B. Sass, L. C. Ang and B. H. J. Juurlink, Brain Res., 1993, 621, 207–214 CrossRef CAS.
- C. Exley, J. Inorg. Biochem., 1999, 76, 133–140 CrossRef CAS.
- C. Exley, Coord. Chem. Rev., 2012, 256, 2142–2146 CrossRef CAS PubMed.
- Z. S. Khachaturian, Ann. N. Y. Acad. Sci., 1994, 747, 1–11 CrossRef CAS.
- A. Campbell and S. C. Bondy, Cell. Mol. Biol., 2000, 46, 721–730 CAS.
- C. H. Guo and C. L. Wang, Clin. Biochem., 2011, 44, 1309–1314 CrossRef CAS PubMed.
- C. H. Guo, P. C. Chen, S. Hsia, G. S. W. Hsu and P. J. Liu, Environ. Toxicol. Pharmacol., 2013, 35, 30–38 CrossRef CAS PubMed.
- D. P. Perl, U. Fogarty, N. Harpaz and D. B. Sachar, Inflammatory Bowel Dis., 2004, 10, 881–883 CrossRef PubMed.
- C. Exley, G. Mamutse, O. Korchazhkina, E. Pye, S. Strekopytov, A. Polwart and C. Hawkins, Mult. Scler., 2006, 12, 533–540 CrossRef CAS PubMed.
- R. K. Gherardi, M. Coquet, P. Cherin, L. Belec, P. Moretto, P. A. Dreyfus, J. F. Pellissier, P. Chariot and F. J. Authier, Brain, 2001, 124, 1821–1831 CrossRef CAS PubMed.
- V. J. Johnson and R. P. Sharma, Neurotoxicology, 2003, 24, 261–268 CrossRef CAS.
- W. J. Lukiw, M. E. Percy and T. P. Kruck, J. Inorg. Biochem., 2005, 99, 1895–1898 CrossRef CAS PubMed.
- L. Tomljenovic and C. A. Shaw, J. Inorg. Biochem., 2011, 105, 1489–1499 CrossRef CAS PubMed.
- Z. Khan, C. Combadière, F. J. Authier, V. Itier, F. Lux, C. Exley, M. Mahrouf-Yorgov, X. Decrouy, P. Moretto, O. Tillement, R. K. Gherardi and J. Cadusseau, BMC Med., 2013, 11, 99 CrossRef CAS PubMed.
- A. Leonard and G. B. Gerber, Mutat. Res., 1988, 196, 247–257 CrossRef CAS.
- A. Banasik, A. Lankoff, A. Piskulak, K. Adamowska, H. Lisowska and A. Wojcik, Environ. Toxicol., 2005, 20, 402–406 CrossRef CAS PubMed.
- P. D. Darbre, J. Inorg. Biochem., 2005, 99, 1912–1919 CrossRef CAS PubMed.
- A. P. Sappino, R. Buser, L. Lesne, S. Gimelli, F. Bena, D. Belin and S. J. Mandriota, J. Appl. Toxicol., 2012, 32, 233–243 CrossRef CAS PubMed.
- P. D. Darbre, F. Manello and C. Exley, J. Inorg. Biochem., 2013 DOI:10.1016/j.jinorgbio.2013.07.005.
- J. L. Greger and M. J. Baier, Food Chem. Toxicol., 1983, 21, 473–477 CrossRef CAS.
- G. V. Iyengar, Radiat. Phys. Chem., 1998, 51, 545–560 CrossRef CAS.
- P. B. Moore, J. P. Day, G. A. Taylor, I. N. Ferrier, L. K. Fifield and J. A. Edwardson, Dementia Geriatr. Cognit. Disord., 2000, 11, 66–69 CrossRef CAS PubMed.
- C. E. Sieniawska, L. C. Jung, R. Olufadi and V. Walker, Ann. Clin. Biochem., 2012, 49, 341–351 CrossRef CAS PubMed.
- S. Davenward, Silicic acid-rich mineral water as a non-invasive method of reducing aluminium body burden in healthy individuals, Alzheimer's and Parkinson's disease, PhD thesis, Keele University, 2013 Search PubMed.
- K. G. McGrath, Med. Hypotheses, 2009, 72, 665–674 CrossRef CAS PubMed.
- G. Crisponi, V. M. Nurchi, V. Bertolasi, M. Remelli and G. Faa, Coord. Chem. Rev., 2012, 256, 89–104 CrossRef CAS PubMed.
- G. Crisponi, V. M. Nurchi, G. Faa and M. Remelli, Monatsh. Chem., 2011, 142, 331–340 CrossRef CAS.
- C. Exley, Coord. Chem. Rev., 2012, 256, 82–88 CrossRef CAS PubMed.
- D. Dobrzyński and C. Exley, Acta Balneologica, 2010, 52, 142–150 Search PubMed.
- C. Exley, J. S. Chappel and J. D. Birchall, J. Theor. Biol., 1991, 151, 417–428 CrossRef CAS.
- C. Exley, Environ. Toxicol. Chem., 2000, 19, 933–939 CrossRef CAS.
- C. Exley and J. D. Birchall, J. Theor. Biol., 1992, 159, 83–98 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
