Themed collection Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules

The ultimate Lewis acid catalyst: using tris(pentafluorophenyl) borane to create bespoke siloxane architectures
Boranes are effective catalysts for bespoke siloxane synthesis via (modified) Piers–Rubinsztajn reactions, enabling complex macromolecular architectures with novel functionality, through careful selection of reaction components and conditions.

Chem. Commun., 2022,58, 7451-7465
https://doi.org/10.1039/D2CC00441K
The modern role of boron as a ‘magic element’ in biomedical science: chemistry perspective
This article discusses in a big-picture manner popular boron chemistries that are a focus in multidisciplinary applications.

Chem. Commun., 2021,57, 13629-13640
https://doi.org/10.1039/D1CC05481C
Substitution at boron in BODIPYs
BODIPYs provide tunable electronic properties key to many applications. This article highlights structural modification at boron.

Chem. Commun., 2022,58, 7351-7359
https://doi.org/10.1039/D2CC02362H
Bringing out the potential of organoboron compounds by designing the chemical bonds and spaces around boron
This feature article highlights our recent study on new organoboron compounds and boron-mediated reactions, developed based on the concept of vacant boron p-orbital engineering in combination with peripheral space design for boron.

Chem. Commun., 2022,58, 4420-4434
https://doi.org/10.1039/D2CC00653G
Multicomponent synthesis of stereogenic-at-boron fluorophores (BOSPYR) from boronic acids, salicylaldehydes, and 2-formylpyrrole hydrazones
A straightforward, one-step MCR yields visible light-absorbing, viscosity-responsive BOSPYR dyes with tunable emissions and inherent chirality at-boron, which open doors for potential applications in biological imaging and chiroptical sensing.

Chem. Commun., 2025,61, 576-579
https://doi.org/10.1039/D4CC03956D
B(C6F5)3-mediated direct intramolecular C7-alkenylation of N-propargylindoles
Metal-free direct C7-alkenylation of N-propargylindoles without directing groups is described. C7-Alkenylation products could further convert into the fused indoles by deprotonation and finally polyaromatic N-heterocycles by hydride abstraction.

Chem. Commun., 2023,59, 10279-10282
https://doi.org/10.1039/D3CC02599C
Nucleophilic strategies to construct –CF2– linkages using borazine-CF2Ar reagents
Using nucleophilic, boron-based –CF2Ar reagents, we demonstrate three methods to form C–CF bonds: (1) nucleophilic aromatic substitution, (2) palladium catalyzed cross-coupling, and (3) nucleophilic substitution.

Chem. Commun., 2022,58, 11705-11708
https://doi.org/10.1039/D2CC01938H
An electron counting formula to explain and to predict hydrogenated and metallated borophenes
An electron counting rule for borophenes based on graphene and MgB2 helps to design metallated borophenes and borophanes, and suggests strategies to release 2D-borophenes from metal surfaces.

Chem. Commun., 2022,58, 9882-9885
https://doi.org/10.1039/D2CC03218J
Towards depeptidized aminoboronic acid derivatives through the use of borylated iminium ions
Herein, we use α-boryl iminium intermediates to access progressively depeptidized branched β-aminoboronic acids. We show the interaction of these compounds with carbohydrates and demonstrate their potential as synthetic building blocks.

Chem. Commun., 2022,58, 5033-5036
https://doi.org/10.1039/D2CC00659F
Azidoborolate anions and azidoborole adducts: isolable forms of an unstable borole azide
An unstable azidoborole derivative was prepared and indirectly identified through trapping in the form of neutral Lewis adducts and, quite surprisingly, in the form of the diazidoborolate complex anion.

Chem. Commun., 2022,58, 4735-4738
https://doi.org/10.1039/D2CC00543C
Electrophilic activation of difunctional aminoboranes: B–N coupling versus intramolecular Cl/Me exchange
Upon electrophilic initiation, difunctional aminoboranes underwent either B–N coupling or an unexpected Cl/Me exchange reaction.

Chem. Commun., 2022,58, 4464-4467
https://doi.org/10.1039/D2CC00976E
Photochromic dithienylethene-containing four-coordinate boron(III) compounds with a spirocyclic scaffold
A series of photochromic four-coordinate spirocyclic boron(III) compounds that display thermally activated upconversion processes from the lower-lying unreactive excited state to the higher-lying photoreactive excited state is reported.

Chem. Commun., 2022,58, 4231-4234
https://doi.org/10.1039/D2CC00107A
Synthesis of bis(2-pyridylthio)methyl zinc hydride and catalytic hydrosilylation and hydroboration of CO2
The zinc hydride compound, [Bptm]ZnH, is a catalyst for hydroboration of CO2 and carbonyl compounds.

Chem. Commun., 2022,58, 4188-4191
https://doi.org/10.1039/D1CC06963B
Potential application of metallacarboranes as an internal reference: an electrochemical comparative study to ferrocene
Metallacarboranes are electrochemical analogs to ferrocene. Here the potential advent of metallacarboranes as internal reference systems is studied in various electrochemical conditions and aqueous and organic solvents with no need for derivatization.

Chem. Commun., 2022,58, 4196-4199
https://doi.org/10.1039/D2CC00424K
Cesium carbonate mediated C–H functionalization of perhalogenated 12-vertex carborane anions
C–H functionalization of undecahalogenated carborane anions, [HCB11X11−] (X = Cl, Br, I), is performed with Cs2CO3 in acetonitrile.

Chem. Commun., 2022,58, 4060-4062
https://doi.org/10.1039/D2CC00173J
o-Carborane-based fluorophores as efficient luminescent systems both as solids and as water-dispersible nanoparticles
A particular fluorene-derivative-based o-carborane π-conjugated system exhibits a strong solid-state luminescence efficiency, while preserving the luminescent properties as nanoparticles homogeneously dispersed in water.

Chem. Commun., 2022,58, 4016-4019
https://doi.org/10.1039/D1CC07211K
New approaches to the functionalization of the 1-carba-closo-decaborate anion
Two new approaches to the functionalization of the 1-carba-closo-decaborate anion resulting in the formation of B–O and B–N bonds were developed.

Chem. Commun., 2022,58, 3775-3778
https://doi.org/10.1039/D1CC06395B
Enantioselective synthesis of cyclic α-aminoboronates via copper-catalyzed dearomative borylation of 4-quinolinols
An enantioselective and regioselective dearomative borylation of 4-quinolinols was developed for synthesis of unprecedented heterocyclic α-amino boronates, a new class of compounds potentially relevant to drug discovery.

Chem. Commun., 2022,58, 3677-3680
https://doi.org/10.1039/D2CC00027J
AuB8−: an Au–borozene complex
Photoelectron spectroscopy and quantum chemistry studies are used to investigate the structure and bonding of AuB8−.

Chem. Commun., 2022,58, 3134-3137
https://doi.org/10.1039/D1CC07303F
Kinetic stabilization allows structural analysis of a benzoborirene
The successful synthesis and first structural characterization by single crystal X-ray crystallography of a highly strained benzoborirene motif, as well as its reactivity towards selected electrophiles and nucleophiles are reported.

Chem. Commun., 2022,58, 2818-2821
https://doi.org/10.1039/D1CC06588B
Utilization of BODIPY-based redox events to manipulate the Lewis acidity of fluorescent boranes
This report describes the incorporation of BODIPY dyes into the ligand framework of a borane to generate a family of molecular boranes that are capable of exhibiting tunable Lewis acidities through BODIPY-based redox events.

Chem. Commun., 2022,58, 2646-2649
https://doi.org/10.1039/D1CC06400B
Nematicidal activity of naphthalimide–boron cluster conjugates
The present work demonstrated the hitherto unknown biological activity of metallacarboranes.

Chem. Commun., 2022,58, 2528-2531
https://doi.org/10.1039/D1CC07075D
Structurally rigidified cobalt bis(dicarbollide) derivatives, a chiral platform for labelling of biomolecules and new materials
A reaction sequence for an easy coupling of the conformationally restrained cobalta bis(dicarbollide)(1−) ion with organic molecules is presented along with electrochemical studies and resolution of enantiomers.

Chem. Commun., 2022,58, 2572-2575
https://doi.org/10.1039/D1CC06979A
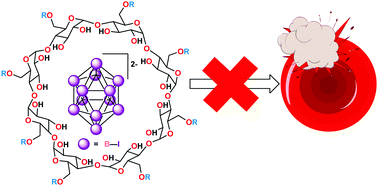
Encapsulation of closo-dodecaiodododecaborate in 2-hydroxypropyl-γ-cyclodextrin prevents hemolysis
Na2B12I12 is a candidate X-ray contrast agent, but produces rapid hemolysis at the concentrations needed for medical imaging. Encapsulation within 2-hydroxypropyl-γ-cyclodextrin effectively protects red blood cells at substoichiometric levels.
Chem. Commun., 2022,58, 2375-2378
https://doi.org/10.1039/D1CC06348K
Binding affinity of aniline-substituted dodecaborates to cyclodextrins
Structure–activity relationships of dodecaborate-substituted nitroanilines as hosts in cyclodextrins have been elucidated.

Chem. Commun., 2022,58, 2363-2366
https://doi.org/10.1039/D1CC06524F
In situ generation of radical initiators using amine-borane complexes for carbohalogenation of alkenes
Atom transfer radical addition of alkyl halides to alkenes was developed using a low amount of a stable initiator, amine borane complexes.

Chem. Commun., 2022,58, 2124-2127
https://doi.org/10.1039/D1CC06390A
Synthesis of isoxazoloazaborines via gold(I)-catalyzed propargyl aza-Claisen rearrangement/borylative cyclization cascade
Isoxazoloazaborines have been synthesized from 4-N-propargylaminoisoxazole via gold(I)-catalyzed propargyl aza-Claisen rearrangement followed by electrophilic borylative cyclization in 27–86% yields.

Chem. Commun., 2022,58, 1942-1945
https://doi.org/10.1039/D1CC07002A
Hydroboration of vinyl halides with mesitylborane: a direct access to (mesityl)(alkyl)haloboranes
The direct access to (mesityl)(alkyl)haloboranes (Mes(Alk)BX) (X = Br, Cl) from mesitylborane dimer and vinyl halides is presented, revealing two accessible pathways, one of which being kinetically favoured.

Chem. Commun., 2022,58, 1589-1592
https://doi.org/10.1039/D1CC06365K
Regiocontrolled synthesis of enantioenriched 2-substituted dehydropiperidines by stereospecific allyl–allyl cross-coupling of a chiral allylic boronate
Regiocontrolled cross-coupling of an optically enriched dehydropiperidinyl boronate with cinnamyl carbonates affords 2-allylated 3,4-dehydropiperidines exclusively with enantiospecificity up to 99%.

Chem. Commun., 2022,58, 1370-1373
https://doi.org/10.1039/D1CC06186K
Refining boron–iodane exchange to access versatile arylation reagents
Aryl(Mes)iodonium salts, which are multifaceted aryl transfer reagents, are synthesized via boron-iodane exchange.

Chem. Commun., 2022,58, 1211-1214
https://doi.org/10.1039/D1CC06341C
Ethyl-, vinyl- and ethynylcyanoborates: room temperature borate ionic liquids with saturated and unsaturated hydrocarbon chains
Salts of the anions [RB(CN)3]− and [RBF(CN)2]− (R = ethyl, vinyl, ethynyl) provide high thermal and electrochemical stabilities. The EMIm-salts are low viscosity room temperature ionic liquids with a wide liquid range.

Chem. Commun., 2022,58, 1223-1226
https://doi.org/10.1039/D1CC06393F
Electrophilic and nucleophilic displacement reactions at the bridgehead borons of tris(pyridyl)borate scorpionate complexes
A ruthenium tris(pyridyl)borate complex is subjected to electrophilic and nucleophilic reactions at the bridgehead borons. These transformations allow for facile tuning of the properties and open up new pathways to functional scorpionate complexes.

Chem. Commun., 2022,58, 977-980
https://doi.org/10.1039/D1CC06181J
Metal-free transfer hydrochlorination of internal C–C triple bonds with a bicyclo[3.1.0]hexane-based surrogate releasing two molecules of hydrogen chloride
A non-protic surrogate that contains two molecules of HCl for the synthesis of alkenyl chlorides from internal alkynes is reported. The HCl transfer is catalyzed by B(C6F5)3 and driven by release of strain and aromatization.
![Graphical abstract: Metal-free transfer hydrochlorination of internal C–C triple bonds with a bicyclo[3.1.0]hexane-based surrogate releasing two molecules of hydrogen chloride](/En/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC06591B&imageInfo.ImageIdentifier.Year=2022)
Chem. Commun., 2022,58, 973-976
https://doi.org/10.1039/D1CC06591B
Amine-boranes reactions promoted by lanthanide(II) ions
This study shows for the first time the catalytic activity in amine-borane dehydrogenation for lanthanide(II) species and unveils the step-by-step transformations of the starting complexes into active catalytic intermediates.

Chem. Commun., 2022,58, 859-862
https://doi.org/10.1039/D1CC06401K
Unlocking metal coordination of diborylamides through ring constraints
A new cyclic diborylamide ligand has been developed and successfully employed to synthesize low-coordinate iron(II) complexes.

Chem. Commun., 2022,58, 867-870
https://doi.org/10.1039/D1CC06458D
[closo-B10H8-1,10-(COOH)2]2−: a building block for functional materials?
A convenient synthesis of [closo-B10H8-1,10-(COOH)2]2− leads to a new class of stable diaminocarbonium ylides.
![Graphical abstract: [closo-B10H8-1,10-(COOH)2]2−: a building block for functional materials?](/En/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC06485A&imageInfo.ImageIdentifier.Year=2022)
Chem. Commun., 2022,58, 851-854
https://doi.org/10.1039/D1CC06485A
Derivatization of an especially electron-rich diborane
Novel symmetric and unsymmetric diboranes with sp3-hybridized boron atoms and especially electron-donating bridging guanidinate substituents are synthesized and their properties analysed. Moreover, dismutation equilibria are disclosed.

Chem. Commun., 2022,58, 693-696
https://doi.org/10.1039/D1CC06359F
Rhodium-catalyzed sequential B(3)-, B(4)-, and B(5)-trifunctionalization of o-carboranes with three different substituents
A Rh-catalyzed one-pot trifunctionalization of o-carboranes with three different substituents via sequential B(3)–, B(4)–, and B(5)–H activation has been developed.

Chem. Commun., 2022,58, 629-632
https://doi.org/10.1039/D1CC05936J
Pd-Catalyzed coupling of benzyl bromides with BMIDA-substituted N-tosylhydrazones: synthesis of trans-alkenyl MIDA boronates
A series of trans-alkenyl MIDA boronates have been prepared by palladium-catalyzed cross-coupling reaction of N-methyliminodiacetyl boronate (BMIDA)-substituted N-tosylhydrazone and benzyl bromides.

Chem. Commun., 2022,58, 399-402
https://doi.org/10.1039/D1CC06170D
Cu-mediated vs. Cu-free selective borylation of aryl alkyl sulfones
Borylation of cyclic aryl–alkyl sulfones gives boronate esters by selective cleavage of C(sp2)–SO2 or C(sp3)–SO2 bonds via Cu-mediated or Cu-free processes.

Chem. Commun., 2022,58, 395-398
https://doi.org/10.1039/D1CC06144E
Intramolecular rotations and electronic states of iron in the iron bis(dicarbollide) complex Fe[(C2B9H11)2] studied by a 57Fe nuclear probe and computational methods
The rotation of iron bis(dicarbollide) complexes was characterized by Mössbauer spectroscopy and computational methods. Unexpected FeII-like character in the 8-dioxane iron bis(dicarbollide) adduct was discovered.
![Graphical abstract: Intramolecular rotations and electronic states of iron in the iron bis(dicarbollide) complex Fe[(C2B9H11)2] studied by a 57Fe nuclear probe and computational methods](/En/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC05111C&imageInfo.ImageIdentifier.Year=2022)
Chem. Commun., 2022,58, 391-394
https://doi.org/10.1039/D1CC05111C
Borole-based half-sandwich complexes of germanium and tin
The synthesis and initial observations regarding the reactivity of borole-based half-sandwich complexes with apical divalent group 14 elements germanium and tin are described.

Chem. Commun., 2022,58, 246-249
https://doi.org/10.1039/D1CC06227A
Nucleophilic borylation of fluorobenzenes with reduced arylboranes
Two challenging but rewarding topics in chemical synthesis are C–F-bond activation and development of B-centered nucleophiles. We have now tackled both subjects by forming aryl–B bonds via SNAr-type reactions on fluorobenzenes under mild conditions.

Chem. Commun., 2022,58, 254-257
https://doi.org/10.1039/D1CC06225E
C,C′-Ru to C,B′-Ru isomerisation in bis(phosphine)Ru complexes of [1,1′-bis(ortho-carborane)]
The transformation of C,C′-bound bis(ortho-carborane) to C,B′-bound is reported for the first time.
![Graphical abstract: C,C′-Ru to C,B′-Ru isomerisation in bis(phosphine)Ru complexes of [1,1′-bis(ortho-carborane)]](/En/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC06119D&imageInfo.ImageIdentifier.Year=2022)
Chem. Commun., 2022,58, 64-67
https://doi.org/10.1039/D1CC06119D
Competitive gold/nickel transmetalation
Transmetalation is a key method for the construction of element–element bonds.

Chem. Commun., 2022,58, 68-71
https://doi.org/10.1039/D1CC06064C
[Mes-B-TMP]+ borinium cation initiated cyanosilylation and catalysed hydrosilylation of ketones and aldehydes
Aryl amino borinium cation can serve as the catalyst for hydrosilylation of ketones and aldehydes.
![Graphical abstract: [Mes-B-TMP]+ borinium cation initiated cyanosilylation and catalysed hydrosilylation of ketones and aldehydes](/En/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC06319G&imageInfo.ImageIdentifier.Year=2021)
Chem. Commun., 2021,57, 13732-13735
https://doi.org/10.1039/D1CC06319G
Harnessing the electronic differences between CAAC-stabilised 1,4-diborabenzene and 9,10-diboraanthracene for synthesis
The oxidation of cyclic alkyl(amino)carbene-stabilised closed-shell 1,4-diborabenzene and the related open-shell singlet biradical 9,10-diboraanthracene with elemental chalcogens yields bicyclic compounds with different lengths of chalcogen bridges.

Chem. Commun., 2021,57, 13526-13529
https://doi.org/10.1039/D1CC05173C
Transborylation of alkenylboranes with diboranes
Exchange of boryl moieties is possible between alkenylboranes and diboron reagents as a stereospecific cross-metathesis with formation of mixed diboron reagents. DFT calculations propose a mechanism with both symmetric and non-symmetric diboron reagents.

Chem. Commun., 2021,57, 13361-13364
https://doi.org/10.1039/D1CC05815K
KB3H8: an environment-friendly reagent for the selective reduction of aldehydes and ketones to alcohols
Selective reduction of aldehydes and ketones to alcohols using KB3H8 was achieved in water and THF.

Chem. Commun., 2021,57, 12776-12779
https://doi.org/10.1039/D1CC05638G
Carbon monoxide bond cleavage mediated by an intramolecular frustrated Lewis pair: access to new B/N heterocycles via selective incorporation of single carbon atoms
The interaction of carbon monoxide with an intramolecular FLP and a strong electrophile leads to rapid cleavage of the CO triple bond and enables the formation of new B/N heterocycles via selective incorporation of single carbon atoms.

Chem. Commun., 2021,57, 12528-12531
https://doi.org/10.1039/D1CC05673E
Lewis acid activation of Weiss’ reagents ([PhI(Pyr)2]2+) with boranes and isolation of [PhI(4-DMAP)]2+
I(III) reagent [PhI(Pyr)2]2+ (Weiss’ reagent) can be activated with borane Lewis acids in which a pyridine ligand is abstracted giving a more reactive derivative of the reagents.
![Graphical abstract: Lewis acid activation of Weiss’ reagents ([PhI(Pyr)2]2+) with boranes and isolation of [PhI(4-DMAP)]2+](/En/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC04725F&imageInfo.ImageIdentifier.Year=2021)
Chem. Commun., 2021,57, 12163-12166
https://doi.org/10.1039/D1CC04725F
Access to adducts of parent iminoborane isomers, HBNH and NBH2, using frustrated Lewis pair chelation
A general frustrated Lewis pair chelation approach was used to access the parent iminoborane isomers HBNH and NBH2.

Chem. Commun., 2021,57, 10895-10898
https://doi.org/10.1039/D1CC04923B
Carboxyboranylamino ethanol: unprecedented discovery of boron agents for neutron capture therapy in cancer treatment
Carboxyboranylamino ethanol is cell membrane permeable and has low cytotoxicity. It shows high molecular docking scores and improved antitumor efficacy in vitro.

Chem. Commun., 2021,57, 10174-10177
https://doi.org/10.1039/D1CC03034E
Thiophene-fused boracycles as photoactive analogues of diboraanthracenes
Photoactive thiophene-fused boracycle analogues of diboraanthracenes.

Chem. Commun., 2021,57, 10170-10173
https://doi.org/10.1039/D1CC03323A
Isolation and reactivity of a gold(I) hydroxytrifluoroborate complex stabilized by anion-π+ interactions
A new ambiphilic phosphine-xanthylium ligand is described, along with its ability to stabilize a catalytically competent Au–μ(OH)–BF3 complex via anion-π+ interactions.

Chem. Commun., 2021,57, 10154-10157
https://doi.org/10.1039/D1CC04105C
A strongly Lewis-acidic and fluorescent borenium cation supported by a tridentate formazanate ligand
A fluorescent, strongly Lewis-acidic borenium cation (Gutmann–Beckett acceptor number >100) was created using a tridentate formazanate ligand. This cation shows a potential utility as a colourimetric reactivity probe.

Chem. Commun., 2021,57, 9530-9533
https://doi.org/10.1039/D1CC03873G
A boron–nitrogen transborylation enabled, borane-catalysed reductive cyanation of enones
The borane-catalysed hydrocyanation of enones has been developed and applied across a series of substrates including those being functional groups susceptible to reduction.

Chem. Commun., 2021,57, 9406-9409
https://doi.org/10.1039/D1CC03649A
Carborane photochromism: a fatigue resistant carborane switch
A dithienylethene molecule involving carborane clusters shows remarkable fatigue resistance and high contrast visual colour changes when irradiated with alternating ultraviolet and visible light.

Chem. Commun., 2021,57, 9466-9469
https://doi.org/10.1039/D1CC03248H
Photoredox B–H functionalization to selective B–N(sp3) coupling of nido-carborane with primary and secondary amines
B–N(sp3) coupling of nido-carborane with organic amines by photoredox catalysis leads to nitrogen-containing nido-carboranes in moderate to good yields.

Chem. Commun., 2021,57, 8580-8583
https://doi.org/10.1039/D1CC03326C
B(C6F5)3-Catalyzed site-selective N1-alkylation of benzotriazoles with diazoalkanes
The site-selective N1-alkylation of benzotriazoles with diazoalkanes is achieved using 10 mol% of B(C6F5)3 as a catalyst.

Chem. Commun., 2021,57, 7758-7761
https://doi.org/10.1039/D1CC03048E
Facile proton-coupled electron transfer enabled by coordination-induced E–H bond weakening to boron
Coordination induced bond weakening (CIBW) leads to facile PCET at various E–H bonds.

Chem. Commun., 2021,57, 6903-6906
https://doi.org/10.1039/D1CC02832D
About this collection
This special collection, Guest Edited by Robert J. Gilliard (University of Virginia), Rosario Núñez (Institut de Ciència de Materials de Barcelona) and Caleb Martin (Baylor University), covers all areas of boron chemistry with an emphasis on synthesis and reactivity. This includes small molecules, boron clusters, frustrated Lewis pairs, borylation, catalysis, and ligand development.